Research Article - (2023) Volume 14, Issue 4
Abstract
Three dimensional bioprinting has been the cutting edge of tissue engineering research that has shown the tremendous potential of fabricating customized desired 3D tissue model only of its appropriate size and dimension. The combination of different nanomaterials with dimensional scales and the 3D bioprinting technologies enabled the manufacturing of various transplantable tissue scaffolds such as multilayered skin, bone, vascular grafts, cardiac tissues, tracheal splints, skull bone and cartilaginous structures with unique, physical, chemical and biological properties similar to the native structure for the treatment of osteosarcoma, cancer bone metastasis, breast cancer and inflammatory diseases as well as tissue regeneration. The exploration of the implications of nanoparticles in engineering bone scaffolds have just commenced and have revealed tremendous growth in the clinical efficacy of 3D bioprinted tissue scaffolds. In this review, we have discussed briefly the integration of nanotechnology and nanobiomaterials with 3D bioprinted tissue engineered scaffolds for engineering tissue and organ implants and the mechanism of 3D printing nanotechnology in addition of various 3D bioprinting strategies.
Keywords
3D bioprinting, Stem cells, Tissue scaffolds, Bone cancer metastasis, Bone regeneration, Therapeutic implants, Hydrogels
Introduction
3-D bioprinting is an innovative developmental technology that focuses on the construction of cellular patterns within a reserved space in which the cellular viability and capability are conserved within the printed gibbet. It is one of the main rapid prototyping technologies of the current research that can fabricate any desired 3D structure via building up material layer by layer with the aid of a digital design. The term 3D Printing (3DP) was first proposed by Professor Sachs from the Massachusetts Institute of Technology (MIT) which is conceptually defined as a process of creating digital designs that facilitates the manufacturing of a broad range of object geometries using biocompatible materials, cells, supporting components i.e., anything that is accessible as a spreadable powder including ceramic metal, metal-ceramic composite and polymeric materials (Campbell TA, Ivanova OS, 2013; Bergmann C, et al., 2010). Therefore, we can say that different materials with varying dimensional scales can be converted into complex 3D functional living tissues using 3D printing strategy. The design reliability, quality, specificity and skill of developing any customized structure design makes this technique of bioprinting to be applicable to regenerative medicine, tissue engineering and tumor therapy to deal with the necessity of clinically translatable tissues and organs for improving survival of the mankind. The bioprinting technology is linked with nanotechnology as the components of the cells are on the nanoscale 100-1000 μm and these cell-biomaterials interactions are microscale events. The 3D bioprinting technology uses nanomaterials such as gold, SiO2 gold nanoshells, water soluble polymers, hydrogels, starch based powders and fibrins for creating various medical structures such as neuron-adhesive patterns, collagen scaffolds, synthetic biodegradable scaffolds and fibrin channels that potentially alleviate the inflammatory response by targeting and treating the problem (Danilevicius P, et al., 2013; Sachs EM, et al., 1989). These 3-D bioprinted products provide high throughput for cell programming, drug testing and toxicology. In 2017, a novel method was designed to fabricate a 3D biomimetic neural scaffold having porous implanted united fibers. The two biofabrication softwares were employed using natural biomaterial Polycaprolactone (PCL) and synthetic biomaterial polycaprolactone mixed with gelatin which provided enhanced neural stem cells adhesion and proliferation. The resultant microfiber scaffold effectively inhibited apoptosis with enhanced biocompatibility and mechanical properties with greater neural length and positioned neural extension of the parent cortical neuron fibers (Utela B, et al., 2008). Other major applications of these 3D bioprinted nanoscale structures include the fabrication of nanofilters, nanorobots and nanobiosensors for a variety of biomedical applications. The application of 3D bioprinting for the treatment of cancer by fabricating in vitro tumor models has been a potential topic of the current research. 3D printed hydrogels and implants loaded with cytostatic drugs are revolutionizing tumor therapies (Melchels FP, et al., 2010). Taking in view the above mentioned applications of 3D bioprinting technology and its interaction with nanotechnology, the present article focuses on 3D bioprinted scaffolds and personalized medicine composed of nanoscale biomaterials as bio-inks applicable for tissue engineering and cancer therapies with increased efficacy and alleviated toxicity derived from a broad biodistribution of the body.
Technique of nanobiomaterial fabrication using 3D bioprinting
The 3D printing nanotechnology is a flexible tool of creating customized 3D tissues and organs engaged for diverse applications in the field of alternative medicine, tumor therapy and tissue engineering. This technique possesses the magical potential to create functional replacement organs and tissue for damaged organs and tissues in patients. In addition, rapid fabrication of specific size and shape of human based tissue models and organoids for true diagnosis, replacement therapies, pathology, drug modeling and development makes this technique a gold standard in the future (Yoo D, 2012). The product development using 3D bioprinting involves pre-bioprinting, bio-printing and post bioprinting. In pre-bioprinting, a model specific for printing using Computed Tomography (CT) and Magnetic Resonance Imaging (MRI) is fabricated. However, the second step of bioprinting involves the usage of bioink (mixture of cells, bioactive nanomolecules and biomaterial) which is then printed in a layer by layer design in a printer cartridge to compose a 3D structure to be used in the replacement of original cells (Guillemot F, et al., 2010; Li X, et al., 2012). Some modifications are made after three dimensional printing of nanobiomaterials which is known as post-bioprinting process. This process includes the remodeling of tissue via chemical and mechanical signals which resultantly forms a well demarcated 3D tissue scaffold. By using this process of designing, high precision and customized fabrication of engineered tissue scaffolds with retained cell patterns and viability of the printed 3D printed structures can be attained (Li X, et al., 2006). In this regard, the study of 3D bioinks is of remarkable value which is the printable forms of biomaterials combined with growth cells and bioactive materials that are able to be printed into 3D form at room temperature irrespectable of cell reliability and authentic mechanical properties. Diverse 3D printing methods have been developed currently which include Stereolithography (SLA), Digital Light Processing (DLP), Selective Laser Sintering (SLS), Fused Filament Fabrication (FFF) and inkjet printing. The most excessively used technique is Inkjet Microextrusion Laser Assisted Printing Technology (IMLPT) which incorporates biomolecules, cells and drugs of the interest with hydrogel pre-polymer solution which is laser printed (Koutsopoulos S, 2012). Some important factors must be taken in consideration while employing 3D bioprinting nanotechnology such as controlled volumes of liquids, surface tension, cellular resolution and viability and nature of biomolecule. A balanced combinatorial effect of these important factors can result in functional tissue engineered nanobiomaterial assisted 3D bioprinted tissues and organs. For example, an observation was made when low molecular weight alginate was used in combination with high molecular weight alginate in ratio of 1:2 that consequently gave improved cell integrity, structure viability and processed growth pattern in comparison with using isolated low molecular weight alginate and high molecular weight alginate. Hence, it is of worth consideration to work upon the viability and processability of printable biomaterials and the technique of 3D bioprinting for achieving successful 3D bioprinted scaffolds and tissue patterns with cell based bioinks (Chen KI, et al., 2011; Lautenschläger F and Piel M, 2013).
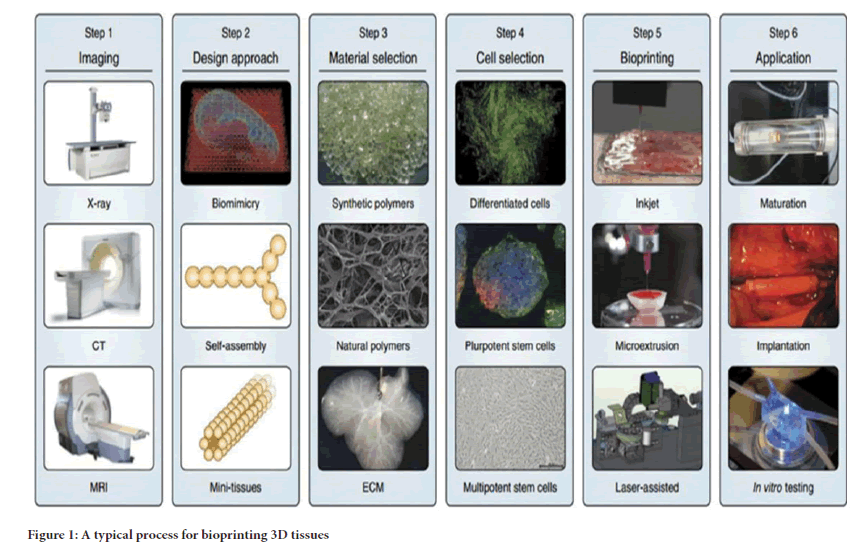
The 3D workflow is demonstrated in three distinct steps. The first step is the uplift of powder supply platform system and lowering one layer down of the fabrication platform. The second step is the aligning and thinning of the polymer powder layer through roller and the third step involves the printing of a liquid binder that binds the adjacent powder together which depends upon the binder droplet-powder particle interactions influenced by powder material, powder size, shape, packing density, binder viscosity, binder surface tension, powder surface treatment and in the same way droplet size, velocity, temperature of the powder and binder are some of the main factors involved to gain the accuracy in the geometry of the new bioprinted object. Repeating steps 1 and 3 side by side ultimately produces a successful 3D product with accurate geometry (Figure 1) (Li X, et al., 2011).
Figure 1: A typical process for bioprinting 3D tissues
The three dimensional bioprinting of nanobiomaterials is typically of two types. One is called as “Inkjet Printing” for which typical printers such as NP 2.1 (GeSim, Germany) and the Z402 (Zcorp, USA). However, the second one is known as the “Nanoimprint Lithography” with the typical printers such as the EVG620 nanoimprinter and the 520 hot embosser (EV Group, Austria) (Shin H, 2007).
Literature Review
Bonding based inkjet printing
The technology of bonding based inkjet printing is typically based upon joining the micro particles of a binder biomaterial by depositing them on the surface of a powder bed through inkjet printer. A hydraulic piston puts pressure on the powder bed to create a new layer of the powder on the surface of the previously formed layer which resultantly gives a fine joined layer on the position of the object formation (Caldorera-Moore M, Peppas NA, 2009; Li X, et al., 2008). After the completion of this step, the unbounded powder is removed and the newly designed model is trampled further by orthodox pre-sintering process. Depending upon the distinct operational modes, the inkjet printing technology is further classified as Drop-On-Demand (DOD) catalog which is based upon the principle that every single droplet is ejected with the help of electrical signals and the continuous ejection of the drop catalog is functional with the wave of electrical impulses (Liu X, et al., 2010). The composition of DOD catalog is associated with the combination of piezoactuated and electrostatic actuated (electromechanical), the thermal actuated (electrothermal) and the electrostatic vacuum. The uninterrupted ejection catalogue is processed by electrofield-controlled inkjet and Hertz-continuous inkjet also known as mutual charged droplet repulsion type. The bonding based inkjet printing technology has diverse range of applications in fabricating many tissue scaffolds with nanobiomaterials. Three dimensional porous scaffolds were prepared using Polylactide-Coglycolide (PLGA) powder assorted with salt particles and suitable organic solvent (Li X, et al., 2010). The 3D hepatocytes were successfully fabricated with improved cell viability allowing patterning of cellular arrays with specified genetic modifications (Kim HN, et al., 2013). Moreover, the 3D microvasculature was fabricated through thermal inkjet printer using human microvascular endothelial cell suspension in thrombin solutions and printed on fibrinogen solutions which worked as a substrate. The newly designed 3D printed microvascular cells possess the property of proliferation within their fibrin channels allowing to form a tubular lining fibrin channels allowing to form a tubular lining (Liu K, et al., 2013).
Bioink-jet printing
The concept of cellular array printing was developed by Yan et al., which aids in the printing of single cells, cellular aggregates and gels to propose the possible organ printing to address the biomedical complications. For this purpose, the bioinks use thermal and piezotip print heads and ink cartridges for printing and patterning cellular arrays in a spatial three dimensional pattern. This bioink technology typically employs thermoreversible polymers, aqueous media or hydrogel precursors combined with living cells. The laser-assisted cell printing technology is another innovative laser based cell printing technique composed of Laser Guidance Direct Write (LGDW). This LGDW technology process the printing of embryonic chick spinal cord cells pattern on a viable glass slide. The newly fabricated neural spinal cells greatly facilitates the proliferation. Some other laser based techniques were also employed such as Laser-Induced Forward Transfer (LIFT) technique and modified inkjet printing to print three dimensional viable brain cells and ocular cells. The recently developed technique based on combination of laser electricity and water is Electro Hydrodynamic Jetting (EHDJ) method which provided successful printing of genetic proteins to compete with gene therapy which corrects the gene sequence in a spatial manner (Li X, et al., 2008; Owens III DE, et al., 2007).
Nanoimprint lithography
The nanoimprint lithography is no doubt a rapid and cost effective technique of designing 3D fabricated nanostructures using biomaterials. The mechanism of Nanoimprint Lithography (NIL) involves the repeated mounding of multiple layers of nanostructures on each other is such a way that a multilayered spacer structure is formed which is linearized via chemical and mechanical polishing so that a confined plane structure can be obtained with efficient viability and sustainability. This technique is novel which is currently in process to attain a stacked multilayered structure by transferring biomaterial several times on the same substrate to compose a 3D printed nanobiomaterial in an efficient and cost effective manner. For example, a 3D gold structure nanobiomaterial was fabricated using nanoimprint lithography technique which is known for its diverse pharmaceutical importance in nanodrugs (Tanaka M, et al., 2012).
Combinatorial impact of 3D bioprinting with nanotechnology
The combination of 3D bioprinting with nanomaterials is the focus of the current interest of the scientists. This combination provides the most sustainable cell material interactions and enhanced tissue scaffolds for medical purposes. A biological cell in the human body is present in the form of multifaceted mixture of pores, ridges and various micro and nano-featured Extracellular Cell Matrix Environment (ECME) which allows efficient cellular crosstalk and molecular signaling upon implantation of the engineered graft. Bikram M, et al., 2007 stimulated osteogenesis of fabricated hBMSCs in Polyethylene Glycol (PEG) Dimethyl Acrylate (PEGDA) scaffold by using bioactive ceramic nanoparticles. In the next step, the hBMSCs which were adjourned with PEGDA were printed on a Bioactive Glass Nanoparticles (BGN) and Hydroxyapatite nanoparticles (HA) under the condition of continuous polymerization reaction which resultantly produced accurate placement of the printed substrates in 3D dimension. The biochemical analysis and cellular interactions depicted high alkaline phosphatase activity, greatest cell viability (86.63 ± 6.02% and highest compressive modulus of 358.91 ± 48.05 kPa after 21 days of culturing in PEGDA- HA group in comparison with PEGDA-BGN group. Furthermore, these results were confirmed with gene analysis through quantitative Polymerase Chain Reaction (PCR) method. Masson trichome staining also confirmed the maximum deposition of collagen in PEGDA-HA group scaffold pattern. These results depicted that the 3D fabricated bioprinted tissue engineered scaffold in combinatorial with nanomaterials provide significant mimicking of the native parent tissue pattern. This technology also promotes the regeneration of the functional tissue in the patient and tissue biomaterial interactions in 3D controlled pattern (Landers R, Mülhaupt R, 2000). Nomura H, et al., 2006 generated nanocluster sized 3D cell-laden hydrogel array using bioprinting-nanomaterial based approach within the Extracellular Matrix (ECM) environment. Two gels gelatin methacrylate (GeIMA and Polyethylene Glycol (PEG) dimethacrylate were used in the ratio of 1:3 to develop a confined pattern. This cell-laden GeIMA/ PEG composition was employed for the screening of Human Periodontal Ligament Stem Cells (PDLSCs) response to the extracellular matrix environment. The cellular viability and proliferation of human PDLSCs in GeIMA/PEG array was found to be significantly upregulated and the surface area decreased by increasing the percentage of PEG (Nomura H, et al., 2006). All these experimental evidences clearly prove that nanomaterial loaded 3D printed scaffolds potentially increase the regeneration of the damaged tissues and organs and path an innovative field towards tissue engineering and regenerative medicine.
Discussion
Integration of three dimensional bioprinting of nanomaterial scaffolds for complex tissue and organ engineering
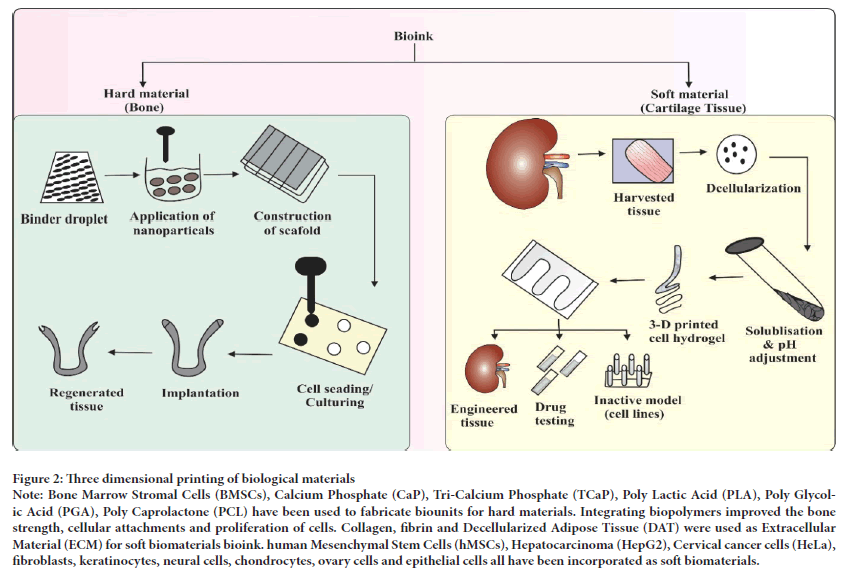
Three dimensional bioprinted bone: The engineering of hard tissues such as bone is somewhat a complex process as it requires a high mechanical strength and porosity which is difficult to attain with traditional techniques. The commonly employed cell lines used for this purpose are bone marrow mesenchymal cell lines which are also in use by traditional techniques. The presently used fast prototyping modality i.e., Fused Deposition Modeling (FDM) carries out bone tissue engineering via employing hard thermoplastic polymers with the property of relatively increased mechanical strength. A biodegradable 3D polymer ceramic scaffold was fabricated using FDM (Lam CX, et al., 2002). Since, FDM is a rapid prototyping technique, it implants the human mesenchymal stem cells joined with fibrin glue into the desired scaffold and left in incubator for culturing for one and a half month. The final biomimetic scaffold pattern fabricated showed fine attachment, migration and osteogenic (bone tissue) differentiation (Xu T, et al., 2006). The human extracellular bone matrix is a nanocomposition of protein based soft hydrogel matrix of collagen, osteopontin, inorganic molecules of calcium phosphate hydroxyhyaluronic acid (nHA (Ca10 (PO4)6(OH)2 and water which is 70% of the bone matrix. The biomimetic nanobiomaterials such as calcium phosphate hydroxyhyaluronic acid, tricalcium phosphate and calcium polyphosphate are frequently used in 3D bioprinting due to their enhanced cytoconductivity, osteocompatibility and bioactive features. Among them, calcium phosphate hydroxyhyaluronic acid is at the forefront of the current research as it is a table top bone biomaterial which is used in a UV laser bioprinting technology to combine a biocompatible Polyethylene Glycol Diacrylate (PEGDA) hydrogel with Hydroxyapatite nanoparticles to attain a customized 3D hydrogel scaffold with increasing diameter of 15 mm and 400 μm thick bioscaffold design (Sanjana NE and Fuller SB, 2004; Li X, et al., 2014). The Figure 2 illustrates the biofabrication of the bone scaffolds using biomaterials. The utilization of TCP biomaterial has also been in observation for 3D bioprinting which is used in 3D sintering bioprinting technique in the form of powder and applies microwaves as heat source which confirms fabrication in the scaf fold. This 3D sintering TCP technology is able to give more microporosity and macroporosity ratio in addition with the formation of new bone in vivo as compared to the conventional source of fabrication utilizing energy source (Yeong WY, et al., 2007). The human skull and bone 3D bioprinted into an actual replica and the skull measured by CT scan. When fabricating a 3D bioprinted human skull, the human skull is first scanned with helical CT to obtain an actual image of the skull and then the size of the skull is set according to the printer nozzle size and maximum volume is attained with the help of inkjet cartilage and printed in layer-by-layer fashion. The skull cells are then interconnected by themselves and co-cultured into a scaffold for subsequent bone tissue formation (Fielding GA, et al., 2012).
Figure 2: Three dimensional printing of biological materials
Note: Bone Marrow Stromal Cells (BMSCs), Calcium Phosphate (CaP), Tri-Calcium Phosphate (TCaP), Poly Lactic Acid (PLA), Poly Glycolic Acid (PGA), Poly Caprolactone (PCL) have been used to fabricate biounits for hard materials. Integrating biopolymers improved the bone strength, cellular attachments and proliferation of cells. Collagen, fibrin and Decellularized Adipose Tissue (DAT) were used as Extracellular Material (ECM) for soft biomaterials bioink. human Mesenchymal Stem Cells (hMSCs), Hepatocarcinoma (HepG2), Cervical cancer cells (HeLa), fibroblasts, keratinocytes, neural cells, chondrocytes, ovary cells and epithelial cells all have been incorporated as soft biomaterials.
Currently, an “Oxford Performance Materials” named company created an alternative substitute for craniofacial bone via SLS to address the bone defect problems by using Polyether Keytone-Ketone Proprietary (OPEKK- IG) biomimetic polymer. The observations proved that OPEKK-IG is mechanically stable, osteoconductive, chemically strong and an enhanced texture in addition to cellular propagation without exerting metabolic stress on the cells. OPEKK-IG is the first clinically approved 3D printed polymer by FDA implanted for the first time in human in March 2013 (Boland T, et al., 2007).
Three dimensional bioprinted cartilage and osteochondral tissue: Osteoarthritis, trauma, osteoporosis and sports injuries are the common bone related clinical problems associated with degenerative and acute cartilage. The number of osteochondral and articular cartilage patients has exceeded to 6 million in the United States due to a number of ankle, wrist and knee fractures.
The multifarious stratified structure and deprived recovering capacity of articular bone and osteocartilage sometimes, make the associated injuries to be permanent. Expanded avascular milieu and complex porous structure presents several obstacles for permanent restoration of the bone (Butscher A, et al., 2011; Li X, et al., 2009). Traditional bone designing methods cannot accurately mimic the innate cartilage structure due to the low porous biomaterial used for fabrication. Three dimensional bioprinting offers patient specific osteochondral construction with natural geometry and mechanical properties. A recently study depicted the combined effect of aligned electrospun fiber scaffolds with inkjet bioprinting to produce a hybrid nanofabricated scaffold which possesses great mechanical movability and improved cartilage structure as compared to the traditionally fabricated design. This promising approach of cartilage regeneration is the currently explored field for osteobiomaterial engineers in the biomedical field. The combination of nanobiomaterials with 3D printing technique more exclusively provides the confined patient specific geometry of the final architecture of the cartilage (Rivron NC, et al., 2009; Li X, et al., 2013).
In recent years, the bacterial nanocellulose is widely used natural biomaterial which uses 3D bioprinting technique to create a mechanically strong bone and cartilage pattern according to the size of the patient that closely matches with the innate geometry of the respective bone and cartilage construction. Actually bacterial nanocellulose increases the adhesion of NIH/3T3 and endothelial cell lines which ensure a tremendous 3D construction of chondrogenic scaffold pattern (Liu C, et al., 2007). Other nanomaterials which can be used in combination with 3D bioprinting include multifaceted carbon nanotubes and nanoencapsulated domains that have been proved to be worthwhile in 3D osteochondral and cartilage fabrication. Osteochondral tissue is present at the junction of bone and cartilage due to which it presents high chemical, mechanical and morphological pitch. The attainability of these factors in 3D printed structures encounter several obstacles, however, the investigation regarding this aspect is still necessary to achieve the aforementioned goals. An in vivo and in vitro study was conducted to explore the osteochondral tissue regeneration by using nanoimprint lithography technique. Two diverse forms of osteogenic progenitor cells and cartilage cells (chondrocytes) were 3D fabricated simultaneously into a complicated alginate hydrogel scaffold with retainable cell viability. The results showed that distinctive endothelial cell layers were adhered successfully with each other which results into a promising osteochondral construct but due to low mechanical strength of alginate, it cannot be used permanently as a substitute for the damaged cartilage (Cheng JX, 2011). However, later on this alginate hydrogel was used mixed with 3D printed Poly Caprolactone (PCL) to design a more supportive biomimetic scaffold with enhanced mechanical strength. This mechanism was based on the principle that PCL scaffold was exploited to function as bone forming scaffold which was loaded with chondrocytes and osteoblasts laden hydrogel layer by layer to complete the 3D fabricated design of osteochondral tissue (Jang MW, et al., 2010; Bajaj P, et al., 2012). After the observation of seven days, upregulated cellular proliferation with high cellular density was observed. Researchers fabricated a 3D printed chondrocyte structure using polyethylene glycol dimethacrylate solution via bio inkjet printing technique and incorporated it at the site of defect in osteochondral plug. The proteoglycan was deposited successfully at the interface of implanted tissue and natural tissue and functioned properly just as the native tissue (Tasoglu S and Demirci U, 2013). A UK lab created a novel 3D printed scaffold to treat the human bone marrow defects. This work was carried out by employing polylactic acid filament with exclusive design of biphasic geometry of the bone to allow rapid stem cell differentiation and facilitates the good mechanical strength and interfacial integration (Sant S, et al., 2012).
Three dimensional bioprinted neural tissue: Neural tissue damage is one of the main issues of the central nervous system patients such as in Alzheimer disease and Parkinson’s disease. 3D bioprinting nanotechnology provides a permanent treatment for the neural maladies by fabricating neural tissues of the controlled patterns and structures. The structure of native neural tissue is somewhat different from the other tissues of the body as it requires extreme evenness of the spacing, specific size and proximity for inducing mutual cell communication and signaling and morphological characteristics. Inkjet bioprinting provided an avenue to develop new neural tissues as well as regenerate the damaged nerve cells via fabricating such a design that is able to allow more efficient cell to cell communication with accurate settlement just as the native neural tissue (Billiet T, et al., 2012). The design is well fabricated by depositing precise quantities of a variety of proteins, cells and growth factors to stimulate the cross talk similar to the natural neural tissue. Such a work was presented in a study in which the inkjet bioprinting technology was used to develop structures of primary embryonic hippocampal and cortical neurons in which the neuronal functional fidelity and cellular characteristics were reserved after printing. Ciocca L, et al., 2009 developed a unique bioink by using a gellan gum hydrogel based surfactant in 2013 which was able to alleviate some of the intrinsic precincts of the transforming consumer printers for the research laboratories. This bioink was novel because not only print reliability it was also comprised upon several cell types from two dissimilar commercially accessible most demanded printing systems and the fluid characteristics of the bioink facilitates the reticence of cell agglutination in the solution (Kain A, et al., 2009).
However, despite well printing of the inkjet bioprinting technique, other most promising bioprinting technologies such as Directed Mirror Device Sintered Laser Technology (DMDSL) are also used for neural tissue engineering. DMDSL uses lasers and ultraviolet rays to allow photo crosstalk between protein molecules or hydrogels. The proteins are adjourned in a bulk of optically active transparent hydrogel which induces the effective bond formation process within the side chains of the amino acids and proteins. This results in the formation of complex structures which facilitates the binding of other bioactive molecules to design a precise pattern of neural scaffold of strong mechanical strength and effective placement of cells for efficient crosstalk and signaling between the neurons (Sachs E, et al., 1990; Marizza P, et al., 2013). Researchers used this approach and Schwann cells to fabricate a neural scaffold formed of a modified natural polymer, glycidyl methacrylated hyaluronic acid mostly occur in the extracellular matrix of the natural neural tissue. DMDSL technique used in this neural tissue fabrication process created the similar neuronal phenotypes and electrophysiology just as the native neuronal tissue with excellent geometries of complex resolution that allows propagation of the cell lines for 24 hours. The resultant neuronal phenotypes were further incorporated with sufficient gradients of nanoparticles to further elucidate the differentiation of the neuronal cells. This augments the photocrosslinkability and nonphotocrosslinkability of the used polymers of glycidyl methacrylated hyaluronic acid to fabricate the construction of the neuronal scaffold with high porosity and mechanical and chemical asset (Waid S, et al., 2012; Keller SS, et al., 2011). Other studies also supported the 3D bioprinting of the nanoneural scaffold. Keller SS, et al., 2011 also employed this DMDSL laser based bioprinting to fabricate an in vitro model of embryonic dorsal root ganglion neurite expansion. PEG polymer and Puramatrix were used to provide a controlled environment for the growth of neurites in the form of channel. The final construct presented excellent mechanical strength when compared with Puramatriz scaffold alone being manipulated from the natural favorable cellular milieu. The graphene nanoplatelets were also used to construct the aligned nerve fabricates of uniform size and well porous structure through SLS technology. The graphene nanoplatelets construct were observed to be highly cytocompatible and improved conductance with pronounced rate of neural regeneration (Bartolo P, et al., 2012; Castrejón-Pita JR, et al., 2008).
Three dimensional bioprinted vascular tissue: The vascular network of the body is of immense importance because it is the system which is mainly responsible for the transportation of the nutrients, oxygen and removal of wastes from the body. The 3 D printing nanotechnology faces a lot of obstacles to create a perfusable 3D vascular network that can efficiently transport nutrients and to all parts of the body and remove wastes. Currently available technologies of 3D bioprinting address these challenges successfully to fabricate a complex vascular network which enhances the cell survival rate. The endothelial cells are guided to form vessels in a pattern similar to native vascular tissue that displays a great promise for tissue regeneration. For example, Woo JH, et al., 2009 was the first researcher who created a bioink in the form of a glass from a mixture of complex carbohydrates (glucose, sucrose and dextran) which was optically transparent. The bioink glass when cooled was adjoined with photocrosslinkable biomaterials so that it can interconnect the surrounding biomaterials. This carbohydrate bioink glass was then extruded using a superheated syringe into an interlocked microfluidic vascular system which resulted in the formation of a scaffold cast around the interconnected vascular network in the form of a blend of 10T1/2 cells and photocrosslinkable extracellular matrix prepolymer. The scaffold with carbohydrate bioink polymer was then immersed in water due to which carbohydrate was completely dissolved leaving behind a resonating scaffold of microvascular structure channel. After this, the human umbilical vein endothelial cells were then perfused through the previously formed channels which were observed to be attached and form a biphasic tissue like architecture. The final 3D printed vascular scaffold was observed to maintain the phenotypic and proteomic expression at a high density similar to the natural vasculature (Baca HK, et al., 2011).
However, for the successful fabrication of the 3D bioprinted vascular systems, the materials should be chosen with great care which is able to mimic the native structure of the vascular tissue. The scarcity of the biomimetic materials available for vascular tissue fabrication enabled the scientists to formulate a new composition of hyaluronic acid methacrylate: Gelatin methacrylate (HA-MA:GE-MA) using hobbyist printer (Fab@Home). They constructed the hydrogel based cellular tubular structure characterized with a cylindrical structure that was printable through the hydrogel printing system that resembled the vascular channels. This 3D fabricated design of vascular channel was more feasible and economical for printing the whole network of cellular conduit that demonstrated it a precursor for further designing a 3D bioprinted organ. More exclusively, this work promoted the potential to tissue engineering for efficient construct of vascularized pathways that incorporate with new native tissue forms in vivo. This 3D printed vascular tissue also opened the path for the fabrication of 3 D bioprinted aortic valve for addressing various cardiovascular issues (Cui X and Boland T, 2009). In this concern, Yan KC, et al., 2009 created a 3D bioprinted aortic valve model via 3D bioplotter loaded with alginate and gelatin hydrogel doped with two inimitable cell types i.e., aortic root sinus smooth muscle cells and aortic valve leaflet interstitial cells. Both the cell types were cultured for seven days and then extruded into gibbet that imitated the form of natural porcine aortic valve. The cell laden cultures were evaluated for cell survival rate and mechanical properties and the observations depicted that 80% of the 3D printed cells survived and the Young’s modulus factor of the scaffolds was significantly decreased progressively from tissue fabrication to scaffold collapse (Mendonça G, et al., 2009; Mironov V, et al., 2009). These observations provided the evidence of strong compliance of the steady mechanical properties of the cellular scaffold model of the biomimetic aortic valve.
Three dimensional bioprinted organs: The widespread clinical applications of 3D organ bioprinting have made this technique a great hope for organ regeneration. Although 3D organ bioprinting is still under experimental practices, researchers look forward to this technique of organ regeneration as clinical treatment of organ damage. Three dimensional tissue and organ bioprinting can be carried out with or without incorporating living cells which are bioprinted directly into the fabricate (Xu T, et al., 2005). Some examples are listed in Table 1. Dr. Xu with his coworkers in their research lab in Wake Forest worked a lot on creating 3D bioprinted organs. They were the first to develop successful conventionally engineered bladder and implant them into seven children and teenagers of terminal myelomeningocle. Pursuing this work, they designed a bladder that was three dimensional bioprinted using inkjet bioprinting technology (Grodzinski JJ, 2006). This technology was used in further modified form by adjoining the nanotechnology to produce a nanofeatured mechanically strong fabricated scaffold that can boost the stem cell proliferation. The inkjet bioprinter was connected with electrospinning needle that produced the printed scaffold and multiple cellular populations into three characteristic layers of the bladder side by side. After printing, the cell population was cultured at 37°C and histologically investigated which confirmed their confined position and cellular proliferation. Therefore, a functional bladder scaffold with all proper cell types was three dimensionally bioprinted in conjunction with nanotechnology was successfully fabricated. Inkjet bioprinting can simultaneously incorporate multiple tissue types to print an organ (Ringeisen BR, et al., 2006; Bergmair I, et al., 2010). Three individual types of stem cells were concurrently bioprinted into a single construct and their process of proliferation and differentiation was investigated in both in vitro and in vivo. The results were astonishing as optimal cell viability and proliferation was observed in the culture. Following in vivo and in vitro study, the scaffold was vascularized into an organ and each cell type showcased histological substantiation of differentiation (Fielding GA, et al., 2012; de Bartolo L,et al., 2012).
| Tissue types | Biomaterials | Bioprinting technologies | Mechanisms | References |
|---|---|---|---|---|
| Hard tissue (Bone) | Ceramics | Fused deposition modeling | The 3D scaffold was bioprinted using MSC hydrogel showing osteogenic differentiation | (Li X, et al., 2012) |
| TCP | Selective laser technology | Microwaves based method was applied to form a porous biomimetic scaffold that accelerates the bone formation | (Li X, et al., 2009) | |
| Cartilage and osteochondral tissue | Collagen fibrin hydrogel | Inkjet bioprinting | The electrospun PCL fabricated scaffold that supported the development of collagen in vitro and in vivo | (Li X, et al., 2014) |
| Alginate hydrogel | Bioplotting | Osteochondral scaffold was designed with developed ECM morphology in the under observed bone and cartilage | (Seyednejad H, et al., 2012) | |
| Neural tissue | Fibrin hydrogel | Inkjet bioprinting | The 3D fibrin hydrogel was employed to create neural mat with adhered well proliferated cells | (Flaibani M and Elvassore N, 2012) |
| Sintered laser technology | Hyaluronic acid hydrogel | Biomimetic nerve ducts were fabricated in biomimetic nerve scaffold to neuronal and axonal growth in vitro | (Li X, et al., 2011) | |
| Vascular tissue | Bioplotting | Hyaluronic-gelatin hydrogel | Cellular constructs were biomimetic that synthesize vascular system with improved cellular viability. | (Kim G, et al., 2008) |
| Aortic root cells and complex organ | Bioplotting | Alginate spheroids | Stem cells were biomimetic in organ like fashion with high cell viability and confined geometry | (Wang JX, et al., 2009) |
| Inkjet bioprinting | Calcium chloride/sodium alginate hydrogel | Cells are fabricated into layer by layer fashion with proliferative capacity and maintained viability and then observed in vivo. | (Wang J, et al., 2012) |
Table 1: 3D bioprinted tissue and organ regeneration.
de Bartolo L, et al., 2012 followed a different approach to fabricate the vascularized adipose derived stromal stem cells scaffold to print an organ. The bioprinted pluripotent stem cells differentiated into cardiac cells and smooth muscle cells, maximally present in the human body which shows promise of providing an avenue to organ regeneration research. The bioplotter is very useful and effective emerging organ printing system used by researchers currently which fabricate the 3D printed organ plot via using spheres of alginate plotted in calcium chloride solution. The bioplotted spheroids were then incubated at 37°C to differentiate systematically which resulted in excellent cell survival and proliferation. After differentiation, the spheroids were packed together in an alginate system in the form a 3D cell culture environment which then fused with the adjacent spheroids into the premeditated geometry. The consequential cell-laden alginate structure formed a composite geometry of microtissue which was very much similar to vascular networks of cartilage, cardiac tissue, renal cell constructs, muscular tissue, potent vascular networks of the body (Mironov V, et al., 2008). One of the most sophisticated and highly developed commercial bioprinters competent for organ printing were created and operated by the company “Organovo”. The company used a very advanced mechanism of organ generation through incorporating bioink infused spherical drops of the cell that consequently mingle with each other over a specific time period and assemble themselves into the biologically similar organ construct. This company paved its path to success by designing 3D fully functional blood vessel structure which could be fibroblasts and endothelial cell fabricates that travel to their respective appropriate space in the lumen when bioprinted by their developed method. The company is also working to develop a fully biologically functional artificial 3D bioprinted liver model that could potentially update pharmaceutical testing system (Cervantes TM, et al., 2013).
Applications of three dimensional bioprinting in cancer and metastasis
Cancer related deaths are the major problem of the current era and tumor metastasis is the center point to be worth noted. The major site of metastasis for a majority of cancers is the bone tissue as the skeleton is the most widespread organ to be affected by metastatic cancer. For example, approximately, 70% of the breast cancer metastasizes into the bone; therefore, it is very important to increase the survival rate of cancer patients by targeting the bone metastasis. Bone is the site of disease that produces the greatest morbidity rate. The conventional treatment strategy for cancer bone metastasis also known as osteosarcoma is the chemotherapy, radiotherapy and surgery which not only kills the metastatic cells but also affects the normal cells survival (Fierz FC, et al., 2008; Sobral JM, et al., 2011). Therefore, a blend of nanoparticles, selective drugs and gene therapy is the current direction to develop more effective treatment strategy for osteosarcoma. It has been known that suitable microenvironment and the enriched nutrient cells pave the direction to tumor cell migration to the bone tissue and subsequent escalation of the tissue. Although osteosarcoma needs more advanced therapies rather than aforementioned conventional therapies because the nanotechnology targeted drug delivery systems are not applicable for the treatment of osteosarcoma (Zhou WY, et al., 2008).
A study reported that an osteosarcoma-associated-cell surface antigen (ALCAM) and artificially engineered anti-ALCAM-hybrid polymerized liposomal nanoparticles immunoconjugate, alpha-AL-HPLN were used to deliver chemotherapeutic drug DXR to osteosarcoma cells. It was observed that this nanotargeted drug osteosarcoma-associated-cell surface antigen (ALCAM) anti-ALCAM-hybrid polymerized liposomal nanoparticles alpha-AL-HPLN hybrid not only showed increased cytotoxicity but also decreased the survival rate of untargeted normal surrounding cells as compared to the usage of isolated conventional drug DXR. However, arsenic trioxide magnetic nanoparticles were also shown to exert the cytotoxic effects on osteosarcomal cells within the vicinity of magnet field on the murine osteosarcoma cells. Calcium phosphate nanoparticles exhibit more pronounced cytotoxic activity when delivered in conjunction with anticancer drug cisplatin to an osteosarcomal model of albino rat (Kruth JP, et al., 2004). The nanoparticles loaded anticancer drugs could increase the retention time of the drug in local tissue or fluids but the activity of increased cytotoxicity rate has demanded to develop more advanced technology to address the bone related tumors i.e., osteosarcoma and osteoarthritis. For instance, polymeric hydrogel was used in combination with chemotherapeutic drug Dextran crosslinked ionically with nanoparticles in synovial fluid in a rabbit model. This self-assembly not only increased the retention time of IL-1 receptor antagonist (IL-1Ra) which decreased the expression of inflammatory cytokine IL-1 responsible for the expression of osteosarcoma related genes but also observed the decreased cell survival rate of the normal osteocells in synovial fluid (Marshall AJ and Ratner BD, 2005). To overcome the aforementioned obstacles, the nanoparticles were used in conjunction with bioactive materials for tissue repair and regeneration to attain the improved tissue-material response after implantation. Two ways are generally adopted create bioactive nanostructured bone scaffolds to treat osteosarcoma. The first one is to synthesize nanoparticles-polymer composite scaffolds and the second is the synthesis of well nanopore sized bioactive glass scaffold. The bioactive nanoparticles-polymer composite scaffold was prepared by incorporating beta tricalcium phosphate, Hap, bioactive glass polymer and CaSiO3 nanoparticles into the scaffold matrix. The final construct showed improved cytocompatibility, chemical stability, mechanical strength, and mineralization ability and degradation capacity with more distinct functionality than that of microcomposites prepared from conventional methods (Colombo P, et al., 2013). Hence, the physiochemical and biological characteristics of the regenerated scaffold are significantly improved by using nanoparticles in conjunction with biopolymers via formation of nanobiopolymers. For the first time, the scientists improved the bioactivity of the conventional bioactive glass for bone regeneration by synthesizing Mesoporous Bioactive Glass (MBG) in 2004 using a blend of supramolecular surfactants with sol-gel method. The CaO-SiO2-P2 O5 composition of bioactive glass was coupled with nanoparticles of SiO2 which resulted into a highly ordered mesoporous structure of pore size ranging from 5-20 nm (Eom SH, et al., 2008). The conventional nonmesoporous bioactive glass was unable to mineralize the bone properly. However, MBG possesses optimal apatite mineralization ability in simulated body fluids, increased surface area and porosity and excellent cytocompatibility. This technique was applied with three dimensional bioprinting technologies to prepare 3D porous bone scaffolds for bone tissue mechanization and drug delivery relevance. Therefore, three methods have been developed to design 3D bioprinted MBG scaffolds. The first MBG scaffold was designed by employing porogen method. Researchers used methyl cellulose as the porogen to develop porous structured MBG scaffold with a bulky pore size of 100 μm. however, the second scaffold was designed by somewhat different method of polymer template which was well mesoporous of the massive pore size ranging from 300-500 nm with varying compositions for pertinent applications in bone tissue engineering. The third type is the formation of MBG scaffolds by polyurethane sponge template method which provided highly interconnected porous structures but they show low mechanical strength, therefore, they are not suitable for successful tissue regeneration (Wu C, et al., 2011). The 3D bioplot technique is very advantageous to control the pore morphology by architecting layer by layer plotting under suitable conditions. The 3D bioplot technique provides excellent mechanical strength and mineralization ability which is 200 times than that of MBG scaffolds prepared using traditional polyurethane polymers. Therefore, MBG scaffold is a better nanobiomaterial which combines drug delivery system with 3D bioprinting technique for efficacious bone regeneration which paved a pathway towards the synthesis of 3D functional tissues and organs (Yoo D, 2013).
In vitro complex 3d bioprinted cancer models
Since, the last decade, in vitro cancer modeling was mostly dependent upon 2D mono-cell cultures and animal models. However, 2D modeling has some limitations over mimicking the tumor microenvironment in humans. The advancement from 2D to complex 3D bioprinted cancer modeling of experimental animals including disease progression, cell-matrix interactions, hypoxic nuclei, leaked torn vasculature and interrupted signaling cascades. 3D bioprinting allows fruitful development of tumor spheroids which are very much similar to natural cellular heterogeneity of in vivo solid tumors described by distinct metabolic and proliferative rate. Such type of in vitro models are very much applicable to high throughput tumor screening and drug testing for therapeutics (Tirella A, et al., 2011). A wide range of 3D bioprinted tumor spheroids have been prepared using MCF-7 and BT474 breast cancer cells, Hela cells for cervical cancer, SU3 stem cell line for glioma using the bioink varying from PEG dimethacrylate, PEG-diacrylate to gelatin-alginate-fibrinogen (Table 2). The 3D constructed tumor spheroid models are able to reproduce enzyme secretion and typical factors specific for every type of tumor like vascular endothelial growth factor and matrix degrading enzyme in addition to resistance of drugs used for conventional antitumor therapy. In addition, to the 3D microtumor environment, the cell-to-cell crosstalk is of worth understanding to mimic the regulatory cascades for controlling the signaling, migration, adhesion and metastasis of the tumor cells. For instance, if we take into account the interactions of cancer cells with immune cells like macrophages through the paracrine system, the 3D bioprinting is able to mimic vessel microenvironment incorporated with macrophages and MDA-MB-231 breast cancer cells in the core of peptide conjugated with alginate fibers. Another application of 3D bioprinting is the study of regulatory feedback mechanism and tumor metastasis in the human ovarian cancer cells (OVCAR-5) which were cultured with natural fibroblasts cells printed on MatrigelTM which was a blend of gelatinous protein produced by Engelbreth-Holm-Swarm mouse sarcoma cells. The resultant construct contained some multicellular acini with recapitulated characteristics of ovarian cancer micronodules in vivo (Faddoul R, et al., 2013). Laser printers are also highly appreciated to synthesize artificial bone by incorporating MDA-MB-231 breast cancer cells cultured simultaneously with human fetal osteoblasts resulting in the formation of multicellular spheroids using hydroxyapatite nanoparticles i.e., PEG and PEG-diacrylate. This biomimetic model of cancer cells showed the increased capacity of tumor migration in the bone structure. Stereolithography is another widely used technique to produce bioprinted Breast Cancer Cells (BrCa) which are also co-cultured with fetal stromal osteoblasts and bone marrow mesenchymal stem cells condensed with gelatin methacrylate hydrogel in nanocrystalline hydroxyapatite. The increased vascular endothelial cells growth with enhanced migration of BrCa cells into the bioprinted fabricate exhibited this technique an applicable technique of 3D bioprinted cancer tissue models. Further research is carried on to deposit different bioinks of interest to integrate extrusion based biomaterials with polymeric scaffolds enhancing the biological features of the in vitro cancer models (Li X, et al., 2012).
| Cancer cell lines | Bioink used | Type of bioprinter | Purpose | References |
|---|---|---|---|---|
| MMCF-7 breast cancer cell line | Gelatin-alginate fibrinogen | Extrusion printer | The in vivo extracellular matrix tumor microenvironment was fabricated and tumor spheroids were formed to achieve necrosis and drug resistance | (Lian Q, et al., 2006) |
| BrCa breast cancer cell line | Gelatin-alginate | CIJ | (Jose RR, et al., 2016) | |
| SU3 gliomal cancer cell line | Peptide conjugated alginated fibers | PAM | (Skardal A, Atala A, 2015) | |
| Hela cervical tumor cell line | Polyethlene glycol diacrylate (MW 700) | PAM | (Lee SJ, et al., 2017) | |
| BT474 breast cancer cell line | Gelatin methacrylate hydrogel with nanocrystalline hydroxyapatite | Extrusion bioprinter | (Chen C, et al., 2016) | |
| Murine RAW 264.7 macrophage cancer cell line | Matrix | PAM | The regulatory pathways of carcinogenesis were biomimetic and hypoxia core and adhesion mat was fabricated to induce artificial cell to cell communication | (Henriksson I, et al., 2017) |
| OVCAR-5 ovarian cancer cell line | Matrigel TM (gelatinous protein mixture secreted by Engelbreth-Holm-Swarm mouse sarcoma cells) | PAM | (Shafiee A and Atala A, 2017) | |
| MCF-7 breast cancer cell line | Gelatin-PEG-dimethacrylate | Sintered laser bioprinter | (Ferris CJ, et al., 2013) | |
| MDA-MB-231 breast cancer cell line | Gelatin-alginate | Laser based bioprinter |
Table 2: Three dimensional printing of in vitro cancer models.
3D bioprinted models of breast refurbishment
Breast cancer is one of the main malicious gynecological disorders in women. The conventional method for the treatment of breast cancer is the plastic surgery after diagnosis through mastectomy. The autologous Deep Inferior Epigastric Perforator (DIEP) flap reconstruction is one of the methods leading to the least donor-site morbidity. This method involves the removal of fat from the skin and abdominal area along with artery and epigastric vein and transplanted to the breast area of the similar patient under observation. This method of modulation of breast is although replicative of the shape of the original chest; however, new alternative method is still aesthetically going ahead. The intricacy that surgeons often face while fabricating a breast scaffold is its form and projection via flap which is solved by extrusion based 3D printers such as FDM. The mirror image of the undamaged breast is taken via 3D laser printer which is then superimposed on the affected breast region in a three dimensional fashion which is better functional as compared to the conventional way of surgery. In this regard, 3D surface imaging such as MRI, CT scan etc. can give better clue of the required tissue projection and volume to be extruded in the affected portion which is more beneficial when a bulky volume of tissue is required to construct a balanced breast design. However, if insufficient tissue is used, it will be unable to form a proper projection of the breast flap and shaping flattened adipocutaneous tissue will be challenging. 3D bioprinted breast scaffold via 3D laser printing and FDM is not only inexpensive but also owes flexible nature with de-epithelialized DIEP flap incorporated into the synthetic 3D mold which alters this scaffold into the symmetrical to contralateral breast with only trivial adjustments (Cervantes TM, et al., 2013).
Three dimensional printed organ implants using nanobiomaterials
3D metal printing using materials like metals and polymers has been successfully used in designing implants mainly for bone and dental prosthesis via manufacturing screws, joints and flat substrates. Bone metastasis is mainly treated with parenteral treatment of chemotherapeutic drugs. But high doses needed to achieve successful treatment at the site of bone tumor mainly results in the elevated toxicity in the rest of the body. Alternative approach to impede this therapeutic failure is by implanting drug loaded bone implants which are very much efficient to release the chemotherapeutic drug directly at the affected places only and hamper the toxic concentration in other body areas. Recently, a titanium implant was manufactured for bone cancer which was loaded with two antitumor drugs i.e., apoptosis inducing ligand and doxorubicin with the help of 3D metal printer (Sant S, et al., 2012). The 3D printed titanium chips were designed with the help of a powder layer which was selectively melted using laser technology. This process was repeated several times until the required thickness of the implant was acquired which was quite mechanically strong and manageable. The fabricated implant was then loaded electrochemically with anode ions to make an anodic ion layer of 188 and 25 μg/cm2 of both doxorubicin and apoptosis induced ligand respectively to imprint microparticles and nanoparticles to increase the biointegration. These 3D drug loaded nanoparticles implant showed anticancer activity against MDA-MB-231-TXSA cancer cells. The E-jet 3D printing technology manufactured an implant using anticancer magnetocaloric polycaprolactone (PCL)/Fe3O4 nanoparticles (Billiet T, et al., 2012). This 3D technology works on the principle of magnet hyperthermia in which the magnetic energy is converted into heat energy leading preferentially to the death of the proliferative cells. The specific use of 7% w/v solution of PCL biomaterial was due to the property of biocompatibility and biological stability of the biomaterial, mixed with magnetic starch Fe3O4 nanoparticles that were E-jet bioprinted to produce PCL/ Fe3O4 mat. The advantage of 3D printed implants over conventional anticancer therapies is the improved surface area with enhanced contact with cancer cells. The 3D therapeutic implant is placed after surgery in the vicinity of the tumor growth obstructing any intravenous induction of the anticancer drugs. In vivo study of tumor growth inhibition in albino mice depicted that therapeutic 3D implants significantly halted the growth of tumor as well as propagate the survival rate following one month of the treatment (Li X, et al., 2008).
The 3D printed biomaterials paved the way of fabrication of four dimensional (4D) bionanomaterials which is basically an amalgam of 3D printing and time factor. 4D printing enables a bioprinted entity to be programmed carefully to maintain the shape alterations depending on the surroundings when subjected to external stimuli such as temperature. 4D nanobioprinting technology was employed in China in 2017 to refurbish a breast implant for the cancer patient. The 3D implant made alterations with passing time and the adipocutaneous fibrous tissue grew into the full breast implant until it replaced the complete damaged organ (Tasoglu S and Demirci U, 2013).
Conclusion
This article summarized the mechanism and applications of 3D bioprinting nanotechnology applying a blend of biomaterials and nanotechnology along with the currently available literature to support the 3DP performance and its applications in tissue engineering and tumor therapy. The 3D bioprinting nanotechnology is one of the most eye-catching biofabrication tools for developing tissue engineered scaffolds for addressing many medical problems including fractures and tumor therapy. Nanotechnology is a potential technique for developing nano featured biomaterials with the property of reinforcement that is able to manipulate cellular microenvironment. Widespread researches have depicted that 3D bioprinted nanofeatured engineered scaffolds can control the properties of cellular proliferation to fabricate tissues and organs. 3D printing of biomaterials such as hydrogels and implants loaded with cytotoxic drugs are transfiguring cancer therapies targeting tumor cells in a confined way of increased efficacy and reduced toxicity. Vascularization is one of the most challenging target to achieve induction of angiogenesis and upregulation of angiogenic growth factors such as Vascular Endothelial Growth Factor (VEGF) via using bioreactors. The 3D bioprinters have the potential to induce 3D vascularization fabrication scaffold by using multiple print heads loaded with variable cell types.
Future Scope of the Study
3D printed tissues and scaffolds are used for screening purposes and appraisal in in vivo model such as albino rat and mice. This is challenging to reach the authorities such as Food and Drug administration (FDA) to meet their requirements to reach the patient for applying this 3D printed artificial scaffolds. However, numerous technologies of 3D bioprinting have been developed so far, still is known regarding 3D printed biomaterials based scaffolds for organ and tissue implants in addition, the interaction of these biomaterials based microenvironment such as stem cells and with nanotechnological principles is very necessary to develop the physiologically functional organs and tissues. Therefore, the field of this 3D bioprinting based nanotechnology is still in early stage. Keeping these points at the forefront, future research projects may be designed to converge both the technologies i.e., nanotechnology and 3D bioprinting to mimic the native structure of the tissues and organs.
References
- Campbell TA, Ivanova OS. 3D printing of multifunctional nanocomposites. Nano Today. 2013; 8(2): 119-120.
- Bergmann C, Lindner M, Zhang W, Koczur K, Kirsten A, Telle R, et al. 3D printing of bone substitute implants using calcium phosphate and bioactive glasses. J Eur Ceram. 2010; 30(12): 2563-2567.
- Danilevicius P, Rekstyte S, Balciunas E, Kraniauskas A, Sirmenis R, Baltriukiene D, et al. Laser 3D micro/nanofabrication of polymers for tissue engineering applications. Opt Laser Technol. 2013; 45: 518-524.
- Sachs EM, HJ CM, Williams PA. Three-dimensional printing techniques, US Patent 5204055. 1989.
- Utela B, Storti D, Anderson R, Ganter M. A review of process development steps for new material systems in three Dimensional Printing (3DP). J Manuf Process. 2008; 10(2): 96-104.
- Melchels FP, Feijen J, Grijpma DW. A review on stereolithography and its applications in biomedical engineering. Biomaterials. 2010; 31(24): 6121-6130.
[Crossref] [Google scholar] [Pubmed]
- Yoo D. New paradigms in internal architecture design and freeform fabrication of tissue engineering porous scaffolds. Med Eng Phys. 2012; 34(6): 762-776.
[Crossref] [Google scholar] [Pubmed]
- Guillemot F, Souquet A, Catros S, Guillotin B, Lopez J, Faucon M, et al. High-throughput laser printing of cells and biomaterials for tissue engineering. Acta Biomater. 2010; 6(7): 2494-2500.
[Crossref] [Google scholar] [Pubmed]
- Li X, Liu H, Niu X, Yu B, Fan Y, Feng Q, et al. The use of carbon nanotubes to induce osteogenic differentiation of human adipose-derived MSCs in vitro and ectopic bone formation in vivo. Biomaterials. 2012; 33(19): 4818-4827.
[Crossref] [Google scholar] [Pubmed]
- Li X, Feng Q, Liu X, Dong W, Cui F. Collagen-based implants reinforced by chitin fibres in a goat shank bone defect model. Biomaterials. 2006; 27(9): 1917-1923.
[Crossref] [Google scholar] [Pubmed]
- Koutsopoulos S. Molecular fabrications of smart nanobiomaterials and applications in personalized medicine. Adv Drug Deliv Rev. 2012; 64(13): 1459-1476.
[Crossref] [Google scholar] [Pubmed]
- Chen KI, Li BR, Chen YT. Silicon nanowire field-effect transistor-based biosensors for biomedical diagnosis and cellular recording investigation. Nano today. 2011; 6(2): 131-154.
- Lautenschläger F, Piel M. Microfabricated devices for cell biology: All for one and one for all. Curr Opin Cell Biol. 2013; 25(1): 116-124.
[Crossref] [Google scholar] [Pubmed]
- Li X, Liu H, Niu X, Fan Y, Feng Q, Cui FZ, et al. Osteogenic differentiation of human adipose-derived stem cells induced by osteoinductive calcium phosphate ceramics. J Biomed Mater Res B Appl Biomater. 2011; 97(1): 10-19.
[Crossref] [Google scholar] [Pubmed]
- Shin H. Fabrication methods of an engineered microenvironment for analysis of cell-biomaterial interactions. Biomaterials. 2007; 28(2): 126-133.
[Crossref] [Google scholar] [Pubmed]
- Caldorera-Moore M, Peppas NA. Micro-and nanotechnologies for intelligent and responsive biomaterial-based medical systems. Adv Drug Deliv Rev. 2009; 61(15): 1391-1401.
[Crossref] [Google scholar] [Pubmed]
- Li X, van Blitterswijk CA, Feng Q, Cui F, Watari F. The effect of calcium phosphate microstructure on bone-related cells in vitro. Biomaterials. 2008; 29(23): 3306-3316.
[Crossref] [Google scholar] [Pubmed]
- Liu X, Li X, Fan Y, Zhang G, Li D, Dong W, et al. Repairing goat tibia segmental bone defect using scaffold cultured with mesenchymal stem cells. J Biomed Mater Res B Appl Biomater. 2010; 94(1): 44-52.
[Crossref] [Google scholar] [Pubmed]
- Li X, Fan Y, Watari F. Current investigations into carbon nanotubes for biomedical application. Biomed Mater. 2010; 5(2): 022001.
[Crossref] [Google scholar] [Pubmed]
- Kim HN, Jiao A, Hwang NS, Kim MS, Kim DH, Suh KY. Nanotopography-guided tissue engineering and regenerative medicine. Adv Drug Deliv Rev. 2013; 65(4): 536-558.
[Crossref] [Google scholar] [Pubmed]
- Liu K, Tian Y, Jiang L. Bio-inspired superoleophobic and smart materials: Design, fabrication, and application. Prog Mater Sci. 2013; 58(4): 503-564.
- Li X, Gao H, Uo M, Sato Y, Akasaka T, Abe S, et al. Maturation of osteoblast-like SaoS2 induced by carbon nanotubes. Biomed Mater. 2008; 4(1): 015005.
[Crossref] [Google scholar] [Pubmed]
- Owens III DE, Eby JK, Jian Y, Peppas NA. Temperature-responsive polymer-gold nanocomposites as intelligent therapeutic systems. J Biomed Mater Res A. 2007; 83(3): 692-695.
[Crossref] [Google scholar] [Pubmed]
- Tanaka M, Mabuchi Y, Hayashi T, Hara M. Subtractive offset printing for fabrication of sub micrometer scale electrodes with gold nanoparticles. Microelectron Eng. 2012; 95: 14-20.
- Bikram M, Gobin AM, Whitmire RE, West JL. Temperature-sensitive hydrogels with SiO2-Au nanoshells for controlled drug delivery. J Control Release. 2007; 123(3): 219-227.
[Crossref] [Google scholar] [Pubmed]
- Landers R, Mülhaupt R. Desktop manufacturing of complex objects, prototypes and biomedical scaffolds by means of computer-assisted design combined with computer-guided 3D plotting of polymers and reactive oligomers. Macromol Mater Eng. 2000; 282(1): 17-21.
- Nomura H, Katayama Y, Shoichet MS, Tator CH. Complete spinal cord transection treated by implantation of a reinforced synthetic hydrogel channel results in syringomyelia and caudal migration of the rostral stump. Neurosurgery. 2006; 59(1): 183-192.
[Crossref] [Google scholar] [Pubmed]
- Lam CX, Mo XM, Teoh SH, Hutmacher DW. Scaffold development using 3D printing with a starch-based polymer. Mater Sci Eng C. 2002; 20(1-2): 49-56.
- Xu T, Gregory CA, Molnar P, Cui X, Jalota S, Bhaduri SB, et al. Viability and electrophysiology of neural cell structures generated by the inkjet printing method. Biomaterials. 2006; 27(19): 3580-3588.
[Crossref] [Google scholar] [Pubmed]
- Sanjana NE, Fuller SB. A fast flexible ink-jet printing method for patterning dissociated neurons in culture. J Neurosci Methods. 2004; 136(2): 151-163.
[Crossref] [Google scholar] [Pubmed]
- Li X, Huang Y, Zheng L, Liu H, Niu X, Huang J, et al. Effect of substrate stiffness on the functions of rat bone marrow and adipose tissue derived mesenchymal stem cells in vitro. J Biomed Mater Res A. 2014; 102(4): 1092-1101.
[Crossref] [Google scholar] [Pubmed]
- Yeong WY, Chua CK, Leong KF, Chandrasekaran M, Lee MW. Comparison of drying methods in the fabrication of collagen scaffold via indirect rapid prototyping. J Biomed Mater Res B Appl Biomater. 2007; 82(1): 260-266.
[Crossref] [Google scholar] [Pubmed]
- Fielding GA, Bandyopadhyay A, Bose S. Effects of silica and zinc oxide doping on mechanical and biological properties of 3D printed tricalcium phosphate tissue engineering scaffolds. Dent Mater. 2012; 28(2): 113-122.
- Boland T, Tao X, Damon BJ, Manley B, Kesari P, Jalota S, et al. Drop-on-demand printing of cells and materials for designer tissue constructs. Mater Sci Eng C. 2007; 27(3): 372-376.
- Butscher A, Bohner M, Hofmann S, Gauckler L, Müller R. Structural and material approaches to bone tissue engineering in powder-based three-dimensional printing. Acta Biomater. 2011; 7(3): 907-920.
[Crossref] [Google scholar] [Pubmed]
- Li X, Liu X, Dong W, Feng Q, Cui F, Uo M, et al. In vitro evaluation of porous poly (l-lactic acid) scaffold reinforced by chitin fibers. J Biomed Mater Res B Appl Biomater. 2009; 90(2): 503-539.
[Crossref] [Google scholar] [Pubmed]
- Rivron NC, Rouwkema J, Truckenmüller R, Karperien M, de Boer J, van Blitterswijk CA. Tissue assembly and organization: Developmental mechanisms in microfabricated tissues. Biomaterials. 2009; 30(28): 4851-4858.
[Crossref] [Google scholar] [Pubmed]
- Li X, Wang L, Fan Y, Feng Q, Cui FZ, Watari F. Nanostructured scaffolds for bone tissue engineering. J Biomed Mater Res A. 2013; 101(8): 2424-2435.
[Crossref] [Google scholar] [Pubmed]
- Liu C, Xia Z, Czernuszka JT. Design and development of three-dimensional scaffolds for tissue engineering. Chem Eng Res Des. 2007; 85(7): 1051-1064.
- Cheng JX. Research on the bio-safety of nanomaterials. Proc Int Conf Comput Sci Netw Technol. 2011; 4: 2716-2719. IEEE.
- Jang MW, Jin BK, Lee SH, Park JH, Ryu JM, Yun SP, et al. Effect of PMMA and cross-linked dextran mixture on bio-safety and volume in rat. Tissue Eng Regen Med. 2010; 7(1): 57-63.
- Bajaj P, Chan V, Jeong JH, Zorlutuna P, Kong H, Bashir R. 3-D biofabrication using stereolithography for biology and medicine. Annu Int Conf IEEE Eng Med Biol Soc. 2012: 6805-6808.
[Crossref] [Google scholar] [Pubmed]
- Tasoglu S, Demirci U. Bioprinting for stem cell research. Trends Biotechnol. 2013; 31(1): 10-19.
[Crossref] [Google scholar] [Pubmed]
- Sant S, Tao SL, Fisher OZ, Xu Q, Peppas NA, Khademhosseini A. Microfabrication technologies for oral drug delivery. Adv Drug Deliv Rev. 2012; 64(6): 496-507.
[Crossref] [Google scholar] [Pubmed]
- Billiet T, Vandenhaute M, Schelfhout J, van Vlierberghe S, Dubruel P. A review of trends and limitations in hydrogel-rapid prototyping for tissue engineering. Biomaterials. 2012; 33(26): 6020-6041.
[Crossref] [Google scholar] [Pubmed]
- Ciocca L, de Crescenzio F, Fantini M, Scotti R. CAD/CAM and rapid prototyped scaffold construction for bone regenerative medicine and surgical transfer of virtual planning: A pilot study. Comput Med Imaging Graph. 2009; 33(1): 58-62.
[Crossref] [Google scholar] [Pubmed]
- Kain A, Mueller C, Reinecke H. High aspect ratio-and 3D-printing of freestanding sophisticated structures. Procedia Chem. 2009; 1(1): 750-753.
- Sachs E, Cima M, Williams P, Brancazio D, Cornie J. Three dimensional printing: Rapid tooling and prototypes directly from a CAD model. CIRP Ann. 1990; 39(1): 201-204.
- Marizza P, Keller SS, Boisen A. Inkjet printing as a technique for filling of micro-wells with biocompatible polymers. Microelectron Eng. 2013; 111: 391-395.
- Waid S, Wanzenboeck HD, Muehlberger M, Bertagnolli E. Optimization of 3D patterning by Ga implantation and Reactive Ion Etching (RIE) for Nanoimprint Lithography (NIL) stamp fabrication. Microelectron Eng. 2012; 97: 105-158.
- Keller SS, Feidenhans N, Fisker-Bødker N, Soulat D, Greve A, Plackett DV, et al. Fabrication of biopolymer cantilevers using nanoimprint lithography. Microelectron Eng. 2011; 88(8): 2294-2296.
- Bartolo P, Kruth JP, Silva J, Levy G, Malshe A, Rajurkar K, et al. Biomedical production of implants by additive electro-chemical and physical processes. CIRP Ann. 2012; 61(2): 635-655.
- Castrejón-Pita JR, Martin GD, Hoath SD, Hutchings IM. A simple large-scale droplet generator for studies of inkjet printing. Rev Sci Instrum. 2008; 79(7): 075108.
[Crossref] [Google scholar] [Pubmed]
- Woo JH, Jo SY, Kang H, Noh I. Modification of the bulk properties of the porous poly (lactide-co-glycolide) scaffold by irradiation with a cyclotron ion beam with high energy for its application in tissue engineering. Biomed Mater. 2009; 4(4): 044101.
[Crossref] [Google scholar] [Pubmed]
- Baca HK, Carnes EC, Ashley CE, Lopez DM, Douthit C, Karlin S, et al. Cell-directed-assembly: Directing the formation of nano/bio interfaces and architectures with living cells. Biochim Biophys Acta. 2011; 1810(3): 259-267.
[Crossref] [Google scholar] [Pubmed]
- Cui X, Boland T. Human microvasculature fabrication using thermal inkjet printing technology. Biomaterials. 2009; 30(31): 6221-6227.
[Crossref] [Google scholar] [Pubmed]
- Yan KC, Paluch K, Nair K, Sun W. Effects of process parameters on cell damage in a 3D cell printing process. Int Mech Eng Congress. 2009; 43758: 75-81.
- Mendonça G, Mendonça DB, Simoes LG, Araujo AL, Leite ER, Duarte WR, et al. The effects of implant surface nanoscale features on osteoblast-specific gene expression. Biomaterials. 2009; 30(25): 4053-4062.
[Crossref] [Google scholar] [Pubmed]
- Mironov V, Visconti RP, Kasyanov V, Forgacs G, Drake CJ, Markwald RR. Organ printing: Tissue spheroids as building blocks. Biomaterials. 2009; 30(12): 2164-2174.
[Crossref] [Google scholar] [Pubmed]
- Xu T, Jin J, Gregory C, Hickman JJ, Boland T. Inkjet printing of viable mammalian cells. Biomaterials. 2005; 26(1): 93-99.
[Crossref] [Google scholar] [Pubmed]
- Li X, Wang L, Fan Y, Feng Q, Cui FZ. Biocompatibility and toxicity of nanoparticles and nanotubes. J Nanomater. 2012; 2012: 1-19.
- Li X, Gao H, Uo M, Sato Y, Akasaka T, Feng Q, et al. Effect of carbon nanotubes on cellular functions in vitro. J Biomed Mater Res A. 2009; 91(1): 132-139.
[Crossref] [Google scholar] [Pubmed]
- Li X, Yang Y, Fan Y, Feng Q, Cui FZ, Watari F. Biocomposites reinforced by fibers or tubes as scaffolds for tissue engineering or regenerative medicine. J Biomed Mater Res A. 2014; 102(5): 1580-1594.
[Crossref] [Google scholar] [Pubmed]
- Seyednejad H, Gawlitta D, Kuiper RV, de Bruin A, van Nostrum CF, Vermonden T, et al. In vivo biocompatibility and biodegradation of 3D-printed porous scaffolds based on a hydroxyl-functionalized poly (e-caprolactone). Biomaterials. 2012; 33(17): 4309-4318.
[Crossref] [Google scholar] [Pubmed]
- Flaibani M, Elvassore N. Gas anti-solvent precipitation assisted salt leaching for generation of micro-and nano-porous wall in bio-polymeric 3D scaffolds. Mater Sci Eng C Mater Biol Appl. 2012; 32(6): 1632-1639.
[Crossref] [Google scholar] [Pubmed]
- Li X, Liu X, Huang J, Fan Y, Cui FZ. Biomedical investigation of CNT based coatings. Surf Coat Technol. 2011; 206(4): 759-766.
- Kim G, Son J, Park S, Kim W. Hybrid process for fabricating 3D hierarchical scaffolds combining rapid prototyping and electrospinning. Macromol Rapid Commun. 2008; 29(19): 1577-1581.
- Wang JX, Fan YB, Gao Y, Hu QH, Wang TC. TiO2 nanoparticles translocation and potential toxicological effect in rats after intraarticular injection. Biomaterials. 2009; 30(27): 4590-4600.
[Crossref] [Google scholar] [Pubmed]
- Wang J, Gao Y, Hou Y, Zhao F, Pu F, Liu X, et al. Evaluation on cartilage morphology after intra-articular injection of titanium dioxide nanoparticles in rats. J Nanomater. 2012: 1.
- Jagur-Grodzinski J. Polymers for tissue engineering, medical devices, and regenerative medicine. Concise general review of recent studies. Polym Adv Technol. 2006; 17(6): 395-418.
- Ringeisen BR, Othon CM, Barron JA, Young D, Spargo BJ. Jet-based methods to print living cells. Biotechnol J. 2006; 1(9): 930-948.
[Crossref] [Google scholar] [Pubmed]
- Bergmair I, Mühlberger M, Hingerl K, Pshenay-Severin E, Pertsch T, Kley EB, et al. 3D materials made of gold using nanoimprint lithography. Microelectron Eng. 2010; 87(5-8): 1008-1010.
[Crossref] [Google scholar] [Pubmed]
- Fielding GA, Bandyopadhyay A, Bose S. Effects of silica and zinc oxide doping on mechanical and biological properties of 3D printed tricalcium phosphate tissue engineering scaffolds. Dent Mater. 2012; 28(2): 113-122.
[Crossref] [Google scholar] [Pubmed]
- de Bartolo L, Leindlein A, Hofmann D, Bader A, de Grey A, Curcio E, et al. Bio-hybrid organs and tissues for patient therapy: A future vision for 2030. Chem Eng Process. 2012; 51: 79-87.
- Mironov V, Kasyanov V, Markwald RR. Nanotechnology in vascular tissue engineering: From nanoscaffolding towards rapid vessel biofabrication. Trends Biotechnol. 2008; 26(6): 338-344.
[Crossref] [Google scholar] [Pubmed]
- Cervantes TM, Bassett EK, Tseng A, Kimura A, Roscioli N, Randolph MA, et al. Design of composite scaffolds and three-dimensional shape analysis for tissue-engineered ear. J R Soc Interface. 2013; 10(87): 20130413.
[Crossref] [Google scholar] [Pubmed]
- Fierz FC, Beckmann F, Huser M, Irsen SH, Leukers B, Witte F, et al. The morphology of anisotropic 3D-printed hydroxyapatite scaffolds. Biomaterials. 2008; 29(28): 3799-3806.
[Crossref] [Google scholar] [Pubmed]
- Sobral JM, Caridade SG, Sousa RA, Mano JF, Reis RL. Three-dimensional plotted scaffolds with controlled pore size gradients: Effect of scaffold geometry on mechanical performance and cell seeding efficiency. Acta biomaterialia. 2011; 7(3): 1009-1018.
[Crossref] [Google scholar] [Pubmed]
- Zhou WY, Lee SH, Wang M, Cheung WL, Ip WY. Selective laser sintering of porous tissue engineering scaffolds from poly (L-lactide)/carbonated hydroxyapatite nanocomposite microspheres. J Mater Sci Mater Med. 2008; 19: 2535-2540.
[Crossref] [Google scholar] [Pubmed]
- Kruth JP, Froyen L, van Vaerenbergh J, Mercelis P, Rombouts M, Lauwers B. Selective laser melting of iron-based powder. J Mater Process Technol. 2004; 149(1-3): 616-622. [Crossref]
- Marshall AJ, Ratner BD. Quantitative characterization of sphere-templated porous biomaterials. AIChE J. 2005; 51(4): 1221-1232.
- Colombo P, Bernardo E, Parcianello G. Multifunctional advanced ceramics from preceramic polymers and nano-sized active fillers. J Eur Ceram. 2013; 33(3): 453-469.
- Eom SH, Senthilarasu S, Uthirakumar P, Hong CH, Lee YS, Lim J, et al. Preparation and characterization of nano-scale ZnO as a buffer layer for inkjet printing of silver cathode in polymer solar cells. Sol Energy Mater. 2008; 92(5): 564-570. [Crossref]
- Wu C, Luo Y, Cuniberti G, Xiao Y, Gelinsky M. Three-dimensional printing of hierarchical and tough mesoporous bioactive glass scaffolds with a controllable pore architecture, excellent mechanical strength and mineralization ability. Acta biomaterialia. 2011; 7(6): 2644-2650.
[Crossref] [Google scholar] [Pubmed]
- Yoo D. New paradigms in hierarchical porous scaffold design for tissue engineering. Mater Sci Eng C Mater Biol Appl. 2013; 33(3): 1759-1772.
[Crossref] [Google scholar] [Pubmed]
- Tirella A, Vozzi F, de Maria C, Vozzi G, Sandri T, Sassano D, et al. Substrate stiffness influences high resolution printing of living cells with an ink-jet system. J Biosci Bioeng. 2011; 112(1): 79-85.
[Crossref] [Google scholar] [Pubmed]
- Lian Q, Li DC, Tang YP, Zhang YR. Computer modeling approach for a novel internal architecture of artificial bone. Comput Aided Des. 2006; 38(5): 507-514.
- Jose RR, Rodriguez MJ, Dixon TA, Omenetto F, Kaplan DL. Evolution of bioinks and additive manufacturing technologies for 3D bioprinting. ACS Biomater Sci Eng. 2016; 2(10): 1662-1678.
[Crossref] [Google scholar] [Pubmed]
- Skardal A, Atala A. Biomaterials for integration with 3-D bioprinting. Ann Biomed Eng. 2015; 43(3): 730-746.
[Crossref] [Google scholar] [Pubmed]
- Lee SJ, Nowicki M, Harris B, Zhang LG. Fabrication of a highly aligned neural scaffold via a table top stereolithography 3D printing and electrospinning. Tissue Eng Part A. 2017; 23(11-12): 491-502.
[Crossref] [Google scholar] [Pubmed]
- Chen C, Bang S, Cho Y, Lee S, Lee I, Zhang S, et al. Research trends in biomimetic medical materials for tissue engineering: 3D bioprinting, surface modification, nano/micro-technology and clinical aspects in tissue engineering of cartilage and bone. Biomater Res. 2016; 20(1): 1-7.
[Crossref] [Google scholar] [Pubmed]
- Henriksson I, Gatenholm P, Hägg DA. Increased lipid accumulation and adipogenic gene expression of adipocytes in 3D bioprinted nanocellulose scaffolds. Biofabrication. 2017; 9(1): 015022.
[Crossref] [Google scholar] [Pubmed]
- Shafiee A, Atala A. Tissue engineering: Toward a new era of medicine. Annu Rev Med. 2017; 68: 29-40.
[Crossref] [Google scholar] [Pubmed]
- Ferris CJ, Gilmore KJ, Beirne S, McCallum D, Wallace GG. M. Bio-ink for on-demand printing of living cells. Biomater Sci. 2013: 224-230.
- Velenis D, Stucchi M, Marinissen EJ, Swinnen B, Beyne E. Impact of 3D design choices on manufacturing cost. IEEE. 2009: 1-5.
- Faddoul R, Reverdy-Bruas N, Blayo A, Khelifi B. Inkjet printing of silver nano-suspensions on ceramic substrates-Sintering temperature effect on electrical properties. Microelectron Eng. 2013; 105: 31-39.
Author Info
Sara Zahid1, Fatima Zahid2, Faisal Gulzar3, Qurban Ali3, Ayesha Zahid1 and Arif Malik1*2Department of Biotechnology, Ibadat International University, Islamabad, Pakistan
3Department of Pharmacy, University of Lahore, Lahore, Pakistan
Citation: Zahid S: 3-D Bioprinted Nanobiomaterials: A Cutting Edge in Tissue Engineering and Tumor Therapy
Received: 20-Mar-2023 Accepted: 04-Apr-2023 Published: 11-Apr-2023, DOI: 10.31858/0975-8453.14.4.229-240
Copyright: This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
ARTICLE TOOLS
- Dental Development between Assisted Reproductive Therapy (Art) and Natural Conceived Children: A Comparative Pilot Study Norzaiti Mohd Kenali, Naimah Hasanah Mohd Fathil, Norbasyirah Bohari, Ahmad Faisal Ismail, Roszaman Ramli SRP. 2020; 11(1): 01-06 » doi: 10.5530/srp.2020.1.01
- Psychometric properties of the World Health Organization Quality of life instrument, short form: Validity in the Vietnamese healthcare context Trung Quang Vo*, Bao Tran Thuy Tran, Ngan Thuy Nguyen, Tram ThiHuyen Nguyen, Thuy Phan Chung Tran SRP. 2020; 11(1): 14-22 » doi: 10.5530/srp.2019.1.3
- A Review of Pharmacoeconomics: the key to “Healthcare for All” Hasamnis AA, Patil SS, Shaik Imam, Narendiran K SRP. 2019; 10(1): s40-s42 » doi: 10.5530/srp.2019.1s.21
- Deuterium Depleted Water as an Adjuvant in Treatment of Cancer Anton Syroeshkin, Olga Levitskaya, Elena Uspenskaya, Tatiana Pleteneva, Daria Romaykina, Daria Ermakova SRP. 2019; 10(1): 112-117 » doi: 10.5530/srp.2019.1.19
- Dental Development between Assisted Reproductive Therapy (Art) and Natural Conceived Children: A Comparative Pilot Study Norzaiti Mohd Kenali, Naimah Hasanah Mohd Fathil, Norbasyirah Bohari, Ahmad Faisal Ismail, Roszaman Ramli SRP. 2020; 11(1): 01-06 » doi: 10.5530/srp.2020.1.01
- Manilkara zapota (L.) Royen Fruit Peel: A Phytochemical and Pharmacological Review Karle Pravin P, Dhawale Shashikant C SRP. 2019; 10(1): 11-14 » doi: 0.5530/srp.2019.1.2
- Pharmacognostic and Phytopharmacological Overview on Bombax ceiba Pankaj Haribhau Chaudhary, Mukund Ganeshrao Tawar SRP. 2019; 10(1): 20-25 » doi: 10.5530/srp.2019.1.4
- A Review of Pharmacoeconomics: the key to “Healthcare for All” Hasamnis AA, Patil SS, Shaik Imam, Narendiran K SRP. 2019; 10(1): s40-s42 » doi: 10.5530/srp.2019.1s.21
- A Prospective Review on Phyto-Pharmacological Aspects of Andrographis paniculata Govindraj Akilandeswari, Arumugam Vijaya Anand, Palanisamy Sampathkumar, Puthamohan Vinayaga Moorthi, Basavaraju Preethi SRP. 2019; 10(1): 15-19 » doi: 10.5530/srp.2019.1.3