Research Article - (2024) Volume 15, Issue 2
Abstract
Ferula assa-foetida(Apiaceae), a naturally occurring medicinal plant found in the Ghardaia region of the Algerian Sahara, has garnered interest for its therapeutic potential. This study aims to partially characterize and assess the antidiabetic activity of water-soluble Polysaccharides isolated from F. assa-foetida gum-resin (PGFA). PGFA were obtained through extraction with distilled water at 80°C for 2 hours, followed by polysaccharide precipitation using three volumes of ethanol 96% and subsequent freeze-drying. The extraction yield thus obtained was 24.3%. The total carbohydrate content was 77% ± 0.22%, comprising 46.35% ± 0.015% neutral carbohydrates and 18.7 ± 0.045% uronic acids. Thin-Layer Chromatography (TLC) analysis of PGFA revealed the presence of galacturonic acid, glucuronic acid, arabinose, galactose, rhamnose and xylose. Evaluation of the antidiabetic activity, specifically Alpha (α)-D-glucosidase inhibitors demonstrated a significant inhibitory effect of PGFA (77.66%) at a concentration of 100 mg/ml-1. This inhibition was compared to acarbose, a positive control, which exhibited strong inhibitory activity (100%) at the same concentration. These findings support for the use of polysaccharides extract from Ferula assa-foetida in preventing diabetic complications.
Keywords
Polysaccharides, Ferula assa-foetida, Gum-resin, Thin-layer chromatography, α-D-glucosidase inhibitors
Introduction
Diabetes Mellitus (DM) caused by hyperglycaemia, has already become a global problem which is threatening thousands of people’s health. Moreover, the long-term presence of hyperglycaemia can cause chronic damage to various tissues and bring serious complications to the human body (Yang JP, et al., 2012; Zhang L, et al., 2016). Particularly, inhibiting the activity of enzymes related to starch digestion (α-glucosidase and α-amylase) is one of the best ways to control blood glucose. Inhibition of glucosidase is one of therapeutic approach in lowering glucose level in the blood, thereby leading towards management of diabetes mellitus (Ha TJ, et al., 2012; Zhang Z, et al., 2016).
Polysaccharides are the macromolecules which are composed of sugars. They constitute of an important class of bioactive compounds with a wide range of pharmacological activities. The utilization of plant polysaccharides, especially exudate gums (gums), is attributed to their diverse structural and functional properties in the realms of food, pharmaceuticals, cosmetics, textiles and biomedical products (Mirhosseini H and Amid BT, 2012).
Numerous species within the Ferula genus have been known since ancient times as sources of gum-resins. F. assa-foetida, a spontaneous medicinal plant belonging to this genus, has been the subject of pharmacological and biological studies, which have exposed its anti-oxidative, antiviral, antifungal, anticancer, antidiabetic, antispasmodic and hypotensive properties (Mahendra P and Bisht S, 2012). F. assa-foetidais employed as a sedative, analgesic, antispasmodic, and diuretic (Bagheri SM, et al., 2014).
The study aims to extract and partially characterize water-soluble polysaccharides from F. assa-foetida gum-resin collected in the Algerian Sahara. Additionally, the study evaluates the anti-diabetic activity of these polysaccharides through in vitrotesting.
Material and Methods
Plant materials and chemicals
Ferula assa-foetidawas collected from Ghardaia (Algeria) in November 2016. The gum-resin was meticulously separated and subsequently stored at room temperature in a dark and dry environment. Standard monosaccharides such as arabinose, rhamnose, galactose, glucose, mannose, glucuronic acid, galacturonic acid, metahydroxydiphenyl, Trifluoroacetic Acid (TFA), α-glucosidase, acarbose and p-Nitrophenyl α-D-Glucopyranoside (p-NPG) from Sigma-Aldrich in Germany were obtained. Additionally, Bovine Serum Albumin (BSA) was used in our experiments.
Extraction of PGFA
The water polysaccharides extraction process followed the methodology described by Wang M, et al., 2013, Chidouh A, et al., 2014 and Chen G, et al., 2016. Twenty grams of powdered gum-resin from Ferula assa-foetida underwent a pretreatment process using petroleum ether for 24 hours at room temperature. Subsequently, the insoluble residue was macerated in distilled water (v/v) for 2 hours at 80°C with continuous agitation. After this maceration, the mixture was filtered. The resulting filtrate was then subjected to centrifugation at 4000 × g for 15 minutes. Following centrifugation, three volumes of 96% ethanol were added to the supernatant and the mixture was stored at -4°C for 24 hours. The precipitate that formed was collected by centrifugation at 10000 × g for 15 minutes and subjected to three washes with acetone. Finally, the precipitate was freeze-dried to obtain the water-soluble polysaccharides fraction, which is named PGFA.
Biochemical composition
The chemical composition of PGFA was comprehensively analyzed. The total sugar content was determined using the phenol-sulfuric method (Dubois M, et al., 1956), while neutral sugar content was assessed through the resorcinol-sulfuric acid assay (Monsigny M, et al.1988). In both cases, glucose was served as the standard for calibration. Total uronic acid content was quantified colorimetrically using the m-hydroxydiphenyl assay, with glucuronic acid as the standard reference (Blumenkrantz Z and Asboe-Hansen, 1973). To determine the protein content, the micro-Bradford method was employed, as outlined by Bradford in 1976, with BSA by using as the standard for calibration.TLC of PGFA
25 grams of PGFA was hydrolyzed with TFA for 4 hours at 100°C, followed by evaporation and recovery with 1 ml of distilled water. The hydrolysate and standard sugars were separated using TLC on silica-gel plates as the stationary phase, employing two distinct mobile phase systems. Regarding the mobile phases utilized, they were as follows, system 1 consisting of chloroform, n-butanol, methanol, acetic acid and water (Yang C, et al., 2010) and system 2 comprised of acetonitrile, ethyl acetate, propanol and water (Han NS and Robyt JF, 1998). Further, spot visualization was carried out and the Retention Factor (RF) values for the separated spots, including the PGFA hydrolysate and various standards, were calculated.
Antidiabetic activity of PGFA
The antihyperglycemic activity of PGFA was investigated by assessing its inhibition of α-glucosidase activity. The α-glucosidase inhibition assay was conducted following the methodology described by Bisht S, et al., 2013 with slight modifications. In dry tubes, 500 µl of α-glucosidase solution (2 IU/l-1) was mixed with 100 µl of each PGFA dilution (2.5 to 100 mg/ml- 1), acarbose (positive control), or ultrapure water (negative control). The mixture was pre-incubated at 37°C for 15 minutes. Subsequently, 100 µl of a p-NPG (p-nitrophenyl-α-D-glucopyranoside) solution (4 mM) was added to each tube. The tubes were shaken and then incubated at 37°C for 20 minutes. To stop the enzymatic reaction, 1 ml of Na CO (0.2 M) was added and the absorbance were measured at 405 nm. All experiments were conducted in triplicate. The results of the inhibition activity were expressed using the following equation:Inhibition (%)=(Acontrol-Asample)/(Acontrol) × 100
Statistical analysis
The data was analyzed using Origin Pro8 software and Microsoft Excel 2007.
Results and Discussion
Biochemical composition
The extraction yield of PGFA was 24.3% (w/w), which exceeded the yields of polysaccharides found in Ferula gumosa Boiss gum, where it was reported as 5%, but was slightly lower than the yield of polysaccharides extracted from Ferula assa-foetidagum harvested in Iran, which was reported as 27.1% (Saeidy S, et al., 2018), and also lower than the polysaccharides extracted from Ferula communis gum, which had a yield of 33.5% (Youmbai A, et al., 2021).
The biochemical analysis of PGFA is presented in Table 1. PGFA consisted of approximately 77 ± 0.22% of total sugars, with neutral sugars accounting for 46.35±0.015% and uronic acid at 18.7 ± 0.045%. Additionally, the protein content was found to be relatively low at 2.06 ± 1.02%. These findings are comparable to the water-soluble polysaccharides extracted from Ferula communis gum resin, which contained 5.29% (m/m) protein, 71.45% (m/m) uronic acid, and 27.90% neutral sugars (Youmbai A, et al., 2021). Saeidy S, et al., 2018, reported that polysaccharides extracted from Ferula assa-foetidagum, which contained 67.39% total sugars and 5.2% uronic acid.
| Extraction yield (% w/w) | Carbohydrate (w/w %) | Proteins (% w/w) | ||
|---|---|---|---|---|
| Total | Neutral | Uronic acid | ||
| 24.3 | 77 ± 0.22 | 46.35 ± 0.015 | 18.7 ± 0.045 | 2.06 ± 1.02 |
Table 1: Biochemical characterization of polysaccharides from F. assa-foetida gum
Partial structural characterization of PGFA by TLC
TLC chromatography analysis was employed to elucidate the composition of the polysaccharide extract, focusing on its constituent sugars. This was accomplished by comparing the RF values of hydrolyzed spots with those of reference standards (Table 2). In system 1 (Figure 1), we observed four distinct RF values for the sugars are 0.166, 0.416, 0.527 and 0.638. These RF values were found to correspond to the following sugars: 0.166 (galacturonic acid), 0.416 (galactose), 0.527 (arabinose) and 0.638 (rhamnose). In contrast, system 2 (Figure 1) exhibited 5 different RF values for the sugars are 0.136, 0.191, 0.520, 0.438 and 0.643. These values were identified as 0.136 (galacturonic acid), 0.191 (glucuronic acid), 0.520 (galactose), 0.438 (arabinose) and 0.643 (rhamnose). These findings collectively suggest that the crude extract of water-soluble polysaccharides from Ferula assa-foetidagum comprises a heteropolysaccharide consisting of galacturonic acid, glucuronic acid, galactose, arabinose, rhamnose, and xylose.
| Sugar names | L-galactose | Glucuronic acid | Ara arabinose | Galactose | Glucose | Mannose | Rhamnose | Xylose |
|---|---|---|---|---|---|---|---|---|
| Standards (system 1) | 0.166 | 0.208 | 0.527 | 0.416 | 0.458 | 0.472 | 0.638 | 0.569 |
| PGFA | 0.166 | / | 0.527 | 0.416 | / | / | 0.638 | / |
| Standards (system 2) | 0.136 | 0.191 | 0.52 | 0.438 | 0.493 | 0.465 | 0.643 | 0,561 |
| PGFA | 0.136 | 0.191 | 0.52 | 0.438 | / | / | 0.643 | / |
Table 2: The RF values of the separated spots of PGFA and the standards using system 1 and 2

Figure 1: Chromatogram of polysaccharide hydrolysates of PGSA using system 1 and system 2
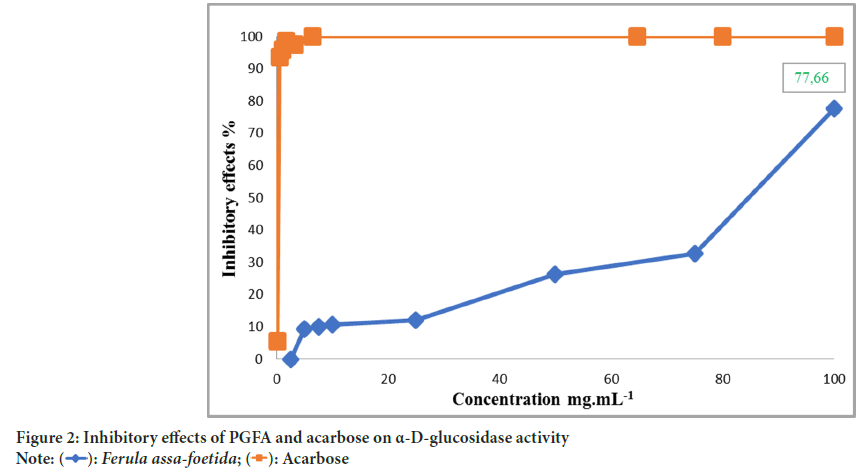
In Figure 2, PGFA exhibited inhibitory effects on α-D-glucosidase activity, albeit with a lower potency compared to acarbose. As the concentration of PGFA increased from 2.5 to 100 mg/ml-1, a dose-response relationship was observed, with the inhibition rate of PGFA increasing. The inhibition rate reached a maximum of 77.66% at a concentration of 100 mg/ml-1.
Figure 2: Inhibitory effects of PGFA and acarbose on α-D-glucosidase activity 
Comparatively, Youmbai A, et al., 2021, reported that the inhibitory efficacy of acarbose reaches its maximum of 100% at a concentration of 6.45 mg/ ml-1, while polysaccharides extracted from Ferula communis gum achieves similar inhibition at 100 mg/ml. Bisht S, et al., 2013, reported that polysaccharides isolated from Acacia tortilis gum had an Half-maximal Inhibitory Concentration (IC50) of 0.5 mg/ml-1 for α-D-glucosidase inhibition. Wang XT, et al., 2016, found even stronger inhibition of α-D-glucosidase at 0.8 mg/ml-1 using heteroglucan extracted from Fagopyrum tartaricum. Jia X, et al., 2017, demonstrated that among three polysaccharide fractions isolated from Rhynchosia minima root, the fraction richest in arabinogalactan displayed the strongest inhibition of α-D-glucosidase activity, with an IC50 value of 8.85 mg/ml-1.
PGFA has shown a significant inhibitory activity against α-D-glucosidase, suggesting its potential as a promising therapeutic agent for the management of blood glucose. This potential could further be harnessed in the health food industry. It is well-established that the effects of polysaccharides may vary depending on factors such as glycosidic linkage, molecular weight, conformation, and degree of branching. Therefore, it is recommended that further research be conducted to investigate the structural characteristics of Ferula assa-foetidagum polysaccharides.
Conclusion
The gum-resin of Ferula assa-foetida is rich in polysaccharides. Structural analysis of the polysaccharide extract by thin-layer chromatography using two separation systems revealed the presence of galacturonic acid, glucuronic acid, arabinose, galactose, rhamnose, and xylose. The polysaccharides from the gum-resin exhibited a significant inhibitory effect on the α-D-glucosidase enzyme at the tested concentrations. This finding could provide a scientific basis for the traditional use of this plant for its anti-diabetic effect.
References
- Yang JP, Hsu T, Lin F, Hsu W, Chen Y. Potential antidiabetic activity of extracellular polysaccharides in submerged fermentation culture of Coriolus versicolor LH1. Carbohydr Polym. 2012; 90(1): 174-180.
[Crossref] [Google Scholar] [Pubmed]
- Zhang L, Tu ZC, Yuan T, Wang H, Xie X, Fu ZF. Antioxidants and α-glucosidase inhibitors from Ipomoea batatas leaves identified by bioassay-guided approach and structure-activity relationships. Food Chem. 2016; 208: 61-67.
[Crossref] [Google Scholar] [Pubmed]
- Ha TJ, Lee JH, Lee MH, Lee BW, Kwon HS, Park CH, et al. Isolation and identification of phenolic compounds from the seeds of Perilla frutescens (L.) and their inhibitory activities against α-glucosidase and aldose reductase. Food Chem. 2012; 135(3): 1397-1403.
[Crossref] [Google Scholar] [Pubmed]
- Zhang Z, Kong F, Ni H, Mo Z, Wan JB, Hua D, et al. Structural characterization, α-glucosidase inhibitory and DPPH scavenging activities of polysaccharides from guava. Carbohydr Polym. 2016; 144: 106-114.
[Crossref] [Google Scholar] [Pubmed]
- Mirhosseini H, Amid BT. A review study on chemical composition and molecular structure of newly plant gum exudates and seed gums. Food Res Int. 2012; 46(1): 387-398.
- Mahendra P, Bisht S. Ferula asafoetida: Traditional uses and pharmacological activity. Pharmacog Rev. 2012; 6(12): 141.
[Crossref] [Google Scholar] [Pubmed]
- Bagheri SM, Dashti-R MH, Morshedi A. Antinociceptive effect of Ferula assa-foetida oleo-gum-resin in mice. Res Pharm Sci. 2014; 9(3): 207-12.
[Google Scholar] [Pubmed]
- Wang M, Jiang C, Ma L, Zhang Z, Cao L, Liu J, et al. Preparation, preliminary characterization and immunostimulatory activity of polysaccharide fractions from the peduncles of Hovenia dulcis. Food Chem. 2013; 138(1): 41-47.
[Crossref] [Google Scholar] [Pubmed]
- Chidouh A, Aouadi S, Heyraud A. Extraction, fractionation and characterization of water-soluble polysaccharide fractions from myrtle (Myrtus communis L.) fruit. Food Hydrocoll. 2014; 35: 733-739.
- Chen G, Yuan Q, Saeeduddin M, Ou S, Zeng X, Ye H. Recent advances in tea polysaccharides: Extraction, purification, physicochemical characterization and bioactivities. Carbohydr Polym. 2016; 153: 663-678.
[Crossref] [Google Scholar] [Pubmed]
- Dubois M, Gilles KA, Hamilton JK, Rebers PT, Smith F. Colorimetric method for determination of sugars and related substances. Anal Chem. 1956; 28(3): 350-356.
- Monsigny M, Petit C, Roche AC. Colorimetric determination of neutral sugars by a resorcinol sulfuric acid micromethod. Anal Biochem. 1988; 175(2): 525-530.
[Crossref] [Google Scholar] [Pubmed]
- Blumenkrantz N, Asboe-Hansen G. New method for quantitative determination of uronic acids. Anal Biochem. 1973; 54(2): 484-489.
[Crossref] [Google Scholar] [Pubmed]
- Yang C, Guan J, Zhang JS, Li SP. Use of HPTLC to differentiate among the crude polysaccharides in six traditional Chinese medicines. J Planar Chromatogr-Mod TLC. 2010; 23(1): 46-49.
- Han NS, Robyt JF. Separation and detection of sugars and alditols on thin layer chromatograms. Carbohydr Res. 1998; 313(2): 135-137.
- Bisht S, Kant R, Kumar V. α-d-Glucosidase inhibitory activity of polysaccharide isolated from Acacia tortilis gum exudate. Int J Biol Macromol. 2013; 59: 214-220.
[Crossref] [Google Scholar] [Pubmed]
- Saeidy S, Nasirpour A, Keramat J, Desbrières J, Le Cerf D, Pierre G, et al. Structural characterization and thermal behavior of a gum extracted from Ferula assa foetida L. Carbohydr Polym. 2018; 181: 426-432.
[Crossref] [Google Scholar] [Pubmed]
- Youmbai A, Mehellou Z, Boual Z, Michaud P, Md Didi OEH. Partial characterization of water-soluble polysaccharides of gums resins of Ferula communis L. (Apiaceae): Biological activities. Int Sem Plant Polysaccharides Arid Environ. 2017.
- Wang XT, Zhu ZY, Zhao L, Sun HQ, Meng M, Zhang JY, et al. Structural characterization and inhibition on α-d-glucosidase activity of non-starch polysaccharides from Fagopyrum tartaricum. Carbohydr Polym. 2016; 153: 679-685.
[Crossref] [Google Scholar] [Pubmed]
- Jia X, Hu J, He M, Zhang Q, Li P, Wan J, et al. α-Glucosidase inhibitory activity and structural characterization of polysaccharide fraction from Rhynchosia minima root. J Funct Foods. 2017; 28: 76-82.
Author Info
Nouhad Amina Righi*, Messaouda Babahamou, Zakaria Boual and Mohamed Didi Ould El HadjCitation: Righi NA: Antidiabetic Potential of Polysaccharide Extract from Ferula assa-foetida Gum Resin Harvested in Algerian Sahara
Received: 31-Jan-2024 Accepted: 15-Feb-2024 Published: 22-Feb-2024, DOI: 10.31858/0975-8453.15.2.59-62
Copyright: This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
ARTICLE TOOLS
- Dental Development between Assisted Reproductive Therapy (Art) and Natural Conceived Children: A Comparative Pilot Study Norzaiti Mohd Kenali, Naimah Hasanah Mohd Fathil, Norbasyirah Bohari, Ahmad Faisal Ismail, Roszaman Ramli SRP. 2020; 11(1): 01-06 » doi: 10.5530/srp.2020.1.01
- Psychometric properties of the World Health Organization Quality of life instrument, short form: Validity in the Vietnamese healthcare context Trung Quang Vo*, Bao Tran Thuy Tran, Ngan Thuy Nguyen, Tram ThiHuyen Nguyen, Thuy Phan Chung Tran SRP. 2020; 11(1): 14-22 » doi: 10.5530/srp.2019.1.3
- A Review of Pharmacoeconomics: the key to “Healthcare for All” Hasamnis AA, Patil SS, Shaik Imam, Narendiran K SRP. 2019; 10(1): s40-s42 » doi: 10.5530/srp.2019.1s.21
- Deuterium Depleted Water as an Adjuvant in Treatment of Cancer Anton Syroeshkin, Olga Levitskaya, Elena Uspenskaya, Tatiana Pleteneva, Daria Romaykina, Daria Ermakova SRP. 2019; 10(1): 112-117 » doi: 10.5530/srp.2019.1.19
- Dental Development between Assisted Reproductive Therapy (Art) and Natural Conceived Children: A Comparative Pilot Study Norzaiti Mohd Kenali, Naimah Hasanah Mohd Fathil, Norbasyirah Bohari, Ahmad Faisal Ismail, Roszaman Ramli SRP. 2020; 11(1): 01-06 » doi: 10.5530/srp.2020.1.01
- Manilkara zapota (L.) Royen Fruit Peel: A Phytochemical and Pharmacological Review Karle Pravin P, Dhawale Shashikant C SRP. 2019; 10(1): 11-14 » doi: 0.5530/srp.2019.1.2
- Pharmacognostic and Phytopharmacological Overview on Bombax ceiba Pankaj Haribhau Chaudhary, Mukund Ganeshrao Tawar SRP. 2019; 10(1): 20-25 » doi: 10.5530/srp.2019.1.4
- A Review of Pharmacoeconomics: the key to “Healthcare for All” Hasamnis AA, Patil SS, Shaik Imam, Narendiran K SRP. 2019; 10(1): s40-s42 » doi: 10.5530/srp.2019.1s.21
- A Prospective Review on Phyto-Pharmacological Aspects of Andrographis paniculata Govindraj Akilandeswari, Arumugam Vijaya Anand, Palanisamy Sampathkumar, Puthamohan Vinayaga Moorthi, Basavaraju Preethi SRP. 2019; 10(1): 15-19 » doi: 10.5530/srp.2019.1.3