Review Article - (2022) Volume 13, Issue 5
Abstract
A drug manufacturer applies for a patent to protect their drug from being copied or sold by other companies. A patent is granted for a period of time during which, only the original manufacturer can research, develop and sell the brand name drug. When the patent expires, other manufacturers can submit an Abbreviated New Drug Application (ANDA) to the FDA for approval to market the generic version. A generic drug is equivalent to its brand name, but usually much less expensive. Generic drugs are approved by the U.S. Food and Drug Administration (FDA), and are deemed to be safe and effective as the brand name product. Generic drugs must meet strict FDA requirements with respect to quality, performance, labeling, manufacturing and bioequivalence. Generic drugs must have same active ingredient and expected to have same effect when used in place of brand name drug. This review aims to provide information on the drugs whose patent is going to expire in the coming years, and expecting their generic version in the market.
Keywords
Abbreviated New Drug Application (ANDA), Off-patent, US Food and Drug Administration (USFDA), Generic drugs
Introduction
Patent
A patent is a property right issued by the United States Patent and Trademark Office (USPTO) to an inventor “to exclude others from making, using, offering for sale, or selling the invention throughout the United States or importing the invention into the United States” for a limited time, in exchange for public disclosure of the invention when the patent is granted.
Generally, the term of a new patent is 20 years from the date on which the application for the patent was filed in the United States (Lal R, 2015).
Patent expiration
When a drug's US patent expires, manufacturers other than the initial developer may take advantage of an abbreviated approval process to introduce lower-priced generic versions. An expired patent no longer affords the inventor or patent owner any protection, the concept becomes available for any organization or individual to freely use. In most uses, generics are clinically equivalent to the original branded drug. Some drugs are straightforward to imitate and produce at low cost. Others, particularly those requiring sterile manufacturing conditions or similarly complex production processes, are much more costly to copy (Gorman L, 2014).
Generic drug
A generic drug is a medication created to be the same as an already marketed brand-name drug in dosage form, safety, strength, route of administration, quality, performance characteristics, and intended use. These similarities help to demonstrate bioequivalence, which means that a generic medicine works in the same way and provides the same clinical benefit as its brand-name version. Generic drugs also tend to cost less than their brand-name counterparts because generic drug applicants do not have to repeat animal and clinical (human) studies that were required of the brand-name medicines to demonstrate safety and effectiveness. This is why the application is called an “abbreviated new drug application” (FDA, 2020).
Standard generic medicines must meet to receive FDA approval
Generic applicants must scientifically demonstrate that their product is performs in the same manner as the innovator drug. One way applicants demonstrate that a generic product performs in the same way as the innovator drug is to measure the time it takes the generic drug to reach the bloodstream in healthy volunteers. This demonstration of “bioequivalence” gives the rate of absorption, or bioavailability, of the generic drug, which can then be compared to that of the innovator drug. To be approved by FDA, the generic version must deliver the same amount of active ingredients into a patient's bloodstream in the same amount of time as the innovator drug.
The "Drug Price Competition and Patent Term Restoration Act of 1984," also known as the Hatch-Waxman Amendments, established bioequivalence as the basis for approving generic copies of drug products. These Amendments permit FDA to approve applications to market generic versions of brand-name drugs without repeating costly and duplicative clinical trials to establish safety and efficacy (FDA, 2020).
The orange book
The publication of Approved Drug Products with Therapeutic Equivalence Evaluations (commonly known as the Orange Book) identifies drug products approved on the basis of safety and effectiveness by the Food and Drug Administration (FDA) under the Federal Food, Drug, and Cosmetic Act (the FD and C Act) and related patent and exclusivity information. All approved products, both innovator and generic, are listed in FDA's Approved Drug Products with Therapeutic Equivalence Evaluations (Orange Book) (FDA, 2020).
Literature Review
Drug patent that are expiring between 2022 to 2025
Desvenlafaxine succinate:
Innovator brand name: PRISTIQ®
Patent number: US6673838
Application number: N021992
Patent expiry date: 03-01-2022
Chemical name: 4-[2-dimethylamino-1-(1-hydroxycyclohexyl) ethyl] phenol (Figure 1)
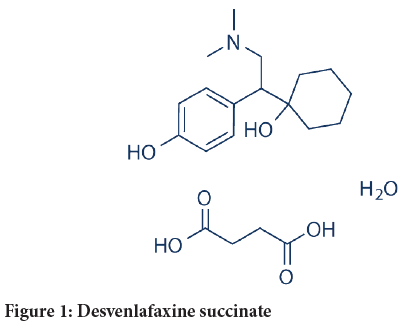
Figure 1: Desvenlafaxine succinate
Empirical formula: C16H25NO2 (free base) and C16H25NO2(C4H6O4) (H2O) (succinate monohydrate).
Molecular weight: Desvenlafaxine succinate monohydrate has a molecular weight of 399.48.
Indication: Major depressive disorder
Active ingredient: Each tablet contains 76 or 152 mg of desvenlafaxine succinate equivalent to 50 or 100 mg of desvenlafaxine, respectively (FDA, 2020; Pharmacompass, 2020; FDA, 2020).
Inactive ingredient: 50 mg tablet consist of hypromellose, microcrystalline cellulose, talc, magnesium stearate and film coating, which consists of polyvinyl alcohol, polyethylene glycol, talc, titanium dioxide, and iron oxides.
100 mg tablet consist of hypromellose, microcrystalline cellulose, talc, magnesium stearate and film coating, which consists of polyvinyl alcohol, polyethylene glycol, talc, titanium dioxide, iron oxide and FD and C yellow #6.
Route: Oral
Dosage form and strength: PRISTIQ® Extended-release tablets are available as 50 and 100 mg tablets.
50 mg, light pink, square pyramid tablet debossed with “W” over “50” on the flat side.
100 mg, reddish-orange, square pyramid tablet debossed with “W” over “100” on the flat side.
Recommended dose: 50 mg once daily with or without food
Mechanism of action: Desvenlafaxine succinate is a potent and selective Serotonin and Norepinephrine Reuptake Inhibitor (SNRI). The clinical efficacy of desvenlafaxine succinate is thought to be related to the potentiation of these neurotransmitters in the central nervous system.
Pharmacodynamics: Desvenlafaxine lacked significant affinity for numerous receptors, including muscarinic cholinergic, H1-histaminergic, or α1-adrenergic receptors in vitro. PRISTIQ also lacked Monoamine Oxidase (MAO) inhibitory activity.
Pharmacokinetics: The single-dose pharmacokinetics of desvenlafaxine is linear and dose-proportional in a dose range of 100 to 600 mg/day. The mean terminal half-life, t1/2, is approximately 11 hours. With once- daily dosing, steady-state plasma concentrations are achieved within approximately 4-5 days. At steady-state, multiple-dose accumulation of desvenlafaxine is linear and predictable from the single-dose pharmacokinetic profile.
Adverse effect: Hypersensitivity, effects on blood pressure, abnormal bleeding, mydriasis, hypomania and mania, serum cholesterol and triglyceride elevation, seizure.
Modafinil:
Innovator brand name: PROVIGIL®
Patent number: US7297346
Application number: N020717
Patent expiry date: 11-29-2023
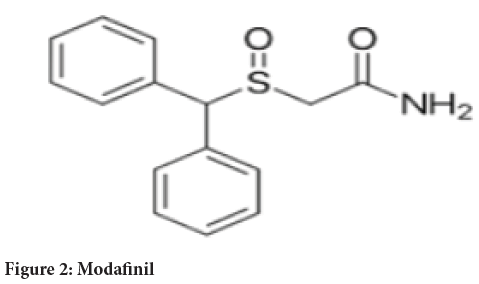
Chemical name: 2-[(diphenylmethyl)sulfinyl]acetamide (Figure 2)
Figure 2: Modafinil
Molecular formula: C15H15NO2S
Molecular weight: 273.35 g/mole
Indication: To improve wakefulness in adult patients with excessive sleepiness associated with narcolepsy, Obstructive Sleep Apnea (OSA), or Shift Work Disorder (SWD) (FDA, 2020; Pharmacompass, 2020; FDA, 2020).
Active ingredient: 100 mg or 200 mg of modafinil
Inactive ingredient: Croscarmellose sodium, lactose monohydrate, magnesium stearate, microcrystalline cellulose, povidone, and pregelatinized starch.
Route: Oral
Dosage form and strength: 100 mg-capsule-shaped, white to off white, tablet, debossed with "PROVIGIL" on one side and "100 mg" on the other. 200 mg capsule-shaped, white to off white, scored tablet, debossed with "PROVIGIL" on one side and "200 mg" on the other.
Recommended dose: The recommended dosage for patients with narcolepsy or OSA is 200 mg taken orally once a day as a single dose in the morning. Doses up to 400 mg/day, given as a single dose, have been well tolerated, but there is no consistent evidence that this dose confers additional benefit beyond that of the 200 mg/day dose. The recommended dosage of PROVIGIL for patients with SWD is 200 mg taken orally once a day as a single dose approximately 1 hour prior to the start of their work shift.
Mechanism of action: Modafinil is not a director indirect-acting dopamine receptor agonist. However, in vitro, modafinil binds to the dopamine transporter and inhibits dopamine reuptake. This activity has been associated in vivo with increased extracellular dopamine levels in some brain regions of animals. In genetically engineered mice lacking the Dopamine Transporter (DAT), modafinil lacked wake-promoting activity, suggesting that this activity was DAT-dependent.
Pharmacokinetics: Modafinil is a 1:1 racemic compound, whose enantiomers have different pharmacokinetics (e.g., the half-life of R-modafinil is approximately three times that of S-modafinil in adult humans). The enantiomers do not interconvert. At steady state, total exposure to R-modafinil is approximately three times that for S-modafinil. The trough concentration (Cmin, ss) of circulating modafinil after once daily dosing consists of 90% of R-modafinil and 10% of S-modafinil. The effective elimination half-life of modafinil after multiple doses is about 15 hours.
Adverse effect: Serious Rash, including stevens-johnson syndrome, angioedema and anaphylaxis reactions, multi-organ hypersensitivity reactions, persistent sleepiness, psychiatric symptoms, effects on ability to drive and use machinery, cardiovascular events.
Canagliflozin:
Innovator brand name: INVOKANA®
Patent number: US8222219
Application number: N204042
Patent expiry date: 04-11-2025
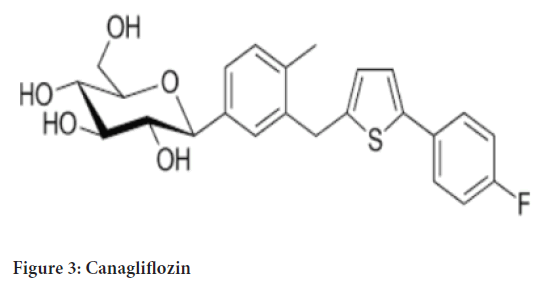
Chemical name: (1S)-1,5-anhydro-1-[3-[[5-(4-fluorophenyl)-2-thienyl] methyl]-4-methylphenyl]-D-glucitol hemihydrate (Figure 3)
Figure 3: Canagliflozin
Molecular formula: C24H25FO5S (1/2 H2O)
Molecular weight: 453.53
Indication: As an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus (FDA, 2020; Pharmacompass, 2020; FDA, 2020).
Active ingredient: 102 and 306 mg of canagliflozin in each tablet strength, corresponding to 100 mg and 300 mg of canagliflozin (anhydrous), respectively.
Inactive ingredient: Inactive ingredients of the core tablet are croscarmellose sodium, hydroxypropyl cellulose, lactose anhydrous, magnesium stearate, and microcrystalline cellulose. The magnesium stearate is vegetable-sourced. The tablets are finished with a commercially available film-coating consisting of the following excipients: Polyvinyl alcohol (partially hydrolyzed), titanium dioxide, macrogol/Polyethylene glycol (PEG), talc, and iron oxide yellow, E172 (100 mg tablet only).
Route: Oral
Dosage form and strength: INVOKANA 100 mg tablets are yellow, capsule-shaped, film-coated tablets with “CFZ” on one side and “100” on the other side.
INVOKANA 300 mg tablets are white, capsule-shaped, film-coated tablets with “CFZ” on one side and “300” on the other side.
Recommended dose: The recommended starting dose is 100 mg once daily, taken before the first meal of the day. In patients tolerating 100 mg once daily who have an estimated Glomerular Filtration Rate (eGFR) of 60 mL/min/1.73 m2 or greater and require additional glycemic control, the dose can be increased to 300 mg once daily.
Mechanism of action: Sodium-Glucose co-transporter 2 (SGLT2), expressed in the proximal renal tubules, is responsible for the majority of the reabsorption of filtered glucose from the tubular lumen. Canagliflozin is an inhibitor of SGLT2. By inhibiting SGLT2, canagliflozin reduces reabsorption of filtered glucose and lowers the Renal Threshold for Glucose (RTG), and thereby increases Urinary Glucose Excretion (UGE).
Pharmacodynamics: Following single and multiple oral doses of canagliflozin in patients with type 2 diabetes, dose dependent decreases in the Renal Threshold for Glucose (RTG) and increases in urinary glucose excretion were observed. From a starting RTG value of approximately 240 mg/dL, canagliflozin at 100 mg and 300 mg once daily suppressed RTG throughout the 24-hour period. Maximal suppression of mean RTG over the 24-hour period was seen with the 300 mg daily dose to approximately 70 to 90 mg/dL in patients with type 2 diabetes in Phase 1 studies. The reductions in RTG led to increases in mean UGE of approximately 100 g/ day in subjects with type 2 diabetes treated with either 100 mg or 300 mg of canagliflozin.
Pharmacokinetics: The pharmacokinetics of canagliflozin is similar in healthy subjects and patients with type 2 diabetes. Following single-dose oral administration of 100 mg and 300 mg, peak plasma concentrations (median Tmax) of canagliflozin occurs within 1 to 2 hours post-dose. Plasma Cmax and AUC (Area under Curve) of canagliflozin increased in a dose-proportional manner from 50 mg to 300 mg.
Adverse effects: Lower limb amputation, hypotension, ketoacidosis, acute kidney injury and impairment in renal function, hyperkalemia, urosepsis and pyelonephritis, hypoglycemia with concomitant use with insulin and insulin secretagogues, genital mycotic infections, hypersensitivity reactions, bone fracture, increases in Low-Density Lipoprotein (LDL-C).
Sitagliptin phosphate:
Innovator brand name: JANUVIA®
Patent number: US7125873
Application number: N021995
Patent expiry date: 07-26-2022
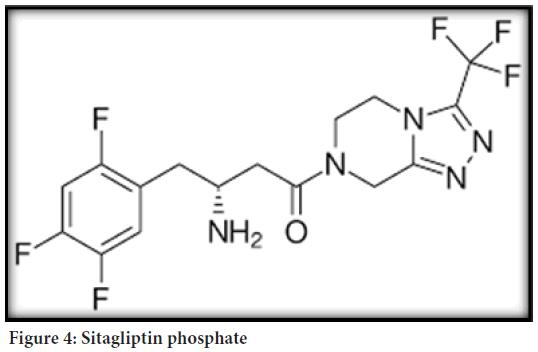
Chemical name: 7-[(3R)-3-amino-1-oxo-4-(2,4,5¬ trifluorophenyl) butyl]-5,6,7,8-tetrahydro-3-(trifluoromethyl)-1,2,4-triazolo[4,3-a] pyrazine phosphate (1:1) monohydrate (Figure 4).
Figure 4: Sitagliptin phosphate
Empirical formula: C16H15F6N5O (H3PO4) (H2O)
Molecular weight: 523.32
Indication: As an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus (FDA, 2020; Pharmacompass, 2020; FDA, 2020).
Active ingredient: Each film-coated tablet of JANUVIA contains 32.13, 64.25, or 128.5 mg of sitagliptin phosphate monohydrate, which is equivalent to 25, 50, or 100 mg, respectively
Inactive ingredient: Microcrystalline cellulose, anhydrous dibasic calcium phosphate, croscarmellose sodium, magnesium stearate, and sodium stearyl fumarate. In addition, the film coating contains the following inactive ingredients: Polyvinyl alcohol, polyethylene glycol, talc, titanium dioxide, red iron oxide, and yellow iron oxide.
Route: Oral
Dosage form and strength: 100 mg tablets are beige, round, film-coated tablets with “277” on one side. 50 mg tablets are light beige, round, film-coated tablets with “112” on one side. 25 mg tablets are pink, round, film-coated tablets with “221” on one side.
Recommended dose: The recommended dose is 100 mg once daily it can be taken with or without food and should be swallowed whole. The tablets must not be split, crushed, or chewed before swallowing.
Mechanism of action: Sitagliptin is a Dipeptidyl Peptidase-4 (DPP-4) inhibitor, which is believed to exert its actions in patients with type 2 diabetes by slowing the inactivation of incretin hormones. Concentrations of the active intact hormones are increased by JANUVIA, thereby increasing and prolonging the action of these hormones. Incretin hormones, including Glucagon-Like Peptide-1 (GLP-1) and Glucose-dependent Insulinotropic Polypeptide (GIP) are released by the intestine throughout the day, and levels are increased in response to a meal. These hormones are rapidly inactivated by the enzyme, DPP-4. The incretins are part of an endogenous system involved in the physiologic regulation of glucose homeostasis. When blood glucose concentrations are normal or elevated, GLP-1 and GIP increase insulin synthesis and release from pancreatic beta cells by intracellular signaling pathways involving cyclic Adenosine Monophosphate (cAMP).
Pharmacodynamics: In patients with type 2 diabetes, administration of JANUVIA led to inhibition of DPP-4 enzyme activity for a 24-hour period. After an oral glucose load or a meal, this DPP-4 inhibition resulted in a 2-to 3-fold increase in circulating levels of active GLP-1 and GIP, decreased glucagon concentrations, and increased responsiveness of insulin release to glucose, resulting in higher C-peptide and insulin concentrations. The rise in insulin with the decrease in glucagon was associated with lower fasting glucose concentrations and reduced glucose excursion following an oral glucose load or a meal.
Pharmacokinetics: The pharmacokinetics of sitagliptin has been extensively characterized in healthy subjects and patients with type 2 diabetes. After oral administration of a 100 mg dose to healthy subjects, sitagliptin was rapidly absorbed, with peak plasma concentrations (median Tmax) occurring 1 to 4 hours post dose. Plasma AUC of sitagliptin increased in a dose-proportional manner.
Adverse effect: In the study of JANUVIA as add-on combination therapy with metformin and rosiglitazone: Upper respiratory tract infection, nasopharyngitis, peripheral edema, and headache.
The add-on to metformin study and the add-on to pioglitazone study-gastrointestinal adverse reactions: Abdominal pain, nausea, and diarrhea.
Linagliptin:
Innovator brand name: TRADJENTA®
Patent number: US8178541
Application number: N201280
Patent expiry date: 08-12-2023
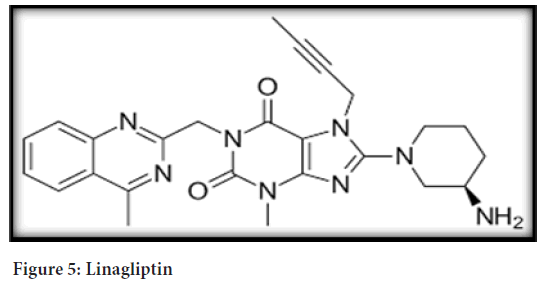
Chemical name: 1H-Purine-2,6-dione, 8-[(3R)-3-amino-1-piperidinyl]- 7-(2-butyn-1-yl)-3,7-dihydro-3-methyl-1-[(4-methyl-2-quinazolinyl) methyl] (Figure 5)
Figure 5: Linagliptin
Empirical formula: C25H28N8O2
Molecular weight: 472.54
Indication: As an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus (FDA, 2020; Pharmacompass, 2020; FDA, 2020).
Active ingredient: Each film-coated tablet of TRADJENTA contains 5 mg of linagliptin free base.
Inactive ingredient: Mannitol, pregelatinized starch, corn starch, copovidone, and magnesium stearate.
In addition, the film coating contains the following inactive ingredients: Hypromellose, titanium dioxide, talc, polyethylene glycol, and red ferric oxide.
Route: Oral
Dosage form and strength: TRADJENTA (linagliptin) 5 mg tablets are light red, round, biconvex, bevel-edged, film-coated tablets with “D5” debossed on one side and the Boehringer Ingelheim logo debossed on the other side.
Recommended dose: The recommended dose is 5 mg once daily. TRADJENTA tablets can be taken with or without food.
Mechanism of action: Linagliptin is an inhibitor of DPP-4, an enzyme that degrades the incretin hormones Glucagon-Like Peptide-1 (GLP-1) and Glucose-dependent Insulinotropic Polypeptide (GIP). Thus, linagliptin increases the concentrations of active incretin hormones, stimulating the release of insulin in a glucose-dependent manner and decreasing the levels of glucagon in the circulation. Both incretin hormones are involved in the physiological regulation of glucose homeostasis.
Pharmacodynamics: Linagliptin binds to DPP-4 in a reversible manner and thus increases the concentrations of incretin hormones. Linagliptin glucose-dependently increases insulin secretion and lowers glucagon secretion thus resulting in a better regulation of the glucose homeostasis. Linagliptin binds selectively to DPP-4 and selectively inhibits DPP-4 but not DPP-8 or DPP-9 activity in vitro at concentrations approximating therapeutic exposures.
Pharmacokinetics: The pharmacokinetics of linagliptin has been characterized in healthy subjects and patients with type 2 diabetes. After oral administration of a single 5-mg dose to healthy subjects, peak plasma concentrations of linagliptin occurred at approximately 1.5 hours post dose (Tmax); the mean plasma Area Under the Curve (AUC) was 139 nmol/h/L and maximum concentration (Cmax) was 8.9 nmol/L. Plasma concentrations of linagliptin decline in at least a biphasic manner with a long terminal half-life (>100 hours), related to the saturable binding of linagliptin to DPP-4. The prolonged elimination phase does not contribute to the accumulation of the drug.
Adverse reaction: Nasopharyngitis, hyperlipidemia (with pioglitazone), cough (with sulfonyl urea), hypertriglyceridemia (with sulfonylurea), weight gain (pioglitazone).
Esomeprazole magnesium; naproxen:
Innovator brand name: VIMOVO®
Patent number: US9345695
Application number: N022511
Patent expiry date: 05-31-2022
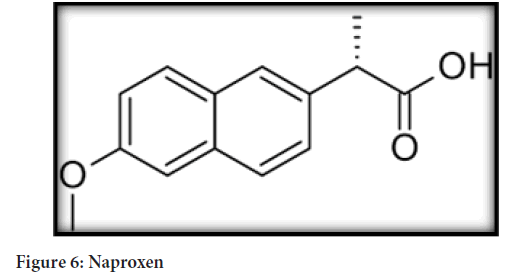
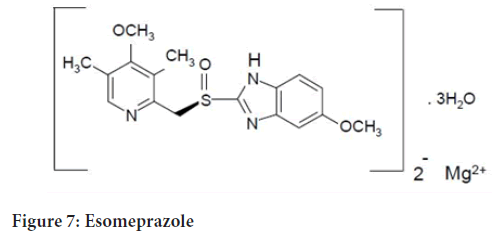
Chemical name: Naproxen-(S)-6-methoxy-α-methyl-2-naphthaleneacetic acid. Esomeprazole-bis(5-methoxy-2-[(S)-[(4-methoxy-3,5-dimethyl-2- pyridinyl)methyl]sulfinyl]-1H-benzimidazole-1-yl) magnesium trihydrate (Figure 6)
Figure 6: Naproxen
Molecular formula: Naproxen-C14H14O3
Esomeprazole-(C17H18N3O3S)2 Mg x 3 H2O (Figure 7)
Figure 7: Esomeprazole
Molecular weight: Naproxen-230.26
Esomeprazole-767.2 MW as a trihydrate and 713.1 MW on an anhydrous basis.
Indication: For the relief of signs and symptoms of osteoarthritis, rheumatoid arthritis and ankylosing spondylitis and to decrease the risk of developing gastric ulcers in patients at risk of developing Non-steroidal anti-inflammatory drugs (NSAID) associated gastric ulcers (FDA, 2020; Pharmacompass, 2020; FDA, 2020).
Active ingredient: Each strength contains either 375 mg of naproxen and 20 mg of esomeprazole (present as 22.3 mg esomeprazole magnesium trihydrate) or 500 mg of naproxen and 20 mg of esomeprazole (present as 22.3 mg esomeprazole magnesium trihydrate).
Inactive ingredient: Carnauba wax, colloidal silicon dioxide, croscarmellose sodium, iron oxide yellow, glyceryl monostearate, hypromellose, iron oxide black, magnesium stearate, methacrylic acid copolymer dispersion, methylparaben, polysorbate 80, polydextrose, polyethylene glycol, povidone, propylene glycol, propylparaben, titanium dioxide, and triethyl citrate.
Route: Oral
Dosage form and strength: VIMOVO is an oval, yellow, delayed-release tablet for oral administration containing either: 375 mg enteric-coated naproxen and 20 mg immediate-release esomeprazole magnesium tablets printed with 375/20 in black, or 500 mg enteric-coated naproxen and 20 mg immediate-release esomeprazole magnesium tablets printed with 500/20 in black.
Recommended dose: The dosage is one tablet twice daily of VIMOVO 375 mg naproxen and 20 mg of esomeprazole or 500 mg naproxen and 20 mg of esomeprazole.
Mechanism of action: VIMOVO consists of an immediate-release esomeprazole magnesium layer and an enteric-coated naproxen core. As a result, esomeprazole is released first in the stomach, prior to the dissolution of naproxen in the small intestine. The enteric coating prevents naproxen release at pH levels below 5.5. The mechanism of action of the naproxen anion, like that of other NSAIDs, is not completely understood but inhibition of cyclooxygenase (COX-1 and COX-2). VIMOVO has analgesic, anti-inflammatory, and antipyretic properties contributed by the naproxen component. Naproxen is a potent inhibitor of prostaglandin synthesis invitro. Naproxen concentrations reached during therapy have produced in vivoeffects. Prostaglandins sensitize afferent nerves and potentiate the action of bradykinin in inducing pain in animal models.
Pharmacodynamics: Serum gastrin effects-The effect of esomeprazole on serum gastrin concentrations was evaluated in approximately 2,700 patients in clinical trials up to 8 weeks and in over 1,300 patients for up to 6-12 months. The mean fasting gastrin level increased in a dose-related manner. This increase reached a plateau within two to three months of therapy and returned to baseline levels within four weeks after discontinuation of therapy.
Adverse effect: Gastric erosion (19%), compared with 38% for equal naproxen dose without PPI, dyspepsia (18%), compared with 27% for equal naproxen dose without PPI, gastritis (17%), diarrhea (6%), abdominal pain (6%), nausea (5%), hiatal hernia (4%), abdominaldistension (4%), flatulence (4%), esophagitis (4%), constipation (3%), headache (3%), dysgeusia (2%), erosive duodenitis (2%), hemorrhagic gastritis (1%), Gastro-Oesophageal Reflux Disease (GERD), duodenal ulcer, erosive esophagitis.
Lacosamide:
Innovatorbrandname:VIMPAT®
Patent number: RE38551
Application number: N022253
Patentexpirydate:-17-2022
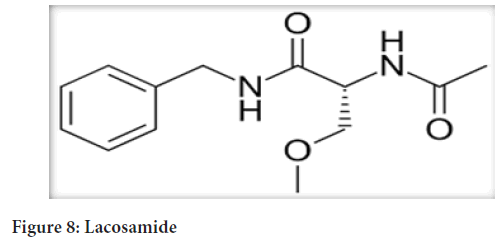
Chemicalname:(R)-2-acetamido-N-benzyl-3- methoxypropionamide (Figure 8)
Figure 8: Lacosamide
Molecularformula: C13H18N2O3
Molecularweight: 250.30
Indication: For the treatment of partial-onset seizures in patients 4 years of age and older. As the safety of VIMPAT injection in pediatric patients has not been established, VIMPAT injection is indicated for the treatment of partial-onset seizures only in adult patients (17 years of age and older) (FDA, 2020; Pharmacompass, 2020; FDA, 2020).
Active and inactive ingredients: VIMPAT Tablets-contains lacosamide as active ingredient and the following inactive ingredients colloidalsilicondioxide, crospovidone, hydroxypropylcellulose, magnesium stearate, microcrystalline cellulose, polyethylene glycol, polyvinyl alcohol, talc, titanium dioxide, and dye pigments as specified below:
VIMPAT tablets are supplied as debossed tablets and contain the following coloring agents: 50 mg tablets: Red iron oxide, black iron oxide, FD and C Blue #2/indigo carmine aluminum lake 100 mg tablets: Yellow iron oxide 150 mg tablets: Yellow iron oxide, red iron oxide, black iron oxide 200 mg tablets: FD and C Blue #2/indigo carmine aluminum lake.
VIMPAT Injection: A clear, colorless, sterile solution containing 10 mg lacosamide per mL for intravenous infusion. One 20 ml vial contains 200 mg of lacosamide drug substance. The inactive ingredients are sodium chloride and water for injection. Hydrochloric acid is used for pH adjustment. VIMPAT injection has a pH of 3.5 to 5.0.
Route: Oral; Intravenous
Dosage form and strength: VIMPAT Tablets-50 mg: Pink, oval, film-coated, debossed with "SP" on one side and "50" on the other. 100 mg: Dark yellow, oval, film-coated, debossed with "SP" on one side and "100" on the other. 150 mg: Salmon, oval, film-coated, debossed with "SP" on one side and "150" on the other. 200 mg: Blue, oval, film-coated, debossed with "SP" on one side and "200" on the other.
VIMPAT Injection-200 mg/20 mL: Clear, colorless sterile solution in single-dose vials. VIMPAT Oral Solution-10 mg/mL: Clear, colorless to yellow or yellow-brown, strawberry-flavored liquid.
Recommended dose: Adults-Indicated as monotherapy or adjunctive therapy for partial onset seizures in adults and children aged ≥ 4 years (Medscape, 2020).
Monotherapy 100 mg PO/IV q12hr (every 12 hours) initially, then based on response and tolerability, increase dose at weekly intervals by 50 mg PO/IV BID; up to a recommended dose of 150-200 mg bis in die (BID) (300-400 mg/day).
Alternate loading dose schedule: 200 mg PO/IV as a single loading dose followed 12 hr later by 100 mg PO/IV BID; then increase dose at weekly intervals by 50 mg BID; up to a recommended dose of 150-200 mg BID (300-400 mg/day).
In patients already taking an Antiepileptic Drug (AED), maintain lacosamide at recommended maintenance dose of 150-200 mg PO BID for at least 3 days before initiating withdrawal of the previous AED.
Adjunctive therapy-Initial: 50 mg PO/IV q12hr.
Based on the response and tolerability, dose can be increased at weekly intervals by 50 mg PO/IV BID; up to a recommended dose of 100-200 mg BID (200-400 mg/day).
Mechanism of action: The precise mechanism by which VIMPAT exerts its antiepileptic effects in humans remains to be fully elucidated. Invitro electrophysiological studies have shown that lacosamide selectively enhances slow inactivation of voltage-gated sodium channels, resulting in stabilization of hyper excitable neuronal membranes and inhibition of repetitive neuronal firing.
Pharmacodynamics: Chronic oral doses of 400 and 800 mg/day were compared with placebo and a positive control (400 mg moxifloxacin). VIMPAT did not prolong QTc interval and did not have a dose-related or clinically important effect on QRS duration. VIMPAT produced a small, dose-related increase in mean PR interval. At steady-state, the time of the maximum observed mean PR interval corresponded with Tmax. The placebo-subtracted maximum increase in PR interval (at Tmax) was 7.3 ms for the 400 mg/day group and 11.9 ms for the 800 mg/day group. For patients who participated in the controlled trials, the placebo-subtracted mean maximum increase in PR interval for a 400 mg/day VIMPAT dose was 3.1 ms in patients with partial-onset seizures and 9.4 ms for patients with diabetic neuropathy.
Pharmacokinetics: VIMPAT is completely absorbed after oral administration with negligible first-pass effect with a high absolute bioavailability of approximately 100%. The maximum lacosamide plasma concentration occurs approximately 1 to 4 hour post-dose after oral dosing, and elimination half-life is approximately 13 hours. Steady state plasma concentrations are achieved after 3 days of twice daily repeated administration.
Adverse effect: Suicidal behaviour and ideation, dizziness and ataxia, cardiac rhythm and conduction abnormalities, syncope, drug reaction with eosinophilia and systemic symtoms/multiorgan hypersensitivity reactions.
Saxagliptin hydrochloride:
Innovatorbrandname: ONGLYZA®
Patent number: RE44186
Application number: N022350
Patent expiry date: 07-31-2023
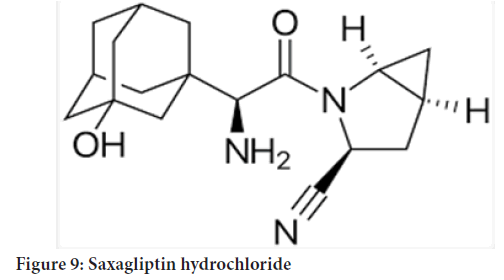
Chemical name: (1S,3S,5S)-2-[(2S)-2-Amino-2-(3¬ hydroxytricyclo[3.3.1.13,7]dec-1-yl)acetyl]-2-azabicyclo[3.1.0]hexane-3-carbonitrile, monohydrate OR(1S,3S,5S)-2-[(2S)-2-Amino-2-(3-hydroxyadamantan-1-yl)acetyl]-2¬ 10 azabicyclo[3.1.0]hexane-3-carbonitrile hydrate (Figure 9)
Figure 9: Saxagliptin hydrochloride
Molecular formula: C18H25N3O2 (H2O)
Molecularweight: 333.43
Indication: As an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus (FDA, 2020; Pharmacompass, 2020; FDA, 2020).
Active ingredient: Each film-coated tablet of ONGLYZA for oral use contains either 2.79 mg saxagliptin hydrochloride (anhydrous) equivalent to 2.5 mg saxagliptin or 5.58 mg saxagliptin hydrochloride (anhydrous) equivalent to 5 mg saxagliptin.
Inactive ingredient: Lactose monohydrate, microcrystalline cellulose, croscarmellose sodium, and magnesium stearate. In addition, the film coating contains the following inactive ingredients: Polyvinyl alcohol, polyethylene glycol, titanium dioxide, talc, and iron oxides.
Route: Oral
Dosage form and Strength: ONGLYZA (saxagliptin) 5 mg tablets are pink, biconvex, round, film-coated tablets with “5” printed on one side and “4215” printed on the reverse side, in blue ink. ONGLYZA (saxagliptin) 2.5 mg tablets are pale yellow to light yellow, biconvex, round, film-coated tablets with “2.5” printed on one side and “4214” printed on the reverse side, in blue ink.
Recommendeddose: 2.5 mg or 5 mg once daily taken regardless of meals.
Mechanism of action: Increased concentrations of the incretin hormones such as Glucagon-Like Peptide-1 (GLP-1) and Glucose-Dependent Insulinotropic Polypeptide (GIP) are released into the bloodstream from the small intestine in response to meals. These hormones cause insulin release from the pancreatic beta cells in a glucose-dependent manner but are inactivated by the Dipeptidyl Peptidase-4 (DPP4) enzyme within minutes. GLP-1 also lowers glucagon secretion from pancreatic alpha cells, reducing hepatic glucose production.
Pharmacodynamics: In patients with type 2 diabetes mellitus, administration of ONGLYZA inhibits DPP4 enzyme activity for a 24-hour period. After an oral glucose load or a meal, this DPP4 inhibition resulted in a 2- to 3-fold increase in circulating levels of active GLP-1 and GIP, decreased glucagon concentrations, and increased glucose-dependent insulin secretion from pancreatic beta cells. The rise in insulin and decrease in glucagon were associated with lower fasting glucose concentrations and reduced glucose excursion following an oral glucose load or a meal.
Pharmacokinetics: The Cmax and AUC values of saxagliptin and its active metabolite increased proportionally in the 2.5 to 400 mg dose range. Following a 5 mg single oral dose of saxagliptin to healthy subjects, the mean plasma AUC values for saxagliptin and its active metabolite were 78 ng/h/ mL and 214 ng/h/mL, respectively. The corresponding plasma Cmax values were 24 ng/mL and 47 ng/mL, respectively. The average variability (%CV) for AUC and Cmax for both saxagliptin and its active metabolite was less than 25%.
Adverse effect: Urinary tract infection, headache, hypersensitivity-related events (eg, urticaria, facial edema), peripheral edema (increased incidence when co-administered with thiazolidinediones), upper respiratory tract infection, gastroenteritis, hypoglycemia.
Simvastatin; sitagliptin phosphate:
Innovatorbrandname: JUVISYNC®
Patent number: US8168637
Application number: N202343
Patent expiry date: 06-26-2022
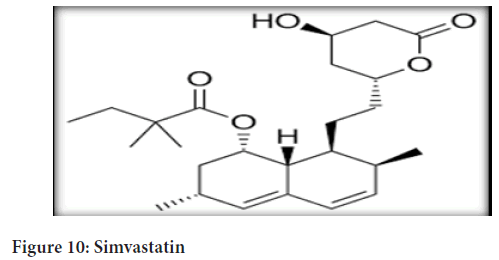
Chemical name: Simvastatin-butanoic acid, 2,2-dimethyl-,1,2,3,7,8,8a- hexahydro-3,7-dimethyl-8-[2-(tetrahydro-4-hydroxy-6-oxo-2H-pyran-2- yl)-ethyl]-1-naphthalenyl ester, [1S-[1α,3α,7β,8β(2S*,4S*),-8aβ]] (Figure 10)
Figure 10: Simvastatin
Sitagliptin phosphate-7-[(3R)-3-amino-1-oxo-4-(2,4,5- trifluorophenyl) butyl]-5,6,7,8-tetrahydro-3-(trifluoromethyl)-1,2,4-triazolo[4,3-a] pyrazine phosphate (1:1) monohydrate.
Molecularformula: C25H38O5; C16H15F6N5O (H3PO4) (H2O)
Molecularweight: 418.57; 523.32
Indication: JUVISYNC™ (sitagliptin and simvastatin) is indicated in patients for whom treatment with both sitagliptin and simvastatin is appropriate (FDA, 2020; Pharmacompass, 2020; FDA, 2020).
Sitagliptin: As an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus.
Activeingredient: Each bilayer tablet of JUVISYNC contains 128.5 mg or 64.25 mg of sitagliptin phosphate monohydrate, which is equivalent to 100 mg or 50 mg of free base, respectively either 10 mg, 20 mg, or 40 mg of simvastatin.
Inactive ingredient: Anhydrous dibasic calcium phosphate, microcrystalline cellulose, croscarmellose sodium, sodium stearyl fumarate, magnesium stearate, ascorbic acid, citric acid monohydrate, lactose monohydrate, and pre-gelatinized corn starch. In addition, the film coating contains the following inactive ingredients for all tablet strengths: Polyvinyl alcohol, polyethylene glycol, talc, titanium dioxide, and red iron oxide. The film coating for the 100 mg/10 mg, 100 mg/20 mg, 100 mg/40 mg, and 50 mg/20 mg tablet strengths also contains yellow iron oxide and black iron oxide. Butylated hydroxyanisole is added as a preservative.
Route: Oral
Dosage form and strength: JUVISYNC 100 mg/10 mg tablets are pinkbeige, bi-convex round, film-coated tablets, coded with the Merck logo and "753" on one side and plain on the other.
JUVISYNC 100 mg/20 mg tablets are pink-beige, bi-convex modified capsule-shaped, filmcoated tablets, coded with the Merck logo and "757" on one side and plain on the other.
JUVISYNC 100 mg/40 mg tablets are orange-beige, bi-convex modified capsule-shaped, filmcoated tablets, coded with the Merck logo and "773" on one side and plain on the other.
Recommended dose: JUVISYNC should be taken as a single daily dose in the evening. JUVISYNC must not be split or divided before swallowing. The recommended starting dose is 100 mg/40 mg per day. For patients already taking simvastatin (10, 20, or 40 mg daily) with or without sitagliptin 100 mg daily, JUVISYNC may be initiated at the dose of 100 mg sitagliptin and the dose of simvastatin already being taken. After initiation or titration of JUVISYNC, lipid levels may be analyzed after 4 or more weeks and dosage adjusted, if needed.
Mechanism of action: Sitagliptin is a DPP-4 inhibitor, which is believed to exert its actions in patients with type 2 diabetes by slowing the inactivation of incretin hormones. Concentrations of the active intact hormones are increased by JUVISYNC, thereby increasing and prolonging the action of these hormones. Incretin hormones, including Glucagon-Like Peptide-1 (GLP-1) and Glucose-dependent Insulinotropic Polypeptide (GIP), are released by the intestine throughout the day, and levels are increased in response to a meal. These hormones are rapidly inactivated by the enzyme, DPP-4. The incretions are part of an endogenous system involved in the physiologic regulation of glucose homeostasis.
Pharmacodynamics: Sitagliptin: In patients with type 2 diabetes, administration of sitagliptin led to inhibition of DPP-4 enzyme activity for a 24- hour period. After an oral glucose load or a meal, this DPP-4 inhibition resulted in a 2- to 3-fold increase in circulating levels of active GLP-1 and GIP, decreased glucagon concentrations, and increased responsiveness of insulin release to glucose, resulting in higher C-peptide and insulin concentrations. The rise in insulin with the decrease in glucagon was associated with lower fasting glucose concentrations and reduced glucose excursion following an oral glucose load or a meal.
Pharmacokinetics: The results of bioequivalence studies in healthy subjects demonstrated that JUVISYNC (sitagliptin and simvastatin) is bioequivalent to coadministration of sitagliptin and simvastatin as individual tablets. Sitagliptin and simvastatin do not have a clinically meaningful pharmacokinetic interaction.
Adverse effect: Most common adverse reactions (incidence ≥ 5%) with simvastatin are: Upper respiratory infection, headache, abdominal pain, constipation, and nausea. Adverse reactions reported in ≥ 5% of patients treated with sitagliptin and more commonly than in patients treated with placebo are: Upper respiratory tract infection, nasopharyngitis and headache. In the add-on to sulfonylurea and add-on to insulin studies, hypoglycemia was also more commonly reported in patients treated with sitagliptin compared to placebo.
Lenalidomide:
Innovatorbrandname: REVLIMID®
Patent number: US9101622
Application number: N021880
Patent expiry date: 05-15-2023
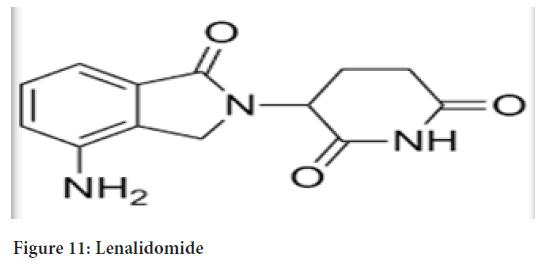
Chemical name: 3(4-amino-1-oxo 1,3-dihydro-2H-isoindol-2-yl) piperidine-2,6-dione (Figure 11)
Figure 11: Lenalidomide
Molecularformula: C13H13N3O3
Molecularweight: 259.3
Indication: Multiple Myeloma-REVLIMID in combination with dexamethasone is indicated for the treatment of patients with Multiple Myeloma (MM) who have received at least one prior therapy (FDA, 2020; Pharmacompass, 2020; FDA, 2020).
For Myelodysplastic Syndromes-REVLIMID is indicated for the treatment of patients with transfusion-dependent anemia due to lowor intermediate-1-risk Myelodysplastic Syndromes (MDS) associated with a deletion 5q cytogenetic abnormality with or without additional cytogenetic abnormalities.
Activeingredient: Lenalidomide
Inactive ingredient: Lactose anhydrous, microcrystalline cellulose, croscarmellose sodium, and magnesium stearate. The 5 mg and 25 mg capsule shell contains gelatin, titanium dioxide and black ink. The 2.5 mg and 10 mg capsule shell contains gelatin, FD and C blue #2, yellow iron oxide, titanium dioxide and black ink. The 15 mg capsule shell contains gelatin, FD and C blue #2, titanium dioxide and black ink.
Route: Oral
Dosage form and strength: REVLIMID 2.5 mg, 5 mg, 10 mg, 15 mg and 25 mg capsules will be supplied through the Rev Assist program. REVLIMID is available in the following capsule strengths: 2.5 mg, 10 mg and 25 mg.
Recommended dose: REVLIMID should be taken orally at about the same time each day, either with or without food. The capsules should be swallowed whole with water. The capsules should not be opened, broken, or chewed.
Multiple myeloma: The recommended starting dose of REVLIMID is 25 mg once daily on days 1-21 of repeated 28-day cycles. The recommended dose of dexamethasone is 40 mg once daily on days 1-4, 9-12, and 17-20 of each 28-day cycle for the first 4 cycles of therapy and then 40 mg once daily orally on days 1-4 every 28 days. Treatment is continued or modified based upon clinical and laboratory findings.
Myelodysplastic syndromes: The recommended starting dose of REVLIMID is 10 mg daily. Treatment is continued or modified based upon clinical and laboratory findings.
Mechanism of action: The mechanism of action of lenalidomide remains to be fully characterized. Lenalidomide possesses immunomodulatory, anti-angiogenic, and antineoplastic properties. Experiments have demonstrated that lenalidomide inhibits the growth of cells derived from patients with multiple myeloma and del (5q) myelodysplastic syndromes in vitro.
Adverse effect: Neutropenia and thrombocytopenia, deep vein thrombosis and pulmonary embolism, allergic reactions, tumor lysis syndrome, tumor flare reactions, hepatotoxicity, second primary malignancies.
Armodafinil:
Innovatorbrandname: NUVIGIL®
Patent number: US7297346
Application number: N021875
Patent expiry date: 11-29-2023
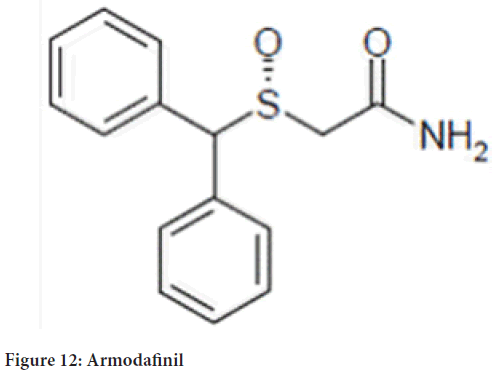
Chemicalname: 2-[(R)(diphenylmethyl)sulfinyl]acetamide
Molecularformula: C15H15NO2S (Figure 12)
Figure 12: Armodafinil
Molecularweight: 273.35
Indication: To improve wakefulness in adult patients with excessive sleepiness associated with Obstructive Sleep Apnea (OSA), narcolepsy, or Shift Work Disorder (SWD).
Activeingredient: 50, 150, 200 or 250 mg of armodafinil.
Inactive ingredient: Croscarmellose sodium, lactose monohydrate, magnesium stearate, microcrystalline cellulose, povidone, and pregelatinized starch (FDA, 2020; Pharmacompass, 2020; FDA, 2020).
Route: oral
Dosage form and strength: 50 mg-round, white to off-white tablet with on one side and "205" on the other. 150 mg-oval, white to off-white tablet with on one side and "215" on the other. 200 mg-rounded, rectangular, white to off-white tablet with on one side and "220" on the other. 250 mg-oval, white to off-white tablet with on one side and "225" on the other.
Recommended dose: Obstructive Sleep Apnea (OSA) and Narcolepsy-150 mg to 250 mg taken orally once a day as a single dose in the morning. In patients with OSA, doses up to 250 mg/day, given as a single dose, have been well tolerated, but there is no consistent evidence that these doses confer additional benefit beyond that of the 150 mg/day dose.
Shift Work Disorder (SWD)-150 mg taken orally once a day as a single dose approximately 1 hour prior to the start of their work shift. Dosage modification in patients with severe hepatic impairment-the dosage should be reduced.
Mechanism of action: Armodafinil is an indirect dopamine receptor agonist; armodafinil bind in vitro to the dopamine transporter and inhibit dopamine reuptake.
Pharmacokinetics: Armodafinil exhibits linear time-independent kinetics following single and multiple oral dose administration. Increase in systemic exposure is proportional over the dose range of 50 to 400 mg. No time-dependent change in kinetics was observed through 12 weeks of dosing. Apparent steady state for armodafinil was reached within 7 days of dosing.
Adverse effect: Serious dermatologic reactions, Drug Reaction with Eosinophilia and System Symptoms (DRESS)/Multiorgan hypersensitivity, angioedema and anaphylaxis reactions, persistent sleepiness, psychiatric symptoms, effects on ability to drive and use machinery, cardiovascular Events.
Rilpivirine hydrochloride:
Innovatorbrandname:EDURANT®
Patent number: US8101629
Application number: N202022
Patent expiry date: 08-09-2022
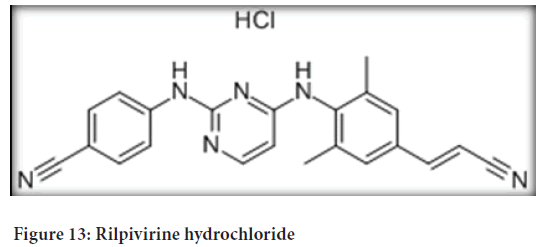
Chemical name: 4-[[4-[[4-[(E)-2-cyanoethenyl]-2,6¬ dimethylphenyl] amino]-2-pyrimidinyl]amino]benzonitrile monohydrochloride (Figure 13)
Figure 13: Rilpivirine hydrochloride
Molecularformula:C22H18N6 (HCl)
Molecularweight: 402.88
Indication: In combination with other antiretroviral agents, is indicated for the treatment of Human Immunodeficiency Virus type 1 (HIV-1) infection in antiretroviral treatment-naïve patients with HIV-1 RNA less than or equal to 100,000 copies/mL at the start of therapy (FDA, 2020; Pharmacompass, 2020; FDA, 2020).
Activeingredient:Rilpivirine hydrochloride
Inactive ingredient: Croscarmellose sodium, lactose monohydrate, magnesium stearate, polysorbate 20, povidone K30 and silicified microcrystalline cellulose. The tablet coating contains hypromellose 2910 6 mPa.s, lactose monohydrate, PEG 3000, titanium dioxide and triacetin.
Route:Oral
Dosage form and strength: 25 mg white to off-white, film-coated, round, biconvex, tablet of 6.4 mm, debossed with “TMC” on one side and “25” on the other side. Each tablet contains 27.5 mg of rilpivirine hydrochloride, which is equivalent to 25 mg of rilpivirine.
Recommended dose: In patients 12 years of age and older and weighing at least 35 kg is one 25 mg tablet once daily taken orally with a meal. EDURANT is not recommended for patients less than 12 years of age.
Mechanism of action: Rilpivirine is a diarylpyrimidine Non-Nucleoside Reverse Transcriptase Inhibitor (NNRTI) of Human Immunodeficiency Virus type 1 (HIV-1) and inhibits HIV-1 replication by non-competitive inhibition of HIV-1 Reverse Transcriptase (RT). Rilpivirine does not inhibit the human cellular DNA polymerases α, β and γ.
Pharmacodynamics: Effects on Electrocardiogram-The effect of EDURANT at the recommended dose of 25 mg once daily on the QTcF interval was evaluated in a randomized, placebo and active (moxifloxacin 400 mg once daily) controlled crossover study in 60 healthy adults, with 13 measurements over 24 hours at steady state. The maximum mean time-matched (95% upper confidence bound) differences in QTc interval from placebo after baseline-correction was 2.0 (5.0) milliseconds (i.e., below the threshold of clinical concern).
Pharmacokinetics: The pharmacokinetic properties of rilpivirine have been evaluated in adult healthy subjects and in adult antiretroviral treatment-naïve HIV-1-infected subjects. Exposure to rilpivirine was generally lower in HIV-1 infected subjects than in healthy subjects (Table 1).
| Parameter | Rilpivirine 25 mg once daily N=679 |
|---|---|
| Area under Curve 24h (ng per h/mL) | |
| Mean ± Standard Deviation (SD) | 2235 ± 851 |
| Median (range) | 2096 (198-7307) |
| C0h (ng/mL) | |
| Mean ± Standard Deviation | 79 ± 35 |
| Median (range) | 73 (2-288) |
Table 1: Population pharmacokinetic estimates of rilpivirine 25 mg once daily in antiretroviral treatment-naïve HIV-1-infected adult subjects (pooled data from phase 3 trials through week 96)
Adverse effect: Skin and hypersensitivity reactions, depressive disorders, hepatotoxicity.
Conclusion
Generic drugs are an effective and FDA approved option for patients. As the studies performed during brand drug development need not to be repeated for generic drug development, it results in lower cost, which provides an affordable option to the patients which may increase the likelihood that patient’s follow-through with doctor-recommended care.
References
- Lal R. Patents and exclusivity. FDA/CDER SBIA Chronicles. 2015; 505(2): 1-3.
- Gorman L. Patent expiration and pharmaceutical prices. National Bureau of Economic Research. 2014.
- FDA. Generic drugs: Questions and answers. US Food and Drug Administration. 2020.
- FDA. Abbreviated New Drug Application (ANDA). US Food and Drug Administration. 2020.
- FDA. Approved drug products with therapeutic equivalence evaluations (Orange book). US Food and Drug Administration. 2020.
- FDA. Orange book: Approved drug products with therapeutic equivalence evaluations. US Food and Drug Administration. 2020.
- Pristiq US patent expiration. Pharmacompass. 2020.
- FDA. FDA label of pristiq (desvenlafaxine succinate). US Food and Drug Administration. 2020.
- Modafinil US patent expiration. Pharmacompass. 2020.
- FDA. FDA label of provigil (modafinil). US Food and Drug Administration. 2020.
- Invokana US patent expiration. Pharmacompass. 2020.
- FDA. FDA label of invokana (canagliflozin). US Food and Drug Administration. 2020.
- Januvia US patent expiration. Pharmacompass. 2020.
- FDA. FDA label of januvia (sitagliptin phosphate). US Food and Drug Administration. 2020.
- Linagliptin US patent expiration. Pharmacompass. 2020.
- FDA. FDA label of tradjenta (linagliptin). US Food and Drug Administration. 2020.
- Vimovo US patent expiration. Pharmacompass. 2020.
- FDA. FDA label of vimovo (esomeprazole magnesium; naproxen). US Food and Drug Administration. 2020.
- Lacosamide US patent expiration. Pharmacompass. 2020.
- FDA. FDA label of vimpat(lacosamide). US Food and Drug Administration. 2020.
- Medscape. Vimpat (lacosamide) dosing, indications, interactions, adverse effects and more. Medscape. 2020.
- Saxagliptin hydrochloride US patent expiration. Pharmacompass. 2020.
- FDA. FDA label of onglyza (saxagliptin hydrochloride). US Food and Drug Administration. 2020.
- Juvisync US patent expiration. Pharmacompass. 2020.
- FDA. FDA label of juvisync (sitagliptin; simvastatin). US Food and Drug Administration. 2020.
- Lenalidomide US patent expiration. Pharmacompass. 2020.
- FDA. FDA label of revlimid(lenalidomide). US Food and Drug Administration. 2020.
- Nuvigil US patent expiration. Pharmacompass. 2020.
- FDA. FDA label of nugivil (armodafinil). US Food and Drug Administration. 2020.
- Rilpivirine hydrochloride US patent expiration. Pharmacompass. 2020.
- FDA. FDA label of edurant (rilpivirine hydrochloride). US Food and Drug Administration. 2020.
Author Info
Tularam Barot1, Bhupendra Prajapati2*, Jeshika Patel1, Shruti Barot1, Nirmal Vashi1 and Priyadarshini Patel12Department of Pharmaceutics, Shree SK Patel College of Pharmaceutical Education and Research, Ganpat University, Gujarat, India
Citation: Barot T: A Review on the Drugs Getting Off-Patent between the Years 2022-2025 in USA and Their Pharmaceutical Properties
Received: 01-Apr-2022 Accepted: 29-Apr-2022 Published: 06-May-2022, DOI: 10.31858/0975-8453.13.5.296-305
Copyright: This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
ARTICLE TOOLS
- Dental Development between Assisted Reproductive Therapy (Art) and Natural Conceived Children: A Comparative Pilot Study Norzaiti Mohd Kenali, Naimah Hasanah Mohd Fathil, Norbasyirah Bohari, Ahmad Faisal Ismail, Roszaman Ramli SRP. 2020; 11(1): 01-06 » doi: 10.5530/srp.2020.1.01
- Psychometric properties of the World Health Organization Quality of life instrument, short form: Validity in the Vietnamese healthcare context Trung Quang Vo*, Bao Tran Thuy Tran, Ngan Thuy Nguyen, Tram ThiHuyen Nguyen, Thuy Phan Chung Tran SRP. 2020; 11(1): 14-22 » doi: 10.5530/srp.2019.1.3
- A Review of Pharmacoeconomics: the key to “Healthcare for All” Hasamnis AA, Patil SS, Shaik Imam, Narendiran K SRP. 2019; 10(1): s40-s42 » doi: 10.5530/srp.2019.1s.21
- Deuterium Depleted Water as an Adjuvant in Treatment of Cancer Anton Syroeshkin, Olga Levitskaya, Elena Uspenskaya, Tatiana Pleteneva, Daria Romaykina, Daria Ermakova SRP. 2019; 10(1): 112-117 » doi: 10.5530/srp.2019.1.19
- Dental Development between Assisted Reproductive Therapy (Art) and Natural Conceived Children: A Comparative Pilot Study Norzaiti Mohd Kenali, Naimah Hasanah Mohd Fathil, Norbasyirah Bohari, Ahmad Faisal Ismail, Roszaman Ramli SRP. 2020; 11(1): 01-06 » doi: 10.5530/srp.2020.1.01
- Manilkara zapota (L.) Royen Fruit Peel: A Phytochemical and Pharmacological Review Karle Pravin P, Dhawale Shashikant C SRP. 2019; 10(1): 11-14 » doi: 0.5530/srp.2019.1.2
- Pharmacognostic and Phytopharmacological Overview on Bombax ceiba Pankaj Haribhau Chaudhary, Mukund Ganeshrao Tawar SRP. 2019; 10(1): 20-25 » doi: 10.5530/srp.2019.1.4
- A Review of Pharmacoeconomics: the key to “Healthcare for All” Hasamnis AA, Patil SS, Shaik Imam, Narendiran K SRP. 2019; 10(1): s40-s42 » doi: 10.5530/srp.2019.1s.21
- A Prospective Review on Phyto-Pharmacological Aspects of Andrographis paniculata Govindraj Akilandeswari, Arumugam Vijaya Anand, Palanisamy Sampathkumar, Puthamohan Vinayaga Moorthi, Basavaraju Preethi SRP. 2019; 10(1): 15-19 » doi: 10.5530/srp.2019.1.3