Research Article - (2022) Volume 13, Issue 11
Abstract
Arboviruses are recognized by the World Health Organization (WHO) as a major public health problem, due to their growing territorial spread and the great need for preventive and control actions, which are increasingly complex. Among the arboviruses that present the greatest circulation is the Zika virus (ZIKV). Thus, this work aims to evaluate the expression of mammalian Target of Rapamycin (mTOR) in tissues of females of the species Mesocricetus auratus infected with the Zika virus. The samples used in this study come from a larger project entitled “Experimental Zika virus infection in golden hamsters (Mesocricetus auratus)” from the sexually transmitted group, previously approved by the Ethics Committee on Animal Use (CEUA) of Evandro Chagas Institute with registration number 24/2016. The samples are a total of 63 samples, divided between ZIKV-infected and non-infected animals. This study showed how Zika virus may be related to mTOR 1 and mTOR 2 expressions. The comparative results to viral load emphasize the correlation between the virus and mTOR. Such correlation is of importance to ZIKV, for protein regulation pathways and virus interference on those pathways may turn out to be a factor related to its pathogenic mechanism.
Keywords
Zika virus (ZIKV), mammalian Target of Rapamycin (mTOR) pathway, mTOR 1, mTOR 2
Introduction
Arboviruses are recognized by the World Health Organization (WHO) as a major public health problem, due to their growing territorial spread and the great need for preventive and control actions, which are increasingly complex. Among the arboviruses that present the greatest circulation is the Zika virus (ZIKV) (Vicenti I, et al., 2020).
ZIKV was first isolated from a female Rhesus monkey in the Zika forest (Uganda) in 1947. It remains in tropical and subtropical ecologies in Africa and Asia, causing mild symptoms in humans (Wikan N and Smith DR, 2016; Vasconcelos PF, 2015).
After its viral isolation in the late 1940s, new cases of infection caused by Zika in humans were detected in Uganda in 1952. The ZIKV belongs to the Flaviridae family and the Flavivirus genus, having a genome of Ribonucleic Acid (RNA) single-stranded and positive polarity (Dick GW, et al., 1952; Medeiros D and Vasconcelos PF, 2019).
The ZIKV is transmitted through the bite of the Aedes aegypti mosquito, and its transmission is considered a major concern for public health, given that these arthropods are distributed in tropical and subtropical areas, covering a considerable contingent of susceptible individuals. In addition to transmission by mosquito bites, other authors have described the transmission of the virus through sexual, perinatal and blood transfusions (Wikan N and Smith DR, 2016; Haddow AD, et al., 2012).
Reports point out that the ZIKV was introduced in Brazil during the 2014 soccer world cup, starting an epidemic in the northeastern capitals that hosted the games. However, other events that took place in Brazil may also have contributed to the entry of the virus in the country, the Pope's visit in 2013 and a canoeing competition in 2014, both of which took place in the city of Rio de Janeiro (Vasconcelos PF, 2015).
This virus causes a febrile illness discretely related to the occurrence of other symptoms, such as headache, rash, malaise, edema and joint pain, sometimes intense. However, Zika infection has also been linked to more severe cases, which include involvement of the central nervous system (Guillain-Barré syndrome, transverse myelitis and meningitis). In 2015, the ZIKV aroused the curiosity of the international scientific community, when it reported that the infection in pregnant women was related to severe cases of microcephaly in newborns (Campos GS, et al., 2015; Schuler-Faccini L, et al., 2016).
Studies involving rodents were of great importance to clarify information about Zika infection, showing that the virus can affect the brain during development, resulting in serious neurological deficiencies in addition to anatomical malformations (Zanluca C, et al., 2015).
Within this context, mTOR pathways have shown great relevance, as notable changes in cell metabolism during the early stages of neuronal stem cell differentiation are crucial for brain development. Studies demonstrate that ZIKV inhibits the mTOR signaling pathway, which causes aberrant autophagy activation, promoting virus replication and propagation (Liang Q, et al., 2016). Thus, this work aims to evaluate the expression of mTOR in tissues of females of the species Mesocricetus auratus infected with the Zika virus.
Methods
Samples and ethical aspects
The samples used in this study come from a larger project entitled “Experimental Zika virus infection in golden hamsters (Mesocricetus auratus)” from the sexually transmitted group, previously approved by the Ethics Committee on Animal Use (CEUA) of Evandro Chagas Institute with registration number 24/2016, according to Animal Research: Reporting of In Vivo Experiments (ARRIVE) guidelines and in accordance with relevant guidelines and regulations.
The samples are a total of 63 samples, divided between ZIKV-infected and non-infected animals as in the table below (Table 1).
| Organ | Days post infection (dpi) | Animals | Number of animals | ID |
|---|---|---|---|---|
| Brain | 3 | Adult female | 6 Infected, 3 not infected | CF-3 dpi |
| Brain | 7 | Adult female | 6 Infected, 3 not infected | CF-7 dpi |
| Brain | 23 | Adult female | 6 Infected, 3 not infected | CF-23 dpi |
| Uterus | 3 | Adult female | 6 Infected, 3 not infected | UT-3 dpi |
| Uterus | 7 | Adult female | 6 Infected, 3 not infected | UT-7 dpi |
| Uterus | 23 | Adult female | 6 Infected, 3 not infected | UT-23 dpi |
| Brain | Infected mother* | New born | 6 Infected, 3 not infected | CN-23 dpi |
Note: *The mother infected in early pregnancy
Table 1: Samples with the organ, days post infection (dpi), animals, number of animals and ID
RNA extraction
To obtain the genetic material, the samples were processed using the Maxwell 16 LEV mRNA Tissue kit (Promega, USA) according to the manufacturer's protocol and then quantified by the qubit 2.0 platform (Invitrogen, USA).
Quantification of viral load
To quantify the viral load, the commercial GoTaqProbe 1-Step RT-qPCR System kit (Promega, USA). Together with the absolute curve quantification method, using a plasmid cloned in pGEM easy vector (Promega, USA) of the genome of the ZIKV using the protocol described by Faye O, et al., (Faye O, et al., 2008).Quantification of mTOR expression
To quantify the machinery of the mRNAs, the 2-step qPCR kit (Promega, USA) was used, where the complementary DNA (cDNA) performed using 100 ng of messenger RNA (mRNA) from each extracted sample. Then, the expression of the targets mTOR 1 and mTOR 2 was performed by quantitative Polymerase Chain Reaction (qPCR) using the following primers: mTOR 1_F (AGGCCGCATTGTCTCTATCAA), mTOR 1_R (GCAGTAAATGCAGGTAGTCATCCA), mTOR 2_F (TCTACCACGACAGCCCGGCA) and mTOR 2_R (TGGGGGCCCCGTTCCATCAT).
Expression control used Glyceraldehyde 3-phosphate dehydrogenase (GAPDH), gene (F-GTTGTCTCCTGCGACTTCA and R-GGTGGTCCAGGGTTTCTTA) and B-Actin (F-CTGTCCCTGTATGCCTCTG and R-ATTGTCACGCACGATTTCC) (Passtoors WM, et al., 2013).
The quantification of the expression level of the mRNAs based on the Cts (Threshold Cycle) obtained, using the 2-ΔΔCt method, through the R-project program (r-project.org) (Livak KJ and Schmittgen TD, 2001).
Statistical analysis
For statistical analysis, the Shapiro-Wilk normality verification test, Analysis of Variance (ANOVA), and Pearson's correlation were performed. All analyses were performed on the Jamovi 2.0 program (https://www.jamovi.org).
Results
During our analysis, it was possible to quantify the viral loads in the animals included in the study. A higher viral load was found both at the females brains (CF) and at their uteri (UT) at 3 dpi. At 7 dpi a significant decrease was already possible (p=0.027) for CF; however, such a reduction is not as remarkable as the uteri (p=1.0) (Figure 1).
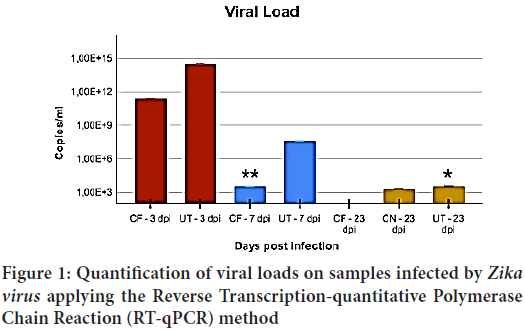
Figure 1: Quantification of viral loads on samples infected by Zika virus applying the Reverse Transcription-quantitative Polymerase Chain Reaction (RT-qPCR) method
At 23 dpi it is possible to verify the absence of viral loads in CF, while the UT group shows a reduction when compared to the 3 dpi mark (p=0.045). The group also includes newborn individuals (CN) presenting a viral load close to that of the UT group at 23 dpi, and no significant variation of viral load was detected within any of those groups during this time span (p=0.80).
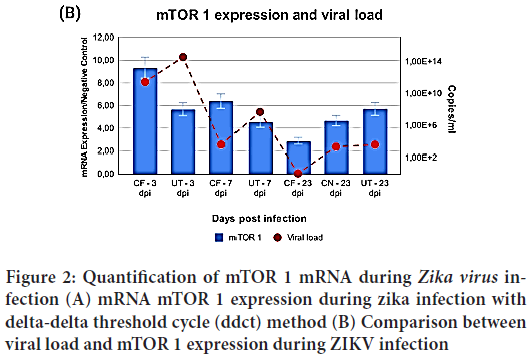
After ascertaining viral loads, the RT-qPCR test for mammalian Target of Rapamycin Complex-1(mTORC) expression was applied on the same groups and samples used for viral load quantification. It was therefore possible to compare both mRNA expression from mTORC1 and viral load (Figure 2). Different viral loads were determined between the groups (p=0.029), and a variation may be seen along the ZIKV infection period.
Figure 2: Quantification of mTOR 1 mRNA during Zika virus infection (A) mRNA mTOR 1 expression during zika infection with delta-delta threshold cycle (ddct) method (B) Comparison between viral load and mTOR 1 expression during ZIKV infection
Through analysis of mTOR 1 numbers it is possible to detect a variation over infection time and it is made clearer when comparing the 3 dpi to the 23 dpi marks, when the CF group figures drop sevenfold (p=0.001) between 3 and 23 dpi. When comparing the same time span, a decrease by a factor of 4 (p=0.049) was noticeable. It is worth emphasizing that all periods were compared to noninfected samples.
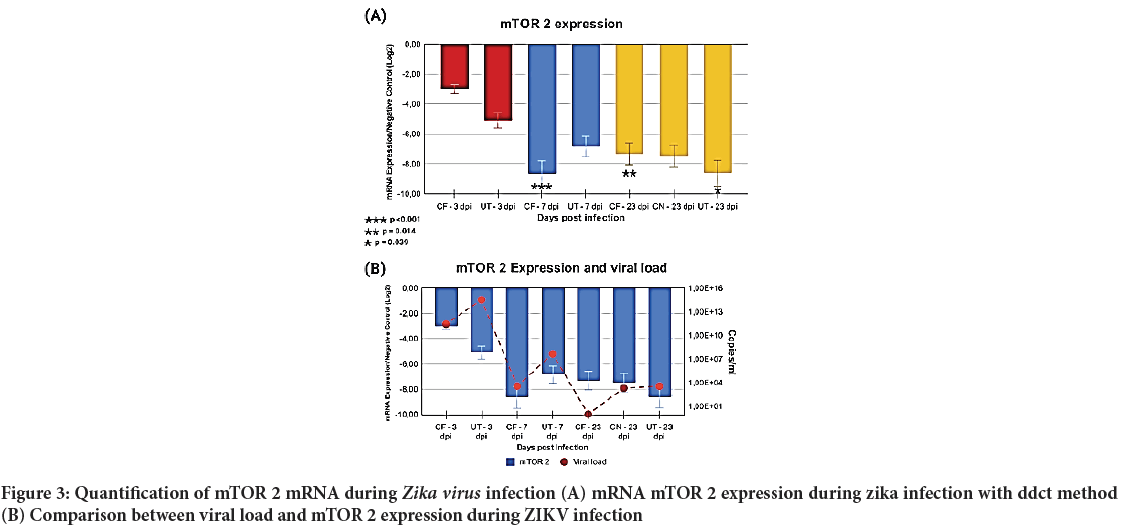
After mTOR 1 analysis, a quantification of mTOR 2 expression was conducted applying the same previous parameters, i.e., infected samples were compared to noninfected animals (control group). mTOR 2 as it turned out was quite different from mTOR 1, as a decrease in mTOR 2 expression was determined over the whole period of study (Figure 3).
Figure 3: Quantification of mTOR 2 mRNA during Zika virus infection (A) mRNA mTOR 2 expression during zika infection with ddct method
(B) Comparison between viral load and mTOR 2 expression during ZIKV
By following the same comparison principle applied to mTORC1, it was possible to establish an important variation in the CF group from 3 to 7 dpi (p<0.001). When comparing 3 to 23 dpi in CF, a noticeable variation was also found (p=0.014). However, CF’s variation from 7 to 23 dpi was not noteworthy.
As for the UT group, the comparison shows only one important variation, between 3 and 23 dpi (p=0.039). Attention should be drawn to the fact that, during the whole period of study, both UT and CF groups showed a decreasing mTOR 2 expression. Such expressions went from 2 down to 9 times lower. The CN group also showed a lower expression than that of the control group, around 6 times lower, similar to the UT-23 dpi group.
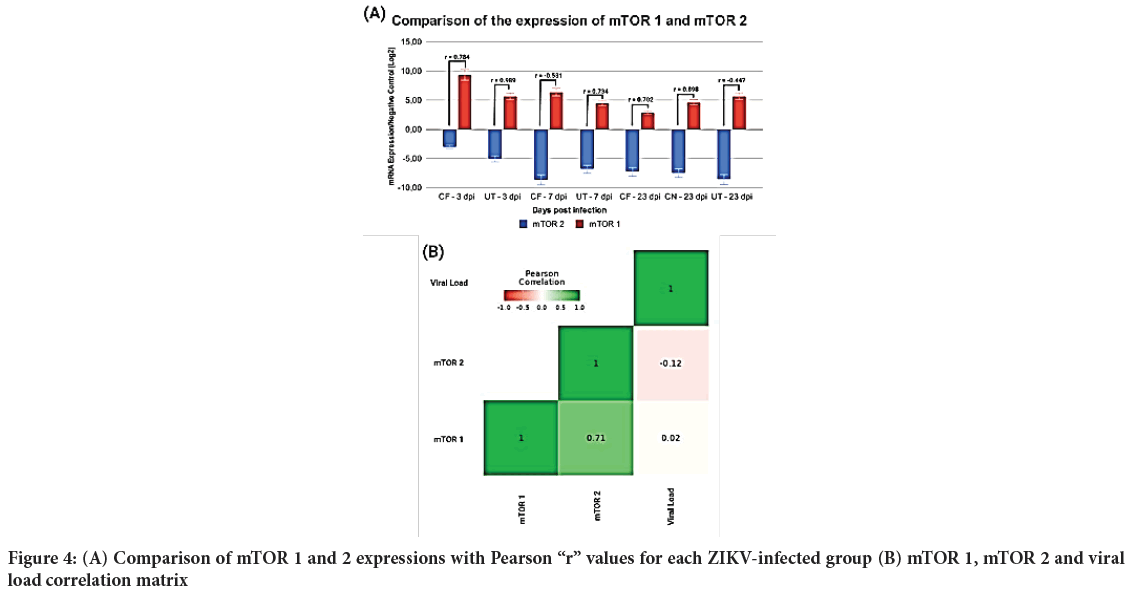
After the individual analyses, mTOR 1 and 2 were evaluated to determine any type of correlation during the ZIKV infection, and the Pearson Correlation test was performed. During this test, apart from the comparison between mTORC1 and 2, mTOR 1, mTOR 2 and viral load were also contrasted (Figure 4).
Figure 4: (A) Comparison of mTOR 1 and 2 expressions with Pearson “r” values for each ZIKV-infected group (B) mTOR 1, mTOR 2 and viral load correlation matrix
It is important to emphasize that sizable variations could be determined between all the studied groups, as well as between the targets, for both expression and viral load.
Discussion
mTOR coordinates many cellular processes in living organisms, regulating homeostasis, protein synthesis, transcription, autophagy, metabolism, brain function and the maturation of different tissues.
The discovery that regulation of mTOR signaling pathways correlates to persisting viral loads suggests that an infection by ZIKV may directly influence mTOR mechanics, promoting the survival of infected cells.
The mTOR signaling shows a vital role in neurological development, including the proliferation of neural stem cells, the development of neural circuits, plasticity and more complex functions.
A study conducted by Liang Q, et al. demonstrated that NS4A and NS4B Non-Structural protein from ZIKV can suppress the AKT-mTOR signaling pathway, which is the main responsible for controlling autophagy stimulation in eukaryotic cells, hence inducing autophagy (Liang Q, et al., 2016; Makker A, et al., 2016).
The Appelberg S, et al. emphasizes that changes to the AKT-mTOR signaling pathway are critical, not only for autophagy regulation, but for cortical development as well, as permanent inhibition may result in microcephaly (Appelberg S, et al., 2020).
This study showed how Zika virus may be related to mTOR 1 and mTOR 2 expressions. The comparative results to viral load emphasize the correlation between the virus and mTOR. Such correlation is of importance to ZIKV, for protein regulation pathways and virus interference on those pathways may turn out to be a factor related to its pathogenic mechanism. Laboratory tests demonstrate a correlation between mTOR 1, mTOR 2 and defensive cells (macrophages and neutrophils), which may suggest that the virus, through modifications to those paths, may induce proliferation of those cells.
It is worth pointing out that mTORC1 may be correlated to the macrophage signaling process, as it is even possible to modulate the inflammatory process during an infection by ZIKV. Correlations exist with the autophagy process and even with the cellular cycle regulation in various flaviviruses, like dengue (Kong W, et al., 2020). Our study demonstrates a significant increase in mTORC1 expression levels during the whole infectious process, which reiterates the occurrence of autophagy inside tissues, as described in literature.
Regarding mTORC2, a significant reduction in expression may be observed along the ZIKV infection process, which is linked to cellular longevity and to maintenance processes in the infected cells. This reasoning becomes more evident when we consider mTORC2 shows increased expression during the cellular maintenance cycle and when attempting cellular repair.
Our study showed a correlation between mTOR 1 and 2 during ZIKV infection, and such correlation appears to be closely connected, not only to infection duration and viral load, but to infected tissues as well, as our experiments involved neural tissue and the reproductive system.
Studies such as Rothan HA, et al. that verified changes in cellular metabolism during ZIKV infection, have correlated higher mTOR 1 expressions during an increase in viral load with a decrease in mTOR 2 in the same period, which corroborates our findings (Rothan HA, et al., 2019).
Our data, however, shows that a negative expression process for mTOR 2 with a positive expression for mTORC1 will occur both in mature neural cells, represented by the CF group, and in newborn cells, represented by CN. It is therefore possible to observe that ZIKV not only acts upon cellular differentiation, but on cellular metabolization and repair as well (Araki K, et al., 2009).
Our results appear to contribute to recent papers pointing to the relevance of mTOR to ZIKV maintenance and replication. Those findings demonstrate how mTOR is not only involved with cellular processing and maintenance.
Processes as autophagy are of great importance during the ZIKV infection, which is made clear by reviewing the results of Chiramel AI and Best SM demonstrates how important mTOR pathways are to the autophagy process inside neural tissues during infection by ZIKV (Chiramel AI and Best SM, 2018).
Collins SL, et al. covered the importance of this pathway for macrophage inflammatory signalling (Collins SL, et al., 2021). It makes clear how the mTOR pathways are a key to ZIKV maintenance and viral replication in very specific types of tissue, as those covered in the present work.
Detailed analyses of mTOR functions for other RNA viruses like Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2), Hepatitis C and Dengue 2 establish the relevance of mTOR pathways for the maintenance of infected cells and demonstrate how a reduced mTOR 2 expression may favor a viral infection, which is noticeable in the groups examined in our study (Lee EB, et al., 2020; Bowman LJ, et al., 2018).
Conclusion
In the present work, we were able to detect an important connection between mTOR 1 and mTOR 2 pathways during a ZIKV infection to cells from the nervous system and to cells from the female reproductive system. Also, those mTOR pathways are most important for the continuation of the ZIKV infection process during the infection period, and that such importance is not limited to cellular autophagy processes but reaches other cellular maintenance functions. It should be highlighted that the difference in expression from mTOR pathways in neural cells not only occurs in young neural cells, like those from newborn individuals, but in mature neural cells as well.
References
- Vicenti I, Dragoni F, Giannini A, Giammarino F, Spinicci M, Saladini F, et al. Development of a cell-based immunodetection assay for simultaneous screening of antiviral compounds inhibiting Zika and dengue virus replication. SLAS Discov. 2020; 25(5): 506-514.
[Crossref] [Google Scholar] [Pubmed]
- Wikan N, Smith DR. Zika virus: History of a newly emerging arbovirus. Lancet Infect Dis. 2016; 16(7): 119-126.
[Crossref] [Google Scholar] [Pubmed]
- Vasconcelos PF. Zika virus disease: A new emerging problem in the Americas? Rev Pan-Amaz Saude. 2015; 6(2): 9-10.
- Dick GW, Kitchen SF, Haddow AJ. Zika virus (I): Isolations and serological specificity. Trans R Soc Trop Med Hyg. 1952; 46(5): 509-520.
[Crossref] [Google Scholar] [Pubmed]
- Medeiros D, Vasconcelos PF. Is the Brazilian diverse environment is a crib for the emergence and maintenance of exotic arboviruses? An Acad Bras Cienc. 2019; 91.
[Crossref] [Google Scholar] [Pubmed]
- Haddow AD, Schuh AJ, Yasuda CY, Kasper MR, Heang V, Huy R, et al. Genetic characterization of Zika virus strains: Geographic expansion of the Asian lineage. PLoS Negl Trop Dis. 2012; 6(2): 1477.
[Crossref] [Google Scholar] [Pubmed]
- Campos GS, Bandeira AC, Sardi SI. Zika virus outbreak, Bahia, Brazil. Emerging Infect Dis. 2015; 21(10): 1885.
[Crossref] [Google Scholar] [Pubmed]
- Schuler-Faccini L, Ribeiro EM, Feitosa IM, Horovitz DD, Cavalcanti DP, Pessoa A, et al. Possible association between Zika virus infection and microcephaly-Brazil, 2015. MMWR Morb Mortal Wkly Rep. 2016; 65(3): 59-62.
[Crossref] [Google Scholar] [Pubmed]
- Zanluca C, Melo VC, Mosimann AL, Santos GI, Santos CN, Luz K. First report of autochthonous transmission of Zika virus in Brazil. Mem Inst Oswaldo Cruz. 2015; 110: 569-572.
[Crossref] [Google Scholar] [Pubmed]
- Liang Q, Luo Z, Zeng J, Chen W, Foo SS, Lee SA, et al. Zika virus NS4A and NS4B proteins deregulate Akt-mTOR signaling in human fetal neural stem cells to inhibit neurogenesis and induce autophagy. Cell Stem Cell. 2016; 19(5): 663-671.
[Crossref] [Google Scholar] [Pubmed]
- Faye O, Faye O, Dupressoir A, Weidmann M, Ndiaye M, Sall AA. One-step RT-PCR for detection of Zika virus. J Clin Virol. 2008; 43(1): 96-101.
[Crossref] [Google Scholar] [Pubmed]
- Passtoors WM, Beekman M, Deelen J, van der Breggen R, Maier AB, Guigas B, et al. Gene expression analysis of mTOR pathway: Association with human longevity. Aging Cell. 2013; 12(1): 24-31.
[Crossref] [Google Scholar] [Pubmed]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods. 2001; 25(4): 402-408.
[Crossref] [Google Scholar] [Pubmed]
- Makker A, Goel MM, Mahdi AA, Bhatia V, Das V, Agarwal A, et al. PI3K/Akt/mTOR signaling and its regulator tumour suppressor genes PTEN and LKB1 in human uterine leiomyomas. The Indian J Med Res. 2016; 143(1): 112.
[Crossref] [Google Scholar] [Pubmed]
- Appelberg S, Gupta S, Svensson AS, Ambikan AT, Mikaeloff F, Saccon E, et al. Dysregulation in Akt/mTOR/HIF-1 signaling identified by proteo-transcriptomics of SARS-CoV-2 infected cells. Emerg Microbes Infect. 2020; 9(1): 1748-1760.
[Crossref] [Google Scholar] [Pubmed]
- Kong W, Mao J, Yang Y, Yuan J, Chen J, Luo Y, et al. Mechanisms of mTOR and autophagy in human endothelial cell infected with dengue Virus-2. Viral Immunol. 2020; 33(1): 61-70.
[Crossref] [Google Scholar] [Pubmed]
- Rothan HA, Fang S, Mahesh M, Byrareddy SN. Zika virus and the metabolism of neuronal cells. Mol Neurobiol. 2019; 56(4): 2551-2557.
[Crossref] [Google Scholar] [Pubmed]
- Araki K, Turner AP, Shaffer VO, Gangappa S, Keller SA, Bachmann MF, et al. mTOR regulates memory CD8 T-cell differentiation. Nature. 2009; 460(7251): 108-112.
[Crossref] [Google Scholar] [Pubmed]
- Chiramel AI, Best SM. Role of autophagy in Zika virus infection and pathogenesis. Virus Res. 2018; 254: 34-40.
[Crossref] [Google Scholar] [Pubmed]
- Collins SL, Oh MH, Sun IH, Chan-Li Y, Zhao L, Powell JD, et al. mTORC1 signaling regulates proinflammatory macrophage function and metabolism. J Immunol. 2021; 207(3): 913-922.
[Crossref] [Google Scholar] [Pubmed]
- Lee EB, Sung PS, Kim JH, Park DJ, Hur W, Yoon SK. microRNA-99a restricts replication of hepatitis C virus by targeting mTOR and de novo lipogenesis. Viruses. 2020; 12(7): 696.
[Crossref] [Google Scholar] [Pubmed]
- Bowman LJ, Brueckner AJ, Doligalski CT. The role of mTOR inhibitors in the management of viral infections: A review of current literature. Transplantation. 2018; 102(2S): S50-S59.
[Crossref] [Google Scholar] [Pubmed]
Author Info
Luan Filipe de Souza Pereira*, Edivaldo Herculano Correa de Oliveira, Karla Fabiane Lopes de Melo, Francisco Canind� Ferreira de Luna, Edna Cristina Santos Franco, Pedro Fernando da Costa Vasconcelos and Samir Mansour Moraes CassebCitation: Pereira LFS: Analysis of the Expression of mTOR in Tissues of Females of Species Mesocricetus auratus Infected by Zika Virus
Received: 20-Oct-2022 Accepted: 03-Nov-2022 Published: 10-Nov-2022, DOI: 10.31858/0975-8453.13.11.756-760
Copyright: This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
ARTICLE TOOLS
- Dental Development between Assisted Reproductive Therapy (Art) and Natural Conceived Children: A Comparative Pilot Study Norzaiti Mohd Kenali, Naimah Hasanah Mohd Fathil, Norbasyirah Bohari, Ahmad Faisal Ismail, Roszaman Ramli SRP. 2020; 11(1): 01-06 » doi: 10.5530/srp.2020.1.01
- Psychometric properties of the World Health Organization Quality of life instrument, short form: Validity in the Vietnamese healthcare context Trung Quang Vo*, Bao Tran Thuy Tran, Ngan Thuy Nguyen, Tram ThiHuyen Nguyen, Thuy Phan Chung Tran SRP. 2020; 11(1): 14-22 » doi: 10.5530/srp.2019.1.3
- A Review of Pharmacoeconomics: the key to “Healthcare for All” Hasamnis AA, Patil SS, Shaik Imam, Narendiran K SRP. 2019; 10(1): s40-s42 » doi: 10.5530/srp.2019.1s.21
- Deuterium Depleted Water as an Adjuvant in Treatment of Cancer Anton Syroeshkin, Olga Levitskaya, Elena Uspenskaya, Tatiana Pleteneva, Daria Romaykina, Daria Ermakova SRP. 2019; 10(1): 112-117 » doi: 10.5530/srp.2019.1.19
- Dental Development between Assisted Reproductive Therapy (Art) and Natural Conceived Children: A Comparative Pilot Study Norzaiti Mohd Kenali, Naimah Hasanah Mohd Fathil, Norbasyirah Bohari, Ahmad Faisal Ismail, Roszaman Ramli SRP. 2020; 11(1): 01-06 » doi: 10.5530/srp.2020.1.01
- Manilkara zapota (L.) Royen Fruit Peel: A Phytochemical and Pharmacological Review Karle Pravin P, Dhawale Shashikant C SRP. 2019; 10(1): 11-14 » doi: 0.5530/srp.2019.1.2
- Pharmacognostic and Phytopharmacological Overview on Bombax ceiba Pankaj Haribhau Chaudhary, Mukund Ganeshrao Tawar SRP. 2019; 10(1): 20-25 » doi: 10.5530/srp.2019.1.4
- A Review of Pharmacoeconomics: the key to “Healthcare for All” Hasamnis AA, Patil SS, Shaik Imam, Narendiran K SRP. 2019; 10(1): s40-s42 » doi: 10.5530/srp.2019.1s.21
- A Prospective Review on Phyto-Pharmacological Aspects of Andrographis paniculata Govindraj Akilandeswari, Arumugam Vijaya Anand, Palanisamy Sampathkumar, Puthamohan Vinayaga Moorthi, Basavaraju Preethi SRP. 2019; 10(1): 15-19 » doi: 10.5530/srp.2019.1.3