Review Article - (2022) Volume 13, Issue 4
A Histopathology Model of Astrocytoma: A Review
Alireza Arabestanino*, Arman Ai and Hasti JalaliAbstract
Neoplastic transformation occurs in all glial cell types of the human nervous system, producing a wide variety of clinic-pathological entities and morphological variants. As the molecular events responsible for astrocytoma formation and progression are being clarified, it is becoming possible to correlate these alterations with the specific histopathological and biological features of astrocytoma, anaplastic astrocytoma and glioblastoma multiforme (Sonoda Y, et al., 2001). Diagnosis, treatment, and prognostication in brain stem astrocytomas have been hindered by the occurrence in the same site of two distinct pathological entities-fibrillary and pilocytic astrocytoma. The small size of the specimens from this region adds an additional confounding factor in tumor classification. Nevertheless, histological assignment to either of these two prognostically different categories is often possible, especially if the importance of this distinction is recognized. In the face of a nonspecific histological diagnosis, e.g. "low-grade astrocytoma', certain radiographic and clinical features may, in combination with the pathological findings, be useful in tumor sub classification.
Keywords
Astrocytoma, Neoplastic, Anaplastic astrocytoma, Glioblastoma
Introduction
Epidemiology
The astrocytomas are the commonest form of gliomas and primary tumors of the brain in man. Tumorigenesis is a multi-step process in which cells become immortalized, proliferate, invade and modulate their environment. Over the past few years, molecular biological studies have provided insights into the basic mechanisms underlying such tumorigenic processes in a variety of tumors (Nishikawa R, et al., 1994), including the common diffuse fibrillary astrocyte. The Epidermal Growth Factor Receptor (EGFR) (Ekstrand AJ, et al., 1992), as well as the Platelet-Derived Growth Factor (PDGF) and its receptors, have been implicated as oncogenes and genes on chromosomes (Varmus HE, 1984) lp, 9p, 10, l2p, 13q, 17p, 19q and 22q as tumor suppressors (Kraus JA, et al., 1995). The World Health Organization (WHO) has recently published current criteria for classification and malignancy grading of all brain tumors (Schiffer D, 1993; Davis FG and Mc- Carthy BJ, 2000). The WHO malignancy grading has been shown to correlate to the biological behavior of the individual types tumor’s, and ranges from malignancy grade I (the least biologically aggressive) to grade IV (the most malignant) (Table 1). Some tumor types have only one grade others up to four (Kleihues P, et al., 1993).
| WHO Grade | WHO designation | St. Anne/Mayo | Histopathology | Associated genetic alterations |
|---|---|---|---|---|
| I | Pilocytic astrocytoma | Pilocytic astrocytoma | Bipolar, piloid cells, Rosenthal fibers, Eosinophilic granular bodies | Accumulation of p53 (>50%) |
| Deletion of 17q/NF1 (<20%) | ||||
| II | Low grade astrocytoma | Astrocytoma grade 1 and 2 | Neuroplastic fibrillary, or gemistocysitc astrocytes; nuclear atypia | p53 accumulation (>40%), p53 mutation (>25%), LOH 17p (>20%) |
| III | Anaplastic astrocytoma | Astrocytoma grade 3 | Neuroplastic fibrillary, or gemistocysitc astrocytes; nuclear atypia, mitotic activity | p53 accumulation (>50%), p53 mutation (>30%), LOH 10 (15%) 19q (40%) |
| IV | Glioblastoma multiforme | Astrocytoma grade 4 | Cellular anaplasia, nuclear atypia, mitoses, vascular proliferation, necrosis | p53 accumulation (40%), p53 mutation (>25%), LOH 10 (>60%), 17p (25%), 19q (24%), p16 deletion (>60%), EGF-R (>30%) and CDK4 (>70%) amplification |
Table 1: Comparison of the World Health Organization (WHO) and St. Anne/Mayo grading system for astrocytomas
The terminology used in this paper will correspond to the most recent WHO report. Astrocytic tumor’s constitute 65%-70% of all gliomas and are malignancy graded on the basis of histological features into grades I-IV (Kleihues P, et al., 1995). The pilocytic astrocytomas (Koeller KK and Rushing EJ, 2004) (malignancy grade I) are the least malignant, occur mainly in children (Jennings MT and Ivengar S, 2001), only very rarely progress to more malignant tumors and have generally a good prognosis. Consequently they will not be discussed further in this text. In contrast, the adult diffuse astrocytic tumor’s frequently show malignant progression. The adult diffuse astrocytomas include the astrocytomas (malignancy grade II), the anaplastic astrocytomas (malignancy grade III) and the glioblastomas (malignancy grade IV), and these will be the main focus of this text. The average survival of patients with an astrocytoma (malignancy grade II) is around 7 years, while patients with anaplastic astrocytomas have a median survival half that time. Glioblastoma patients have a very poor prognosis with average survival reported between 9 and 11 months despite modern therapy. Grade II tumors have a peak incidence between 25 and 50, while the glioblastomas have a peak incidence between 45 and 70 years. Glioblastomas are the most common form and are divided into those that develop from a previously diagnosed astrocytoma or anaplastic astrocytoma (secondary glioblastomas) and those that appear to develop de novo that is with no evidence of their having been an earlier tumor of lesser malignancy grade. There are clinical and molecular data to support the hypothesis that these tumors may develop in different ways. In this prospective, we develop a histopathological model which summarizes known molecular genetic abnormalities (Ichimura K, et al., 2000) and relates these two key histological and biological phenomena that characterize human astrocytomas.
Literature Review
Biological and histopathological of astrocytoma
The malignancy stages of astrocytic tumors are displayed graphically in Figure 1. Tumors of the cerebral hemispheres of young adults and on Computerized Tomographic (CT) scans which appear as ill-defined, non-enhancing masses are diagnosed as low grade astrocytomas. At the microscopic level, tumors with these radiographic features show normal brain infiltrated with dispersed small astrocytic cell with somewhat enlarged and irregular nuclei, although the cell density may not be markedly different from normal tissue (Figure 1). Cells from some of these tumors have been shown to have some cytogenetic abnormalities; usually numerical changes of chromosomes 7, 10, 22, and Y. Some astrocytomas remain dormant, some enlarge slowly, and some progress to medium or high grade tumors. As can be seen in Figure 1, these progressing tumors acquire distinct phenotypic features. For example, the higher stage recurrent tumor in the case shown in became enlarged and enhanced on CT. As well, progression is marked morphologically by increased cellularity, nuclear pleomorphism, and endothelial proliferation; and, in glioblastoma, the most malignant stage of the disease, necrosis (Figure 1). Cells from tumors with either medium or high grade histology have shown some consistent cytogenetic aberrations including loss of chromosome 10, gains of chromosome 7, various aberrations of chromosomes 9 and 22, and double minute chromosomes (Sugawa N, et al., 1990). Although some late stage tumors have clearly evolved from less malignant precursors, a large number seem to arise de novo. Whether de novo glioblastomas have evolved differently or just more rapidly and sub clinically, is debatable. Further, there is a curious relationship between age, tumor stage, and clinical behavior of astrocytic tumors (Schmidt E, et al., 1999). As a general rule, astrocytic tumors increase in frequency with advancing age, the proportion of malignant to benign tumors increases, and tumors of any stage tend to behave more aggressively in older patients. The basis for this relationship is unclear (Abdollahzadeh M, et al., 1994).
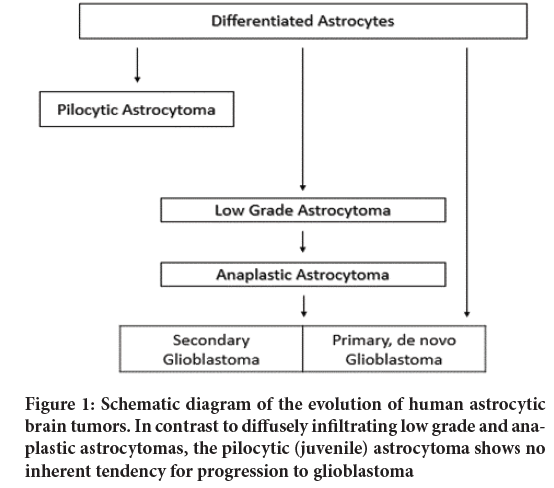
Figure 1: Schematic diagram of the evolution of human astrocytic brain tumors. In contrast to diffusely infiltrating low grade and anaplastic astrocytomas, the pilocytic (juvenile) astrocytoma shows no inherent tendency for progression to glioblastoma
Astrocytoma genetics
The assumption that astrocytomas arise from astrocytes is supported by two observations. First, astrocytoma cells have the morphologic characteristics of astrocytes and express the astrocyte marker, Glial Fibrillary Acidic Protein (GFAP). Second, mature astrocytes appear to retain the ability to divide, a feature characteristic of cells capable of neoplastic changes. On the other hand, these correlations have limitations especially when one considers mixed gliomas, tumors with more than one differentiated element. One hypothesis is that astrocytomas arise from initiated periventricular stem cells and that further genetic damage to these pluripotent precursors gives the tumors their individual peculiarities. This argument is particularly relevant when considering the relationship of the relatively less differentiated glioblastomas to other members of the pathway to date, evidence for the progressive nature of astrocytic malignancy has relied solely on the clinical and morphological analyses exemplified in. One of the cardinal features of advanced malignancy, the wide regional heterogeneity of cell types (both in terms of differentiation of the cells and their degrees of anaplasia) seems to suggest an ongoing evolution of cellular populations. Though apparently clonal tumors (Nowell PC, 1976; James CD, et al., 1988) appear to acquire divergent genetic characteristics through this evolution, resulting in genotypically and phenotypically diverse populations in the same tumor. In order to address some of these aspects of astrocytoma biology and to determine the underlying basis for progression of these tumors, we have undertaken a genetic lineage analysis. We have taken advantage of the wealth of information indicating the involvement of two classes of genetic aberrations in growth dysregulation (Mishima K, et al., 2001) and other phenotypes in the malignant cell. On one hand, the overexpression and genetic rearrangement of cellular or viral alleles of proto-oncogenes has been shown to occur with genetically dominant effects on the acquisition of malignant characteristics. On the other hand, the loss of alleles of genes presumably responsible for the maintenance of the normal growth state has also been shown to occur in tumors, relative to constitutional genotypes. Thus, the malignant phenotype appears to be the result of a combination and accumulation of events which act at loci controlling both the positive and negative regulation of cell growth (Furnari FB, et al., 1998). The clues we have followed in mapping these loci have come, in the main, from cytogenetics (Knudson AG, 1986). For example, the previously mentioned presence of double minute chromosomes and polysomy of chromosome 7 accomplishes genomic amplification of the epidermal growth factor receptor (EGFR) (Lorimer IA, et al., 1995) gene which is located on chromosome 7q. The incidence of EGFR amplification in gliomas (Humphrey PA, et al., 1988; Ekstrand AJ, et al., 1994) is in the range of 40% E131, and is exclusive to the more malignant stages. The aforementioned aberrations of chromosome 9p appear to result in deletions of the interferon (IFN) (Miyakoshi J, et al., 1990) 01 and p loci and these aberrations include most of the malignancy stages (James CD, et al., 1988). The observation of numerical changes of chromosomes 10 and 17 in glioblastoma has been refined and extended through the comparison of alleles at loci on these chromosomes in normal and tumor tissues of the various stages. These latter analyses showed that loss of alleles caused mainly by mitotic recombination of chromosome 17 homologues occurred in the low, medium, and high grade tumors (James CD, et al., 1989). Point mutations of the p53 gene (located on chromosome 17p) have been detected in glioblastomas and are now being analyzed in lower grade tumors as well (Louis DN, 1994). Allelic comparisons of chromosome 10 loci have shown a nearly obligate loss of one entire homologue in glioblastomas but not in lower stage tumors. Examples of the data supporting each of these inferences of genetic change are shown in. Although the functional influence of these changes on the attainment of malignancy is yet subject to experimental testing, they can be used to make several inferences about the process. Excepting alterations of chromosome 17p, these abnormalities have been restricted to glioblastoma and anaplastic astrocytoma, representatives of the malignant end of the spectrum. These data when combined with the biological evidence for malignant progression of fibrillary astrocytomas suggest that losses of genetic information for chromosome 10, deletion of the IFN loci, and other less frequent events are likely to be more closely related to tumor progression than initiation.
Clinical signs and symptoms
Signs and symptoms of grade I and grade II astrocytomas are subtle because the brain is able to temporarily adapt to the presence of a slow-growing tumor. Symptoms of grade III and grade IV astrocytomas may be sudden and debilitating. Symptoms can result from increased pressure within the brain and may include headaches, vision changes and nausea or vomiting. Symptoms may also occur based on the location of the tumor due to interference with normal brain function and include focal seizures, difficulty with speaking, loss of balance and weakness, paralysis or loss of sensation of one side of the body. Fatigue and depression are common in individuals with an astrocytoma. Desmoplastic Infantile Astrocytoma (DIA) is a very rare grade I astrocytoma. This tumor tends to occur in the cerebral hemispheres and is usually diagnosed in children less than two years of age. Symptoms may include an increased head size, bulging soft spots (fontanelles) in the skull, eyes that focus downward and seizures. A related tumor, desmoplastic infantile ganglioglioma, is a mixed astrocytic and neuronal tumor, but is otherwise similar to DIA. Subependymal giant cell astrocytoma occurs in the ventricles of the brain and is almost always associated with a genetic condition called tuberous sclerosis. Other rare neuroepithelial tumors (Aldape K, et al., 2000) include Pleomorphic Xanthoastrocytoma (PXA) and ganglioglioma (a mixed glial-neuronal tumor).
Results and Discussion
Astrocytic tumors
Astrocytomas are defined as tumors composed predominantly of neoplastic astrocytes. If not further specified, the term applies to diffusely infiltrative neoplasms which, according to their biologic behavior, are subdivided into low grade astrocytoma (WHO Grade II), anaplastic astrocytoma (WHO Grade III), and glioblastoma multiforme (WHO Grade IV). A distinctly different entity is the pilocytic astrocytoma which is decidedly different in terms of its location, age distribution, biologic behavior, and genetic basis.
Pilocytic astrocytoma (Malignancy grade I): This is the most frequent brain tumor in childhood, with a peak incidence between the ages of 8 to 13 years. In contrast to diffuse astrocytic tumors, the pilocytic astrocytoma is a more circumscribed, slowly growing lesion which very rarely undergoes progression to anaplasia. It therefore corresponds to WHO grade I. Pilocytic astrocytomas are typically located in midline structures, e.g., the optic nerve, the third ventricle, the thalamus, the median temporal lobe, the brain stem, and the cerebellum. Location in the cerebral hemispheres is much less common. Total surgical resection is difficult in some locations but relatively easy in others, such as the cerebellum. Subtotal resection is often followed by slow recurrence but some lesions remain stable over a course of several years. Macroscopically, the pilocytic astrocytoma is homogeneous, with occasional cysts and well circumscribed borders. It is histologically characterized by bipolar, fusiform, or “poloid cells with dense fibrillation. Particularly common is a biphasic pattern in which pilocytic areas are intimately associated with a loosely structured microcystic component. Hyperchromatic, bizarre nuclei, glomerid vascular proliferation, and invasion of the leptomeninges are common but do not see malignancy. Elongated eosinophilic, club-shaped structures (Rosenthal fibers), and eosinophilic intracytoplasmic protein droplets (“granular bodies”) are histopathological hallmarks of this neoplasm. Glial Fibrillary Acidic Protein (GFAP) expression is always demonstrable, although to a varying degree. The histogenesis of pilocytic astrocytomas has remained enigmatic. Its morphological characteristics and predilection for certain brain areas of children and adolescents may reflect an origin from a distinct precursor cell, but no such cell type has been identified to date. More likely is the possibility, that pilocytic astrocytomas do not have a distinct cell of origin but that their biological and morphological features reflect the acquisition of genetic alterations different from those of other gliomas. This explanation is supported by the observation that pilocytic astrocytomas, especially in the optic nerve, frequently occur in association with neurofibromatosis von Recklinghausen (NF1 syndrome) and that some sporadic pilocytic astrocytomas show loss of genetic material from chromosome 17q, the region in which the NF1 gene is located.
Astrocytomas (malignancy grade II): WHO Grade II molecular genetic events. The formation of WHO grade II astrocytomas is associated with at least three alterations-
• inactivation of the pS3 tumor suppressor gene
• activation of the PDGF system
• loss of a tumor suppressor gene on chromosome 22q
The pS3 gene on chromosome 17p encodes the p53 protein, which has an integral role in a number of cellular processes, including apoptosis, cell cycle arrest, response to DNA damage and angiogenesis. Inactivation of pS3, usually with mutation of one copy and chromosomal loss of the remaining allele, occurs in approximately one-third of astrocytomas, anaplastic astrocytomas and glioblastoma multiforme (GBM) (Simpson JR, et al., 1993). These mutations are primarily missense mutations and target the evolutionarily-conserved domains in exons 5, 7 and 8 (Nigro JM, et al., 1989). Particular hot spots for mutations include codons 175, 248 and 273, in which C to T transitions are most likely the result of spontaneous deamination of 5-methylcytosine residues. These mutations affect p53 residues that are crucial for DNA binding, presumably leading to loss of p53-mediated transcriptional activity. The MDM2 and p14ARF genes have been studied in small numbers of these tumors and no abnormalities have been reported. Recent studies of the TP53 related gene, P73, have not identified any mutations. Other findings considered significant include overexpression of the PDGFRA gene. Loss of alleles from 6q, 10p, 13q, and 22q occur in some astrocytomas. There is no evidence to suggest that there is mutation of the single retained tumor suppressor gene RB1 allele at 13q14.2 or the NF2 tumor suppressor gene on 22q (Myers MP, et al., 1998; Myers MP, et al., 1997). Deletion mapping of chromosomes 6 and 10 shows losses on 6q and the distal end of 10p in a significant number of astrocytomas (Humphrey PA, et al., 1990). The potential tumor suppressor genes in all of these regions remain unknown. There are no consistently reported amplified genes or regions of the genome reported in astrocytomas (Steck PA, et al., 1997). The changes found in the astrocytomas form the baseline for progression in the adult diffuse astrocytic tumor series. Epigenetic changes such as hypermethylation of tumor suppressor gene promoters may also play an important role in transcriptional silencing of important cancer genes and the development of astrocytomas. This has not been studied in any detail as yet.
Anaplastic (Malignant) astrocytomas (malignancy grade III): An astrocytoma with focal or diffuse anaplasia, e.g., a tendency to increased cellularity, pleomorphism, and nuclear atypia. In contrast to grade II astrocytomas it displays mitotic activity. Anaplastic astrocytomas may arise from low-grade astrocytomas but are also frequently diagnosed at first biopsy, without indication of a less malignant precursor lesion. The fraction of tumor cells with immunoreactivity for GFAP varies (Burns KL, et al., 1998). Anaplastic astrocytomas show an inherent and often rapid tendency to progress to glioblastoma. The numbers of cases of anaplastic astrocytomas studied is also limited. There are also some practical problems in studying a series of anaplastic astrocytomas. In sparsely sampled tumor’s the diagnosis can only indicate a minimum malignancy grade- there could be regions fulfilling the criteria for glioblastoma elsewhere. Thus in a series of anaplastic astrocytomas there may be some glioblastomas while there will be no anaplastic astrocytomas in a glioblastoma series as once the criteria for glioblastoma are fulfilled the tumor cannot be classified as anything other than a glioblastoma. However, this applies only to a clinical diagnosis that is always based on the worst findings in a tumor. Naturally in a tumor that is progressing from one malignancy grade to another there will remain tumor cell populations of the lesser malignancy grade that might be included in a sample molecular analysis. Thus the findings have to be interpreted with care and relatively large series are necessary to enable a correct interpretation of the findings. Cytogenetics, comparative genomic hybridization and molecular genetic techniques all show that the losses of alleles on 6q, 10p, 13q, 17p and 22q, as seen in the astrocytoma malignancy grade II and they occur at similar or higher frequencies in the anaplastic astrocytomas. Mutations of the TP53 gene also occur at approximately the same frequency (Cho Y, et al., 1994). Thus in the anaplastic astrocytomas the p53 pathway is also non-functional and in the majority of cases (approximately 67%) and this is due to mutations of the TP53 gene. With the sole exception of losses of alleles on 19q (targeted gene(s) unknown) there are no conclusively demonstrated abnormalities specific to this malignancy grade. Around 20% of anaplastic astrocytomas show similar genetic abnormalities to those found in glioblastomas and discussed below.
Glioblastomas (malignancy grade IV): This is the most frequent and malignant brain tumor and typically affects adults (Winger MJ, et al., 1989), with a peak incidence between 45 and 60 years. The clinical history is usually short (less than 3 months) unless the neoplasms have developed from low grade or anaplastic astrocytoma (secondary glioblastoma). Patients typically present with nonspecific neurological symptoms, such as headache and personality changes, or with rapid development of life threatening intracranial pressure. After incomplete surgical resection and radiotherapy, the mean survival time is in the range of 6-9 months. The histological criteria for glioblastomas are well defined and these tumors if adequately sampled are generally easy to differentiate from the astrocytomas of lower malignancy grade. Despite this, glioblastomas developing from tumors of lesser malignancy grade (secondary glioblastomas) may retain in the primary tumor populations of cells that represent the different stages that the tumor went through during its progression. Secondary glioblastomas have only been studied in relatively small numbers for a myriad of reasons. However, the high frequency de novo glioblastomas has permitted their study in considerable numbers. Glioblastomas show the greatest numbers of genetic abnormalities among the astrocytic tumor’s and clear patterns of abnormality in these tumors are emerging. In addition to the targeting of the p53 pathway as is seen in the astrocytomas and anaplastic astrocytomas, the mechanisms controlling cellular entry into the S-phase of the cell cycle are also rendered inoperative. In contrast to the astrocytomas and anaplastic astrocytomas, the glioblastomas abrogate the p53 pathway in different ways. Some have no wild-type TP53 gene, as found in the astrocytomas of lower malignancy grade (approximately 37%), but note that this incidence is much lower. Others have wild-type TP53 genes but mutate genes coding for proteins that control cellular levels of p53. The mutations found lead to the rapid brake-down of the wild-type protein resulting in a cell with little or no wild-type p53.
Clinical management and therapy
The use of molecular findings in providing useful prognostic information in astrocytic tumor’s has yet to be realized. There have been many studies providing results that are frequently difficult to interpret (Figure 2). The age of the patient at diagnosis and the histopathological information still provide the most useful data. We should not be disheartened by this as our understanding of the workings of the normal and malignant cell are as yet rudimentary. Many of the molecular and genetic findings outlined above have also resulted in attempts to experimentally design therapies to block or reinstate the cellular mechanisms activated or deranged by the mutations observed (McCormack BM, et al., 1992). Some have even got to the stage of phase I and II trials. While many have built on careful observation of human tumor tissue and extensive experimental manipulation in laboratory tests no clear therapeutic breakthrough has yet occurred. However, we can expect that the more we understand the complex mechanisms of the normal cell as well as the aberrations that occur in the malignant cell we will be able to find ways of specifically treating malignant disease, leaving the normal surrounding brain less, if not entirely, undamaged.
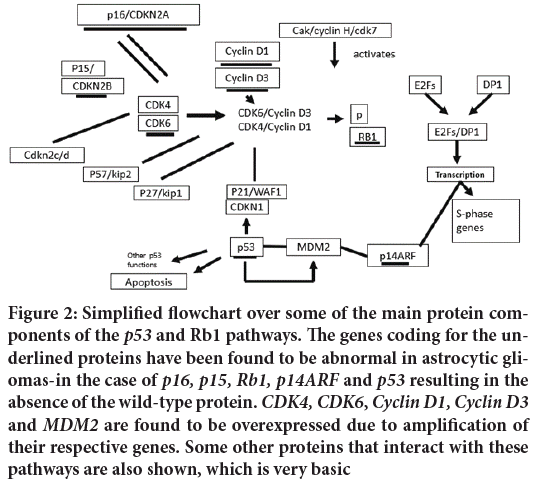
Figure 2: Simplified flowchart over some of the main protein components of the p53 and Rb1 pathways. The genes coding for the underlined proteins have been found to be abnormal in astrocytic gliomas-in the case of p16, p15, Rb1, p14ARF and p53 resulting in the absence of the wild-type protein. CDK4, CDK6, Cyclin D1, Cyclin D3 and MDM2 are found to be overexpressed due to amplification of their respective genes. Some other proteins that interact with these pathways are also shown, which is very basic
Conclusion
Although the model presented here is undoubtedly very simple, it is a simple and logical model of the formation and histopathology of emerging astrocytomas. Some histological and pathological signs of astrocytomas can be partially explained molecularly-biologically. Pathological changes can also be caused by a number of biological and genetic pathways. So far, many studies have shown little correlation between biological and genetic parameters and patient prognosis, but there is no clear association with tumor behavior. However, the ability of molecular and pathological research to evaluate biological events in human astrocytomas increases the likelihood that a new approach to diagnosis and treatment based on understanding biological phenomena will emerge.
References
- Nishikawa R, Ji XD, Harmon RC, Lazar CS, Gill GN, Cavenee WK, et al. A mutant epidermal growth factor receptor common in human glioma confers enhanced tumor tumorigenicity. Proc Natl Acad Sci. 1994; 91(16): 7727-7731.
[Crossref] [Google scholar] [Pubmed]
- Ekstrand AJ, Sugawa N, James CD, Collins VP. Amplified and rearranged epidermal growth factor receptor genes in human glioblastomas reveal deletions of sequences encoding portions of the N-and/or C-terminal tails. Proc Natl Acad Sci. 1992; 89(10): 4309-4313.
[Crossref] [Google scholar] [Pubmed]
- Varmus HE. The molecular genetics of cellular oncogenes. Annu Rev Genet. 1984; 18(1): 553-612.
[Crossref] [Google scholar] [Pubmed]
- Kraus JA, Koopmann J, Kaskel P, Maintz D, Brandner S, Schramm J, et al. Shared allelic losses on chromosomes lp and 19q suggest a common origin of oligodendroglioma and oligoastrocytoma. J Neuropathol Exp Neurol. 1995; 54(1): 91-95.
[Crossref] [Google scholar] [Pubmed]
- Schiffer D. Brain tumors: Pathology and its biological correlates. Springer. 1993.
- Davis FG, McCarthy BJ. Epidemiology of brain tumors. Curr Opin Neurol. 2000; 13(6): 635-640.
[Crossref] [Google scholar] [Pubmed]
- Kleihues P, Burger PC, Scheithauer BW. Histological typing of tumors of the central nervous system: World health organization international histological classification of tumors. Springer. 1993.
- Kleihues P, Soylemezoglu F, Schäuble B, Scheithauer BW, Burger PC. Histopathology, classification, and grading of gliomas. Glia. 1995; 15(3): 211-221.
[Crossref] [Google scholar] [Pubmed]
- Koeller KK, Rushing EJ. Pilocytic astrocytoma: Radiologic-pathologic correlation. Radiographics. 2004; 24, 1693-708.
- Jennings MT, Ivengar S. Pharmacotherapy of malignant astrocytomas of children and adults: Current strategies and future trends. CNS Drugs. 2001; 15(9): 719-743.
[Crossref] [Google scholar] [Pubmed]
- Ichimura K, Bolin MB, Goike HM, Schmidt EE, Moshref A, Collins VP. Deregulation of the p14ARF/MDM2/p53 pathway is a prerequisite for human astrocytic gliomas with G1-S transition control gene abnormalities. Cancer Res. 2000; 60(2): 417-424.
[Google scholar] [Pubmed]
- Sugawa N, Ekstrand AJ, James CD, Collins VP. Identical splicing of aberrant epidermal growth factor receptor transcripts from amplified rearranged genes in human glioblastomas. Proc Natl Acad Sci. 1990; 87(21): 8602-8606.
[Crossref] [Google scholar] [Pubmed]
- Schmidt E, Ichimura E, Goike HM, Moshref A, Liu L, Collins VP. Mutational profile of the PTEN/MMAC1 gene in primary human astrocytic tumors and xenografts. J Neuropathol Exp Neurol. 1999; 58(11): 1170-1183.
[Crossref] [Google scholar] [Pubmed]
- Abdollahzadeh M, Hoffman HJ, Blazer SI, Becker LE, Humphreys RP, Drake JM, et al. Benign cerebellar astrocytoma in childhood: Experience at the hospital for sick children 1980-1992. Childs Nerv Syst. 1994; 10: 380-383.
[Crossref] [Google scholar] [Pubmed]
- Nowell PC. The clonal evolution of tumor cell populations: Acquired genetic lability permits stepwise selection of variant sublines and underlies tumor progression. Science. 1976; 194: 23.
[Crossref] [Google scholar] [Pubmed]
- James CD, Carlbom E, Dumanski JP, Hansen M, Nordenskjold M, Collins VP, et al. Clonal genomic alterations in glioma malignancy stages. Cancer Res. 1988; 48(19): 5546-5551.
[Google scholar] [Pubmed]
- Mishima K, Johns TG, Luwor RB, Scott AM, Stockert E, Jungbluth AA, et al. Growth suppression of intracranial xenografted glioblastomas overexpressing mutant epidermal growth factor receptors by systemic administration of monoclonal antibody (mAb) 806, a novel monoclonal antibody directed to the receptor. Cancer Res. 2001; 61(14): 5349-5354.
[Google scholar] [Pubmed]
- Furnari FB, Huang HJ, Cavenee WK. The phosphoinositol phosphatase activity of PTEN mediates a serum-sensitive G1 growth arrest in glioma cells. Cancer Res. 1998; 58(22): 5002- 5008.
[Google scholar] [Pubmed]
- Knudson AG. Genetics of human cancer. Annu Rev Genet. 1986; 20: 231-251.
[Crossref] [Google scholar] [Pubmed]
- Lorimer IA, Wikstrand CJ, Batra SK, Bigner DD, Pastan I. Immunotoxins that target an oncogenic mutant epidermal growth factor receptor expressed in human tumors. Clin Cancer Res. 1995; 1(8): 859-864.
[Google scholar] [Pubmed]
- Humphrey PA, Wong AJ, Vogelstein B, Friedman HS, Werner MH, Bigner DD, et al. Amplification and expression of the epidermal growth factor receptor gene in human glioma xenografts. Cancer Res. 1988; 48(8): 2231-2238.
[Crossref] [Google scholar] [Pubmed]
- Ekstrand AJ, Longo N, Hamid ML, Olson JJ, Liu L, Collins VP, et al. Functional characterization of an EGF receptor with a truncated extracellular domain expressed in glioblastomas with EGFR gene amplification. Oncogene. 1994; 9(8): 2313-2320.
[Google scholar] [Pubmed]
- Miyakoshi J, Dobler KD, Turner AJ, McKean JD, Petruk K, Allen PB, et al. Absence of IFNA and IFNB genes from human malignant glioma cell lines and lack of correlation with cellular sensitivity to interferons. Cancer Res. 1990; 50(2): 278.
[Google scholar] [Pubmed]
- James CD, Carlbom E, Nordenskjold M, Collins VP, Cavenee WK. Mitotic recombination of chromosome 17 in astrocytomas. Proc Natl Acad Sci. 1989; 86(8): 2858-2862.
[Crossref] [Google scholar] [Pubmed]
- Louis DN. The p53 gene and protein in human brain tumors. J Neuropathol Exp Neurol. 1994; 53: 11-21.
[Crossref] [Google scholar] [Pubmed]
- Aldape K, Simmons ML, Davis RL, Miike R, Wiencke J, Barger G, et al. Discrepancies in diagnoses of neuroepithelial neoplasms: The San Francisco Bay area adult glioma study. Cancer. 2000; 88(10): 2342-2349.
[Crossref] [Google scholar] [Pubmed]
- Simpson JR, Horton J, Scott C, Curran WJ, Rubin P, Fischbach J, et al. Influence of location and extent of surgical resection on survival of patients with glioblastoma multiforme: Results of three consecutive Radiation Therapy Oncology Group (RTOG) clinical trials. Int J Radiat Oncol Biol Phys. 1993; 26(2): 239-244.
[Crossref] [Google scholar] [Pubmed]
- Nigro JM, Baker SJ, Preisinger AC, Jessup JM, Hostetter R, Cleary K, et al. Mutations in the p53 gene occur in diverse human tumour types. Nature. 1989; 342(6250): 705-708.
[Crossref] [Google scholar] [Pubmed]
- Myers MP, Pass I, Batty IH, Kaay J, Stolarov JP, Hemmings BA, et al. The lipid phosphatase activity of PTEN is critical for its tumor suppressor function. Proc Natl Acad Sci. 1998; 95(23): 13513-13518.
[Crossref] [Google scholar] [Pubmed]
- Myers MP, Stolarov JP, Eng C, Li J, Wang SI, Wigler WH, et al. PTEN, the tumor suppressor from human chromosome 10q23, is a dual-specificity phosphatase. Proc Natl Acad Sci. 1997; 94(17): 9052-9057.
[Crossref] [Google scholar] [Pubmed]
- Humphrey PA, Wong AJ, Vogelstein B, Zalutsky MR, Fuller GN, Archer GE, et al. Anti-synthetic peptide antibody reacting at the fusion junction of deletion-mutant epidermal growth factor receptors in human glioblastoma. Proc Natl Acad Sci. 1990; 87(11): 4207-4211.
[Crossref] [Google scholar] [Pubmed]
- Steck PA, Pershouse MA, Jasser SA, Yung WKA, Lin H, Ligon AH, et al. Identification of a candidate tumor suppressor gene, MMAC1, at chromosome 10q23.3 that is mutated in multiple advanced cancers. Nat Genet. 1997; 15(4): 356-362.
[Crossref] [Google scholar] [Pubmed]
- Burns KL, Ueki K, Jhung SL, Koh J, Louis DN. Molecular genetic correlates of p16, cdk4 and pRb immunohistochemistry in glioblastomas. J Neuropathol Exp Neurol. 1998; 57(2): 122-130.
[Crossref] [Google scholar] [Pubmed]
- Cho Y, Gorina S, Jeffrey PD, Pavletich NP. Crystal structure of a p53 tumor suppressor-DNA complex: Understanding tumorigenic mutations science. 1994; 265: 346- 355.
[Crossref] [Google scholar] [Pubmed]
- Winger MJ, Macdonald DR, Cairncross JG. Supratentorial anaplastic gliomas in adults. The prognostic importance of extent of resection and prior low-grade glioma. J Neurosurg. 1989; 71(4): 487-493.
[Crossref] [Google scholar] [Pubmed]
- McCormack BM, Miller DC, Budzilovich GN, Voorhees GJ, Ransohoff J. Treatment and survival of low-grade astrocytoma in adults-1977-1988. Neurosurgery. 1992; 31(4): 636- 642.
[Crossref] [Google scholar] [Pubmed]
Author Info
Alireza Arabestanino*, Arman Ai and Hasti JalaliCitation: Arabestanino A: A Histopathology Model of Astrocytoma: A Review
Received: 08-Mar-2022 Accepted: 29-Mar-2022 Published: 05-Apr-2022, DOI: 10.31858/0975-8453.13.4.229-234
Copyright:
ARTICLE TOOLS
- Dental Development between Assisted Reproductive Therapy (Art) and Natural Conceived Children: A Comparative Pilot Study Norzaiti Mohd Kenali, Naimah Hasanah Mohd Fathil, Norbasyirah Bohari, Ahmad Faisal Ismail, Roszaman Ramli SRP. 2020; 11(1): 01-06 » doi: 10.5530/srp.2020.1.01
- Psychometric properties of the World Health Organization Quality of life instrument, short form: Validity in the Vietnamese healthcare context Trung Quang Vo*, Bao Tran Thuy Tran, Ngan Thuy Nguyen, Tram ThiHuyen Nguyen, Thuy Phan Chung Tran SRP. 2020; 11(1): 14-22 » doi: 10.5530/srp.2019.1.3
- A Review of Pharmacoeconomics: the key to “Healthcare for All” Hasamnis AA, Patil SS, Shaik Imam, Narendiran K SRP. 2019; 10(1): s40-s42 » doi: 10.5530/srp.2019.1s.21
- Deuterium Depleted Water as an Adjuvant in Treatment of Cancer Anton Syroeshkin, Olga Levitskaya, Elena Uspenskaya, Tatiana Pleteneva, Daria Romaykina, Daria Ermakova SRP. 2019; 10(1): 112-117 » doi: 10.5530/srp.2019.1.19
- Dental Development between Assisted Reproductive Therapy (Art) and Natural Conceived Children: A Comparative Pilot Study Norzaiti Mohd Kenali, Naimah Hasanah Mohd Fathil, Norbasyirah Bohari, Ahmad Faisal Ismail, Roszaman Ramli SRP. 2020; 11(1): 01-06 » doi: 10.5530/srp.2020.1.01
- Manilkara zapota (L.) Royen Fruit Peel: A Phytochemical and Pharmacological Review Karle Pravin P, Dhawale Shashikant C SRP. 2019; 10(1): 11-14 » doi: 0.5530/srp.2019.1.2
- Pharmacognostic and Phytopharmacological Overview on Bombax ceiba Pankaj Haribhau Chaudhary, Mukund Ganeshrao Tawar SRP. 2019; 10(1): 20-25 » doi: 10.5530/srp.2019.1.4
- A Review of Pharmacoeconomics: the key to “Healthcare for All” Hasamnis AA, Patil SS, Shaik Imam, Narendiran K SRP. 2019; 10(1): s40-s42 » doi: 10.5530/srp.2019.1s.21
- A Prospective Review on Phyto-Pharmacological Aspects of Andrographis paniculata Govindraj Akilandeswari, Arumugam Vijaya Anand, Palanisamy Sampathkumar, Puthamohan Vinayaga Moorthi, Basavaraju Preethi SRP. 2019; 10(1): 15-19 » doi: 10.5530/srp.2019.1.3






