Review Article - (2023) Volume 14, Issue 1
Antibiotics Modulate Variable Immunological Responses in Sepsis-A Narrative Review
Naveed Saleem*Abstract
Sepsis is a serious and life-threatening medical emergency associated with dysregulated host immune responses to infection. Like cerebral vascular or acute cardiovascular incidents, sepsis is considered a time-dependent condition having severe and longterm consequences on human health. Apart from organ support, prompt administration of appropriate antimicrobial therapy is crucial to limit the burden of complications related to sepsis in Intensive Care Unit (ICU) patients.
The management of septic patients requires comprehensive and multi-disciplinary strategies for an adequate diagnosis. Most of the ICU population receives empirical antibiotic therapy without having a confirmed diagnosis. The misuse of antibiotics in Intensive Care Units (ICU) may increase the possibility of developing multidrug resistance along with considerable ecological side effects. The first doses of empirical anti-microbial therapy are slightly higher, regardless of the presence or absence of organ dysfunction, which may upregulate the production of circulating pro-and-anti-inflammatory mediators, having negative effects on the general well-being of the patients. This notion supports the introduction of individualized antimicrobial approaches based on local patterns of resistance to ensure the appropriate dosage of empirical therapy, as well as to limit the emergence of multidrug resistance in advanced-care patients. The adequacy and treatment duration must be viewed at regular intervals for effective de-escalation, and novel diagnostic approaches must be introduced to improve the quality of care in the ICU population.
Keywords
Bactericidal, Bacteriostatic, Antibiotic(s), Antimicrobial Therapy
Introduction
Sepsis is one of the major health problems with higher rates of morbidity and mortality in the modern era of critical care management (Remick DG, 2007). Theoretically, it is defined as “life-threatening organ dysfunction due to a dysregulated host response to an infection” (Singer M, et al., 2016). It is a critical public health concern since it affects millions of people worldwide each year and remains a dominant cause of Multiple Organ Dysfunction Syndromes (MODS) in acute care settings (Remick DG, 2007). The overall mortality rates are quite higher (ranging from 15% to 55%) among critically ill patients in intensive care units (Rudd KE, et al., 2020).
In recent years, the administration of appropriate antimicrobial therapy, nutritional support and the availability of aggressive and invasive surgical interventions for advanced and life-threatening ailments may have improved clinical outcomes (Lepper P, et al., 2002). All the aforementioned factors may consider as potential leading causes of morbidity and mortality in the advanced care units (Lepper P, et al., 2002). Therefore, a better understanding of the pathophysiology of sepsis is essential to improve disease outcomes. Although the process of sepsis in severely ill patients has been discussed extensively in the past few decades, the chain of events responsible for pathophysiological alterations still is unclear.
Sepsis is quite a common problem in acute care settings; therefore, antibiotics are frequently administrated for the management of bacteremia and sepsis (Huttunen R and Aittoniemi J, 2011). Along with the infection’s treatment, antibiotics can also be used prophylactically to limit the spread of microbes in critically ill patients (Huttunen R and Aittoniemi J, 2011). Early and effective treatment by antibiotics limits the inflammatory sequelae and reduces the severity of ongoing organ dysfunction during acute illnesses (Huttunen R and Aittoniemi J, 2011). Moreover, early antibiotics administration may be associated with unpredicted effects on the clinical presentation possibly due to exacerbated inflammatory and immunological responses (Pankey GA and Sabath LD, 2004). Therefore, unnecessary exposure to antimicrobial drugs must be avoided, as it may promote the (over)growth of other non-susceptible organisms.
In the last few decades, it has become obvious that antibiotics play a key role in the pathophysiological events of bacteremia, sepsis, and septic shock (Spyridaki A, et al., 2012). This is due to their ability to liberate biologically active components of the cell wall and other microbial compounds, because of the destruction of the pathogens (Spyridaki A, et al., 2012). This aspect of antimicrobial therapy has been proved in most of the in vivo, in vitroas well as in clinical trials.
The literature search on various electronic databases such as PubMed, Embase, and Cochrane library was completed in August 2022 to critically re-evaluate the data regarding the release of pro-inflammatory mediators and toxic bacterial products by certain antibiotics. To support this notion, it is mandatory to see the effects of antibiotics on the liberation of endotoxin and cytokines in previously conducted preclinical and clinical trials for planning future experimental studies or treatment protocols.
Literature Review
Overview of sepsis and immune dysregulation
The World Health Organization (WHO) has recognized Infectious diseases as one of the major and leading causes of death worldwide, both in developed as well as less-developed countries (Chousterman BG, et al., 2017). In the last few decades, conceptual and technical advances have accelerated the understanding of various mechanisms, which are involved in the host’s response to an infection. However, the ‘’big picture’’ about the virulence of the micro-organisms and the host responses is still not clear.
Theoretically, it is expected that sepsis is a clinical syndrome having bimodal nature of presentation (Chousterman BG, et al., 2017). Primarily, it is characterized by a brief inflammatory phase, which is named the “Systematic Inflammatory Response Syndrome” (SIRS) (Chousterman BG, et al., 2017). This early phase activates the host’s innate immunological responses, in the presence of different micro-organisms, and bacterial and cellular products (Behrens EM and Koretzky GA, 2017). Later, it is followed by a sustained anti-inflammatory response, known as “Compensatory Anti-inflammatory Response Syndrome” (CARS) which represents the compensatory immune response, to limit the systemic activated inflammatory and immunological responses, ultimately leading to tissue injury and multiple organ failure due to immune paralysis (Behrens EM and Koretzky GA, 2017). The late-onset anti-inflammatory response proves that sepsis can be linked with a state of immunosuppression, which may last for a considerable period (weeks or months) following the initial onset of SIRS, as explained in Figure 1.
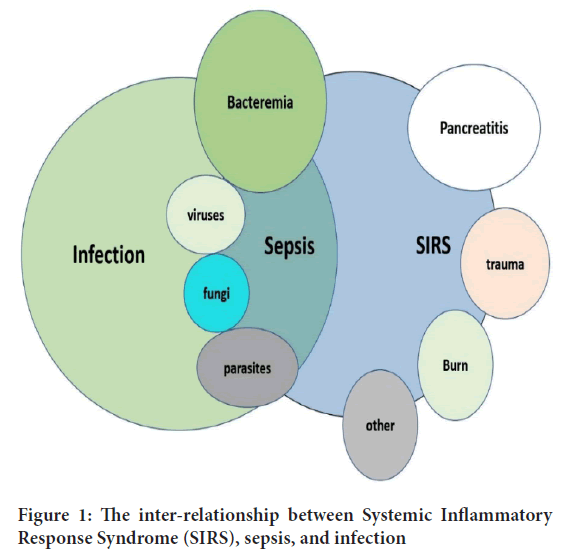
Figure 1: The inter-relationship between Systemic Inflammatory Response Syndrome (SIRS), sepsis, and infection
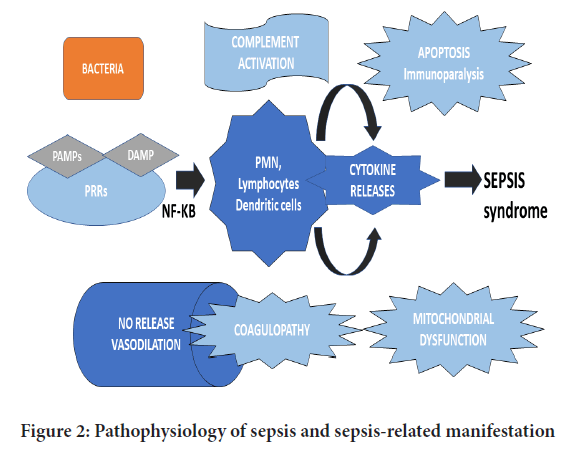
The equilibrium between the pro and anti-inflammatory responses is crucial for patients with severe sepsis (Netea MG, et al., 2003). SIRS is presumed to be associated with the release of pro-inflammatory cytokines such as Interleukins-1 (IL-1), IL-6 and TNF-α (Netea MG, et al., 2003). On the contrary, CARS may be attributed due to the biological effects of anti-inflammatory mediators such as IL-1ra, IL-10 and soluble Tumour Necrosis Factor Receptor-1 (sTNFR-1) (Netea MG, et al., 2003). Higher proportions of IL-1β, IL-6, IL-8 and Tumour Necrosis Factor-α (TNF-α) are associated with poor prognostic outcomes in septic patients (Netea MG, et al., 2003). Subsequently, the anti-inflammatory molecules which are produced in greater amounts during sepsis, predict the severity of the illness. Besides, it signifies the overall clinical and survival outcomes. Therefore, the imbalance between pro and counter-inflammatory mediators in the septic state demonstrates the severity of the systemic responses to the infection (Tisoncik JR, et al., 2012). Furthermore, it results in overwhelming stimulation and production of cytokines into systematic circulation, which could have harmful effects on patients with acute and life-threatening ailments, as illustrated in Figure 2.
Figure 2: Pathophysiology of sepsis and sepsis-related manifestation
Relationship between pro-inflammatory and toxic microbial products and sepsis
The virulence of the pathogenic organisms is not only the contributing factor to cellular and tissue destruction in response to an infection (Tisoncik JR, et al., 2012). Pathogens-Associated Microbial Products (PAMPs) such as lipoteichoic acid in gram-positive bacteria and endotoxins Lipopolysaccharide (LPS) in gram-negative bacteria play a pivotal role in the pathogenesis of sepsis (Gogos CA, et al., 2004). The microbe-derived constituents are associated with the production of various mediators by host immune systems, which create an imbalance in-between the immunological responses ultimately leading to tissue damage (Gogos CA, et al., 2004). Therefore, this review enlightens the clinical significance of the micro-organisms or microbial products to elicit a strong systemic inflammatory response, which will extend within the entire body due to the circulation of host immune cells and soluble pro-inflammatory cytokines.
The proinflammatory activity of Lipopolysaccharide (LPS)
In the past few decades, endotoxins (LPS) have been considered a key player in the pathogenesis of gram-negative septicemia (Huttunen R and Aittoniemi J, 2011). Moreover, its significance in a variety of clinical disorders such as peritonitis, infections, and trauma has been thoroughly investigated in many trials (Lepper P, et al., 2002). Lipopolysaccharides (LPS), which are a major component of the outer membrane of gram-negative micro-organisms, exhibit a strong pro-inflammatory response to host defence mechanisms (Nau R and Eiffert H, 2005). Lipid A mediates the inflammatory activities of LPS by facilitating the extensive production of a variety of pro-inflammatory mediators such as Interleukins (IL-1β. IL-6, IL-8) and TNF-α from human peripheral mononuclear blood lymphocytes and monocytes, as well as from the expression of adhesion molecules on endothelial cells (Nau R and Eiffert H, 2005). Moreover, LPS elicits many clinical manifestations, that mimic septic shock (Nau R and Eiffert H, 2005).
In recent years, the mechanisms responsible for cellular activation and host inflammatory responses by LPS have been extensively studied. Both the LPS Binding Proteins (LPB) and the LPS receptor CD14 are considered key mediators for the activation of inflammatory cell pathways (Netea MG, et al., 2003). Although, CD14 is not a transmembrane protein (Netea MG, et al., 2003). Therefore, other transmembrane signaling molecules such as the Toll-Like Receptor (TLR4) act as one of the trans-membrane components of the LPS-receptor complex and facilitate the transduction of LPS signaling (Netea MG, et al., 2003). This activation of the signaling cascade favours the transcription of the genes encoding inflammatory cytokines (Netea MG, et al., 2003).
The proinflammatory activity of Teichoic and Lipoteichoic-acid
Teichoic and Lipoteichoic Acids (TA, LTA) are the components of the cell walls of gram-positive pathogens and constitute up to 50% of their dry weight (Tisoncik JR, et al., 2012). Lipoteichoic acid favours the anchoring of the wall to the underlying cellular structures, while teichoic acid facilities the adhesion and supply of magnesium ions to the micro-organisms. Besides, it contributes to resistance to autolysis and the structural changes within the DNA of pneumococcal pathogens (Tisoncik JR, et al., 2012). The key structure of lipoteichoic acid is like gram-positive organisms, but LTAs are considered heterogeneous in molecular details and contemplated as the counterparts of the LPS of gram-negative organisms regarding the induction of inflammation (Tisoncik JR, et al., 2012).
LTAs possess variable biological features, which are mediated by the induction of many Interleukins such as IL-1, IL-6, IL-8, 12 and cytokines having pro-inflammatory characteristics (Gogos CA, et al., 2004). In animal models, the administration of LTAs is associated with the release of TNF-α, and the induction of inducible nitric oxide synthase, which favours circulatory failure (Nau R and Eiffert H, 2005). Furthermore, it can act either as an agonist or as an antagonist of LPS depending upon the origin of the LTAs.
Antibiotics modulate the release of pro-inflammatory and toxic microbial products
In recent years, it is quite apparent that antibiotics may play a pivotal role in the pathophysiological events of sepsis and septic-associated manifestations (Pankey GA and Sabath LD, 2004). This is mainly because of their ability to release immunologically active components of the microbial cell wall and other toxic products due to the rapid destruction of the micro-organisms (Spyridaki A, et al., 2012). Moreover, certain antibiotics may have harmful effects by inducing endotoxin release, particularly during gram-negative bacteremia (Gogos CA, et al., 2004). A few in vivostudies demonstrate that exposure of gram-negative microorganisms to certain antimicrobial agents can result in the release of both endotoxin and cytokines (Lepper P, et al., 2002).
In an experimental model of gram-negative sepsis, it is reported that there is an accelerated inflammatory response after the administration of antibiotics (Lepper P, et al., 2002). A recent clinical study demonstrated the immediate impairment of clinical conditions within a few hours after antibiotics in a large proportion of acute care patients with sepsis (Gia marellos-Bourboulis EJ, et al., 2006). This phenomenon is justified by antibiotic-induced endotoxin and cytokines released during the process of pathogen killing.
Jackson et al. reported that there was a discrepancy in the endotoxin release with the different classes of antibiotics (Vianna RC, et al., 2004). The antimicrobial agents having a high affinity for Penicillin-Binding Protein 3 (PBP-3), such as cephalosporins and piperacillin-tazobactam associated with an increase in inflammatory response and organ dysfunction due to massive production of endotoxin (Vianna RC, et al., 2004). On the contrary, antibiotics such as aminoglycosides are linked with lesser variations in inflammatory cytokines (Vianna RC, et al., 2004).
Clinical efficacy of bacteriostatic and bactericidal antibiotics
Adequate antimicrobial therapy is considered a cornerstone pharmacological regime in modern medical practices, as it provides appropriate management of bacteremia and sepsis (Pankey GA and Sabath LD, 2004) and lessens the burden of complications in patients with serious and life-threatening diseases. In current medical practices, the broader classification of antibiotics is a successful concept to distinguish antibiotics that inhibit the visible bacterial growth- “bacteriostatic” from the antimicrobials that kill or destroy the micro-organisms i.e. “bactericidal” (Pankey GA and Sabath LD, 2004). At present, this classification is adopted in most clinical guidelines worldwide (Pankey GA and Sabath LD, 2004), which needs to be followed while managing patients having infectious and life-threatening ailments.
Bactericidal antimicrobials are traditionally considered superior to bacteriostatic antimicrobials, as the former directly kills pathogens while bacteriostatic antimicrobial therapy halts the growth of the micro-organisms (Wald-Dickler N, et al., 2018; Saleem N, et al., 2022). The formal definition of a bactericidal antibiotic is a ratio of Minimum Bactericidal Concentration (MBC) to Minimum Inhibitory Concentration (MIC) <4, whereas a bacteriostatic agent has an MBC: MIC ratio>4 (Wald-Dickler N, et al., 2018; Saleem N, et al., 2022). This definition however is arbitrary with certain bacteriostatic antibiotics being able to kill pathogens at higher concentrations (Wald-Dickler N, et al., 2018; Saleem N, et al., 2022). Antibiotics including linezolid and vancomycin demonstrate bactericidal activity against some bacteria but bacteriostatic activity against others at higher concentrations (Saleem N, et al., 2022; Rubinstein E and Keynan Y, 2014; Clemett D and Markham A, 2000).
To understand, different classes of antibiotics are associated with variable levels of circulating endotoxins and cytokines (Chuang YC, et al., 2014). Several in vivoand in vitrostudies demonstrated that the use of certain antimicrobials leads to significantly higher levels of free endotoxins, whereas others produce relatively lower concentrations (Chuang YC, et al., 2014). Therefore, the levels of LPS, and TA, LTA, PGs and other microbial products are not dependent on the type and the number of invading pathogens (Lepper P, et al., 2002), and also, relate to the type of antibiotics being used, and differences based on the mode of action of certain antibiotics (Gogos CA, et al., 2004). For instance, certain β-lactam antibiotics are more potent inducers of free endotoxin levels than others (Gogos CA, et al., 2004).
Several studies have explained that certain antibiotics have the theoretical potential of either up-regulation or down-regulation of cytokine-induced organ dysfunction or endotoxemia. Bactericidal antibiotics such as Ampicillin-sulbactam and cefamandole seem to accelerate the production of pro-inflammatory mediators (Nemeth J, et al., 2015). Conversely, certain bacteriostatic, such as erythromycin and vancomycin are linked with the down-regulation of antibiotic-induced organ dysfunction (Nemeth J, et al., 2015). In a rat-based septic model, Peng ZY, et al., 2012 stated that bactericidal antibiotics resulted in a temporary increase in an inflammatory response, which accelerated the deterioration of renal functions. However, the resolution of inflammation and acute kidney dysfunction was much quicker and correlated well with better clinical and survival outcomes (Peng ZY, et al., 2012).
In vivo and ex vivo studies demonstrate antibiotics attenuate the immunological response
For gram-negative septicemia, the release of endotoxin has been thoroughly studied in various studies. Rusmin S and deLuca PP, 1975 demonstrated the release of bi-layered membrane vesicles containing endotoxin from micro-organisms, which are retained on intravenous inline filters after antibiotic administration. Usually, antibiotic treatment results in higher concentrations of endotoxin within the supernatants of cultures containing bacteria (Rusmin S and deLuca PP, 1975). This indicates the liberation of endotoxin after exposure of gram-negative pathogens to antimicrobial therapy is based on the type and dose of the therapeutic agents (Lepper P, et al., 2002). Generally, a high concentration of antibiotics releases a lesser quantity of endotoxin than others, which is close to the Minimum Inhibitory Concentration (MIC) (Lepper P, et al., 2002).
In vitro model, β-lactam agents, particularly PBP-2 specific drugs such as imipenem, induce the formation of spheroplast which initiate lesser production of endotoxin in comparison with PBP-3 specific agents such as ceftazidime, cefuroxime and aztreonam leading to the formation of filamentous bacterial forms (Byl B, et al., 2001). Sjölin J, et al., 2000 conducted the in vitrostudy, to explain the concept of combination antibiotics administration which arrested the inflammation and organ dysfunction more effectively than single treatment with higher doses (Sjölin J, et al., 2000). The combination of tobramycin and cefuroxime resulted in a correspondent reduction in endotoxin-liberating capacity, in comparison with that of tobramycin alone (Sjölin J, et al., 2000). Therefore, there is a strong correlation between microbial shape and cell wall morphology before lysis and the amount of endotoxin being released (Lepper P, et al., 2002).
Peng ZY, et al., 2012 conducted an animal-based study to explore the association between antimicrobial therapy, sepsis-induced acute kidney injury and disease outcomes. Cecal Ligation and Puncture (CLP)-induced septic rats with acute renal injury were used to demonstrate that bactericidal antibiotics caused a transient worsening of inflammation, which exacerbated the renal functions (Peng ZY, et al., 2012). The induction of acute kidney injury during sepsis was correlated with the temporary activation of pro-inflammatory mediators (Peng ZY, et al., 2012). Despite shorttermed and rapid clinical deterioration, the resolution of inflammation and improvement within the renal profile was quite evident among the animals receiving antibiotics indicating significantly improved survival outcomes (Peng ZY, et al., 2012).
Ceftazidime, lincomycin and clindamycin use for the treatment in E. coliseptic rat models is linked with a 20-folds increase of immunologically active bacterial products. Shenep JL and Mogan KA, 1984 analyzed that the untreated group had a concentration of free endotoxin corresponding to the levels of bacteremia (Shenep JL and Mogan KA, 1984). On the other hand, in an animal-treated group with antibiotics, the plasma concentrations of endotoxin marked up to 2000 folds despite having relatively lower levels of bacteremia (Shenep JL and Mogan KA, 1984). Therefore, endotoxemia caused by gram-negative sepsis may depend upon the class of antimicrobial therapy, but not in correlation with rates of bacterial killings (Lepper P, et al., 2002).
Clinical studies demonstrating antibiotics induce an immunological response
Usually, patients with sepsis or septic shock are not considered homogeneous representatives of the clinical population due to a lack of universal diagnostic and management guidelines (Klompas M, et al., 2018). At present, there are not enough prospective studies based on a well-defined and severe form of human sepsis having an adequate higher population of the patient to address the question of whether antibiotics instigate variable immunological and inflammatory events among patients having sepsis and sepsis-related complications.
The data from recently published studies suggest there is a variation in immunological responses concerning antibiotic treatment, which may have clinical relevance. Two well-defined randomized trials of antibiotic-induced endotoxemia among patients suffering from gram-negative sepsis are available (Byl B, et al., 2001; Luchi M, et al., 2000). The study population was sufficiently homogenous to limit the risks of selection bias and to support the statistical outcomes. In one group of patients with urosepsis, the clinical advantages of imipenem were noticed over ceftazidime with more sharp and rapid defervescence corresponding with the limited release of biologically active bacterial products (Luchi M, et al., 2000). Simpson AJ, et al., 2000 studied patients suffering from melioidosis, a disease quite prevalent in the Malaysian peninsula and caused by gram-negative rods i.e. Burkholderia pseudomalle(Simpson AJ, et al., 2000). Patients treated with imipenem showed lower systemic endotoxin levels after the first administration of the antibiotic (Simpson AJ, et al., 2000). However, the overall survival outcomes were virtually the same in both treated groups (Simpson AJ, et al., 2000).
Although the above-mentioned studies show that the contribution of specific antibiotics is quite negligible for endotoxin liberation among the patients (Byl B, et al., 2001; Luchi M, et al., 2000; Simpson AJ, et al., 2000). Mock CN, et al., 1995 stated that the selection of antimicrobial therapy had influential effects of PBP-3 specific versus non-PBP-3 specific antibiotics such as aztreonam, ceftazidime or cefotaxime on the clinical and survival outcomes in surgical patients having sepsis (Mock CN, et al., 1995). The overall hospital mortality was 17% among patients receiving PBP-3 specific antibiotics and the death rate was 8% in the group receiving other antimicrobial therapy (Mock CN, et al., 1995).
Discussion and Conclusion
In nutshell, severe sepsis and septic shock are life-threatening conditions that require immediate and aggressive management. Paradoxically, antibiotics may evoke circulatory collapse in some individuals due to the extensive release of inflammatory mediators. Moreover, there is a shred of increasing evidence from in vitro, animal, and clinical studies that antibiotics attenuate the release of biologically active and degraded microbial products having immunomodulatory properties. Therefore, adequately powered randomized trials with appropriate and reproducible criteria must require enhancing the generalizability by focusing on both the risks and benefits of antimicrobial chemotherapy.
Due to the diversity of underlying disease, as well as the complexity of the syndrome and multitude of causative agents, it is quite difficult to prove an advantage of a certain antibiotics therapy in terms of clinical outcomes, unless the recruitment of patients must be done carefully to lower the risks of heterogenicity. Furthermore, it is highly unlikely that mono-therapeutic approaches may overcome the spectrum of diseases associated with sepsis. Therefore, sequential antimicrobial therapy having different modes of action could help to achieve optimal drug effects and limit organ dysfunction by downregulating the production of inflammatory cytokines. The decision regarding empirical therapy should be dependent upon the clinical condition, antibiotic resistance patterns, and preferred modes of delivery rather than perceived differences in efficacy. Differences in efficacy between antibiotics should be considered, but this should be per antibiotic rather than per their classification as bactericidal or bacteriostatic antibiotics.
Conflicts of Interest
This narrative review was conducted as a part of a literature search related to a PhD project.
References
- Remick DG. Pathophysiology of sepsis. Am J Pathol. 2007; 170(5): 1435-1444.
[Crossref] [Google Scholar] [Pubmed]
- Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA. 2016; 315(8): 801-810.
[Crossref] [Google Scholar] [Pubmed]
- Rudd KE, Johnson SC, Agesa KM, Shackelford KA, Tsoi D, Kievlan DR, et al. Global, regional, and national sepsis incidence and mortality, 1990-2017: Analysis for the Global Burden of Disease Study. Lancet. 2020; 395(10219): 200-211.
[Crossref] [Google Scholar] [Pubmed]
- Lepper P, Held TK, Schneider E, Bölke E, Gerlach H, Trautmann M. Clinical implications of antibiotic-induced endotoxin release in septic shock. Intensive Care Med. 2002; 28(7): 824-833.
[Crossref] [Google Scholar] [Pubmed]
- Huttunen R, Aittoniemi J. New concepts in the pathogenesis, diagnosis and treatment of bacteremia and sepsis. J Infect. 2011; 63(6): 407-419.
[Crossref] [Google Scholar] [Pubmed]
- Pankey GA, Sabath LD. Clinical relevance of bacteriostatic versus bactericidal mechanisms of action in the treatment of Gram-positive bacterial infections. Clin Infect Dis. 2004; 38(6): 864-870.
[Crossref] [Google Scholar] [Pubmed]
- Spyridaki A, Raftogiannis M, Antonopoulou A, Tsaganos T, Routsi C, Baziaka F, et al. Effect of clarithromycin in inflammatory markers of patients with ventilator-associated pneumonia and sepsis caused by Gram-negative bacteria: Results from a randomized clinical study. Antimicrob Agents Chemother. 2012; 56(7): 3819-3825.
[Crossref] [Google Scholar] [Pubmed]
- Chousterman BG, Swirski FK, Weber GF. Cytokine storm and sepsis disease pathogenesis. Semin Immunopathol. 2017; 39(5): 517-528.
[Crossref] [Google Scholar] [Pubmed]
- Behrens EM, Koretzky GA. Cytokine storm syndrome: Looking toward the precision medicine era. Arthritis Rheumatol. 2017; 69(6): 1135-1143.
[Crossref] [Google Scholar] [Pubmed]
- Netea MG, Van Der Meer JW, Van Deuren M, Kullberg BJ. Proinflammatory cytokines and sepsis syndrome: Not enough, or too much of a good thing?. Trends Immunol. 2003; 24(5): 254-258.
[Crossref] [Google Scholar] [Pubmed]
- Tisoncik JR, Korth MJ, Simmons CP, Farrar J, Martin TR, Katze MG. Into the eye of the cytokine storm. Microbiol Mol Biol Rev. 2012; 76(1): 16-32.
[Crossref] [Google Scholar] [Pubmed]
- Gogos CA, Skoutelis A, Lekkou A, Drosou E, Starakis I, Marangos MN, et al. Comparative effects of ciprofloxacin and ceftazidime on cytokine production in patients with severe sepsis caused by gram-negative bacteria. Antimicrob Agents Chemother. 2004; 48(8): 2793-2798.
[Crossref] [Google Scholar] [Pubmed]
- Nau R, Eiffert H. Minimizing the release of proinflammatory and toxic bacterial products within the host: A promising approach to improve outcome in life-threatening infections. FEMS Immunol Med Microbiol. 2005; 44(1): 1-6.
[Crossref] [Google Scholar] [Pubmed]
- Giamarellos-Bourboulis EJ, Mega A, Pavleas I, Archontoulis N, Rigas K, Vernikos P, et al. Impact of carbapenem administration on systemic endotoxemia in patients with severe sepsis and Gram-negative bacteremia. J Chemother. 2006; 18(5): 502-506. [Crossref]
[Google Scholar] [Pubmed]
- Vianna RC, Gomes RN, Bozza FA, Amâncio RT, Bozza PT, David CM, et al. Antibiotic treatment in a murine model of sepsis: Impact on cytokines and endotoxin release. Shock. 2004; 21(2): 115-120. [Crossref]
[Google Scholar] [Pubmed]
- Wald-Dickler N, Holtom P, Spellberg B. Busting the myth of “static vs. cidal”: A systemic literature review. Clin Infect Dis. 2018; 66(9): 1470-1474.
[Crossref] [Google Scholar] [Pubmed]
- Saleem N, Ryckaert F, Snow TA, Satta G, Singer M, Arulkumaran N. Mortality and clinical cure rates for pneumonia: A systematic review, meta-analysis, and trial sequential analysis of randomized control trials comparing bactericidal and bacteriostatic antibiotic treatments. Clin Microbiol Infect. 2022.
[Crossref] [Google Scholar] [Pubmed]
- Rubinstein E, Keynan Y. Vancomycin revisited-60 years later. Front Public Health. 2014; 2: 217.
[Crossref] [Google Scholar] [Pubmed]
- Clemett D, Markham A. Linezolid. Drugs. 2000; 59(4): 815-827.
[Crossref] [Google Scholar] [Pubmed]
- Chuang YC, Wang JT, Lin HY, Chang SC. Daptomycin versus linezolid for treatment of vancomycin-resistant enterococcal bacteremia: Systematic review and meta-analysis. BMC Infect Dis. 2014; 14(1): 1-10.
[Crossref] [Google Scholar] [Pubmed]
- Nemeth J, Oesch G, Kuster SP. Bacteriostatic versus bactericidal antibiotics for patients with serious bacterial infections: Systematic review and meta-analysis. J Antimicrob Chemother. 2015; 70(2): 382-395.
[Crossref] [Google Scholar] [Pubmed]
- Peng ZY, Wang HZ, Srisawat N, Wen X, Rimmelé T, Bishop J, et al. Bactericidal antibiotics temporarily increase inflammation and worsen acute kidney injury in experimental sepsis. Crit Care Med. 2012; 40(2): 538.
[Crossref] [Google Scholar] [Pubmed]
- Rusmin S, deLuca PP. Effect of antibiotics and osmotic change on the release of endotoxin by bacteria retained on intravenous inline filters. Am J Hosp Pharm. 1975; 32(4): 378-380.
[Crossref] [Google Scholar] [Pubmed]
- Byl B, Clevenbergh P, Kentos A, Jacobs F, Marchant A, Vincent J, et al. Ceftazidime-and imipenem-induced endotoxin release during treatment of gram-negative infections. Eur J Clin Microbiol Infect Dis. 2001; 20(11): 804-807.
[Crossref] [Google Scholar] [Pubmed]
- Sjölin J, Goscinski G, Lundholm M, Bring J, Odenholt I. Endotoxin release from Escherichia coli after exposure to tobramycin: Dose‐dependency and reduction in cefuroxime‐induced endotoxin release. Clin Microbiol Infect. 2000; 6(2): 74-81.
[Crossref] [Google Scholar] [Pubmed]
- Shenep JL, Mogan KA. Kinetics of endotoxin release during antibiotic therapy for experimental gram-negative bacterial sepsis. J Infect Dis. 1984; 150(3): 380-388.
[Crossref] [Google Scholar] [Pubmed]
- Klompas M, Calandra T, Singer M. Antibiotics for sepsis-finding the equilibrium. JAMA. 2018; 320(14): 1433-1434.
[Crossref] [Google Scholar] [Pubmed]
- Luchi M, Morrison DC, Opal S, Yoneda K, Slotman G, Chambers H, et al. A comparative trial of imipenem versus ceftazidime in the release of endotoxin and cytokine generation in patients with gram-negative urosepsis. J Endotoxin Res. 2000; 6(1): 25-31.
[Crossref] [Google Scholar] [Pubmed]
- Simpson AJ, Opal SM, Angus BJ, Prins JM, Palardy JE, Parejo NA, et al. Differential antibiotic-induced endotoxin release in severe melioidosis. J Infect Dis. 2000; 181(3): 1014-1019.
[Crossref] [Google Scholar] [Pubmed]
- Mock CN, Jurkovich GJ, Dries DJ, Maier RV. Clinical significance of antibiotic endotoxin-releasing properties in trauma patients. Arch Surg. 1995; 130(11): 1234-1241.
[Crossref] [Google Scholar] [Pubmed]
Author Info
Naveed Saleem*Citation: Saleem N: Antibiotics Modulate Variable Immunological Responses in Sepsis-A Narrative Review
Received: 02-Dec-2022 Accepted: 27-Dec-2022 Published: 03-Jan-2023, DOI: 10.31858/0975-8453.14.1.1-5
Copyright: This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
ARTICLE TOOLS
- Dental Development between Assisted Reproductive Therapy (Art) and Natural Conceived Children: A Comparative Pilot Study Norzaiti Mohd Kenali, Naimah Hasanah Mohd Fathil, Norbasyirah Bohari, Ahmad Faisal Ismail, Roszaman Ramli SRP. 2020; 11(1): 01-06 » doi: 10.5530/srp.2020.1.01
- Psychometric properties of the World Health Organization Quality of life instrument, short form: Validity in the Vietnamese healthcare context Trung Quang Vo*, Bao Tran Thuy Tran, Ngan Thuy Nguyen, Tram ThiHuyen Nguyen, Thuy Phan Chung Tran SRP. 2020; 11(1): 14-22 » doi: 10.5530/srp.2019.1.3
- A Review of Pharmacoeconomics: the key to “Healthcare for All” Hasamnis AA, Patil SS, Shaik Imam, Narendiran K SRP. 2019; 10(1): s40-s42 » doi: 10.5530/srp.2019.1s.21
- Deuterium Depleted Water as an Adjuvant in Treatment of Cancer Anton Syroeshkin, Olga Levitskaya, Elena Uspenskaya, Tatiana Pleteneva, Daria Romaykina, Daria Ermakova SRP. 2019; 10(1): 112-117 » doi: 10.5530/srp.2019.1.19
- Dental Development between Assisted Reproductive Therapy (Art) and Natural Conceived Children: A Comparative Pilot Study Norzaiti Mohd Kenali, Naimah Hasanah Mohd Fathil, Norbasyirah Bohari, Ahmad Faisal Ismail, Roszaman Ramli SRP. 2020; 11(1): 01-06 » doi: 10.5530/srp.2020.1.01
- Manilkara zapota (L.) Royen Fruit Peel: A Phytochemical and Pharmacological Review Karle Pravin P, Dhawale Shashikant C SRP. 2019; 10(1): 11-14 » doi: 0.5530/srp.2019.1.2
- Pharmacognostic and Phytopharmacological Overview on Bombax ceiba Pankaj Haribhau Chaudhary, Mukund Ganeshrao Tawar SRP. 2019; 10(1): 20-25 » doi: 10.5530/srp.2019.1.4
- A Review of Pharmacoeconomics: the key to “Healthcare for All” Hasamnis AA, Patil SS, Shaik Imam, Narendiran K SRP. 2019; 10(1): s40-s42 » doi: 10.5530/srp.2019.1s.21
- A Prospective Review on Phyto-Pharmacological Aspects of Andrographis paniculata Govindraj Akilandeswari, Arumugam Vijaya Anand, Palanisamy Sampathkumar, Puthamohan Vinayaga Moorthi, Basavaraju Preethi SRP. 2019; 10(1): 15-19 » doi: 10.5530/srp.2019.1.3