Research Article - (2024) Volume 15, Issue 4
Abstract
Aim: The purpose of this study was to clarify the malignancy grades and clinic pathological characteristics of poorly differentiated adenocarcinoma of colorectal cancer.
Material and methods: A total of 520 patients diagnosed with differentiated adenocarcinoma in p-Stage I-III who underwent curative resection at our hospital between 2011 and 2018 were included in this study of these, 19 patients were diagnosed with Poorly Differentiated Adenocarcinoma (PDA). All PDA cases were sub-classified into solid- and non-solid PDAs based on tumor morphology confirmed by Hematoxylin and Eosin (H&E) stained specimens. In addition, immunostaining was additionally performed for solid PDA, medullary carcinoma, and endocrine cell cancer. The clinic pathological characteristics of each tumor were statistically analyzed.
Results: Compared to Highly Differentiated Adenocarcinomas/Moderately Differentiated Adenocarcinomas (HDA/MDA), PDA was more common in women (p=0.015), larger in size (p=0.033), more frequently located in the right colon (p=0.001), deeper in invasion (p<0.001), venous invasion (p<0.001) and lymph node metastasis (p<0.001). Immunostaining results showed that 5 patients (38.5%) of medullary carcinoma and 5 patients (38.5%) of neuroendocrine neoplasm were included in the solid PDA.
Conclusion: Our study suggests the importance of incorporating immune histological examination for the diagnosis of solid PDA in the future.
Keywords
Colorectal cancer, Clinicopathology, Adenocarcinoma
Introduction
Colorectal cancer is the 2nd leading cause of cancer-related death in Japan (National Cancer Registry). The histopathological subtypes of colorectal cancer have different clinic pathological malignancy grades, which significantly affect recurrence and survival (Funahashi K, et al., 1994; Compton CC, et al., 2000). In particular, Poorly Differentiated Adenocarcinoma (PDA) of colorectal cancer is relatively rare, accounting for about 1.7%-7.7% (Nakamura T, et al., 2002; Ueno H, et al., 2021), of all colorectal cancers and therefore has not been sufficiently investigated clinically. In the recent years, several studies have reported that the histological morphology of the tumor invasive front2nd correlates with metastasis, recurrence and oncological outcomes. Specifically, tumor budding can be defined as a single tumor cells or tumor clusters of upto 4 cells at the invasive front, while Poorly Differentiated Clusters (PDC) as clusters of 5 or more tumor cells lacking glandular structure (Basile D, et al., 2022; Bonetti RL, et al., 2016). These Tumor, Node, Metastasis (TMN) stage independent prognostic factors have been shown to be useful in determining the need for additional resection in early-stage colorectal cancer and in deciding the postoperative adjuvant chemotherapy in advanced colorectal cancer (Basile D, et al., 2022; Bonetti RL, et al., 2016; Nakamura T, et al., 2008; Konishi T, et al., 2018). On the other hand, there are cases that are morphologically difficult to differentiate by Hematoxylin and Eosin (H&E) staining alone, although they are diagnosed as the most predominant histological types according to the Japanese classification of colorectal carcinoma (Shida D, et al., 2019; Ishida H, et al., 2016) and the World Health Organization (WHO) tumor classification (WHO, 2019; WHO, 2000). Medullary carcinoma is a relatively new disease concept, described for the 1st time in the 3rd edition of the WHO tumor classification, published in 2000 (WHO, 2000) and has been included in the Japanese classification of colorectal carcinoma since 2013 (Shida D, et al., 2019). In addition, some cases diagnosed with PDA of colorectal cancer may include Neuro Endocrine Neoplasm (NEN) with poor prognosis (Miura S, et al., 2008; Kouchi Y, et al., 2003). It was reported that PDA of colorectal cancer had a poor prognosis compared to HDA and MDA (Nakamura T, et al., 2002; Ueno H, et al., 2021; Miura S, et al., 2008; Osada S, et al., 1997), but when solid type PDA (por1) and non-solid type PDA (por2) were compared. The prognosis of solid PDA was as favorable as that of MDA (Nakamura T, et al., 2002).
In this study, we sub-classified poorly differentiated adenocarcinoma of colorectal cancer into solid- and non-solid groups and compared them, but found no statistical significant difference in Overall Survival (OS) between the two groups (Figure 1). Thus, in order to differentiate solid PDA from medullary carcinoma and neuroendocrine neoplasm, which are difficult to differentiate by H&E staining alone, we re-examined the clinic pathological characteristics and prognostic factors of PDA using immunohistochemistry.
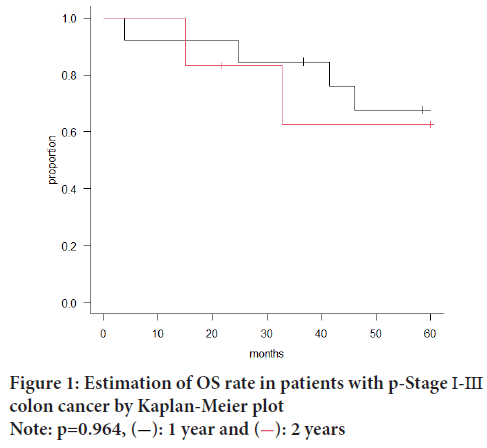
Figure 1: Estimation of OS rate in patients with p-Stage Ⅰ-Ⅲ
colon cancer by Kaplan-Meier plot
Note: p=0.964,  : 1 year and
: 1 year and  : 2 years
: 2 years
Materials and Methods
Study design and patients cohort
The sample size for this study was determined by the number of patients whose pathology could be re-reviewed at our institution. All the surgeries were performed by a team of physicians with more than 20 years of surgical experience. We examined the factors such as age, gender, family history, maximum tumor diameter, tumor location, depth of invasion, lymphatic invasion, venous invasion, lymph node metastasis, recurrence rate, recurrence pattern and OS. During the study period, we found 24 deaths due to other primary malignancies, 29 deaths due to benign diseases other than cancer deaths including death from old age and 3 deaths due to unknown causes. This study was approved by the Ethics Committee of Dokkyo Medical University Hospital (Approval No: R-52-6J). Further, written informed consent was also obtained from the patient for publication and any accompanying images. Inclusion criteria: Patients who were diagnosed with differentiated adenocarcinoma and underwent surgery between January, 2011 and December, 2018 and patients with pStage I-III colorectal cancer were included in this study. Exclusion criteria: Patients those that received preoperative chemotherapy or radiotherapy (n=10), pTis (n=27), transanal endoscopic microsurgery (n=1), non-curative resection (n=5) and synchronous disease stage IV (n=91) were excluded from the study.
Histologic evaluation
For all patients diagnosed with PDA during the study period, whole-mount sections of the tumor including the invasive front were microscopically examined using H&E staining. The pathological evaluation was performed by two expert pathologists with expertise in pathological diagnosis. Immunostaining was performed to -differentiate medullary carcinoma from endocrine cell cancer among tumors lacking glandular ductal structure. MutL protein Homolog 1 (MLH1), Deoxyribonucleic Acid (DNA) Mismatch repair protein (MSH2), MSH6 and Postmeiotic Segregation increased 2 (PMS2) were used to test for mismatch repair protein deficiency, while chromogranin A, synaptophysin and Insulinoma-associated 1 (INSM1) were used to detect endocrine cells. Similarly, Mucin-5AC (MUC-5AC) was used to detect the expression of gastric-type mucin. All the immunostaining results were judged by one of the experts.
Staging and surveillance protocol
Preoperative diagnosis was performed using contrast-enhanced computed tomography scans of the chest, abdomen and pelvis. Further, colonoscopy, magnetic resonance imaging and positron emission tomography techniques were also followed. According to the colorectal cancer treatment guidelines (Ishiguro M, et al., 2013), postoperative adjuvant chemotherapy was administered to patients diagnosed with high-risk p-Stage II/III colorectal cancer based on the histological evaluation of surgical specimens. Postoperative surveillance was conducted for all colorectal cancer patients.
Statistical analysis
The primary endpoint of the study was OS. Patients were followed up for 5 years after surgery or until death and OS was estimated using Kaplan-Meier plot. Patients who were alive without recurrence at the final follow-up were censored. Each pathological parameter was evaluated for its association with OS using the log-rank test. Hazard Ratio (HR) and 95% Confidence Interval (CI) were calculated using the Cox proportional hazards model. Continuous variables were analyzed using either the Mann-Whitney U test or the Kruskal-Wallis test. Categorical variables were analyzed using either Fisher’s exact test or the Chi-square (χ2) test. Easy R (EZR) version 1.61 was used for all statistical analysis and all tests were two-tailed with p<0.05 indicating a statistically significant difference.
Result
The median observation period was 64 (2-147) months while the median age was 70 (25-93) years with 326 men (62.7%) and 194 women (37.3%). Among all the patients 342 had colon cancer (65.8%) and 178 had rectal cancer (34.2%). Of these, 312 were MDA (60.0%), 189 were HDA (36.3%) and 19 were PDA (3.7%). There was 1 patient (0.2%) of familial adenomatous polyposis and 2 patients (0.4%) with ulcerative colitis. The pathological stage distribution for each adenocarcinoma included, 189 HDAs who had 85 patients with p-Stage I (16.3%), 62 patients with p-Stage II (11.9%), 42 patients having p-Stage III (8.1%); 312 MDAs had 63 patients with p-Stage I (12.1%), 132 patients of p-Stage II (25.4%) and 117 patients of p-Stage III (22.5%). Similarly, 19 patients had no case of p-Stage I (0%), 6 patients with p-Stage II (1.2%) and 13 patients with p-Stage III (2.5%). The rate of PDA found in advanced cancer was significantly higher (p<0.001). Postoperative adjuvant chemotherapy was administered to p-Stage II (76 (38.5%)) and p-Stage III patients (116 (67.4%)). When compared to HDA and MDA, PDA showed higher incidence in women (p=0.015) with larger tumor size (p=0.033), higher incidence in the right colon (p=0.001), deeper invasion (p<0.001), higher positive lymphatic vessel invasion (p<0.001), higher lymph node metastases (p<0.001) and higher proportion of patients receiving postoperative adjuvant chemotherapy (p<0.001) (Table 1).
| Pathological characteristics | tub1 (n=189) | tub2 (n=312) | por (n=19) | p |
|---|---|---|---|---|
| Gender (male:female) | 123:66 | 197:115 | 6:13 | 0.015 |
| Age (mean ± SD*) | 68.7 ± 10.9 | 69.9 ± 10.6 | 72.4 ± 15.5 | 0.119 |
| Tumor size (mean ± SD*) | 42.6 ± 26.2 | 45.2 ± 20.7 | 51.9 ± 23.2 | 0.033 |
| Location of cancer | ||||
| Cecum | 12 (6.3%) | 17 (5.4%) | 4 (21.1%) | 0.413 |
| Ascending colon | 35 (18.5%) | 49 (15.7%) | 7 (36.8%) | |
| Transverse colon | 16 (8.5%) | 37 (11.9%) | 3 (15.8%) | |
| Descending colon | 12 (6.3%) | 14 (4.5%) | 0 | |
| Sigmoid colon | 54 (28.6%) | 80 (25.6%) | 2 (10.5%) | |
| Rectum | 60 (31.7%) | 115 (36.9%) | 3 (15.8%) | |
| Right side colon | 63 (33.3%) | 103 (33.0%) | 14 (73.7%) | 0.001 |
| Left side colon | 126 (66.6%) | 209 (67.0%) | 5 (26.3%) | |
| Depth of invasion | ||||
| Shallower than MP** | 98 (51.9%) | 76 (24.4%) | 3 (15.8%) | <0.001 |
| Deeper than MP** | 91 (48.1%) | 236 (75.6%) | 16 (84.2%) | |
| Lymphatic invasion | ||||
| Negative | 129 (68.3%) | 125 (40.0%) | 4 (21.1%) | <0.001 |
| Positive | 60 (31.7%) | 187 (60.0%) | 15 (78.9%) | |
| Venous invasion | ||||
| Negative | 99 (52.4%) | 95 (30.4%) | 5 (26.3%) | <0.001 |
| Positive | 90 (47.6%) | 217 (69.6%) | 14 (73.7%) | |
| Lymph node metastasis | ||||
| Negative | 146 (77.2%) | 195 (62.5%) | 6 (31.6%) | <0.001 |
| Positive | 43 (22.8%) | 117 (37.5%) | 13 (68.4%) | |
| Adjuvant chemotherapy | ||||
| Negative | 137 (72.5%) | 174 (55.8%) | 9 (47.4%) | <0.001 |
| Positive | 52 (27.5%) | 138 (44.2%) | 10 (52.6%) | |
Note: (*): Standard Deviation (SD) and (**): Muscularis Propria (MP)
Table 1: Base-line characteristics of the patients
Microscopic examination of H&E stained specimens from all 19 patients diagnosed with PDA revealed 13 patients of solid PDA and 6 patients of non-solid PDA. We found no statistically significant differences in clinical pathological characteristics between the solid- and non-solid PDA groups (Table 2) or in their OS (Figure 1). In all the patients with HDA, MDA, and PDA in p-Stage I-III, patients having PDA had the poorest OS (p=0.013) (Figure 2).
| Pathological characteristics | Por1 (n=13) | Por2 (n=6) | p |
|---|---|---|---|
| Gender (male:female) | 5:8 | 1:5 | 0.675 |
| Age (mean ± SD*) | 75.9 ± 9.9 | 64.8 ± 23 | 0.403 |
| Tumor size (mean ± SD*) | 50.7 ± 25.4 | 54.5 ± 19.5 | 0.539 |
| Location of cancer | |||
| Cecum | 3 (23.1%) | 1 (16.7%) | 0.315 |
| Ascending colon | 3 (23.1%) | 4 (66.7%) | |
| Transverse colon | 3 (23.1%) | 0 | |
| Descending colon | 0 | 0 | |
| Sigmoid colon | 1 (7.7%) | 1 (16.7%) | |
| Rectum | 3 (23.1%) | 0 | |
| Right side colon | 9 (69.2%) | 5 (83.3%) | 1 |
| Left side colon | 4 (30.8%) | 1 (16.7%) | |
| Depth of invasion | |||
| Shallower than MP** | 3 (23.1%) | 0 | 0.517 |
| Deeper than MP** | 10 (76.9%) | 6 (100%) | |
| Lymphatic invasion | |||
| Negative | 4 (30.8%) | 1 (16.7%) | 1 |
| Positive | 9 (69.2%) | 5 (83.3%) | |
| Venous invasion | |||
| Negative | 4 (30.8%) | 1 (16.7%) | 1 |
| Positive | 9 (69.2%) | 5 (83.3%) | |
| Lymph node metastasis | |||
| Negative | 4 (30.8%) | 2 (33.3%) | 1 |
| Positive | 9 (69.2%) | 4 (66.7%) | |
| Adjuvant chemotherapy | |||
| Negative | 7 (53.8%) | 2 (33.3%) | 0.628 |
| Positive | 6 (46.2%) | 4 (66.7%) | |
Note: (*): Standard Deviation (SD) and (**): Muscularis Propria (MP)
Table 2: Comparison of clinical pathological characteristics between solid- and non-solid PDA groups
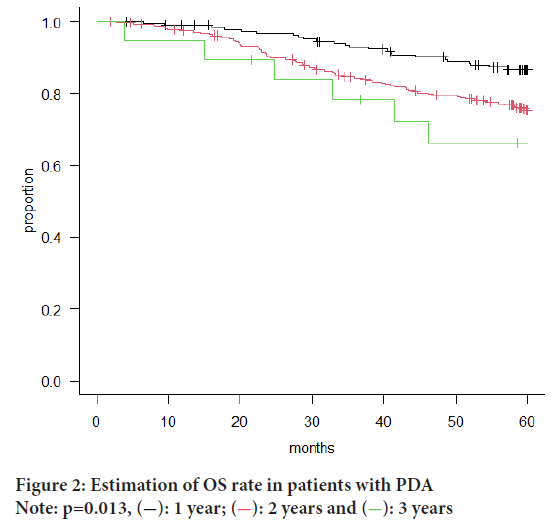
Figure 2: Estimation of OS rate in patients with PDA
Note: p=0.013,  : 1 year;
: 1 year;  : 2 years and
: 2 years and  : 3 years
: 3 years
Cox’s regression analysis was performed to examine HDA, MDA and PDA in p-Stage I-III and based on the analysis, depth of tumor depth, lymphatic vessel invasion and postoperative adjuvant chemotherapy were identified as independent prognostic factors. We found the invasion depth (HR: 2.82, 95% CI: 1.72-4.65 and p<0.001), lymph node metastasis (HR: 1.72, 95% CI: 1.17-2.52 and p=0.005) and postoperative adjuvant chemotherapy (HR: 0.58, 95% CI: 0.39-0.85 and p=0.005).
Among the 13 patients diagnosed as solid PDA, immunostaining results showed that 5 patients (38.5%) were medullary carcinoma and 5 patients (38.5%) were endocrine tumor. All the endocrine tumors were Mixed Neuroendocrine-non-neuroendocrine Neoplasm (MiNEN) and 2 out of 5 endocrine tumors were identified as Microsatellite Instability (MSI)-high due to deficiency of MLH1 and PMS2. Solid PDA showed poor expression of gastric-type mucin and no weakening in expression of mismatch repair gene products was observed. The clinical pathological features of medullary carcinoma were that it tended to be more common in older women, all were located in the right colon and the maximum tumor size was generally larger. Furthermore, compared to solid PDA and MiNEN, medullary carcinoma showed significantly lower lymph node metastasis (p=0.028) in lower proportion of patients who received postoperative adjuvant chemotherapy (p=0.039). It was clarified that medullary carcinoma and MiNEN were more common in the right colon, while solid PDA was more common in the left colon (p=0.003).
Discussion
In this study, we aimed to compare PDA with HDA and MDA of colorectal cancer and to clarify the malignancy grades and clinical-pathological characteristics. All cases of PDA were detected at an advanced stage, i.e., p-Stage II or higher which had deep invasion with significantly higher rates of vascular invasion and lymph node metastasis. Previous studies have shown that the rate of lymph node metastasis in PDA (44%-79%) is higher than that in other differentiated adenocarcinomas (28%-51%) (Nakamura T, et al., 2002; Ueno H, et al., 2021; Miura S, et al., 2008; Sato H, et al., 1998; Ueno H, et al., 2014) and the same is true in the present study. These results may indicate the high degree of malignancy in PDA. However, it has been pointed out that there are tumors with relatively good prognosis even among PDA with poor prognosis and various sub-classifications have been proposed (Nakamura T, et al., 2002; Miura S, et al., 2008; Osada S, et al., 1997). Solid PDA is considered to have a better prognosis than non-solid PDA (Nakamura T, et al., 2002; Goi T, et al., 2004; Masuda H, et al., 2005). In this study, there was no statistically significant difference in the prognosis between solid- and non-solid PDAs. Upon re-examination of H&E stained specimens of the solid PDA cases, it was found that other carcinomas such as medullary carcinoma and endocrine cell carcinoma, which are morphologically similar to solid PDA, might be included in the solid PDA cases.
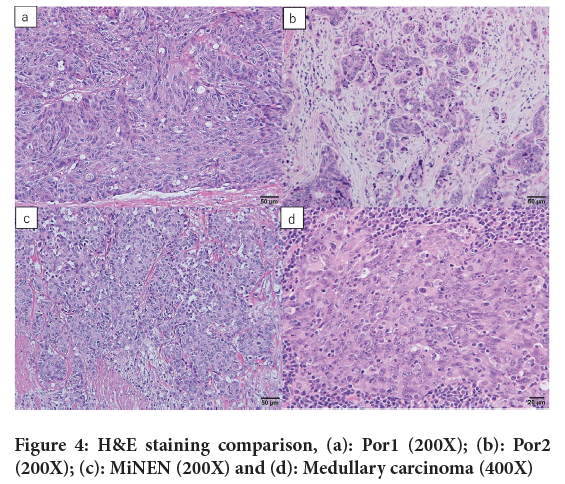
PDA is an adenocarcinoma with poor lumen formation or being positive for intracellular mucins despite negative ductal formation (Arai T, et al., 2013; Shida D, et al., 2019) (Figure 3). Medullary carcinoma was considered as a subtype of PDA and was treated as the same tumor as PDA before 2013 in Japan. Pathologically, medullary carcinoma resembles solid Pancreatic Ductal Adenocarcinoma (PDAC) in its lack of a glandular ductal structure and its trabecular and medullary growth patterns. However, it is distinguished by the frequent presence of inflammatory cell infiltration, predominantly by neutrophils and lymphocytes within and at the periphery of the tumor. Immunostaining findings characteristically show deficiency in MLH1 and Caudal-related homeobox transcription factor 2 (CDX-2) (Masuda H, et al., 2005). Neuroendocrine neoplasm, particularly large cell type endocrine cell cancer, is morphologically similar to solid PDA due to its solid, pavement stone-like growth pattern of cancer cells and lack of lumen formation and therefore, immunohistological staining is required for differentiation (Ueno H, et al., 2021) (Figure 4). In our study, immunostaining results revealed that 10 out of 13 (78%) patients of solid PDA included medullary carcinoma and endocrine tumor, suggesting that the true incidence of solid PDA may be even lower than previously reported (Nakamura T, et al., 2002; Ueno H, et al., 2021).
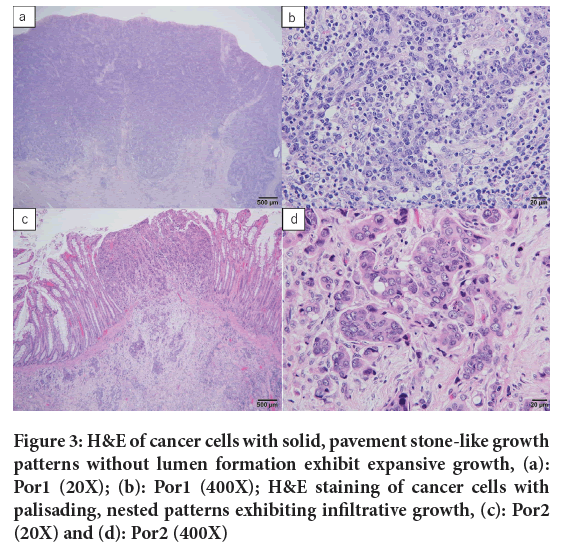
Figure 3: H&E of cancer cells with solid, pavement stone-like growth patterns without lumen formation exhibit expansive growth, (a): Por1 (20X); (b): Por1 (400X); H&E staining of cancer cells with palisading, nested patterns exhibiting infiltrative growth, (c): Por2 (20X) and (d): Por2 (400X)
Figure 4: H&E staining comparison, (a): Por1 (200X); (b): Por2 (200X); (c): MiNEN (200X) and (d): Medullary carcinoma (400X)
Knox RD, et al., 2015, reported that medullary carcinoma accounted for 91 patients (2.8%) out of 3295 surgical resection patients between 1998 and 2012 and a different meta-analysis also reported a prevalence of 2.7% (Pyo JS, et al., 2016). It is estimated that medullary carcinoma accounts for about 2%-3% of all colorectal cancers. Since about 2/3rd of those diagnosed with PDA in the elderly >65 years of age are considered to have medullary carcinoma where the possibility of medullary carcinoma should be the 1st consideration for PDA occurring in the right colon in women >80 years of age (Arai T, et al., 2004). Medullary carcinoma in our study was more common in the elderly women in the right colon, although the prevalence was less than previous reports, i.e., 5 patients out of 520 (0.96%). It has been reported that many cases of PDA show MSI (Okuno M, et al., 1989; Kawaba ta Y, et al., 1999), which may be attributed to the inclusion of medullary carcinoma in these studies. Furthermore, among advanced or recurrent colorectal cancers, MSI-high tumors are known to be resistant to 5-Fluorouracil (5-FU) based chemotherapy (Ribic CM, et al., 2003; Kim GP, et al., 2007; Barratt PL, et al., 2002) and no consensus has been reached on the effectiveness of chemotherapy based on oxaliplatin and irinotecan. As the efficacy of anti-Programmed Cell Death Ligand 1 (PD-L1) antibody drugs for MSI-high tumors has been reported for which appropriate diagnosis and postoperative treatment strategies are necessary (Le DT, et al., 2015).
While neuroendocrine neoplasms displaying histologically neuroendocrine patterns are classified as highly differentiated in the WHO classification (WHO, 2019), they were further categorized into Neuroendocrine Tumors (NETs) Grade 1 (G1)-G3 in this study according to the mitotic figures based on proliferative capacity and Kiel-67 (Ki-67) index. Poorly differentiated tumors with a Ki-67 index >20% were categorized as Neuroendocrine Carcinomas (NECs) and tumors with ≥ 30% of both non-neuroendocrine and neuroendocrine components were categorized as MiNEN. Notably, all the patients in our study were classified as MiNEN. MiNEN is considered to be synonymous with the disease previously referred to as Mixed Adeno-Neuroendocrine Carcinoma (MANEC) (WHO, 2019) and most MiNENs are deemed to be high-grade tumors equivalent to NET G3. However, some MiNEN cases have a mixture of NET G2 and adenocarcinoma that have relatively good prognoses (Kasahara K, et al., 2020). Ki-67 index testing was not performed in this study, but patients with relatively good prognosis were included; further investigation is under consideration. Since no treatment strategy has been established for primary colorectal NEC, chemotherapy with a combination of platinum-based drugs and etoposide or irinotecan, similar to that used for small cell lung cancer is recommended (Japan neuroendocrine tumor society, 2019). As the treatment strategies for PDA and endocrine tumors are quite different, differential diagnosis is necessary as in the case with medullary carcinoma mentioned earlier.
Conclusion
The above findings indicate that PDA is a highly malignant tumor, often diagnosed at an advanced stage which is associated with poor prognosis. There are few reports on the pathological features of solid PDA, so contrary to previous reports the tumor size may be smaller and may occur predominantly in the left colon. Furthermore, solid PDA is morphologically similar to medullary carcinoma and neuroendocrine neoplasm in H&E stained specimens alone, making differentiation difficult. Therefore, immunohistological examination should be performed in all cases whenever possible. Since PDA is very rare, further examination and accumulation of cases will be necessary in the future.
References
- Cancer statistics. Cancer information service, National Cancer Center, Japan (National Cancer Registry, Ministry of Health, Labour and Welfare).
- Funahashi K, Miki T, Ohgai T, Suzuki Y, Koike J, Kubota S, et al. A study on clinicopathological findings and prognosis of colorectal cancers in terms of the histological type. J Jpn Pract Surg Soc. 1994; 55(8): 1960-1966.
- Compton CC, Fielding LP, Burgart LJ, Conley B, Cooper HS, Hamilton SR, et al. Prognostic factors in colorectal cancer: College of American pathologists consensus statement 1999. Arch Pathol Lab Med. 2000; 124(7): 979-994.
[Crossref] [Google Scholar] [Pubmed]
- Nakamura T, Otani Y, Mitomi H, Kanazawa H, Nemoto K, Kokuba, et al. Clinicopathologic characteristics of poorly differentiated adenocarcinoma of the colorectum: Prognostic significance of solid and non-solid types of poorly differentiated adenocarcinoma. Dis Colon Rectum. 2002; 55(1): 16-21.
- Ueno H, Kajiwara Y, Ajioka Y, Sugai T, Sekine S, Ishiguro M, et al. Histopathological atlas of desmoplastic reaction characterization in colorectal cancer. Jpn J Clin Oncol. 2021; 51(6): 1004-1012.
[Crossref] [Google Scholar] [Pubmed]
- Basile D, Broudin C, Emile JF, Falcoz A, Pages F, Mineur L, et al. Tumor budding is an independent prognostic factor in stage III colon cancer patients: A post-hoc analysis of the IDEA-France phase III trial (PRODIGE-GERCOR). Ann Oncol. 2022; 33(6): 628-637.
[Crossref] [Google Scholar] [Pubmed]
- Bonetti RL, Barresi V, Bettelli S, Domati F, Palmiere C. Poorly Differentiated Clusters (PDC) in colorectal cancer: What is and ought to be known. Diagn Pathol. 2016; 11: 1-4.
[Crossref] [Google Scholar] [Pubmed]
- Nakamura T, Mitomi H, Kanazawa H, Ohkura Y, Watanabe M. Tumor budding as an index to identify high-risk patients with stage II colon cancer. Dis Colon Rectum. 2008; 51(5): 568-572.
[Crossref] [Google Scholar] [Pubmed]
- Konishi T, Shimada Y, Lee LH, Cavalcanti MS, Hsu M, Smith JJ, et al. Poorly differentiated clusters predict colon cancer recurrence: An in-depth comparative analysis of invasive-front prognostic markers. Am J Surg Pathol. 2018; 42(6): 705-714.
[Crossref] [Google Scholar] [Pubmed]
- Shida D, Kanemitsu Y, Hamaguchi T, Shimada Y. Introducing the eighth edition of the tumor-node-metastasis classification as relevant to colorectal cancer, anal cancer and appendiceal cancer: A comparison study with the seventh edition of the tumor-node-metastasis and the Japanese classification of colorectal, appendiceal, and anal carcinoma. Jpn J Clin Oncol. 2019; 49(4): 321-328.
[Crossref] [Google Scholar] [Pubmed]
- Ishida H, Yamaguchi T, Tanakaya K, Akagi K, Inoue Y, Kumamoto K. Japanese society for cancer of the colon and rectum. Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines. 2016.
- WHO Classification of Tumours Editorial Board. WHO classification of tumours. Digestive system tumours. 2019.
- Digestive system tumours. WHO classification of tumours, 3th Edition. 2000.
- Miura S, Yoshidome H, Shida T, Kimura F, Shimizu H, Otsuka M, et al. Clinical implications of unusual NeuroD and mASH1 expression in a patient with primary large-cell neuroendocrine carcinoma of the duodenum: Report of a case. Surg Today. 2008; 38: 857-861.
[Crossref] [Google Scholar] [Pubmed]
- Kouchi Y, Jimbou M, Shigeta M, Inokuchi T, Fujita Y, Miyahara M, et al. Neuroendocrine carcinoma of the colon: Report of 3 cases. Jpn J Gastroenterol Surg. 2003; 36: 503-508.
- Osada S, Tanemura H, Ohshita H. Clinicopathological study of poorly differentiated and signet-ring cell carcinoma of the colon and rectum. J Jpn Pract Surg Soc. 1997; 58(1): 22-27.
- Ishiguro M, Watanabe T, Kotake K, Sugihara K. Japanese society for cancer of the colon and rectum guidelines 2010 for the treatment of colorectal cancer: Comparison with Western guidelines. Colorectal Cancer. 2013; 2(2): 179-190.
- Sato H, Maruta M, Maeda K, Utsumi T, Toyama K, Okumura Y, et al. Clinicopathological study of poorly differentiated adenocarcinomas in the colon and rectum. J Jpn Surg Assoc. 1998; 59(5): 1214-1221.
- Ueno H, Hase K, Hashiguchi Y, Shimazaki H, Tanaka M, Miyake O, et al. Site-specific tumor grading system in colorectal cancer: Multicenter pathologic review of the value of quantifying poorly differentiated clusters. Am J Surg Pathol. 2014; 38(2): 197-204.
[Crossref] [Google Scholar] [Pubmed]
- Goi T, Hirono Y, Katayama K, Yamaguchi A. Microsatellite instability and survival rate in the solid or nonsolid types of poorly differentiated colorectal adenocarcinoma. Int Surg. 2004; 89(2): 100-106.
[Google Scholar] [Pubmed]
- Masuda H, Abe Y, Takayama T. Microsatellite instability in poorly differentiated colorectal adenocarcinoma, particularly in relation to two subtypes. Hepatogastroenterology. 2005; 52(61): 82-85.
[Google Scholar] [Pubmed]
- Arai T, Sakurai U, Sawabe M, Honma N, Aida J, Ushio Y, et al. Frequent microsatellite instability in papillary and solid-type, poorly differentiated adenocarcinomas of the stomach. Gastric Cancer. 2013; 16: 505-512.
[Crossref] [Google Scholar] [Pubmed]
- Knox RD, Luey N, Sioson L, Kedziora A, Clarkson A, Watson N, et al. Medullary colorectal carcinoma revisited: A clinical and pathological study of 102 cases. Ann Surg Oncol. 2015; 22: 2988-2996.
[Crossref] [Google Scholar] [Pubmed]
- Pyo JS, Sohn JH, Kang G. Medullary carcinoma in the colorectum: A systematic review and meta-analysis. Hum Pathol. 2016; 53: 91-96.
[Crossref] [Google Scholar] [Pubmed]
- Arai T, Esaki Y, Sawabe M, Honma N, Nakamura KI, Takubo K. Hypermethylation of the hMLH1 promoter with absent hMLH1 expression in medullary-type poorly differentiated colorectal adenocarcinoma in the elderly. Modern Pathol. 2004; 17(2): 172-179.
[Crossref] [Google Scholar] [Pubmed]
- Okuno M, Ikehara T, Nagayama M, Kato Y, Ohira M, Nishimori T, et al. Clinicopathologic features of poorly differentiated adenocarcinoma of the colon. J Japanese Pract Surg Soc. 1989; 50(7): 1307-1312.
- Kawabata Y, Tomita N, Monden T, Ohue M, Ohnishi T, Sasaki M, et al. Molecular characteristics of poorly differentiated adenocarcinoma and signet‐ring cell carcinoma of colorectum. Int J Cancer. 1999; 84(1): 33-88.
[Crossref] [Google Scholar] [Pubmed]
- Ribic CM, Sargent DJ, Moore MJ, Thibodeau SN, French AJ, Goldberg RM, et al. Tumor microsatellite-instability status as a predictor of benefit from fluorouracil-based adjuvant chemotherapy for colon cancer. N Engl J Med. 2003; 349(3): 247-257.
[Crossref] [Google Scholar] [Pubmed]
- Kim GP, Colangelo LH, Wieand HS, Paik S, Kirsch IR, Wolmark N, et al. Prognostic and predictive roles of high-degree microsatellite instability in colon cancer: A national cancer Institute-national surgical adjuvant breast and bowel project collaborative study. J Clin Oncol. 2007; 25(7): 767-772.
[Crossref] [Google Scholar] [Pubmed]
- Barratt PL, Seymour MT, Stenning SP, Georgiades I, Walker C, Birbeck K, et al. DNA markers predicting benefit from adjuvant fluorouracil in patients with colon cancer: A molecular study. Lancet. 2002; 360(9343): 1381-1391.
[Crossref] [Google Scholar] [Pubmed]
- Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015; 372(26): 2509-2520.
[Crossref] [Google Scholar] [Pubmed]
- Kasahara K, Katsumata K, Enomoto M, Wada T, Nagakawa Y, Tsuchida A, et al. A case of a rectal mixed neuroendocrine-non-neuroendocrine neoplasm coexisting with a mixed adenocarcinoma and G2 neuroendocrine neoplasm. J Jpn Surg Assoc. 2020; 81: 931-937
[Crossref]
- Japan neuroendocrine tumor society. Guidelines for pancreatic and gastroenteric neuroendocrine tumors. 2019.
Author Info
Takahiro Kono*Received: 21-Mar-2024 Accepted: 05-Apr-2024 Published: 12-Apr-2024, DOI: 10.31858/0975-8453.15.4.140-145
Copyright: This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
ARTICLE TOOLS
- Dental Development between Assisted Reproductive Therapy (Art) and Natural Conceived Children: A Comparative Pilot Study Norzaiti Mohd Kenali, Naimah Hasanah Mohd Fathil, Norbasyirah Bohari, Ahmad Faisal Ismail, Roszaman Ramli SRP. 2020; 11(1): 01-06 » doi: 10.5530/srp.2020.1.01
- Psychometric properties of the World Health Organization Quality of life instrument, short form: Validity in the Vietnamese healthcare context Trung Quang Vo*, Bao Tran Thuy Tran, Ngan Thuy Nguyen, Tram ThiHuyen Nguyen, Thuy Phan Chung Tran SRP. 2020; 11(1): 14-22 » doi: 10.5530/srp.2019.1.3
- A Review of Pharmacoeconomics: the key to “Healthcare for All” Hasamnis AA, Patil SS, Shaik Imam, Narendiran K SRP. 2019; 10(1): s40-s42 » doi: 10.5530/srp.2019.1s.21
- Deuterium Depleted Water as an Adjuvant in Treatment of Cancer Anton Syroeshkin, Olga Levitskaya, Elena Uspenskaya, Tatiana Pleteneva, Daria Romaykina, Daria Ermakova SRP. 2019; 10(1): 112-117 » doi: 10.5530/srp.2019.1.19
- Dental Development between Assisted Reproductive Therapy (Art) and Natural Conceived Children: A Comparative Pilot Study Norzaiti Mohd Kenali, Naimah Hasanah Mohd Fathil, Norbasyirah Bohari, Ahmad Faisal Ismail, Roszaman Ramli SRP. 2020; 11(1): 01-06 » doi: 10.5530/srp.2020.1.01
- Manilkara zapota (L.) Royen Fruit Peel: A Phytochemical and Pharmacological Review Karle Pravin P, Dhawale Shashikant C SRP. 2019; 10(1): 11-14 » doi: 0.5530/srp.2019.1.2
- Pharmacognostic and Phytopharmacological Overview on Bombax ceiba Pankaj Haribhau Chaudhary, Mukund Ganeshrao Tawar SRP. 2019; 10(1): 20-25 » doi: 10.5530/srp.2019.1.4
- A Review of Pharmacoeconomics: the key to “Healthcare for All” Hasamnis AA, Patil SS, Shaik Imam, Narendiran K SRP. 2019; 10(1): s40-s42 » doi: 10.5530/srp.2019.1s.21
- A Prospective Review on Phyto-Pharmacological Aspects of Andrographis paniculata Govindraj Akilandeswari, Arumugam Vijaya Anand, Palanisamy Sampathkumar, Puthamohan Vinayaga Moorthi, Basavaraju Preethi SRP. 2019; 10(1): 15-19 » doi: 10.5530/srp.2019.1.3