Review Article - (2023) Volume 14, Issue 7
An Insight into Synthetic and Pharmacological Profile of Sulphanilamide
Gagandeep Kaur1, Hardeep Kaur1, Suman Lata1* and Divya Dhawal Bhandari2Abstract
Sulphanilamide, known as a sulpha drug, possesses diverse pharmacological activities, making it a valuable chemotherapeutic agent for combating both bacteria gram-positive and gram-negative bacteria. This compound exhibits a broad spectrum of effects, including anti-hypoglycemic, diuretic, anti-hypertensive, antifungal, antibacterial, anti-inflammatory, and antiretroviral properties. Given that there is increasing prevalence of antibiotic resistance among existing drugs, there is a pressing need to develop novel derivatives of sulphanilamide to effectively combat bacterial and fungal infections.
One key mechanism by which sulphanilamide derivatives combats microorganisms is through their competitive inhibition of folic acid synthesis enzymes. This action serves to hinder the growth of microorganisms. Consequently, in this comprehensive review, we highlight the synthesis of novel derivatives of sulphanilamide that exhibit the aforementioned pharmacological effects. By focusing on these newly synthesized derivatives, we aim to address the urgent need for effective therapeutic agents against bacteria and fungi in light of the growing resistance to conventional antibiotics.
Keywords
Sulphanilamide, Sulpha drugs, Sulphonyl chlorides, Antibiotics
Introduction
Sulphanilamides, also known as sulphonamides, represent a crucial class of synthetic antimicrobial drugs with an extensive variety of effectiveness against bacterial infections. These compounds feature distinctive six or five membered heterocyclic rings and contain the SO2NH2 group, making them organo-sulphur compounds. They exhibit potent activity against pyogenic bacterial infections caused by both gram-positive and gram-negative bacteria, including E.coli, Enterobacter, Salmonella, Klebsiella, Shigella, and Nocardia. Notably, they display remarkable efficacy against gram-positive bacteria for instance S.pneumoniae and S.pyogenes. Their usefulness against gram-negative bacteria, like E.coli and K.pneumoniae, is comparatively lower. Despite their active antibacterial properties, sulphanilamides face significant challenges posed by antibiotic resistance.
In addition to their antimicrobial activity, sulphanilamide derivatives exhibit a plethora of biological actions spanning antihypertensive (Banerjee M, et al., 2005), translation initiation inhibition (Clercq E, 2001), antimicrobial (Genç Y, et al., 2008; Özbek N, et al., 2007), anticancer (Mun J, et al., 2012; El-Sayed NS, et al., 2011; Scozzafava A, et al., 2002), cyclooxygenase-2 inhibition (Talley JJ, et al., 2000), anticonvulsant (Maryanoff BE, et al., 1987), anti-HIV (Stranix BR, et al., 2006; Soukarieh F, et al., 2016), antimigraine (Ong JJ and De Felice M, 2018), carbonic anhydrase inhibition (Talley JJ, et al., 2000), hypoglycemic protease inhibition (Roush WR, et al., 1998), antidiabetic (Naim MJ, et al., 2018), and herbicidal (Haughn GW, et al., 1988) effects. Sulpha drugs have demonstrated promising activity against breast cancer cells (Mirian M, et al., 2011). Azetolamide and methazolamide, two sulpha drugs, are widely used as antiglaucoma agents (Renzi G, et al., 2000). Numerous other sulpha derivatives find application in the treatment of conjunctivitis (Gibson JR, et al., 1983), acne (Del Rosso JQ, 2009), toxoplasmosis (Aspinall TV, et al., 2002), and urinary tract infections (Arredondo-García JL, et al., 2004; McCarty JM, et al., 1994).
By highlighting the multifaceted pharmacological effects and diverse therapeutic applications of sulphanilamide and its derivatives, this unique review aims to underscore the immense potential of these compounds in addressing various medical challenges and fostering novel treatment approaches.
The mechanism of action of sulphanilamide is by inhibition of the enzyme dihydropteroate synthase. This particular enzyme acts in the folic acid bacterial synthesis (Chopra I and Roberts M, 2001) (Figure 1). Folic acid serves as a vital precursor for the synthesis of DNA, RNA, and other essential molecules within bacterial cells.
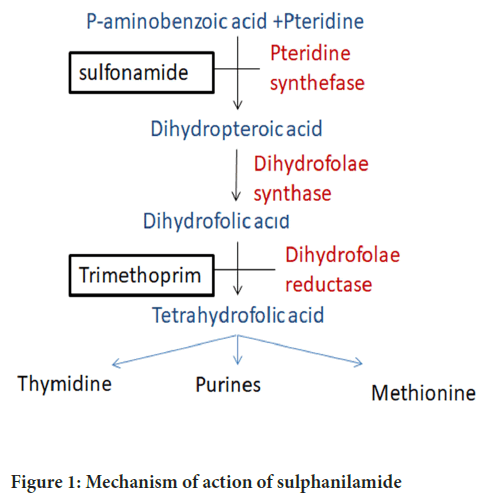
Figure 1: Mechanism of action of sulphanilamide
By inhibiting dihydropteroate synthase, sulphanilamide effectively disrupts the synthesis of folic acid. This inhibition, in turn, hampers the growth and replication of bacteria. As a result, the bacterial cells are unable to produce sufficient folic acid, which is necessary for various cellular processes and essential components. Consequently, the inhibition of folic acid synthesis by sulphanilamide ultimately impedes bacterial growth and impairs their ability to proliferate effectively.
Literature Review
Sulphanilamide holds a significant place in the annals of medical history, as its discovery marked a pivotal milestone in the development of antibiotics. It served as a trailblazer, laying the foundation for the subsequent exploration and creation of other antibiotics that revolutionized the field of medicine. Despite its diminishing usage as an antibiotic in contemporary practice, sulphanilamide continues to be a subject of scientific investigation due to its potential therapeutic applications.
Researchers continue to explore the diverse potential uses of sulphanilamide in various areas, including the treatment of cancer and parasitic infections (Fischbach MA and Walsh CT, 2009). The molecule’s pharmacological properties and mechanisms of action make it a candidate worth investigating for these challenging medical conditions.
However, it is essential to acknowledge that bacterial resistance to sulphanilamide has emerged as a significant issue in current years. Antibiotic abuse and overuse have hampered the efficiency of sulphanilamide and other antibiotics by fostering the emergence of resistant bacterial strains. Consequently, sulphanilamide is now seldom utilized as a first-line treatment for bacterial infections, with alternative antibiotics being preferred to combat resistant strains.
While the prominence of sulphanilamide as an antibiotic has waned, its historical significance and ongoing research into its therapeutic potential underscore its lasting impact on the field of medicine and the development of antimicrobial agents.
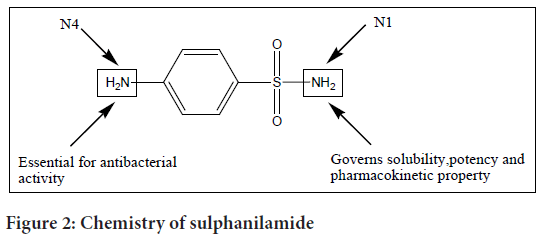
Chemistry of sulphanilamide
Sulphanilamide consists of a sulphonyl group (S(=O)2) connected to an amine group (NH2). A sulphanilamide compound’s typical formula is RSO2NH2, where R stands for different organic alkyl groups (Figure 2).
Figure 2: Chemistry of sulphanilamide
Sulpha drugs are a class of medications that belong to the sulphonamide family. Different sulpha drugs diverge in the nature of N1 (sulphonamide nitrogen) substitution, which influences properties such as solubility, potency, and pharmacokinetics.
Sulpha drugs exert their antimicrobial effects by interfering with the metabolism of bacteria and fungi. They were considered ground breaking medications before the discovery of penicillin and continue to be used in certain applications today. Sulphanilamides are a versatile class of compounds with various applications, making the development of general and effective methods for their synthesis desirable. Over the years, several synthetic methods have been reported which can provide a brief overview of some common and recent approaches. These methods typically involve the use of sulfonyl chloride, transition metals as catalysts, or Grignard reagents.
Discussion
Synthesis of sulphanilamide from thiols
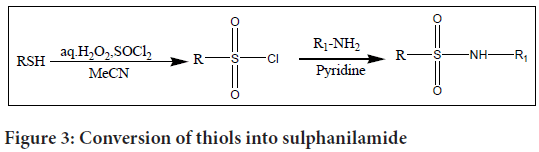
A common technique for making sulphanilamide is the sulphonylation of amines with chlorides in the incidence of a base. In this synthesis method, sulphonyl chlorides are attacked by ammonia, primary, or secondary amines nucleophilically when a base is present. Although effective, this approach depends on the availability of sulphonyl chlorides, which can occasionally be difficult to handle or store.
There are several ways to create sulphonyl chlorides from the equivalent thiols. Blowing chlorine gas (Cl2) into an aqueous acid solution or a biphasic mixture containing the thiol is one such method. In order to pro duce sulphonyl chlorides, sulphonic acids can also be treated with chlorinating chemicals like thionyl chloride (SOCl2), phosphorus oxychloride (POCl3), or phosphorus pentachloride (PCl5).
H2O2-SOCl2 has recently been found to directly oxidise thiols into sulphanilamides (Figure 3). The matching sulphanilamides were produced using this approach in good yields and with incredibly fast reaction times.
Figure 3: Conversion of thiols into sulphanilamide
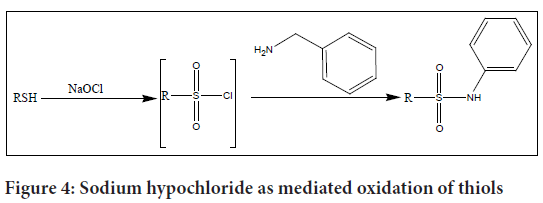
Wright et al. established a unique approach for the synthesis of a sulphonyl chloride in situ utilising sodium hypochlorite, often known as commercial bleach, to produce sulphanilamide from thiols (Wright SW and Hallstrom KN, 2006) (Figure 4). This methodology offers several advantages, including the ready availability of the reagents and the ability to control the amount of oxidant used.
Figure 4: Sodium hypochloride as mediated oxidation of thiols
In their study, the researchers first oxidize the thiol using sodium hypochlorite, generating the corresponding sulphonyl chloride. The use of sodium hypochlorite as an oxidant allows for a controlled oxidation process. After that the sulphonyl chloride reacted with benzylamine to produce sulphanilamide with upto high efficiency 98% yield.
This method provides a convenient and practical approach to sulphanilamide synthesis, utilizing easily accessible reagents and enabling control over the oxidation process. Sodium hypochlorite is used as an oxidizing agent offers advantages in terms of simplicity and cost-effectiveness.
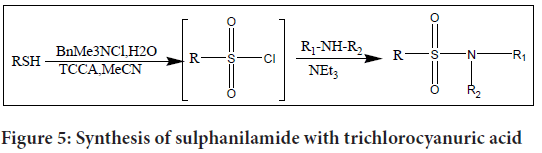
Trichlorocyanuric Acid (TCCA) and benzyltrimethyl ammonium chloride were added to water to produce a regulated quantity of chlorine in an aprotic solvent, especially acetonitrile (MeCN), to create a modified approach for the manufacture of sulphanilamides. Compared to using hypochlorite, this modification has the benefit of manufacturing high-purity chlorine.
The research team induced the reaction in their study by mixing water with trichlorocyanuric acid and benzyltrimethyl ammonium chloride, which resulted in the regulated production of chlorine. The aprotic solvent MeCN was used to carry out the reaction. As an oxidising agent, the procedure generates high-purity chlorine (Figure 5).
Figure 5: Synthesis of sulphanilamide with trichlorocyanuric acid
The inclusion of the ensuing amine into a one-pot reaction is a noteworthy innovation. By adding the amine directly into the reaction mixture, the sulphonyl chloride is generated in situ. This streamlined approach allows for the efficient synthesis of sulphanilamide within a short timeframe, typically under one hour.
This modified method offers the advantages of high-purity chlorine production and the convenience of conducting the entire sulphanilamide synthesis in a one-pot reaction.
Synthesis of sulphanilamide from transition metal catalysts
Extensive research has been done on the synthesis of sulphanilamide utilising transition metal catalysts, notably through cross-coupling processes for C-N bond formation. The palladium-catalyzed Buchwald-Hartwig reaction, often known as N-arylation, is a well-known example (Green RA and Hartwig JF, 2014). The N-arylation of sulphanilamides has been investigated using several transition metal catalysts.
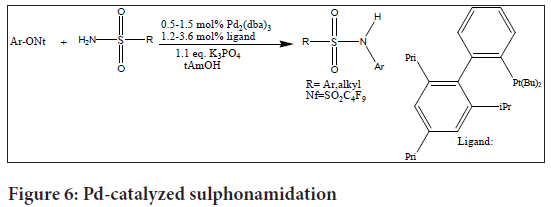
Palladium (Pd), one of these catalysts, has received much research. For instance, the ideal base-solvent combination for Pd-catalyzed sulphonamidation of aryl nonafluorobutanesulphonates has been discovered to consist of the biaryl phosphine ligand t-BuXPhos, K3PO4 as the base, and tert-amyl alcohol as the solvent. These reactional circumstances have demonstrated high tolerance for a range of functional groupings. The drawback of this approach is that 2,6-disubstituted aryl nonaflates do not engage in the reaction effectively (Anderson KW, et al., 2003) (Figure 6).
Figure 6: Pd-catalyzed sulphonamidation
This Pd-catalyzed sulphonamidation technique offers a flexible and effective way to synthesize sulphanilamides by using the right ligands and reaction conditions. However, while employing this approach, it is important to take into account the unique restrictions on 2,6-disubstituted aryl nonaflates.
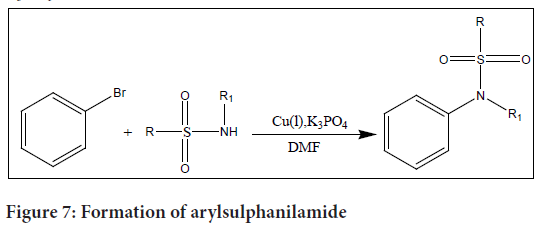
Investigators conducted a research on the coupling reactions between aryl bromides/iodides and copper (I) to create sulphanilamides. They found that employing an amino acid as a ligand offers a number of benefits, including simple removal following the reaction, throughout the optimization process. N-methylglycine and N,N-dimethylglycine were determined to have the maximum efficacy when combined with Cu(I) after a number of amino acids were examined.
The researchers used N,N-DimethylFormamide (DMF) as the solvent and K3PO4 as the base in their optimised reaction conditions. These conditions enabled the generation of the desired N-arylsulphalinamides with high yields, up to 99%.
With the aid of aryl bromides/iodides and copper (I) catalysts, this process provides a useful and effective way for the synthesis of sulphanilamides. Amino acids can be used as ligands, which has advantages such simple ligand removal after reactions. In the synthesis of N-arylsulphanilamides, the mixture of N-methylglycine or N,N-dimethylglycine with Cu(I) catalyst, K3PO4 base, and DMF solvent has outstanding performance (Figure 7).
Figure 7: Formation of arylsulphanilamide
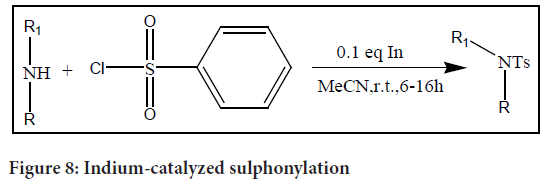
The indium-catalyzed sulphonylation of amines is a simple and effective technique for the production of sulphanilamide. This process enables the synthesis of a wide variety of sulphanilamides with high yields. In terms of substrate range, it exhibits generality by working with anilines that are less nucleophilic and sterically hindered. Additionally, this technique may be used to create sulphonic esters from sulphonyl chlorides and alcohols, thus it is not just restricted to the manufacture of sulphanilamide.
The indium-catalyzed sulphonylation reaction offers a versatile and effective approach to access a wide variety of sulphanilamide compounds (Figure 8). It demonstrates good reactivity even with challenging substrates, enabling the synthesis of sulphanilamide that are otherwise difficult to access using other methods. Additionally, the application of this methodology extends beyond sulphanilamide synthesis to the preparation of sulphonic esters, providing further synthetic versatility.
Figure 8: Indium-catalyzed sulphonylation
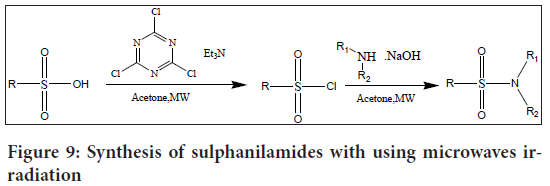
Sulphonyl chlorides, which serve as important intermediates in sulphanilamide synthesis, can be obtained from sulphonic acids. Microwave irradiation is a facile and useful technique for this synthesis that has demonstrated excellent functional group tolerance and high yields (Porcheddu A and De Luca L, 2014) (Figure 9).
Figure 9: Synthesis of sulphanilamides with using microwaves irradiation
Furthermore, the synthesis of sulphanilamides can also be accomplished using classical heating methods, resulting in good to excellent yields.
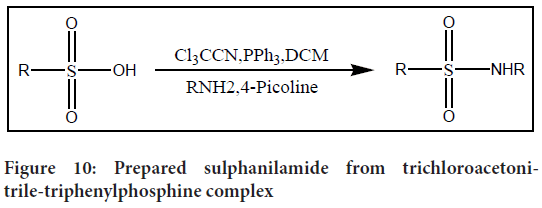
For the production of sulphanilamides, the trichloroacetonitrile-triphenylphosphine combination (Cl3CCN/PPh3) was used. It was found that using a 3:3:1 ratio of Cl3CCN, PPh3, and sulphonic acid with dichloromethane as the solvent resulted in the highest yield. However, the reproducibility of yields in different solvents and ratios was not consistent. Notably, this methodology offers the advantage of being applicable not only to aromatic sulphonyl chlorides but also to heterocyclic and aliphatic sulphonyl chlorides (Figure 10).
Figure 10: Prepared sulphanilamide from trichloroacetonitrile-triphenylphosphine complex
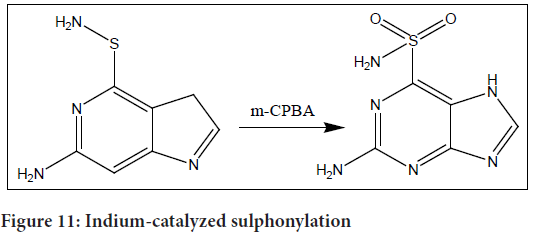
These diverse synthetic approaches provide flexibility in the synthesis of sulphanilamides, allowing for the utilization of different reaction conditions and substrates, expanding the scope of accessible sulphanilamide derivatives. Revankar GR, et al., 1990 used moderate and targeted oxidants to establish a novel method for the synthesis of 2-amino-9H-purin-6-sulfonamide. The oxidation of 2-amino-9H-purin-6-sulfenamide utilising meta-Chloroperoxybenzoic acid (m-CPBA) as the oxidant was the main focus of their work.
The researchers found that using one equivalent of meta-chloroperoxybenzoic acid resulted in a 48% yield of the target compound (Figure 11). When higher amounts of m-CPBA (4 equivalents) were employed, a slightly improved yield of 53% was obtained.
Figure 11: Indium-catalyzed sulphonylation
This methodology offers a mild and selective approach for the synthesis of 2-amino-9H-purin-6-sulphanilamide. By utilizing m-CPBA as the oxidant, the researchers were able to achieve the desired sulphanilamide product with reasonable yields.
Conclusion
In conclusion, several techniques have been published and thoroughly researched for the synthesis of sulphanilamide derivatives. Due to their wide spectrum of biological actions, these compounds are regarded as crucial “scaffolds” in medicinal chemistry for the synthesis of drugs. Additionally, sulphanilamide derivatives find practical applications in the organic chemistry industry, including the production of health products, food colorants, and more. Given their significance in both medicinal and industrial fields, it is crucial to continue research efforts aimed at synthesizing novel compounds containing the sulphonamide group. By exploring new synthetic methodologies and designing innovative derivatives, scientists can contribute to expanding the knowledge and potential applications of sulphalinamide compounds. Such research projects can lead to the development of improved drugs, as well as novel functional materials with beneficial properties for various industries.
References
- Banerjee M, Poddar A, Mitra G, Surolia A, Owa T, Bhattacharyya B. Sulfonamide drugs binding to the colchicine site of tubulin: Thermodynamic analysis of the drug-tubulin interactions by isothermal titration calorimetry. J Med Chem. 2005; 48(2): 547-555.
[Crossref] [Google Scholar] [Pubmed]
- Clercq E. New developments in anti-HIV chemotherapy. Curr Med Chem. 2001; 8(13): 1543-1572.
[Crossref] [Google Scholar] [Pubmed]
- Genç Y, Özkanca R, Bekdemir Y. Antimicrobial activity of some sulfonamide derivatives on clinical isolates of Staphylococus aureus. Ann Clin Microbiol Antimicrob. 2008; 7: 1-6.
[Crossref] [Google Scholar] [Pubmed]
- Özbek N, Katırcıoğlu H, Karacan N, Baykal T. Synthesis, characterization and antimicrobial activity of new aliphatic sulfonamide. Bioorg Med Chem. 2007; 15(15): 5105-5109.
[Crossref] [Google Scholar] [Pubmed]
- Mun J, Jabbar AA, Devi NS, Yin S, Wang Y, Tan C, et al. Design and in vitro activities of N-alkyl-N-[(8-R-2, 2-dimethyl-2 H-chromen-6-yl) methyl] heteroarylsulfonamides, novel, small-molecule hypoxia inducible factor-1 pathway inhibitors and anticancer agents. J Med Chem. 2012; 55(15): 6738-6750.
[Crossref] [Google Scholar] [Pubmed]
- El-Sayed NS, El-Bendary ER, El-Ashry SM, El-Kerdawy MM. Synthesis and antitumor activity of new sulfonamide derivatives of thiadiazolo [3, 2-a] pyrimidines. Eur J Med Chem. 2011; 46(9): 3714-3720.
[Crossref] [Google Scholar] [Pubmed]
- Scozzafava A, Mastrolorenzo A, Supuran CT. Sulfonamides and sulfonylated derivatives as anticancer agents. Curr Cancer Drug Targets. 2002; 2(1): 55-75.
[Crossref] [Google Scholar] [Pubmed]
- Talley JJ, Bertenshaw SR, Brown DL, Carter JS, Graneto MJ, Kellogg MS, et al. N-[[(5-methyl-3-phenylisoxazol-4-yl)-phenyl] sulfonyl] propanamide, sodium salt, parecoxib sodium: A potent and selective inhibitor of COX-2 for parenteral administration. J Med Chem. 2000; 43(9): 1661-1663.
[Crossref] [Google Scholar] [Pubmed]
- Maryanoff BE, Nortey SO, Gardocki JF, Shank RP, Dodgson SP. Anticonvulsant O-alkyl sulfamates. 2,3:4,5-Bis-O-(1-methylethylidene)-beta-D-fructopyranose sulfamate and related compounds. J Med Chem. 1987; 30(5): 880-887.
[Crossref] [Google Scholar] [Pubmed]
- Stranix BR, Lavallée JF, Sévigny G, Yelle J, Perron V, LeBerre N, et al. Lysine sulfonamides as novel HIV-protease inhibitors: Nε-acyl aromatic α-amino acids. Bioorg Med Chem Lett. 2006; 16(13): 3459-3462.
[Crossref] [Google Scholar] [Pubmed]
- Soukarieh F, Nowicki MW, Bastide A, Pöyry T, Jones C, Dudek K, et al. Design of nucleotide-mimetic and non-nucleotide inhibitors of the translation initiation factor eIF4E: Synthesis, structural and functional characterisation. Eur J Med Chem. 2016; 124: 200-217.
[Crossref] [Google Scholar] [Pubmed]
- Ong JJ, De Felice M. Migraine treatment: Current acute medications and their potential mechanisms of action. Neurotherapeutics. 2018; 15: 274-290.
[Crossref] [Google Scholar] [Pubmed]
- Roush WR, Gwaltney SL, Cheng J, Scheidt KA, McKerrow JH, Hansell E. Vinyl sulfonate esters and vinyl sulfonamides: potent, irreversible inhibitors of cysteine proteases. J Am Chem Soc. 1998; 120(42): 10994-10995.
- Naim MJ, Alam O, Alam MJ, Hassan MQ, Siddiqui N, Naidu VG, et al. Design, synthesis and molecular docking of thiazolidinedione based benzene sulphonamide derivatives containing pyrazole core as potential anti-diabetic agents. Bioorg Chem. 2018; 76: 98-112.
[Crossref] [Google Scholar] [Pubmed]
- Haughn GW, Smith J, Mazur B, Somerville C. Transformation with a mutant Arabidopsis acetolactate synthase gene renders tobacco resistant to sulfonylurea herbicides. Mol Genet Genom. 1988; 211: 266-271.
- Mirian M, Zarghi A, Sadeghi S, Tabaraki P, Tavallaee M, Dadrass O, et al. Synthesis and cytotoxic evaluation of some novel sulfonamide derivatives against a few human cancer cells. Iran J Pharm Res. 2011; 10(4): 741.
[Google Scholar] [Pubmed]
- Renzi G, Scozzafava A, Supuran CT. Carbonic anhydrase inhibitors: Topical sulfonamide antiglaucoma agents incorporating secondary amine moieties. Bioorg Med Chem Lett. 2000; 10(7): 673-676.
[Crossref] [Google Scholar] [Pubmed]
- Gibson JR. Trimethoprin-polymyxin B ophthalmic solution in the treatment of presumptive bacterial conjunctivitis-A multicentre trial of its efficacy versus neomycin-polymyxin B-gramicidin and chloramphenicol ophthalmic solutions. J Antimicrob Chemother. 1983; 11(3): 217-221.
[Crossref] [Google Scholar] [Pubmed]
- Del Rosso JQ. The use of sodium sulfacetamide 10%-sulfur 5% emollient foam in the treatment of acne vulgaris. J Clin Aesthet Dermatol. 2009; 2(8): 26.
[Google Scholar] [Pubmed]
- Aspinall TV, Joynson DH, Guy E, Hyde JE, Sims PF. The molecular basis of sulfonamide resistance in Toxoplasma gondii and implications for the clinical management of toxoplasmosis. J Infect Dis. 2002; 185(11): 1637-1643.
[Crossref] [Google Scholar] [Pubmed]
- Arredondo-García JL, Figueroa-Damián R, Rosas A, Jáuregui A, Corral M, Costa A, et al. Comparison of short-term treatment regimen of ciprofloxacin versus long-term treatment regimens of trimethoprim/sulfamethoxazole or norfloxacin for uncomplicated lower urinary tract infections: A randomized, multicentre, open-label, prospective study. J Antimicrob Chemother. 2004; 54(4): 840-843.
[Crossref] [Google Scholar] [Pubmed]
- McCarty JM, Richard G, Huck W, Tucker RM, Tosiello RL, Shan M, et al. A randomized trial of short-course ciprofloxacin, ofloxacin, or trimethoprim/sulfamethoxazole for the treatment of acute urinary tract infection in women. Am J Med. 1999; 106(3): 292-299.
[Crossref] [Google Scholar] [Pubmed]
- Chopra I, Roberts M. Tetracycline antibiotics: Mode of action, applications, molecular biology, and epidemiology of bacterial resistance. Microbiol Mol Biol Rev. 2001; 65(2): 232-260.
[Crossref] [Google Scholar] [Pubmed]
- Fischbach MA, Walsh CT. Antibiotics for emerging pathogens. Science. 2009; 325(5944): 1089-1093.
[Crossref] [Google Scholar] [Pubmed]
- Wright SW, Hallstrom KN. A convenient preparation of heteroaryl sulfonamides and sulfonyl fluorides from heteroaryl thiols. J Org Chem. 2006; 71(3): 1080-1084.
[Crossref] [Google Scholar] [Pubmed]
- Green RA, Hartwig JF. Palladium-catalyzed amination of aryl chlorides and bromides with ammonium salts. Org Lett. 2014; 16(17): 4388-4391.
- Anderson KW, Mendez-Perez, M, Priego J, Buchwald SL. Palladium-catalyzed sulfonamidation of aryl nonaflates. J Org Chem. 2003; 68(25): 9563-9573.
[Crossref] [Google Scholar] [Pubmed]
- Porcheddu A, De Luca L. Microwave-assisted synthesis of sulfonamides. Future Sci. 2014: 120-133.
- Revankar GR, Hanna NB, Imamura N, Lewis AF, Larson SB, Finch RA, et al. Synthesis and in vivo antitumor activity of 2-amino-9H-purine-6-sulfenamide,-sulfinamide, and-sulfonamide and related purine ribonucleosides. J Med Chem. 1990; 33(1): 121-128.
[Crossref] [Google Scholar] [Pubmed]
Author Info
Gagandeep Kaur1, Hardeep Kaur1, Suman Lata1* and Divya Dhawal Bhandari22Department of Pharmacy, University Institute of Pharmaceutical Sciences, Punjab, India
Citation: Gagandeep K: An Insight into Synthetic and Pharmacological Profile of Sulphanilamide
Received: 26-Jun-2023 Accepted: 21-Jul-2023 Published: 28-Jul-2023, DOI: 10.31858/0975-8453.14.7.474-478
Copyright: This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
ARTICLE TOOLS
- Dental Development between Assisted Reproductive Therapy (Art) and Natural Conceived Children: A Comparative Pilot Study Norzaiti Mohd Kenali, Naimah Hasanah Mohd Fathil, Norbasyirah Bohari, Ahmad Faisal Ismail, Roszaman Ramli SRP. 2020; 11(1): 01-06 » doi: 10.5530/srp.2020.1.01
- Psychometric properties of the World Health Organization Quality of life instrument, short form: Validity in the Vietnamese healthcare context Trung Quang Vo*, Bao Tran Thuy Tran, Ngan Thuy Nguyen, Tram ThiHuyen Nguyen, Thuy Phan Chung Tran SRP. 2020; 11(1): 14-22 » doi: 10.5530/srp.2019.1.3
- A Review of Pharmacoeconomics: the key to “Healthcare for All” Hasamnis AA, Patil SS, Shaik Imam, Narendiran K SRP. 2019; 10(1): s40-s42 » doi: 10.5530/srp.2019.1s.21
- Deuterium Depleted Water as an Adjuvant in Treatment of Cancer Anton Syroeshkin, Olga Levitskaya, Elena Uspenskaya, Tatiana Pleteneva, Daria Romaykina, Daria Ermakova SRP. 2019; 10(1): 112-117 » doi: 10.5530/srp.2019.1.19
- Dental Development between Assisted Reproductive Therapy (Art) and Natural Conceived Children: A Comparative Pilot Study Norzaiti Mohd Kenali, Naimah Hasanah Mohd Fathil, Norbasyirah Bohari, Ahmad Faisal Ismail, Roszaman Ramli SRP. 2020; 11(1): 01-06 » doi: 10.5530/srp.2020.1.01
- Manilkara zapota (L.) Royen Fruit Peel: A Phytochemical and Pharmacological Review Karle Pravin P, Dhawale Shashikant C SRP. 2019; 10(1): 11-14 » doi: 0.5530/srp.2019.1.2
- Pharmacognostic and Phytopharmacological Overview on Bombax ceiba Pankaj Haribhau Chaudhary, Mukund Ganeshrao Tawar SRP. 2019; 10(1): 20-25 » doi: 10.5530/srp.2019.1.4
- A Review of Pharmacoeconomics: the key to “Healthcare for All” Hasamnis AA, Patil SS, Shaik Imam, Narendiran K SRP. 2019; 10(1): s40-s42 » doi: 10.5530/srp.2019.1s.21
- A Prospective Review on Phyto-Pharmacological Aspects of Andrographis paniculata Govindraj Akilandeswari, Arumugam Vijaya Anand, Palanisamy Sampathkumar, Puthamohan Vinayaga Moorthi, Basavaraju Preethi SRP. 2019; 10(1): 15-19 » doi: 10.5530/srp.2019.1.3