Review Article - (2023) Volume 14, Issue 3
Box A of HMGB1 Producing DNA Gaps: A Remedy for the DNA Protection and Rejuvenation Effects in Age- and DNA Damage-Associated Diseases
Rathasapa Patarat1,2, Wilunplus Khumsri1,2, Sikrit Denariyakoon3 and Apiwat Mutirangura1*Abstract
The world is becoming an aging society at an unprecedented pace, and the health and socioeconomic burden from age-associated diseases continue to rise. Age-associated conditions are causing physical and functional deterioration toward organs failure. Understanding the aging process is the key to developing therapeutic protocols for many Non-Communicable Diseases (NCDs). Recently, persistent DNA damage has been proposed to drive the human aging process. However, the underlying mechanisms of the DNA damage accumulation in the elderly have not been clearly understood. Our research showed the DNA protective role of the naturally occurring DNA gaps called Youth-associated genome-stabilizing DNA gaps (Youth-DNA-gaps). The reduction of the DNA gaps in the elderly accumulates DNA damage. Box A of HMGB1 protein acts as molecular scissors producing Youth-DNA-gaps. Introducing the Box A expression plasmids into the cell resulted in DNA stabilization and rejuvenation. The role of the DNA gap is to relieve the double helix torsion stress and protected DNA from damage. The accumulation of DNA damage also led to chronic inflammation and cellular senescence, which led to the development of many NCDs, such as Diabetes Mellitus, Alzeimer’s disease, Heart Failure, Chronic Obstructive Pulmonary Disease, radiation insult, and cancer. These Box A-produced DNA gaps can revitalize organ functions, rejuvenate senescent cells, and clear organ fibrosis. Therefore, Box A of HMGB1 protein is a genomic stabilizing molecule that can rejuvenate DNA and may be used as a therapeutic agent to cure various NCDs.
Keywords
DNA damage, DNA protection, HMGB1, Rejuvenation, Senescence-associated diseases, Youth-DNA-gaps
Abbreviations
AD: Alzheimer’s Disease; ATM: Ataxia-Telangiectasia Mutated protein kinase; Aβ: Amyloid-beta; CCL4: Chemokine (C-C motif) Ligand 4; COPD: Chronic Obstructive Pulmonary Disease; D-gal: D-galactose; DAMPs: Damage-Associated Molecular Patterns; DDR: DNA Damage Response; DM: Diabetes Mellitus; GDF15: Growth Differentiation Factor 15; HF: Heart Failure; IKK: IkappaB Kinase; IL-1: Interleukin-1; IL-6: Interleukin-6; IL-7: Interleukin-7; IL-8: Interleukin-8; IL-13: Interleukin-13; IL-15: Interleukin-15; JNK: c-Jun N-terminal Kinases; MDSCs: Myeloid-Derived Suppressor Cells; NCDs: Non-Communicable Diseases; NFTs: Neurofibrillary Tangles; OPN: Osteopontin; P53: Tumor protein 53; PARP: Poly (ADP-ribose) Polymerase; PGC- 1: Peroxisome proliferator-activated receptor-Gamma Coactivator-1; RIND-EDSBs: Replication-Independent Endogenous DNA Double-Strand Breaks; SASPs: Senescence cells release senescence-Associated Secretory Phenotypes; TAMs: Tumor-Associated Macrophages; TNF-α: Tumor Necrosis Factor-alpha; WBCs: White Blood Cells; Youth-DNA-GAPs: Youth-associated genome-stabilizing DNA Gaps; γH2AX: Phosphorylation of histone H2AX
Introduction
The accumulation of endogenous DNA damage has been proposed to be the driver of the molecular pathogenesis process of age-associated diseases (Schumacher B, et al., 2021; Milic M, et al., 2015; Yousefzadeh M, et al., 2021). Therefore, understanding the cause of DNA damage in the elderly is crucial knowledge leading to a breakthrough technology in coping with aging society health problems. Persistent DNA damage drives cells to enter cellular senescence (Olivieri F, et al., 2015; Fagagna FD, et al., 2003). Senescence cells release Senescence-Associated Secretory Phenotypes (SASPs) to signal to immune cells to remove these senescent cells and, in turn, repair aging tissues (Lopes-Paciencia S, et al., 2019; He S and Sharpless NE, 2017; Ritschka B, et al., 2017). However, the SASPs, including Damage-Associated Molecular Patterns (DAMPs), promote inflammation (Rea IM, et al., 2018; Roh JS and Sohn DH, 2018). Senescent cells themselves stop dividing and undergo morphological changes (Hernandez-Segura A, et al., 2018). Therefore, the constant presence of DNA damage in aging cells causes the prolonged presence of both inflammation and senescent cells, resulting in degeneration of the organ structure and function (Wei W and Ji S, 2018). The removal of senescent cells using senolytic therapeutics is one approach to rejuvenating aging organs (Tchkonia T, et al., 2015; Kirkland JL and Tchkonia T, 2020; van Deursen JM, 2019). However, this approach cannot stop genomic instability, which continues the aging process. Preventing DNA damage is a theoretical approach that may yield more sustainable good clinical outcomes.
Literature Review
Recently, we reported novel findings regarding the mechanisms underlying DNA durability and the cellular rejuvenation of HM-GB1-produced DNA gaps, which could allow us to develop a new testing approach and treatment strategy for biological aging (Yasom S, et al., 2022). Furthermore, these discoveries also provided an unprecedented potential pharmacological intervention to cure age and/or DNA damage-associated diseases. I wrote this review to persuade worldwide research groups to translate our discoveries’ new knowledge to benefit humankind.
The crucial question we successfully answered was how the elderly accumulate more DNA damage than the young. We also presented a new DNA protection role of a widely known protein-i.e., HMGB1. Many findings have been made regarding the roles of secretory HMGB1 in inducing inflammation (Yang H, et al., 2020; Andersson U and Tracey KJ, 2011). Although some studies found that intranuclear HMGB1 can prevent DNA damage, the mechanism of action has remained a puzzle (Giavara S, et al., 2005; Han G, et al., 2021; Funayama A, et al., 2013). Our recent report proved that HMGB1 created a DNA gap that can protect the DNA strand from damage by increasing the DNA flexibility in moving to relieve torsion force from twist waves resulting from DNA activity (Yasom S, et al., 2022). These gaps are similar to tiny spaces in a railway track that maintain their stability by releasing the heat and stress generated between two rails.
Thus, our recent finding is the first report of a naturally occurring DNA protection process preventing aging (Figure 1).
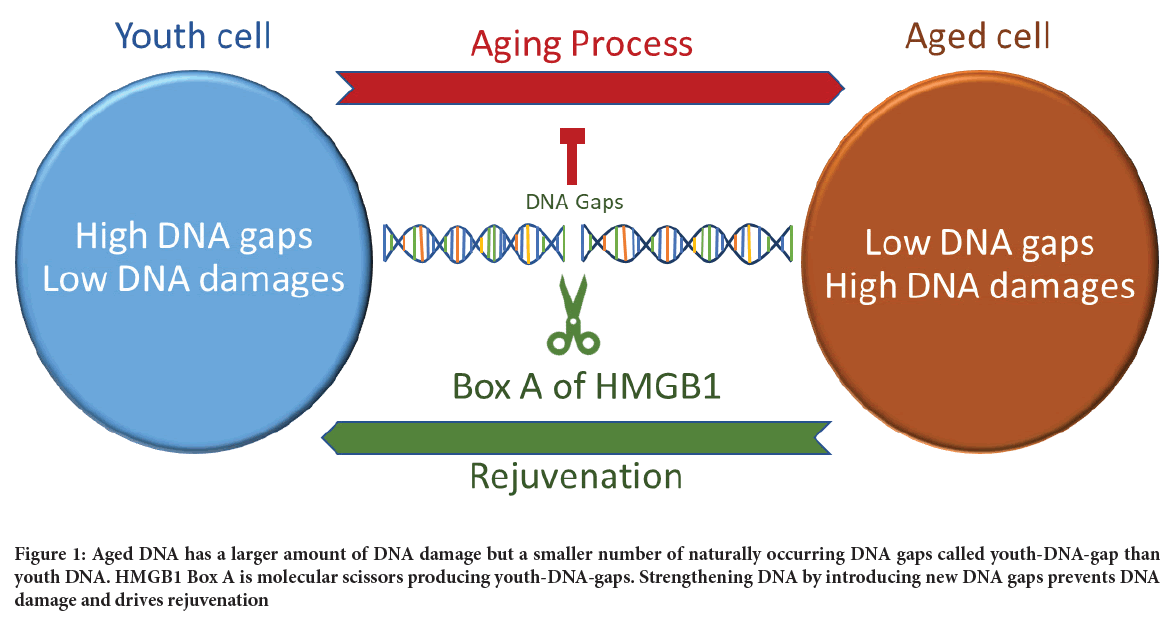
Figure 1: Aged DNA has a larger amount of DNA damage but a smaller number of naturally occurring DNA gaps called youth-DNA-gap than youth DNA. HMGB1 Box A is molecular scissors producing youth-DNA-gaps. Strengthening DNA by introducing new DNA gaps prevents DNA damage and drives rejuvenation
Our findings provide the opportunity to develop a novel concept for aging DNA treatment. We first reported the presence of DNA gaps in 2008 (Pornthanakasem W, et al., 2008). Gaps were detectable in all phases of the cell cycle, so we named them the gap, Replication-Independent Endogenous DNA Double-Strand Breaks (RIND-EDSBs) (Kongruttanachok N, et al., 2010). After several follow-up studies, we concluded that the DNA gaps are not DNA damage. In contrast, these gaps are evolutionarily conserved, essential DNA modifications in all eukaryotic cells (Pongpanich M, et al., 2014). Therefore, we revised the name to physiological RIND-EDSBs. Our previous report showed a reduction in the DNA gaps in aging yeast that consequently caused spontaneous DNA shearing (Thongsroy J, et al., 2018). Therefore, based on this function, we finalized the name of the gaps as “Youth-associated genome-stabilizing DNA gaps (Youth-DNA-gaps)” (Mutirangura A, 2019).
Discussion
Previously, we reported four phenomena in the aging prevention role of Youth-DNA-gaps in mammals, spanning from cellular study to bedside approaches. The four phenomena included Youth-DNA-gap reduction in aging cells, Youth-DNA-gap production and maintenance, DNA damage prevention by Youth-DNA-gaps, and finally, rejuvenation actions of Box A of HMGB1, which is the Youth-DNA-gaps-producing molecule. Here, we discuss candidate diseases and conditions that may be treated by Box A because DNA damage and senescence play roles in their pathogenesis.
Youth-DNA-gap
We detected a reduction in Youth-DNA-gaps in the elderly, naturally aging rats, D-galactose (D-gal)-induced aging rats, and senescent cells caused by different chemicals. The results showed that Youth-DNA-gap reduction is a DNA change found in eukaryotic cells in a wide range of species (e.g., yeast, rat, and human). This reduction can result from many senescence causes, including chemical induction and natural aging.
We proved that the Youth-DNA-gap complex comprises HMGB1-produced DNA gap, SIRT1 deacetylated histone and AGO4 methylated DNA (Yasom S, et al., 2022; Watcharanurak P and Mutirangura A, 2022).
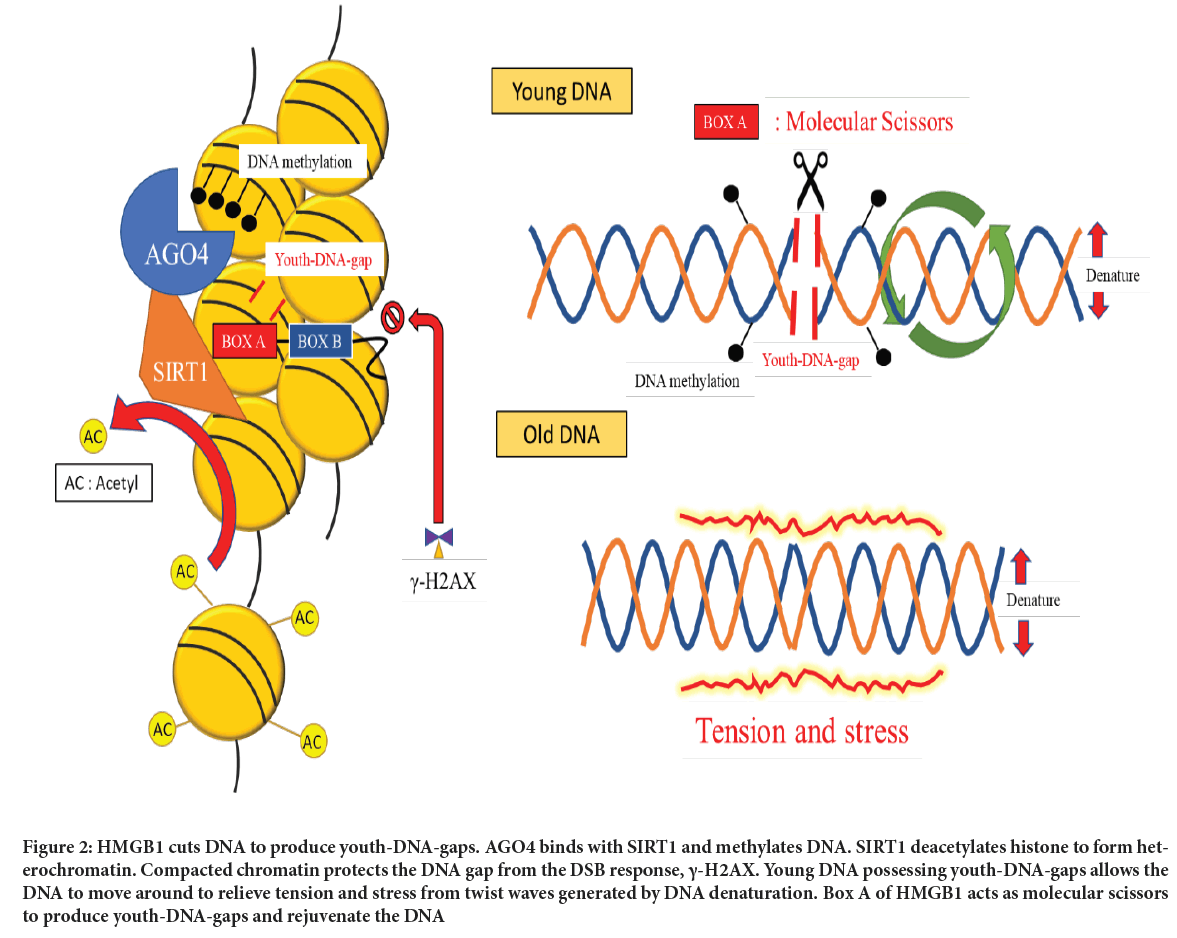
HMGB1 Box A is molecular scissors producing Youth-DNA-gaps, while SIRT1 causes chromatin compaction to prevent gaps in the DNA doublestrand break response. To avoid the random generation of DNA gaps, AGO4, possessing a small RNA sequence to bind to Intersperse Repetitive Sequences (IRSs), interacts with SIRT1 to locate Youth-DNA-gap complexes within methylated IRSs (Chalertpet K, et al., 2019). Understanding how cells produce Youth-DNA-gap allowed us to invent a DNA technology that can generate these cellular gaps to study their roles. Cell lines overexpressing Box A showed low endogenous DNA damage, high resistance to DNA breaks by radiation and low levels of the DNA Damage Response (DDR) signaling pathway. The report presented an unprecedented biological process and the first-ever technology for increasing DNA durability through this unique mechanism of action. We speculate that the DNA gap allows DNA movement to relieve torsion force from the twist wave created by DNA activity. As a result, chemical bonds of DNA possessing DNA gaps are not weakened by the torsion force from the DNA twist wave (Figure 2).
Figure 2: HMGB1 cuts DNA to produce youth-DNA-gaps. AGO4 binds with SIRT1 and methylates DNA. SIRT1 deacetylates histone to form heterochromatin. Compacted chromatin protects the DNA gap from the DSB response, γ-H2AX. Young DNA possessing youth-DNA-gaps allows the DNA to move around to relieve tension and stress from twist waves generated by DNA denaturation. Box A of HMGB1 acts as molecular scissors to produce youth-DNA-gaps and rejuvenate the DNA
Importance of DNA gaps and cellular senescence
Our previous report found an inverse correlation between the number of Youth-DNA-gaps and both chronological aging and the number of senescence cells. When we increased DNA gaps by Box A part of HMGB1 expression plasmids, senescence cells were rejuvenated. Therefore, senescence cells in the elderly are caused by Youth-DNA-gap reduction, promoting DNA damage.
Rejuvenation by Box A-produced DNA gaps
DNA damage can trigger cellular aging. In contrast, reducing DNA damage by Box A-produced DNA gaps revitalized cells. Our most exciting findings indicated that introducing DNA gaps by Box A could effectively improve aging features in the senescent cells and restore two rat models, natural aging and D-gal-induced aging rats, to be closer to young rats. We could ultimately rejuvenate learning and memory functions, improve liver function, reduce visceral fat and lower the senescent cells of aging rats. This treatment can also cure liver fibrosis. These results demonstrated the potential impact of Box A benefits for treating aging-associated diseases through improving DNA stability.
Knowledge and techniques from the Box A-produced DNA gap study provide an endless opportunity for biomedical research, including molecular pathogenesis of diseases and biomarkers and treatment of genomic instability-related conditions.
How does Box A expression plasmid reverse the once thought irreversible senescence process?
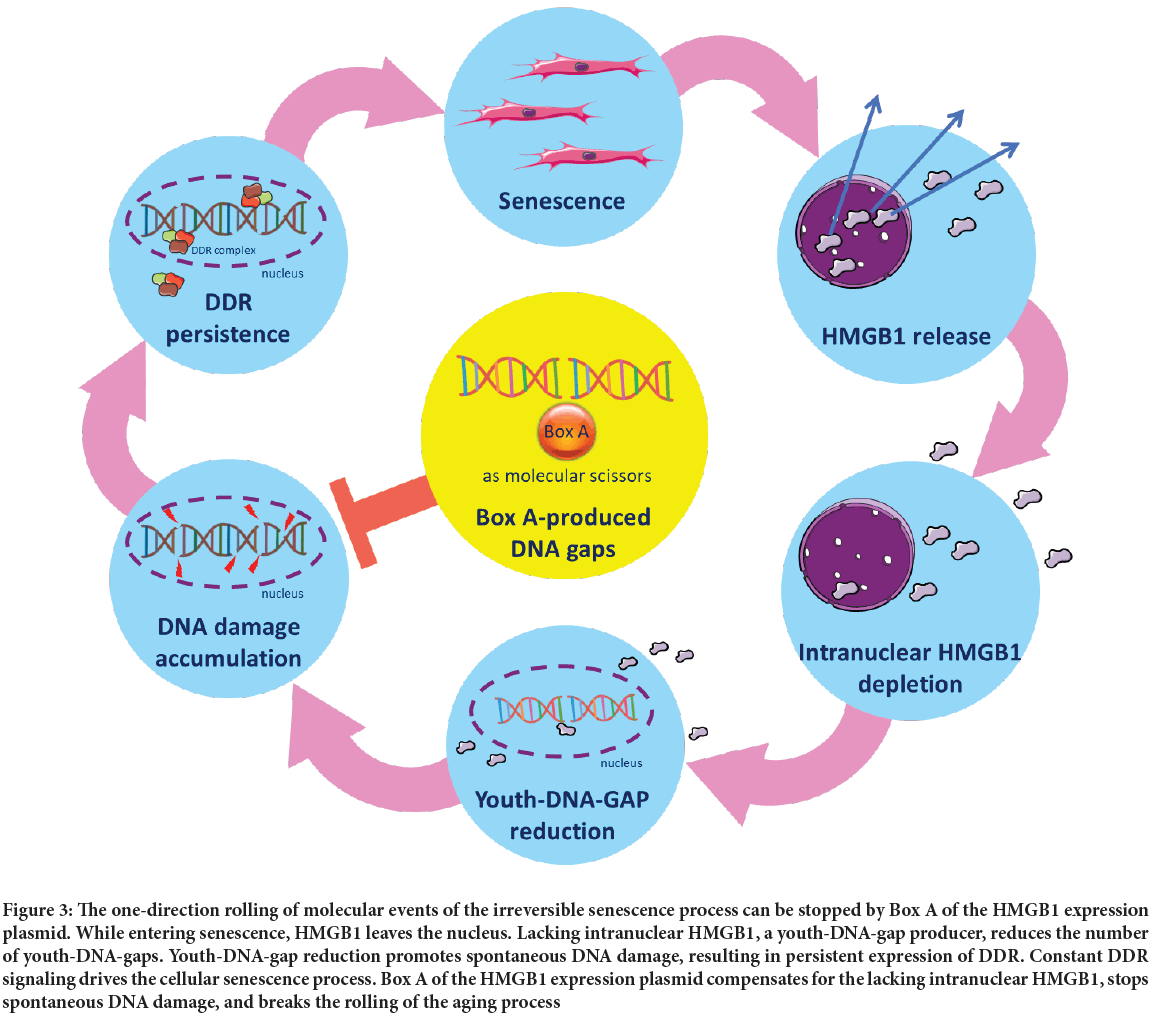
The Youth-DNA-gap reduction mechanism may be the reason why cellular senescence is a progressive process. While most HMGB1 is in the nucleus, one of the early events of senescence is HMGB1 translocation to the cytoplasm and its release into the extracellular space (Davalos AR, et al., 2013; Chaichalotornkul S, et al., 2015). The movement of HMGB1 limits intranuclear HMGB1 and results in Youth-DNA-gap reduction (Thongsroy J, et al., 2013). Persistent reduction of Youth-DNA-gaps continuously promotes endogenous DNA damage and DDR that drives cellular senescence; therefore, the senescence process is naturally irreversible due to the lack of the Youth-DNA-gap producer. To increase the number of DNA gaps, we used a Box A-expressing plasmid to produce these gaps to stabilize the DNA strand, subsequently reducing endogenous DNA damage and DDR and, in turn, causing rejuvenation (Figure 3).
Figure 3: The one-direction rolling of molecular events of the irreversible senescence process can be stopped by Box A of the HMGB1 expression plasmid. While entering senescence, HMGB1 leaves the nucleus. Lacking intranuclear HMGB1, a youth-DNA-gap producer, reduces the number of youth-DNA-gaps. Youth-DNA-gap reduction promotes spontaneous DNA damage, resulting in persistent expression of DDR. Constant DDR signaling drives the cellular senescence process. Box A of the HMGB1 expression plasmid compensates for the lacking intranuclear HMGB1, stops spontaneous DNA damage, and breaks the rolling of the aging process
In our recent study, a Box A-expressing plasmid reversed organ dysfunction and fibrosis (Yasom S, et al., 2022). When cells become senescent, cell growth is arrested, and their structure and function are altered. Senescent cells secrete SASP proteins, promoting inflammation and enforcing senescence in both autocrine and paracrine manners (Wei W and Ji S, 2018). This phenomenon drives the aging process, resulting in a loss of cellular function. Additionally, inflammation can alter the extracellular environment and, in turn, deteriorate organ structure and function. Box A-expressing plasmid rejuvenates cells and tissues and consequently recovers organ function. Although the fibrosis depletion mechanisms have not yet proved, this finding is exciting due to the promising possibility of Box A in removing abnormal macromolecules in Non-Communicable Diseases (NCDs), such as amyloid plaques in Alzheimer’s Disease (AD) or sclerotic plaques of the aging artery.
Biomedical research implications
DNA damage and cellular senescence are associated with a large number of NCDs and clinical conditions, such as degenerative diseases, cancers, Diabetes Mellitus (DM), DNA damage due to environmental insults, and hereditary DNA repair defects (Ruthsatz M and Candeias V, 2020; Campisi J, 2013; Herranz N and Gil J, 2018; Palmer AK, et al., 2019; Centner AM, et al., 2020; Saenen ND, et al., 2019; Knoch J, et al., 2012; Basu AK, 2018; Włodarczyk M and Nowicka G, 2019; Shimizu I, et al., 2014; Valavanidis A, et al., 2013). Due to the association with DNA damage and cellular senescence, similar to the cellular aging process described in Figure 3, the deprivation of intranuclear HMGB1 causing Youth-DNA-gap reduction may play an important role, and a Box A-expressing plasmid may modify the pathogenesis processes of the diseases and conditions.
SASP can promote secondary senescence in infiltrating White Blood Cells (WBCs) (Admasu TD, et al., 2021). Therefore, we should observe epigenetic changes in WBCs of patients with senescent cell accumulation in internal organs. Epigenetic changes in WBCs have been observed in NCDs associated with aging. For example, genome-wide hypomethylation has been found in circulating WBCs of age-associated NCDs, even in some local diseases, such as osteoporosis (Jintaridth P, et al., 2013). Youth-DNA-gaps are highly located within the hypermethylated genome in healthy conditions; however, DNA methylation is limited in the WBCs of pathological conditions, including many NCD patients (Pornthanakasem W, et al., 2008; Thongsroy J and Mutirangura A, 2022). The degree of genome-wide methylation is negatively correlated with the severity of NCDs (Jintaridth P, et al., 2013; Thongsroy J and Mutirangura A, 2022). A recent study demonstrated that the senescence of immune cells could facilitate the whole body’s aging process (Yousefzadeh MJ, et al., 2021). Therefore, Youth-DNA-gap reduction in WBCs may affect the deterioration of disease target organs. Box A treatment may be an essential research tool to determine whether immune senescence is involved in disease or condition pathogenesis, and immune cell rejuvenation will revitalize the entire body of the elderly.
In an animal study, Box A treatment of aging rats showed a decrease in senescent cells and liver fibrosis. This suggests good potential for Box A to heal the degeneration of internal organs in NCDs. Pathologic changes in NCDs are reported both in cells and in the extracellular space. The aggregation of senescent cells has been reported in various organs of NCDs, such as the brain in neurodegenerative diseases (Gillispie GJ, et al., 2021; Han X, et al., 2020), the heart in cardiac diseases including Heart Failure (Shimizu I and Minamino T, 2019), the lung in Chronic Obstructive Pulmonary Disease or COPD (Barnes PJ, 2017), fatty liver and cirrhosis (Papatheodoridi AM, et al., 2020; Huda N, et al., 2019), and kidney failure (Docherty MH, et al., 2019). The other common pathological lesions of NCDs include the deposition of fat cells, loss of ground substance and the accumulation of abnormal macromolecules, such as fatty liver, increased visceral fat, loss of bone mass in osteoporosis, amyloid in AD, lung fibrosis, liver cirrhosis, and sclerotic plaques of arteries. The accumulation of senescent cells, fat cells, fibrosis, and abnormal macromolecules results in NCD organ deterioration. Senescent cells stop dividing and have an altered metabolism and cytoskeleton arrangement. Therefore, increased senescent cells in internal organs cause cellular dysfunction and loss of cell/tissue mass. These aged cells release SASP, which subsequently induces and promotes inflammation. This prolonged inflammation can consequently cause fatty changes and the accumulation of abnormal macromolecules. Box A can rejuvenate aged cells by reversing the morphology and limiting the signaling proteins of senescence cells, promoting cell division in the cell cycle, and resetting aging cells back to normal function. Consequently, chronic inflammation and pathological features could be halted, and these pathological molecules may be removed by functional phagocytosis of rejuvenated WBCs.
Alzheimer’s disease
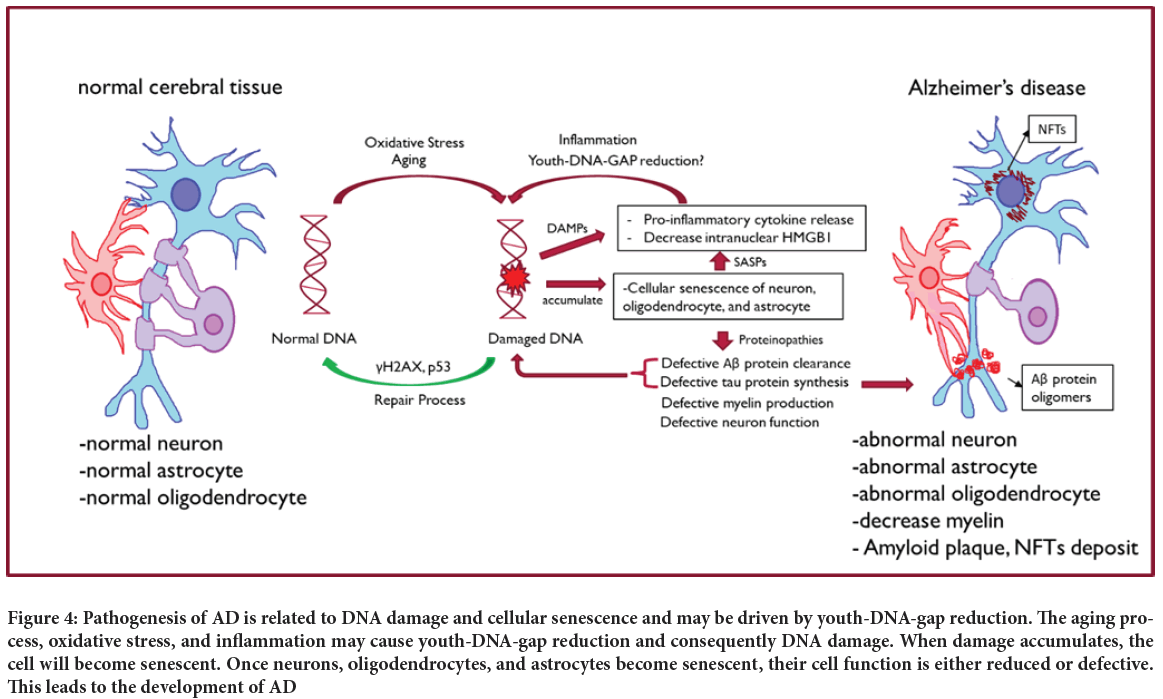
AD is a neurodegenerative disease common in the aging population. The pathogenesis of AD is related to DNA damage and cellular senescence (Zhang P, et al., 2019); hence, Youth-DNA-gap may play a crucial role in AD pathogenesis prevention (Figure 4). The prevalence of AD increases with age, suggesting that AD is connected with the aging process. The pathology of the brain in AD includes cortical atrophy, enlarged sulci, atrophied gyri, decreased brain volume (mostly white matter), atrophied amygdala and hippocampus, granulovacuolar degeneration, Hirano bodies, amyloid plaques, and Neurofibrillary Tangles (NFTs) (Deture MA and Dickson DW, 2019). All of these changes can be explained by the aging process and cellular senescence. The senescence process involved in the neuron microenvironment is multifactor. Many pro-senescence causes might be the root of developing AD, such as oxidative stress from neurons’ high metabolic demands, telomere shortening from epigenetic, mutagenic, or oncogenic substances, ionizing radiation, proto-oncogenes from hereditary disease, deposition of amyloid or tau protein, and defective DNA damage repair (Manoharan S, et al., 2016; Saez-Atienzar S, Masliah E, 2020; Brion JP, et al., 2001; Musi N, et al., 2018; Bevelacqua JJ, Mortazavi SMJ, 2018; Cai Z, et al., 2013; Gouras GK, et al., 2015). Most of these causes lead to or involve DNA damage, which activates the DDR and plays a significant role in cellular senescence.
DNA breaks signal phosphorylation of histone H2AX (γH2AX), which leads to the activation of p53. Activated p53 leads to cell growth arrest. This process protects the integrity of the genome and allows for complete DNA repair (Mijit M, et al., 2020). If the cell can clear all of the damage, then growth arrest will stop and the cell will return to normal. However, if the cell cannot remove all of the damage, then the cell will enter a senescence state. Alterations accompany this state of the cell in terms of protein expression, such as the release of SASPs (Birch J and Gil J, 2020). These SASPs cause inflammation and increase oxidative damage to neurons viathe activation of pro-inflammatory cytokine release and often worsen the condition of the brain parenchyma. Furthermore, damaged cells also release DAMPs, which propagate inflammation in the surrounding tissue. DAMPs will be continuously released until the inflammation resolves or no damaged cells remain.
HMGB1 is an intranuclear protein that is also a DAMP molecule. HMGB1 translocate out of the cell nucleus, and induces neurite degeneration via TLR4-MARCKS (Fujita K, et al., 2016). Once the stress subsides, the HMGB1 protein will relocate back to the nucleus and resume its duty. However, in chronic inflammation, the HMGB1 protein is continuously released, resulting in an overall decrease in intranuclear HMGB1 and consequently should result in Youth-DNA-gap reduction.
Amyloid-beta (Aβ) peptide production and plaque formation are related to senescence of neurons or astrocytes in Aβ protein clearance and catabolism (Paroni G, et al., 2019). This peptide is also found in the healthy aging brain, but when it passes a certain threshold (hereditary AD<sporadic AD), the plaque will express its ‘neurotoxicity’ property. The mechanism underlying the neurotoxicity of Aβ plaques is hypothesized to involve an oligomer of Aβ, which has been shown to reduce neuron potentiation and decrease synaptic activity (Murphy MP and LeVine H III, 2010; Forest KH and Nichols RA, 2019; Allsop D and Mayes J, 2014). Moreover, Aβ plaques are associated with astrocyte dysfunction (Acosta C, et al., 2017). Defective astrocytes impair synaptic plasticity, dysregulate neuronal metabolism, and increase neuroinflammation.
Neurofibrillary Tangles (NFT) are formed by tau protein. The normal function of the tau protein is to control and stabilize microtubules (Guo T, et al., 2017). In AD, the tau protein is misfolded or mismodified post-translationally due to dysfunction or dysregulation of the genes associated with it (Mi K and Johnson GV, 2006). This leads to the formation of NFTs in AD patients. Dysfunctional tau protein leads to a decrease in synaptic function and axonal transport of the neuron (Chong FP, et al., 2018). Accumulation of the tau protein also triggers the NF-κB cascade and upregulates SASP genes, leading to inflammation and senescence. Furthermore, a report suggests that NFT and SASP lead to oligodendrocyte and cerebrovascular senescence, which results in decreased cerebral blood flow, causing the brain to become atrophied (Bryant AG, et al., 2020; Cai Z and Xiao M, 2016) (Figure 4).
Figure 4: Pathogenesis of AD is related to DNA damage and cellular senescence and may be driven by youth-DNA-gap reduction. The aging process, oxidative stress, and inflammation may cause youth-DNA-gap reduction and consequently DNA damage. When damage accumulates, the cell will become senescent. Once neurons, oligodendrocytes, and astrocytes become senescent, their cell function is either reduced or defective. This leads to the development of AD
Heart Failure (HF)
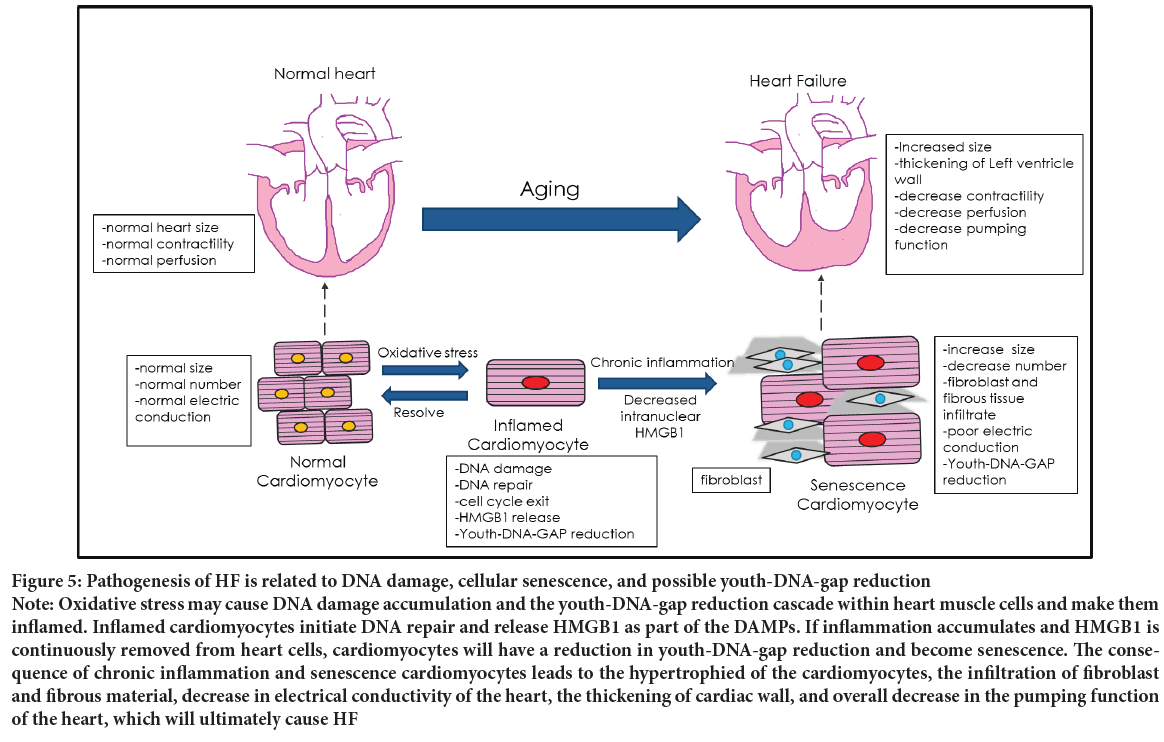
Both DNA damage and senescence play a crucial role in the pathogenesis of Heart Failure (HF) (Figure 5). Therefore, HF may be driven by Youth- DNA-gap reduction and could be treatable by Box A. The characteristics of Heart Failure are cardiac signs and symptoms (breathlessness, systemic swelling, pulmonary cracking, engorged neck vein, etc.) caused by a structural and/or functional cardiac abnormality. Heart Failure is a common long-term illness affecting the elderly worldwide. The incidence of Heart Failure increases with patient age, which suggests that the aging process is one of the causes of Heart Failure (Malik A, et al., 2022; Savarese G and Lund LH, 2017).
HF, impaired myocardial perfusion, is mainly caused by acute or chronic myocardial ischemia (Tanai E and Frantz S, 2018). Acute myocardial infarction is caused by either thrombi or emboli obstructing the coronary artery, but chronic myocardial ischemia is related to inflammation and cellular senescence. The pathology of Heart Failure patients’ cardiac tissue includes left ventricular wall thickening, cardiomyocyte enlargement, decreased heart muscle cell numbers, increased collagen deposits, aggregation of fibrous material, etc (Shinmura K, 2016). These features resemble other organs’ chronic inflammation changes, suggesting a link between the aging heart and chronic inflammation (Figure 5).
Figure 5: Pathogenesis of HF is related to DNA damage, cellular senescence, and possible youth-DNA-gap reduction Note: Oxidative stress may cause DNA damage accumulation and the youth-DNA-gap reduction cascade within heart muscle cells and make them inflamed. Inflamed cardiomyocytes initiate DNA repair and release HMGB1 as part of the DAMPs. If inflammation accumulates and HMGB1 is continuously removed from heart cells, cardiomyocytes will have a reduction in youth-DNA-gap reduction and become senescence. The consequence of chronic inflammation and senescence cardiomyocytes leads to the hypertrophied of the cardiomyocytes, the infiltration of fibroblast and fibrous material, decrease in electrical conductivity of the heart, the thickening of cardiac wall, and overall decrease in the pumping function of the heart, which will ultimately cause HF
Chronic inflammation of cardiomyocytes is mainly proposed to come from oxidative stress (Chen MS, et al., 2022). These oxidative stresses usually come from cellular metabolism, but stress, substance use, glycemic status, lipidemia status, and arteriosclerotic status also cause oxidative stress to change. Oxidative stress reduces Youth-DNA-gaps (Yasom S, et al., 2022). As a result, the pathogenesis of HF may be similar to the senescence process described in Figure 3. When cardiomyocytes become senescent, they stop dividing to replace dead cells and instead synthesize fibrous tissue to replace them. This fibrosis causes structural changes in the cardiac parenchyma, which affects heart pumping function. When the heart’s capacity has fallen to a particular level, the body will compensate for the cardiac function in multiple ways (Jackson G, et al., 2000; Francis GS, Cohn JN, 1990). Nevertheless, these compensatory mechanisms are limited. Once the body cannot cope with the cardiac dysfunction, the patient will develop HF (Figure 5).
Moreover, senescent cells are accompanied by the secretion of SASPs. These molecules are generally pro-inflammatory cytokines that will affect the nearby cells and the organ at large if enough SASPs accumulate. The SASPs involved in HF are Interleukin-6 (IL-6), Interleukin-15 (IL-15), Tumor Necrosis Factor-alpha (TNF-α), Chemokine (C-C motif) ligand 4 (CCL4), Growth Differentiation Factor 15 (GDF15), and Osteopontin (OPN) (Malaquin N, et al., 2019; Schafer MJ, et al., 2020). These molecules alter the balance between the synthesis and destruction of cardiomyocytes.
They will decrease the synthesis and increase the breakdown of the cardiac tissue, which further causes the deterioration of the cardiac structure and will ultimately result in a decline in heart-pumping function (Figure 5).
Additionally, some studies have reported the role of the HMGB1 protein as the protective factor against cardiac hypertrophy (Takahashi T, et al., 2019). The studies reported that the intranuclear HMGB1 protein quantity is crucial to the DNA protection of the cardiomyocyte. The decreased in the intranuclear HMGB1 level will result in the increase of hypertrophic cardiomyocytes, fibrosis, serum Brain Natriuretic Peptide (BNP) levels. This suggests the involvement of Youth-DNA-gaps as the main mechanism for inhibiting cardiac hypertrophy by protecting DNA from damages.
Diabetes Mellitus (DM)
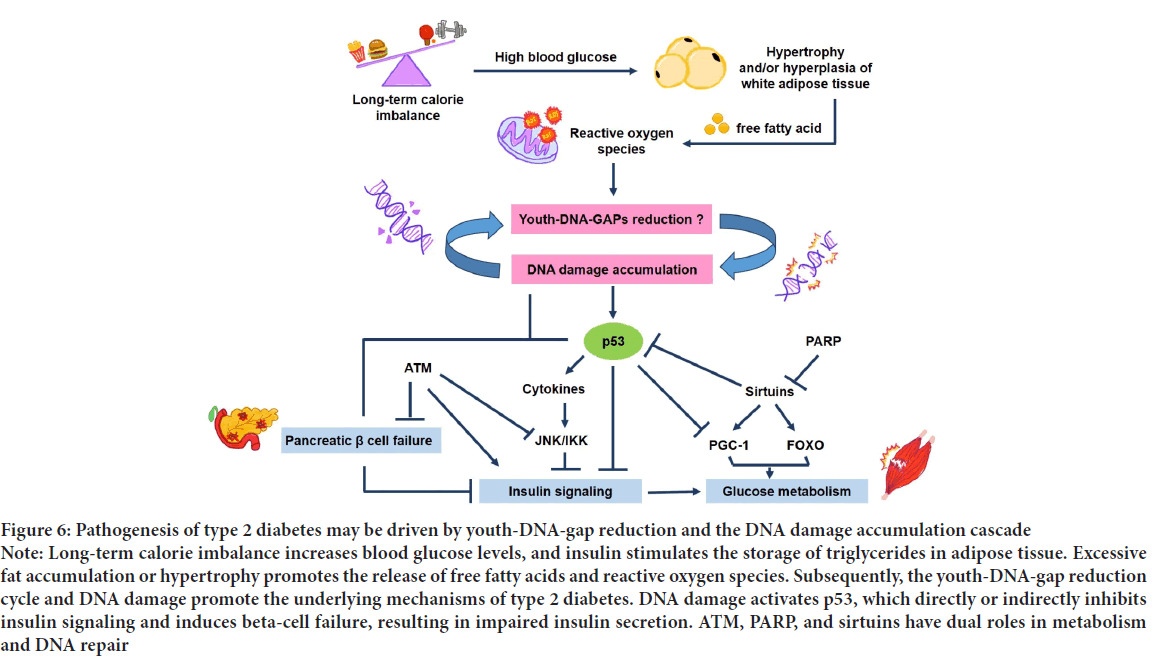
Type 2 DM is a group of metabolic diseases characterized by chronic hyperglycemia resulting from reduced peripheral glucose utilization and impaired beta-cell function (American Diabetes Association, 2013). It is caused by defects in insulin action and insulin secretion (Galicia-Garcia U, et al., 2020). The role of the insulin hormone is to control the balance between endogenous glucose production and tissue glucose uptake (Wilcox G, 2005). An increased plasma glucose concentration stimulates insulin release from pancreatic beta cells to stimulate glucose uptake by the peripheral tissue, primarily skeletal muscle, and suppress endogenous glucose production (deFronzo RA, et al., 1985; Petersen KF and Shulman GI, 2002). However, long-term sugar-heavy meals or calorie imbalances increase blood glucose levels, and insulin stimulates the storage of triglycerides in adipose tissue (hypertrophy and/or hyperplasia of white adipose tissue). Moreover, excess fat accumulation promotes the release of free fatty acids into the circulation from adipocytes. It induces mitochondrial dysfunction to release reactive oxygen species, which may be a critical factor in modulating DNA damage accumulation in response to insulin resistance and tissue damage to develop type 2 DM (Kim JY, et al., 2007; Graciano MF, et al., 2011). DM patients have Alu hypomethylation, and Youth-DNA-gaps are localized within the hypermethylated genome, so DNA damage may be due to Youth-DNA-gap reduction (Figure 6).
Figure 6: Pathogenesis of type 2 diabetes may be driven by youth-DNA-gap reduction and the DNA damage accumulation cascade Note: Long-term calorie imbalance increases blood glucose levels, and insulin stimulates the storage of triglycerides in adipose tissue. Excessive fat accumulation or hypertrophy promotes the release of free fatty acids and reactive oxygen species. Subsequently, the youth-DNA-gap reduction cycle and DNA damage promote the underlying mechanisms of type 2 diabetes. DNA damage activates p53, which directly or indirectly inhibits insulin signaling and induces beta-cell failure, resulting in impaired insulin secretion. ATM, PARP, and sirtuins have dual roles in metabolism and DNA repair
Patients with DM type 2 have a poor healing process, body degeneration, Alu hypomethylation, increased production of reactive oxygen species, significant DNA damage, and DDR. DNA damage also provokes insulin resistance, limiting muscle, fat, and liver responses to insulin, preventing glucose utilization, and causing metabolic syndrome, cardiovascular disease, stroke, kidney disease, eye problems, cancer, and AD. DNA damage has been proposed to promote insulin resistance by activating p53, resulting in JNK/IKK inhibiting insulin signaling. This process alters B cell function to limit insulin secretion. P53 also modulates the coordination of metabolic regulators and DNA repairs, such as PGC-1, ATM, PARP, and sirtuins. The signal transduction pathway deregulates the expression of glucose transporters and influences glucose homeostasis. Moreover, DDR causes senescence, inflammation, a decrease in regeneration, impairment of cellular metabolism, and suppression of endocrine function (Yasom S, et al., 2022). Therefore, the disturbed systemic metabolic homeostasis of DM patients may be driven by Youth-DNA-gap reduction and the DNA damage accumulation cascade (Figure 6).
Environmental insults
Burns, radiation, smoke, and alcohol can promote HMGB1 release and consequently limit the production of Youth-DNA-gaps (Heijink IH, et al., 2015; Johnson KE, et al., 2013; He SJ, et al., 2017; Wang X, et al., 2015). Youth-DNA-gap reduction can be caused by global DSB repair and/or HMGB1 release. These two mechanisms allow us to predict that environmental insults can cause a reduction in Youth-DNA-gaps. These results show that many kinds of ecological insults can reduce Youth-DNA-gaps. Our recent paper demonstrated that D-gal promoted extensive liver fibrosis, while Box A in D-gal-induced animals effectively treated liver fibrosis. Moreover, Box A producing DNA gap is the only known technology that can increase DNA durability and prevent radiation induced DNA breaks. This result indicates that Box A plasmid transfection may be an efficient approach to cure pathologic lesions derived from toxic environmental insults.
Chronic Obstructive Pulmonary Disease (COPD)
Chronic Obstructive Pulmonary Disease (COPD) is a chronic pulmonary disease characterized by deteriorative changes of the lung, resulting in decreased lung capacity. Smokers have a very high probability of developing COPD, so smoking is one of COPD’s most critical causative factors (Laniado-Laborín R, 2009). Statistics show that most COPD patients are mid-adult and elderly, and disease severity also progresses with the patient’s age (Orvoen-Frija E, et al., 2010; Devine JF, 2008). This suggests that smoke and the aging process are involved in the development of COPD.
The pathology of a COPD patient’s lung resembles the repetitive destruction and repair of the lung tissue. The key features of COPD pathology are airway remodeling, airway narrowing, hypersecretion of mucus, recruitment of inflammatory cells, decreased normal alveoli, accumulation of fibrous tissue (O’Reilly S, 2016).
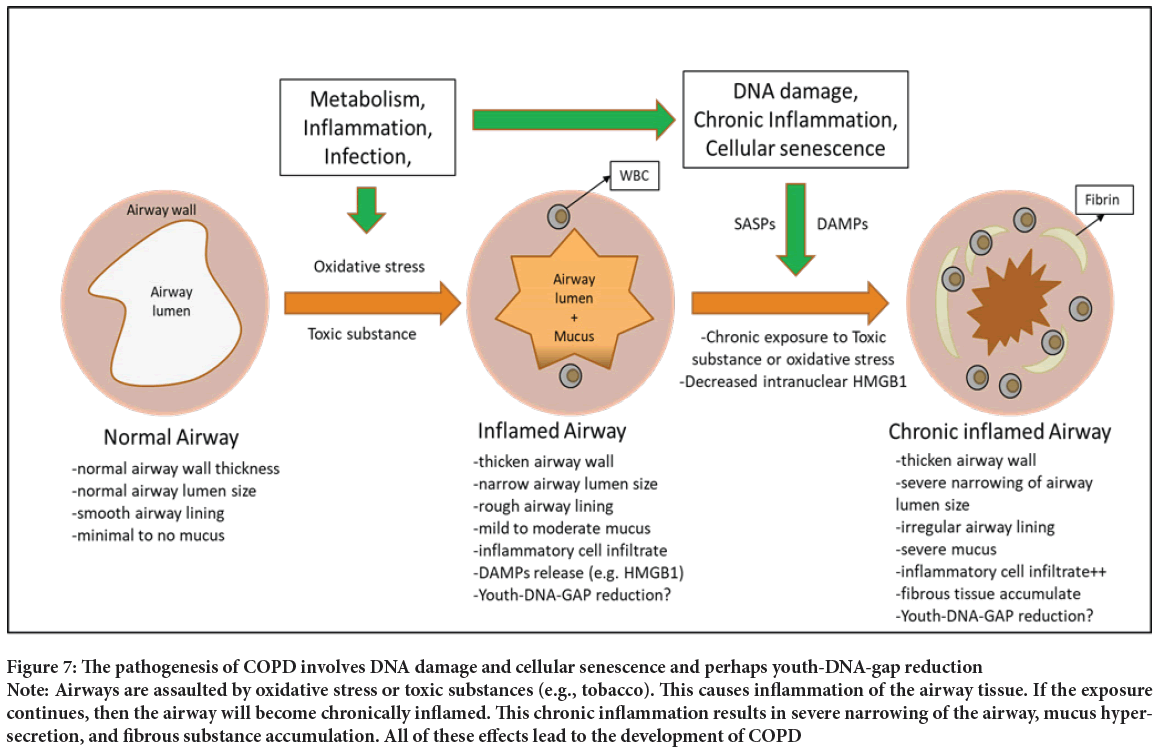
The repetitive destruction of the lung mainly comes from exposure to toxic substances, especially cigarettes and occupational hazards. Once these poisonous substances are inhaled, they damage the airway and lung parenchymal cells. Impaired/dead cells release DAMP molecules and cause inflammatory cell recruitment to the damaged site (Seong SY and Matzinger P, 2004). Prolonged exposure leads to prolonged inflammation in the lung tissue, which generates oxidative stress and replicative stress in the pulmonary cells. These stresses produce changes through DNA damage and cellular senescence, such as Youth-DNA-gap reduction (Figure 7).
Figure 7: The pathogenesis of COPD involves DNA damage and cellular senescence and perhaps youth-DNA-gap reduction Note: Airways are assaulted by oxidative stress or toxic substances (e.g., tobacco). This causes inflammation of the airway tissue. If the exposure continues, then the airway will become chronically inflamed. This chronic inflammation results in severe narrowing of the airway, mucus hypersecretion, and fibrous substance accumulation. All of these effects lead to the development of COPD
Toxic substances in smoke cause damage to the cell membrane, organelles, and DNA (Ames BN, et al., 1993). DNA damage is usually checked by the DNA repair process (Scott TL, et al., 2014). However, after chronic inflammation and repetitive assault by toxic substances, the repair process cannot keep up, resulting in COPD pathogenesis (Figure 7).
Senescent cells will further aggravate the situation of the lung. Senescence cells are often accompanied by the release of multiple SASP molecules, including IL-1, IL-6, IL-7, IL-8, IL-13, IL-15, etc (Coppé JP, et al., 2010). These SASP molecules are mostly pro-inflammatory and further promote the inflammation of the lung, resulting in more oxidative stress to the lung parenchyma (Figure 7).
To summarize, the pathogenesis of COPD is a chronic inflammation of the lung causing repetitive DNA damage and cellular senescence, which alters the airways and lung’s cell function and then results in structural changes and a decreased gas-exchange function of the lung (Figure 7).
Congenital DNA repair defects
There is a specific group of patients in whom Box A expression plasmids may help reduce both morbidity and mortality. Individuals with DNA repair defects accumulate endogenous DNA damage due to poor DNA repair function, and DNA damage causes premature aging characteristics and early death (Foo MX, et al., 2019). Thus, increased DNA durability by Box A-produced DNA gaps may limit DNA lesions and, in turn, slow down the premature aging process.
Cancer implications
Cancer cells possess fewer DNA gaps than other cells. Therefore, a reduction in Youth-DNA-gap may be the underlying mechanism driving genomic instability in cancer. A possible future application is using Box A to prevent cancer. However, many possible outcomes from using Box A are possible because cancer may alter the composition of Youth-DNA-gap formation molecules and cellular responses to DDR signaling. For example, some cancers have depleted intranuclear SIRT1 (Alves-Fernandes DK and Jasiulionis MG, 2019). Therefore, instead of producing Youth-DNA-gaps, HMGB1 in cancers should cause H2AX-associated pathologic EDSBs. Therefore, Box A may act as a cancer cell-specific H2AX-associated DSB induction therapy and selectively kill cancer patients’ cancer cells while rejuvenating normal cells. Moreover, most cancers have corrupted DDRs, such as p53 mutations (Olivier M, et al., 2010). In these cases, Youth-DNA- gaps can be formed properly. Box A may reduce DNA damage and consequently the corrupted DDR. As a result, cancer cell growth will be affected. However, if the Youth-DNA-gap formation molecules and DDR of the cancer cells have not been changed, Box A may promote cancer growth similar to that of normal cells. Alternatively, many reports have demonstrated that immune cells in cancer patients are senescent and efficiently depleted, unable to fight cancer. Therefore, it may be possible to use Box A to rejuvenate and improve the function of immune cells for cancer treatment.
Cancer-specific T cells are the most effective host defense mechanism to eradicate growing cancer cells. The cooperation of antigen-presenting cells and effector T cells is well known to drive cancer-immune responses. While the immune response is rising, the immune regulatory process is counterbalanced. As a result, regulatory immune cells overwhelm effector functions and support developing cancer cells (Wculek SK, et al., 2020). This is due to cancer and immune cross-talk, which causes the dysfunction of immune cells (Li CJ, et al., 2017). There is evidence that cancer and stromal cells release chemotaxis or cytokines to exclude T cells and edit T cell function. The orchestration of immunosuppressive cell composition, including cancer stromal cells and myeloid cells, promotes the host immune editing process. For example, human monocytes can be differentiated markedly from various phenotypes, including Tumor-Associated Macrophages (TAMs), Myeloid-Derived Suppressor Cells (MDSCs), and regulatory dendritic cells (Youn JI, et al., 2008). This depends on external stimuli, cytokines, and growth factors that mediate epigenetic machinery (Garcia-Gomez A, et al., 2018). A recent study showed methylation alterations in cancer stromal cells (Bin-Alee F, et al., 2020). This epigenetic machinery also triggers the plasticity of immune cells and causes cancer-associated T cell dysfunction, including T cell exhaustion and T cell senescence. T cell exhaustion is triggered by immune checkpoint molecules, while T cell senescence leads to DNA damage. There is evidence that cancer-associated regulatory T cells induce T cell senescence by increasing DNA damage markers. These senescent T cells lose proliferative function and have altered cytokine secretory function (Liu X, et al., 2018; Pangrazzi L and Weinberger B, 2020). Normally, T cell senescence is a terminally differentiated effector T cell. This is the natural T cell differentiation that is markedly evident over sixty years of age (Saule P, et al., 2006). However, the early presence of senescent immune cells is found in cancer. This type of T cell maintains the expression of pro-inflammatory cytokines, but exocytosis and granzyme production are defective. Protecting DNA damage in immune cells can be an interesting strategy to maintain immune function and prevent cancer development. However, T cell senescence usually occurs in cancer patients. Likewise, a previous study showed a distinctively high proportion of tumor-infiltrating T cells (Filaci G, et al., 2007). Hence, T cell rejuvenation is a necessity for cancer immunotherapy. This extrapolates the potential implication of in vitro senescent reversal of tumor-infiltrating lymphocytes, which are senescent cancer-specific lymphocytes.
Chemotherapy is one of the cancer treatment backbones. The chemotherapy mechanism causes damage to dividing cells, such as cell cycle inhibition and DNA damage. Therefore, several chemotherapy-induced cellular senescence models are commonly used. Some of these agents are cell cycle-specific inhibitors, such as antimetabolites and topoisomerase inhibitors. The others are noncell cycle-specific agents that mainly cause DNA strand damage. Alkylating agents and platinum-based chemotherapy are the primary classes of chemotherapy causing DNA damages (Onyema OO, et al., 2015). The alkylating drugs act by adding an alkyl group to the guanine base, causing DNA strand breaks or interstrand crosslinks. These induce replication failure and apoptosis. The commonly used alkylating agents include cyclophosphamide and mitomycin. Platinum-based chemotherapy, including cisplatin, carboplatin, and oxaliplatin, is an alkylating-like agent. These cause DNA adducts and strand breaks. Currently, these agents are commonly used in cancer patients. Unfortunately, longterm toxicities also occur in cancer survivors. DNA damage-associated toxicities include secondary cancer, myelodysplastic syndrome, and neur opathy. Moreover, the accumulation of drugs that cause DNA damage in normal tissue also deteriorates organ function. In addition, the quality of life is eventually affected. Notably, HMGB1 was found to be a factor responsible for cisplatin resistance by shielding DNA (Cheung-Ong K, et al., 2013). Conversely, HMGB1 could be used to prevent chemotherapy-induced toxicities in normal tissue.
Radiation is also one of the cancer treatment backbones. This modality directly causes DNA double-strand breaks, which are fatal situations for dividing cells. In cancer, cell cycle checkpoints are also defective, and DNA repair mechanisms are not accomplished. Accordingly, the accumulation of DNA damage with repair defects causes cancer cell death (Huang RX and Zhou PK, 2020). Although radiation is dependent on therapeutic fields and radiation doses, radiation effects on normal adjacent tissue are inevitable. In other words, radiation accelerates cellular senescence (Nguyen HQ, et al., 2018). The rejuvenation of irradiated tissue could normalize the healing process and reduce long-term toxicities. These toxicities cause secondary cancer and tissue fibrosis. Interestingly, the alteration of intracellular HMGB1 is affected by the DNA damage burden. A study showed that X-ray radiation affected the translocation of HMGB1 and triggered the extracellular release of HMGB1 (Wang L, et al., 2016). Moreover, one study showed that HMGB1 knockout sensitized cancer cell lines to radiation effects, while HMGB-1 overexpression and Box A caused resistance to radiation (Zhu X, et al., 2020). Box A expression could be an effective model for preventing radiation side effects and normalizing the post-radiation tissue healing process.
Conclusion
Box A-produced DNA gaps have been demonstrated to increase DNA durability and rejuvenate aging phenotypes. The accumulation of endogenous DNA damage driving cellular senescence by Youth-DNA-gap reduction may be the molecular pathogenesis of aging-associated diseases resulting from DNA damage. This is an excellent opportunity to test whether the Box A-produced DNA gap can cure these currently incurable common diseases and conditions.
Acknowledgements
We would like to thank Department of Anatomy, Chulalongkorn University for equipment and also to the Center of Excellence, Faculty of Medicine, Chulalongkorn University for equipment and personnel.
Funding
This work was supported by the National Research Council of Thailand (NRCT); National Science and Technology Development Agency (Grant number FDA-CO-2561-8477-TH); Ratchadapiseksomphot Fund for Postdoctoral Fellowship and Development of New Faculty Staff, Chulalongkorn University and the Thailand Research Fund (grant number RTA6280004).
References
- Schumacher B, Pothof J, Vijg J, Hoeijmakers JH. The central role of DNA damage in the ageing process. Nature. 2021; 592(7856): 695-703.
[Crossref] [Google Scholar] [Pubmed]
- Milic M, Frustaci A, del Bufalo A, Sánchez-Alarcón J, Valencia-Quintana R, Russo P, et al. DNA damage in non-communicable diseases: A clinical and epidemiological perspective. Mutat Res. 2015; 776: 118-127.
[Crossref] [Google Scholar] [Pubmed]
- Yousefzadeh M, Henpita C, Vyas R, Soto-Palma C, Robbins P, Niedernhofer L. DNA damage-how and why we age?. Elife. 2021; 10: e62852.
[Crossref] [Google Scholar] [Pubmed]
- Olivieri F, Albertini MC, Orciani M, Ceka A, Cricca M, Procopio AD, et al. DNA damage response (DDR) and senescence: Shuttled inflamma-miRNAs on the stage of inflamm-aging. Oncotarget. 2015; 6(34): 35509.
[Crossref] [Google Scholar] [Pubmed]
- Fagagna FD, Reaper PM, Clay-Farrace L, Fiegler H, Carr P, Von Zglinicki T, et al. A DNA damage checkpoint response in telomere-initiated senescence. Nature. 2003; 426(6963): 194-198.
[Crossref] [Google Scholar] [Pubmed]
- Lopes-Paciencia S, Saint-Germain E, Rowell MC, Ruiz AF, Kalegari P, Ferbeyre G. The senescence-associated secretory phenotype and its regulation. Cytokine. 2019; 117: 15-22.
[Crossref] [Google Scholar] [Pubmed]
- He S, Sharpless NE. Senescence in health and disease. Cell. 2017; 169(6): 1000-1011.
[Crossref] [Google Scholar] [Pubmed]
- Ritschka B, Storer M, Mas A, Heinzmann F, Ortells MC, Morton JP, et al. The senescence-associated secretory phenotype induces cellular plasticity and tissue regeneration. Genes Dev. 2017; 31(2): 172-183.
[Crossref] [Google Scholar] [Pubmed]
- Rea IM, Gibson DS, McGilligan V, McNerlan SE, Alexander HD, Ross OA. Age and age-related diseases: Role of inflammation triggers and cytokines. Front Immunol. 2018; 9: 586.
[Crossref] [Google Scholar] [Pubmed]
- Roh JS, Sohn DH. Damage-associated molecular patterns in inflammatory diseases. Immune Netw. 2018; 18(4): e27.
[Crossref] [Google Scholar] [Pubmed]
- Hernandez-Segura A, Nehme J, Demaria M. Hallmarks of cellular senescence. Trends Cell Biol. 2018; 28(6): 436-453.
[Crossref] [Google Scholar] [Pubmed]
- Wei W, Ji S. Cellular senescence: Molecular mechanisms and pathogenicity. J Cell Physiol. 2018; 233(12): 9121-9135.
[Crossref] [Google Scholar] [Pubmed]
- Tchkonia T, Zhu Y, van Deursen J, Campisi J, Kirkland JL. Cellular senescence and the senescent secretory phenotype: Therapeutic opportunities. J Clin Invest. 2013; 123(3): 966-972.
[Crossref] [Google Scholar] [Pubmed]
- Kirkland JL, Tchkonia T. Senolytic drugs: From discovery to translation. J Intern Med. 2020; 288(5): 518-536.
[Crossref] [Google Scholar] [Pubmed]
- van Deursen JM. Senolytic therapies for healthy longevity. Science. 2019; 364(6441): 636-637.
[Crossref] [Google Scholar] [Pubmed]
- Yasom S, Watcharanurak P, Bhummaphan N, Thongsroy J, Puttipanyalears C, Settayanon S, et al. The roles of HMGB1-produced DNA gaps in DNA protection and aging biomarker reversal. FASEB Bioadv. 2022; 4(6): 408-434.
[Crossref] [Google Scholar] [Pubmed]
- Yang H, Wang H, Andersson U. Targeting inflammation driven by HMGB1. Front Immunol. 2020; 11: 484.
[Crossref] [Google Scholar] [Pubmed]
- Andersson U, Tracey KJ. HMGB1 is a therapeutic target for sterile inflammation and infection. Annu Rev Immunol. 2011; 29: 139-162.
[Crossref] [Google Scholar] [Pubmed]
- Giavara S, Kosmidou E, Hande MP, Bianchi ME, Morgan A, d'Adda di Fagagna F, et al. Yeast Nhp6A/B and mammalian Hmgb1 facilitate the maintenance of genome stability. Curr Biol. 2005; 15(1): 68-72.
[Crossref] [Google Scholar] [Pubmed]
- Han G, Ling R, Sun C, Wang X, Zhou Y, Yu L, et al. HMGB1 knockdown increases the radiosensitivity of esophageal squamous cell carcinoma by regulating the expression of molecules involved in DNA repair. Oncol Lett. 2021; 22(1): 503.
[Crossref] [Google Scholar] [Pubmed]
- Funayama A, Shishido T, Netsu S, Narumi T, Kadowaki S, Takahashi H, et al. Cardiac nuclear high mobility group box 1 prevents the development of cardiac hypertrophy and heart failure. Cardiovasc Res. 2013; 99(4): 657-664.
[Crossref] [Google Scholar] [Pubmed]
- Pornthanakasem W, Kongruttanachok N, Phuangphairoj C, Suyarnsestakorn C, Sanghangthum T, Oonsiri S, et al. LINE-1 methylation status of endogenous DNA double-strand breaks. Nucleic Acids Res. 2008; 36(11): 3667-3675.
[Crossref] [Google Scholar] [Pubmed]
- Kongruttanachok N, Phuangphairoj C, Thongnak A, Ponyeam W, Rattanatanyong P, Pornthanakasem W, et al. Replication independent DNA double-strand break retention may prevent genomic instability. Mol Cancer. 2010; 9: 1-4.
[Crossref] [Google Scholar] [Pubmed]
- Pongpanich M, Patchsung M, Thongsroy J, Mutirangura A. Characteristics of replication-independent endogenous double-strand breaks in Saccharomyces cerevisiae. BMC Genomics. 2014; 15(1): 750.
[Crossref] [Google Scholar] [Pubmed]
- Thongsroy J, Patchsung M, Pongpanich M, Settayanon S, Mutirangura A. Reduction in replication-independent endogenous DNA double-strand breaks promotes genomic instability during chronological aging in yeast. FASEB J. 2018; 32(11): 6252-6260.
[Crossref] [Google Scholar] [Pubmed]
- Mutirangura A. Is global hypomethylation a nidus for molecular pathogenesis of age-related noncommunicable diseases?. Epigenomics. 2019; 11(6): 577-579.
[Crossref] [Google Scholar] [Pubmed]
- Watcharanurak P, Mutirangura A. Human RNA-directed DNA methylation methylates high-mobility group box 1 protein-produced DNA gaps. Epigenomics. 2022; 14(12): 741-756.
[Crossref] [Google Scholar] [Pubmed]
- Chalertpet K, Pin-On P, Aporntewan C, Patchsung M, Ingrungruanglert P, Israsena N, et al. Argonaute 4 as an Effector Protein in RNA-Directed DNA Methylation in Human Cells. Front Genet. 2019; 10: 645.
[Crossref] [Google Scholar] [Pubmed]
- Davalos AR, Kawahara M, Malhotra GK, Schaum N, Huang J, Ved U, et al. p53-dependent release of Alarmin HMGB1 is a central mediator of senescent phenotypes. J Cell Biol. 2013; 201(4): 613-629.
[Crossref] [Google Scholar] [Pubmed]
- Chaichalotornkul S, Nararatwanchai T, Narkpinit S, Dararat P, Kikuchi K, Maruyama I, et al. Secondhand smoke exposure-induced nucleocytoplasmic shuttling of HMGB1 in a rat premature skin aging model. Biochem Biophys Res Commun. 2015; 456(1): 92-97.
[Crossref] [Google Scholar] [Pubmed]
- Thongsroy J, Matangkasombut O, Thongnak A, Rattanatanyong P, Jirawatnotai S, Mutirangura A. Replication-independent endogenous DNA double-strand breaks in Saccharomyces cerevisiae model. PLoS One. 2013; 8(8): e72706.
[Crossref] [Google Scholar] [Pubmed]
- Ruthsatz M, Candeias V. Non-communicable disease prevention, nutrition and aging. Acta Biomed. 2020; 91(2): 379-388.
[Crossref] [Google Scholar] [Pubmed]
- Campisi J. Aging, cellular senescence, and cancer. Annu Rev Physiol. 2013; 75: 685-705.
[Crossref] [Google Scholar] [Pubmed]
- Herranz N, Gil J. Mechanisms and functions of cellular senescence. J Clin Invest. 2018; 128(4): 1238-1246.
[Crossref] [Google Scholar] [Pubmed]
- Palmer AK, Gustafson B, Kirkland JL, Smith U. Cellular senescence: At the nexus between ageing and diabetes. Diabetologia. 2019; 62(10): 1835-1841.
[Crossref] [Google Scholar] [Pubmed]
- Centner AM, Bhide PG, Salazar G. Nicotine in senescence and atherosclerosis. Cells. 2020; 9(4): 1035.
[Crossref] [Google Scholar] [Pubmed]
- Saenen ND, Martens DS, Neven KY, Alfano R, Bové H, Janssen BG, et al. Air pollution-induced placental alterations: An interplay of oxidative stress, epigenetics, and the aging phenotype?. Clin Epigenet. 2019; 11(1): 1-4.
[Crossref] [Google Scholar] [Pubmed]
- Knoch J, Kamenisch Y, Kubisch C, Berneburg M. Rare hereditary diseases with defects in DNA-repair. Eur J Dermatol. 2012; 22(4): 443-455.
[Crossref] [Google Scholar] [Pubmed]
- Basu AK. DNA damage, mutagenesis and cancer. Int J Mol Sci. 2018; 19(4): 970.
[Crossref] [Google Scholar] [Pubmed]
- Włodarczyk M, Nowicka G. Obesity, dna damage, and development of obesity-Related diseases. Int J Mol Sci. 2019; 20(5):1146.
[Crossref] [Google Scholar] [Pubmed]
- Shimizu I, Yoshida Y, Suda M, Minamino T. DNA damage response and metabolic disease. Cell Metab. 2014; 20(6): 967-977.
[Crossref] [Google Scholar] [Pubmed]
- Valavanidis A, Vlachogianni T, Fiotakis K, Loridas S. Pulmonary oxidative stress, inflammation and cancer: Respirable particulate matter, fibrous dusts and ozone as major causes of lung carcinogenesis through reactive oxygen species mechanisms. Int J Environ Res Public Health. 2013; 10(9): 3886-3907.
[Crossref] [Google Scholar] [Pubmed]
- Admasu TD, Rae M, Stolzing A. Dissecting primary and secondary senescence to enable new senotherapeutic strategies. Ageing Res Rev. 2021; 70: 101412.
[Crossref] [Google Scholar] [Pubmed]
- Jintaridth P, Tungtrongchitr R, Preutthipan S, Mutirangura A. Hypomethylation of Alu elements in post-menopausal women with osteoporosis. PLoS One. 2013; 8(8): e70386.
[Crossref] [Google Scholar] [Pubmed]
- Thongsroy J, Mutirangura A. The association between Alu hypomethylation and the severity of hypertension. PLoS One. 2022; 17(7): e0270004.
[Crossref] [Google Scholar] [Pubmed]
- Yousefzadeh MJ, Flores RR, Zhu Y, Schmiechen ZC, Brooks RW, Trussoni CE, et al. An aged immune system drives senescence and ageing of solid organs. Nature. 2021; 594(7861): 100-105.
[Crossref] [Google Scholar] [Pubmed]
- Gillispie GJ, Sah E, Krishnamurthy S, Ahmidouch MY, Zhang B, Orr ME. Evidence of the cellular senescence stress response in mitotically active brain cells-implications for cancer and neurodegeneration. Life (Basel). 2021; 11(2): 153.
[Crossref] [Google Scholar] [Pubmed]
- Han X, Zhang T, Liu H, Mi Y, Gou X. Astrocyte senescence and alzheimer's disease: A review. Front Aging Neurosci. 2020; 12: 148.
[Crossref] [Google Scholar] [Pubmed]
- Shimizu I, Minamino T. Cellular senescence in cardiac diseases. J Cardiol. 2019; 74(4): 313-319.
[Crossref] [Google Scholar] [Pubmed]
- Barnes PJ. Senescence in COPD and its comorbidities. Annu Rev Physiol. 2017; 79: 517-539.
[Crossref] [Google Scholar] [Pubmed]
- Papatheodoridi AM, Chrysavgis L, Koutsilieris M, Chatzigeorgiou A. The role of senescence in the development of nonalcoholic fatty liver disease and progression to nonalcoholic steatohepatitis. Hepatology. 2020; 71(1): 363-374.
[Crossref] [Google Scholar] [Pubmed]
- Huda N, Liu G, Hong H, Yan S, Khambu B, Yin XM. Hepatic senescence, the good and the bad. World J Gastroenterol. 2019; 25(34): 5069-5081.
[Crossref] [Google Scholar] [Pubmed]
- Docherty MH, O'Sullivan ED, Bonventre JV, Ferenbach DA. Cellular senescence in the kidney. J Am Soc Nephrol. 2019; 30(5): 726-736.
[Crossref] [Google Scholar] [Pubmed]
- Zhang P, Kishimoto Y, Grammatikakis I, Gottimukkala K, Cutler RG, Zhang S, et al. Senolytic therapy alleviates Aβ-associated oligodendrocyte progenitor cell senescence and cognitive deficits in an Alzheimer's disease model. Nat Neurosci. 2019; 22(5): 719-728.
[Crossref] [Google Scholar] [Pubmed]
- Deture MA, Dickson DW. The neuropathological diagnosis of Alzheimer's disease. Mol Neurodegener. 2019; 14(1): 32.
[Crossref] [Google Scholar] [Pubmed]
- Manoharan S, Guillemin GJ, Abiramasundari RS, Essa MM, Akbar M, Akbar MD. The role of reactive oxygen species in the pathogenesis of alzheimer's disease, parkinson's disease, and huntington's disease: A mini review. Oxid Med Cell Longev. 2016; 2016: 8590578.
[Crossref] [Google Scholar] [Pubmed]
- Saez-Atienzar S, Masliah E. Cellular senescence and Alzheimer disease: The egg and the chicken scenario. Nat Rev Neurosci. 2020; 21(8): 433-444.
[Crossref] [Google Scholar] [Pubmed]
- Brion JP, Anderton BH, Authelet M, Dayanandan R, Leroy K, Lovestone S, et al. Neurofibrillary tangles and tau phosphorylation. Biochem Soc Symp. 2001; (67): 81-88.
[Crossref] [Google Scholar] [Pubmed]
- Musi N, Valentine JM, Sickora KR, Baeuerle E, Thompson CS, Shen Q, et al. Tau protein aggregation is associated with cellular senescence in the brain. Aging Cell. 2018; 17(6): e12840.
[Crossref] [Google Scholar] [Pubmed]
- Bevelacqua JJ, Mortazavi SMJ. Alzheimer's Disease: Possible mechanisms behind neurohormesis induced by exposure to low doses of ionizing radiation. J Biomed Phys Eng. 2018;8(2):153-156.
[Google Scholar] [Pubmed]
- Cai Z, Yan LJ, Ratka A. Telomere shortening and Alzheimer's disease. Neuromolecular Med. 2013; 15(1): 25-48.
[Crossref] [Google Scholar] [Pubmed]
- Gouras GK, Olsson TT, Hansson O. β-Amyloid peptides and amyloid plaques in Alzheimer's disease. Neurotherapeutics. 2015; 12(1): 3-11.
[Crossref] [Google Scholar] [Pubmed]
- Mijit M, Caracciolo V, Melillo A, Amicarelli F, Giordano A. Role of p53 in the regulation of cellular senescence. Biomolecules. 2020; 10(3): 420.
[Crossref] [Google Scholar] [Pubmed]
- Birch J, Gil J. Senescence and the SASP: Many therapeutic avenues. Genes Dev. 2020; 34(23-24): 1565-1576.
[Crossref] [Google Scholar] [Pubmed]
- Fujita K, Motoki K, Tagawa K, Chen X, Hama H, Nakajima K, et al. HMGB1, a pathogenic molecule that induces neurite degeneration via TLR4-MARCKS, is a potential therapeutic target for Alzheimer's disease. Sci Rep. 2016; 6: 31895.
[Crossref] [Google Scholar] [Pubmed]
- Paroni G, Bisceglia P, Seripa D. Understanding the amyloid hypothesis in Alzheimer's disease. J Alzheimers Dis. 2019; 68(2): 493-510.
[Crossref] [Google Scholar] [Pubmed]
- Murphy MP, LeVine H III. Alzheimer's disease and the amyloid-beta peptide. J Alzheimers Dis. 2010; 19(1): 311-323.
[Crossref] [Google Scholar] [Pubmed]
- Forest KH, Nichols RA. Assessing neuroprotective agents for Aβ-induced neurotoxicity. Trends Mol Med. 2019; 25(8): 685-695.
[Crossref] [Google Scholar] [Pubmed]
- Allsop D, Mayes J. Amyloid β-peptide and Alzheimer's disease. Essays Biochem. 2014; 56: 99-110.
[Crossref] [Google Scholar] [Pubmed]
- Acosta C, Anderson HD, Anderson CM. Astrocyte dysfunction in Alzheimer disease. J Neurosci Res. 2017; 95(12): 2430-2447.
[Crossref] [Google Scholar] [Pubmed]
- Guo T, Noble W, Hanger DP. Roles of tau protein in health and disease. Acta Neuropathol. 2017; 133(5): 665-704.
[Crossref] [Google Scholar] [Pubmed]
- Mi K, Johnson GV. The role of tau phosphorylation in the pathogenesis of Alzheimer's disease. Curr Alzheimer Res. 2006; 3(5): 449-463.
[Crossref] [Google Scholar] [Pubmed]
- Chong FP, Ng KY, Koh RY, Chye SM. Tau proteins and tauopathies in Alzheimer's disease. Cell Mol Neurobiol. 2018; 38(5): 965-980.
[Crossref] [Google Scholar] [Pubmed]
- Bryant AG, Hu M, Carlyle BC, Arnold SE, Frosch MP, Das S, et al. Cerebrovascular senescence is associated with tau pathology in Alzheimer's disease. Front Neurol. 2020; 11: 575953.
[Crossref] [Google Scholar] [Pubmed]
- Cai Z, Xiao M. Oligodendrocytes and Alzheimer's disease. Int J Neurosci. 2016; 126(2): 97-104.
[Crossref] [Google Scholar] [Pubmed]
- Malik A, Brito D, Vaqar S, Chhabra L. Congestive Heart Failure. StatPearls. 2022.
[Google Scholar] [Pubmed]
- Savarese G, Lund LH. Global public health burden of heart failure. Card Fail Rev. 2017; 3(1): 7-11.
[Crossref] [Google Scholar] [Pubmed]
- Tanai E, Frantz S. Pathophysiology of heart failure. Compr Physiol. 2015; 6(1): 187-214.
[Crossref] [Google Scholar] [Pubmed]
- Shinmura K. Cardiac senescence, heart failure, and frailty: A triangle in elderly people. Keio J Med. 2016; 65(2): 25-32.
[Crossref] [Google Scholar] [Pubmed]
- Chen MS, Lee RT, Garbern JC. Senescence mechanisms and targets in the heart. Cardiovasc Res. 2022; 118(5): 1173-1187.
[Crossref] [Google Scholar] [Pubmed]
- Jackson G, Gibbs CR, Davies MK, Lip GY. ABC of heart failure. Pathophysiology. BMJ. 2000; 320(7228): 167-170.
[Crossref] [Google Scholar] [Pubmed]
- Francis GS, Cohn JN. Heart failure: Mechanisms of cardiac and vascular dysfunction and the rationale for pharmacologic intervention. FASEB J. 1990; 4(13): 3068-3075.
[Crossref] [Google Scholar] [Pubmed]
- Malaquin N, Tu V, Rodier F. Assessing functional roles of the senescence-Associated Secretory Phenotype (SASP). Methods Mol Biol. 2019; 1896: 45-55.
[Crossref] [Google Scholar] [Pubmed]
- Schafer MJ, Zhang X, Kumar A, Atkinson EJ, Zhu Y, Jachim S, et al. The senescence-associated secretome as an indicator of age and medical risk. JCI Insight. 2020; 5(12): e133668.
[Crossref] [Google Scholar] [Pubmed]
- Takahashi T, Shishido T, Kinoshita D, Watanabe K, Toshima T, Sugai T, et al. Cardiac nuclear high-mobility group Box 1 ameliorates pathological cardiac hypertrophy by inhibiting DNA damage response. JACC Basic Transl Sci. 2019; 4(2): 234-247.
[Crossref] [Google Scholar] [Pubmed]
- American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2013; 36(1): S67-74.
[Crossref] [Google Scholar] [Pubmed]
- Galicia-Garcia U, Benito-Vicente A, Jebari S, Larrea-Sebal A, Siddiqi H, Uribe KB, et al. Pathophysiology of Type 2 diabetes mellitus. Int J Mol Sci. 2020; 21(17): 6275.
[Crossref] [Google Scholar] [Pubmed]
- Wilcox G. Insulin and insulin resistance. Clin Biochem Rev. 2005; 26(2): 19-39.
[Google Scholar] [Pubmed]
- deFronzo RA, Gunnarsson R, Björkman O, Olsson M, Wahren J. Effects of insulin on peripheral and splanchnic glucose metabolism in noninsulin-dependent (type II) diabetes mellitus. J Clin Invest. 1985; 76(1): 149-155.
[Crossref] [Google Scholar] [Pubmed]
- Petersen KF, Shulman GI. Cellular mechanism of insulin resistance in skeletal muscle. J R Soc Med. 2002; 95(42): 8-13.
[Crossref] [Google Scholar] [Pubmed]
- Kim JY, van de Wall E, Laplante M, Azzara A, Trujillo ME, Hofmann SM, et al. Obesity-associated improvements in metabolic profile through expansion of adipose tissue. J Clin Invest. 2007; 117(9): 2621-2637.
[Crossref] [Google Scholar] [Pubmed]
- Graciano MF, Valle MM, Kowluru A, Curi R, Carpinelli AR. Regulation of insulin secretion and reactive oxygen species production by free fatty acids in pancreatic islets. Islets. 2011; 3(5): 213-223.
[Crossref] [Google Scholar] [Pubmed]
- Yasom S, Khumsri W, Boonsongserm P, Kitkumthorn N, Ruangvejvorachai P, Sooksamran A, et al. B1 siRNA increases de novo DNA methylation of B1 elements and promotes wound healing in diabetic rats. Front Cell Dev Biol. 2022; 9: 802024.
[Crossref] [Google Scholar] [Pubmed]
- Heijink IH, Pouwels SD, Leijendekker C, de Bruin HG, Zijlstra GJ, van der Vaart H, et al. Cigarette smoke-induced damage-associated molecular pattern release from necrotic neutrophils triggers proinflammatory mediator release. Am J Respir Cell Mol Biol. 2015; 52(5): 554-62.
[Crossref] [Google Scholar] [Pubmed]
- Johnson KE, Wulff BC, Oberyszyn TM, Wilgus TA. Ultraviolet light exposure stimulates HMGB1 release by keratinocytes. Arch Dermatol Res. 2013; 305(9): 805-815.
[Crossref] [Google Scholar] [Pubmed]
- He SJ, Cheng J, Feng X, Yu Y, Tian L, Huang Q. The dual role and therapeutic potential of high-mobility group box 1 in cancer. Oncotarget. 2017; 8(38): 64534-64550.
[Crossref] [Google Scholar] [Pubmed]
- Wang X, Chu G, Yang Z, Sun Y, Zhou H, Li M, et al. Ethanol directly induced HMGB1 release through NOX2/NLRP1 inflammasome in neuronal cells. Toxicology. 2015; 334: 104-110.
[Crossref] [Google Scholar] [Pubmed]
- Laniado-Laborín R. Smoking and Chronic Obstructive Pulmonary Disease (COPD). Parallel epidemics of the 21 century. Int J Environ Res Public Health. 2009; 6(1): 209-224.
[Crossref] [Google Scholar] [Pubmed]
- Orvoen-Frija E, Benoit M, Catto M, Chambouleyron M, Duguet A, Emeriau JP, et al. La Bronchopneumopathie Chronique Obstructive (BPCO) du sujet âgé en huit questions/réponses Chronic Obstructive Pulmonary Disease (COPD) in the elderly. Rev Mal Respir. 2010; 27(8): 855-873.
[Crossref] [Google Scholar] [Pubmed]
- Devine JF. Chronic obstructive pulmonary disease: An overview. Am Health Drug Benefits. 2008; 1(7): 34-42.
[Google Scholar] [Pubmed]
- O'Reilly S. Chronic obstructive pulmonary disease. Am J Lifestyle Med. 2016; 11(4): 296-302.
- Seong SY, Matzinger P. Hydrophobicity: An ancient damage-associated molecular pattern that initiates innate immune responses. Nat Rev Immunol. 2004; 4(6): 469-478.
[Crossref] [Google Scholar] [Pubmed]
- Ames BN, Shigenaga MK, Hagen TM. Oxidants, antioxidants, and the degenerative diseases of aging. Proc Natl Acad Sci USA. 1993; 90(17): 7915-7922.
[Crossref] [Google Scholar] [Pubmed]
- Scott TL, Rangaswamy S, Wicker CA, Izumi T. Repair of oxidative DNA damage and cancer: Recent progress in DNA base excision repair. Antioxid Redox Signal. 2014; 20(4): 708-726.
[Crossref] [Google Scholar] [Pubmed]
- Coppé JP, Desprez PY, Krtolica A, Campisi J. The senescence-associated secretory phenotype: The dark side of tumor suppression. Annu Rev Pathol. 2010; 5: 99-118.
[Crossref] [Google Scholar] [Pubmed]
- Foo MX, Ong PF, Dreesen O. Premature aging syndromes: From patients to mechanism. J Dermatol Sci. 2019; 96(2): 58-65.
[Crossref] [Google Scholar] [Pubmed]
- Alves-Fernandes DK, Jasiulionis MG. The role of SIRT1 on DNA damage response and epigenetic alterations in cancer. Int J Mol Sci. 2019; 20(13): 3153.
[Crossref] [Google Scholar] [Pubmed]
- Olivier M, Hollstein M, Hainaut P. TP53 mutations in human cancers: Origins, consequences, and clinical use. Cold Spring Harb Perspect Biol. 2010; 2(1): a001008.
[Crossref] [Google Scholar] [Pubmed]
- Wculek SK, Cueto FJ, Mujal AM, Melero I, Krummel MF, Sancho D. Dendritic cells in cancer immunology and immunotherapy. Nat Rev Immunol. 2020; 20(1): 7-24.
[Crossref] [Google Scholar] [Pubmed]
- Li CJ, Liao WT, Wu MY, Chu PY. New Insights into the role of autophagy in tumor immune microenvironment. Int J Mol Sci. 2017; 18(7): 1566.
[Crossref] [Google Scholar] [Pubmed]
- Youn JI, Nagaraj S, Collazo M, Gabrilovich DI. Subsets of myeloid-derived suppressor cells in tumor-bearing mice. J Immunol. 2008; 181(8): 5791-5802.
[Crossref] [Google Scholar] [Pubmed]
- Garcia-Gomez A, Rodríguez-Ubreva J, Ballestar E. Epigenetic interplay between immune, stromal and cancer cells in the tumor microenvironment. Clin Immunol. 2018; 196: 64-71.
[Crossref] [Google Scholar] [Pubmed]
- Bin-Alee F, Arayataweegool A, Buranapraditkun S, Mahattanasakul P, Tangjaturonrasme N, Mutirangura A, et al. Evaluation of lymphocyte apoptosis in patients with oral cancer. J Appl Oral Sci. 2020; 28: e20200124.
[Crossref] [Google Scholar] [Pubmed]
- Liu X, Mo W, Ye J, Li L, Zhang Y, Hsueh EC, et al. Regulatory T cells trigger effector T cell DNA damage and senescence caused by metabolic competition. Nat Commun. 2018; 9(1): 249.
[Crossref] [Google Scholar] [Pubmed]
- Pangrazzi L, Weinberger B. T cells, aging and senescence. Exp Gerontol. 2020; 134: 110887.
[Crossref] [Google Scholar] [Pubmed]
- Saule P, Trauet J, Dutriez V, Lekeux V, Dessaint JP, Labalette M. Accumulation of memory T cells from childhood to old age: Central and effector memory cells in CD4(+) versus effector memory and terminally differentiated memory cells in CD8(+) compartment. Mech Ageing Dev. 2006; 127(3): 274-281.
[Crossref] [Google Scholar] [Pubmed]
- Filaci G, Fenoglio D, Fravega M, Ansaldo G, Borgonovo G, Traverso P, et al. CD8+ CD28- T regulatory lymphocytes inhibiting T cell proliferative and cytotoxic functions infiltrate human cancers. J Immunol. 2007; 179(7): 4323-4334.
[Crossref] [Google Scholar] [Pubmed]
- Onyema OO, Decoster L, Njemini R, Forti LN, Bautmans I, De Waele M, et al. Chemotherapy-induced changes and immunosenescence of CD8+ T-cells in patients with breast cancer. Anticancer Res. 2015; 35(3): 1481-1489.
[Google Scholar] [Pubmed]
- Cheung-Ong K, Giaever G, Nislow C. DNA-damaging agents in cancer chemotherapy: Serendipity and chemical biology. Chem Biol. 2013; 20(5): 648-659.
[Crossref] [Google Scholar] [Pubmed]
- Huang RX, Zhou PK. DNA damage response signaling pathways and targets for radiotherapy sensitization in cancer. Signal Transduct Target Ther. 2020; 5(1): 60.
[Crossref] [Google Scholar] [Pubmed]
- Nguyen HQ, To NH, Zadigue P, Kerbrat S, de La Taille A, Le Gouvello S, et al. Ionizing radiation-induced cellular senescence promotes tissue fibrosis after radiotherapy. A review. Crit Rev Oncol Hematol. 2018; 129: 13-26.
[Crossref] [Google Scholar] [Pubmed]
- Wang L, He L, Bao G, He X, Fan S, Wang H. Ionizing Radiation Induces HMGB1 Cytoplasmic Translocation and Extracellular Release. Guo Ji Fang She Yi Xue He Yi Xue Za Zhi. 2016; 40(2): 91-99.
[Google Scholar] [Pubmed]
- Zhu X, Cong J, Lin Z, Sun J, Yang B, Li A. Inhibition of HMGB1 overcomes resistance to radiation and chemotherapy in nasopharyngeal carcinoma. Onco Targets Ther. 2020; 13: 4189-4199.
[Crossref] [Google Scholar] [Pubmed]
Author Info
Rathasapa Patarat1,2, Wilunplus Khumsri1,2, Sikrit Denariyakoon3 and Apiwat Mutirangura1*2Department of Biomedical Sciences, Graduate School, Chulalongkorn University, Bangkok, Thailand
3The Queen Sirikit Center for Breast Cancer, King Chulalongkorn Memorial Hospital, The Thai Red Cross Society, Bangkok, Thailand
Citation: Patarat R: Box A of HMGB1 Producing DNA Gaps: A Remedy for the DNA Protection and Rejuvenation Effects in Age- and DNA Damage-Associated Diseases
Received: 15-Feb-2023 Accepted: 10-Mar-2023 Published: 17-Mar-2023, DOI: 10.31858/0975-8453.14.3.164-176
Copyright: This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
ARTICLE TOOLS
- Dental Development between Assisted Reproductive Therapy (Art) and Natural Conceived Children: A Comparative Pilot Study Norzaiti Mohd Kenali, Naimah Hasanah Mohd Fathil, Norbasyirah Bohari, Ahmad Faisal Ismail, Roszaman Ramli SRP. 2020; 11(1): 01-06 » doi: 10.5530/srp.2020.1.01
- Psychometric properties of the World Health Organization Quality of life instrument, short form: Validity in the Vietnamese healthcare context Trung Quang Vo*, Bao Tran Thuy Tran, Ngan Thuy Nguyen, Tram ThiHuyen Nguyen, Thuy Phan Chung Tran SRP. 2020; 11(1): 14-22 » doi: 10.5530/srp.2019.1.3
- A Review of Pharmacoeconomics: the key to “Healthcare for All” Hasamnis AA, Patil SS, Shaik Imam, Narendiran K SRP. 2019; 10(1): s40-s42 » doi: 10.5530/srp.2019.1s.21
- Deuterium Depleted Water as an Adjuvant in Treatment of Cancer Anton Syroeshkin, Olga Levitskaya, Elena Uspenskaya, Tatiana Pleteneva, Daria Romaykina, Daria Ermakova SRP. 2019; 10(1): 112-117 » doi: 10.5530/srp.2019.1.19
- Dental Development between Assisted Reproductive Therapy (Art) and Natural Conceived Children: A Comparative Pilot Study Norzaiti Mohd Kenali, Naimah Hasanah Mohd Fathil, Norbasyirah Bohari, Ahmad Faisal Ismail, Roszaman Ramli SRP. 2020; 11(1): 01-06 » doi: 10.5530/srp.2020.1.01
- Manilkara zapota (L.) Royen Fruit Peel: A Phytochemical and Pharmacological Review Karle Pravin P, Dhawale Shashikant C SRP. 2019; 10(1): 11-14 » doi: 0.5530/srp.2019.1.2
- Pharmacognostic and Phytopharmacological Overview on Bombax ceiba Pankaj Haribhau Chaudhary, Mukund Ganeshrao Tawar SRP. 2019; 10(1): 20-25 » doi: 10.5530/srp.2019.1.4
- A Review of Pharmacoeconomics: the key to “Healthcare for All” Hasamnis AA, Patil SS, Shaik Imam, Narendiran K SRP. 2019; 10(1): s40-s42 » doi: 10.5530/srp.2019.1s.21
- A Prospective Review on Phyto-Pharmacological Aspects of Andrographis paniculata Govindraj Akilandeswari, Arumugam Vijaya Anand, Palanisamy Sampathkumar, Puthamohan Vinayaga Moorthi, Basavaraju Preethi SRP. 2019; 10(1): 15-19 » doi: 10.5530/srp.2019.1.3