Research Article - (2022) Volume 13, Issue 6
Abstract
Diabetes is a metabolic condition that affects how the body utilizes digested food for growth and energy. The majority of the food we consume is broken down into glucose, which is the form of sugar in our blood. Glucose is the body’s primary fuel source. The solubility of glibenclamide (glibenclamide), metformin, and sitagliptin was evaluated in triplicate in different pH using a water bath shaker at 37°C using the shake- flask technique. The quantity of medicine accessible for absorption is determined by the drug release. Each drug’s physiochemical characteristics have a substantial impact on release along the G.I.T. For each medication, calibration curve and solubility measurements were performed. In the duodenum and the small intestine, glibenclamide was released more efficiently and fast than metformin and sitagliptin, which had higher pKa values than glibenclamide, i.e., the metformin and sitagliptin were released more quickly and efficiently in pH 1.2 and pH 5.8. Glibenclamide is absorbed from the stomach, if not completely.
Keywords
Diabetes, Solubility, In-vitro release, Antidiabetic drugs, Dissolution
Introduction
Diabetes is something that almost everyone knows about. Diabetes is a severe, lifelong ailment that affects an estimated 23.6 million individuals in the United States or 7.8 percent of the population. 17.9 million have been diagnosed, whereas 5.7 million are yet to be diagnosed. Diabetes was diagnosed in around 1.6 million persons aged 20 and above in 2007 (American diabetes association, 2013). Diabetes is a metabolic condition that affects how the body utilizes digested food for growth and energy. The majority of the food we consume is broken down into glucose, which is the form of sugar in our blood. Glucose is the body’s primary fuel source. Glucose enters the circulation after digestion and is needed by cells for growth and energy. Insulin is required for glucose to enter cells. Insulin is a hormone produced by the pancreas; a large gland located beneath the stomach. When humans eat, the pancreas creates the appropriate quantity of insulin to transport glucose from the blood into the cells. However, in persons with diabetes, the pancreas either generates little or no insulin, or the cells may not react properly to the insulin that is generated. Glucose accumulates in the blood, overflows into the urine, and exits the body via the urine. Even if the blood contains enormous levels of glucose, the body loses its primary source of fuel (Ramachandran A, et al., 2010). Type 1 diabetes is caused by a failure of the pancreas to create enough insulin, and Type 2 diabetes is caused by an inability of the body to effectively utilize insulin to control blood sugar levels. Many organs, including the nerves, kidneys, eyes, and blood vessels, can be damaged by uncontrolled diabetes, which results in high blood sugar, or hyperglycemia. Approximately 1.6 million people worldwide died as a direct result of diabetes in 2016, according to the World Health Organization (WHO). Between 2000 and 2016, there was a 5% increase in diabetes-related premature deaths (Charoo NA, et al., 2022).Type 1 diabetes is an auto-immune condition. When the immune system, the body’s first line of defense against infection, turns on itself, an autoimmune illness result. In diabetes, the pancreas’ beta cells, which produce insulin, are targeted by the immune system and destroyed. Consequently, the pancreas is rendered inoperable. It is impossible for diabetics with type 1 diabetes to live without daily insulin injections (Daneman D, 2006). The exact cause of the immune system’s attack on beta cells is unknown at this time, but scientists believe it is caused by autoimmune, genetic, and/or environmental factors, including viruses; about 5% to 10% of people with diabetes in the United States have type 1 diabetes. However, it is most common among children and young adults. Diabetes is usually diagnosed within a few months, but beta cell destruction can begin years earlier. It is possible that patients will experience symptoms such as excessive thirst and urination as well as constant hunger, weight loss, dizziness, and exhaustion. Diabetic ketoacidosis, also known as diabetic coma, can occur if a person with type 1 diabetes is not diagnosed and given insulin (DiMeglio LA, et al., 2018). In terms of diabetes, type 2 is the most frequent form. Diabetics with type 2 account for the vast majority (90%-95%). The development of gestational diabetes mellitus is influenced by factors such as obesity, family history, previous gestational diabetes, physical inactivity, and ethnicity. Type 2 diabetes affects an estimated 80 percent of the population because of their weight (DeFronzo RA, et al., 2015). There are several unknown reasons why the pancreas is unable to produce enough insulin for the body when it is diagnosed with type 2 diabetes. This is known as insulin resistance. After a few years, the body’s ability to produce insulin decreases. Type 2 diabetes has the same end result and the body is unable to utilize its principal fuel adequately. Symptoms of type 2 diabetes develop over time. Type 2 diabetes takes longer to develop than type 1. Some of the symptoms include fatigue, frequent urination, increased thirst and hunger, weight loss, blurred vision, and a slow rate of wound or sore healing. It’s possible that some people don’t display any signs of disease (Olokoba AB, et al., 2012). Gestational diabetes can develop late in pregnancy for certain women. After the birth of a child, gestational diabetes usually disappears. However, women with gestational diabetes may develop type 2 diabetes during the next five to ten years. In the United States, 3% to 8% of pregnant women have gestational diabetes. Pregnancy hormones or a deficiency of insulin can induce gestational diabetes. No symptoms may be seen in pregnant women with gestational diabetes (Jovanovic L and Pettitt DJ, 2001).
Literature Review
Diabetes diagnosis
Diagnosis of diabetes is most commonly in children and non-pregnant women which can be made by using a fasting blood glucose test. Taking the test in the morning provides the best results (Emerging Risk Factors Collaboration, et al., 2001). OGTT (Oral Glucose Tolerance Test) blood glucose levels are used to determine whether a woman has Gestational Diabetes Mellitus (GDM). Diagnosing diabetes in pregnancy is easier because blood glucose levels are typically lower during pregnancy. Before consuming a beverage containing glucose, a woman’s blood sugar levels are checked. After that, levels are checked every two and three hours for the next two and a half to three hours. Fasting levels of 95 mg/ml, 180 mg/ml, 155 mg/ml, or 140 mg/ml after one hour are all indicators of gestational diabetes, as are those of 95 or 180 mg/ml after two hours (Kjos SL and Buchanan TA 1999). Diabetes is on the rise in the United States, as evidenced by a number of different trends and indicators. It’s important to remember that an increasing number of people are reaching retirement age. Hispanics/Latinos, along with other historically underserved groups, are the nation’s fastest-growing demographic. Finally, the American population is becoming increasingly obese and sedentary. In the United States, the Centers for Disease Control and Prevention recently predicted that one in three people born in 2000 will have diabetes. As predicted by the CDC, the number of Americans with diabetes is expected to rise by 165 percent by 2050 (Caspersen CJ, et al., 2012).
Oral hypoglycemic
Diabetes under control reduces the risk of consequences such as kidney failure, blindness, heart disease, and limb amputation that are linked with uncontrolled diabetes. The most common kind of medicine is hypoglycemic treatment, which may be accomplished with oral hypoglycemics. Patients with type 1 diabetes mellitus need insulin injections because their bodies do not manufacture enough (or any) insulin. Non-injective insulin delivery has proved impossible due to the breakdown of the insulin protein in the digestive system. Vaccines for type I diabetes were invented based on glutamate decarboxylase enzyme, but they are not investigated to be licensed by pharmaceutical companies (Rother KI, 2007). Any combination of nutrition, physical activity, and weight loss can be used as a form of diabetes treatment for type 2 diabetics. Obesity is a major contributor to insulin resistance in those with type 2 diabetes, and it is particularly prevalent in those with the disease. Tissue insulin sensitivity is increased by weight loss and exercise. According to a 2008 research, a rising number of people with type 2 diabetes are receiving more sophisticated and expensive diabetic therapies. The data was studied from 1994 to 2007, and it was shown that the mean number grew rapidly from 1994 to 2007 (Peñalver JJM, et al., 2016). Patient education and adherence to medication are critical in illness management. Improper medication and insulin administration might result in hazardous hypo-or hyperglycemic episodes (Coppola A, et al., 2016).
Sulphonyl urea drugs
Glibenclamide is the most famous drug of this family chemically as the name suggests, glibenclamide is dione derivative of sulphonamide (Figure 1). Second generation sulphonylureas (-2-methoxy benzamide) inhibit ATP-sensitive potassium channels in beta cells of the pancreas. There is an increase in intracellular calcium in beta cells due to the depolarization caused by this inhibition, which leads to the release of insulin from the beta cells (Luzi L and Pozza G 1997). Sulphonylurea class oral hypoglycemic glibenclamide is used to treat noninsulin-dependent diabetes mellitus. A lack of bioavailability has been attributed to its poor dissolution properties1 in the past (Gianotto EA, et al., 2007). In order to lower high blood glucose levels, glibenclamide stimulates the pancreas to release more insulin. Sulphonylureas, of which glibenclamide is a member, are a class of medications. It is possible to have hypoglycemia (low blood glucose) or hyperglycemia if you do not properly control your blood glucose (high blood glucose). Your heart, eyes, circulation, and kidneys can all be affected if your blood sugar levels are too high (Adrogué HJ, 1992). Biopharmaceutical Classification System: Glibenclamide (pKa=5.3) is an acid that is practically insoluble in water and acidic environments but is highly permeable (class 2) (BCS). Complete, uniform, and rapid bioavailability is achieved through the oral route. Glibenclamide therapy is usually started with a dose of 2.5 mg once a day. The maximum dose that should be taken each day is 20 milligrams (Ahad HA, et al., 2010).
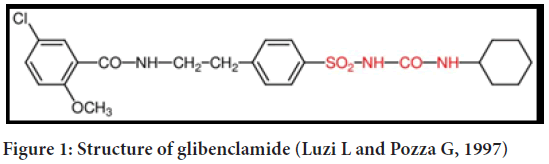
Figure 1: Structure of glibenclamide (Luzi L and Pozza G, 1997)
Biguanides
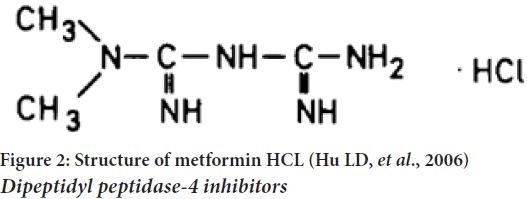
Metformin is the most famous drug of this class. Type 2 diabetic patients are unable to control their blood sugar levels with diet alone. A 20%-30% oral dose is recovered in the feces and low bioavailabilities of 40% without taking any meals associated with rapid elimination characterize metformin’s gastrointestinal absorption. Metformin’s hepatic metabolism appears to be negligible in humans, as evidenced by the fact that it decreases in concentration with increasing dose. It is possible to reduce the frequency of dosing and improve patient compliance by using a modified-release tablet dosage form (Hu LD, et al., 2006). Type 2 diabetes is treated with the antihyperglycemic medication metformin HCl. It is an anti-hyperglycemic medication that is both unique and widely utilized around the world. No other oral anti-hyperglycemic drugs have a similar chemical or pharmacological profile. One of the drug’s primary mechanisms of action appears to be its ability to inhibit glyconeogenesis. C4H11N5.HCl is the chemical formula for metformin HCl. (Dibbern HW, et al., 2002) (Figure 2).
Figure 2: Structure of metformin HCL (Hu LD, et al., 2006) Dipeptidyl peptidase-4 inhibitors
Dipeptidyl peptidase-4 inhibitors
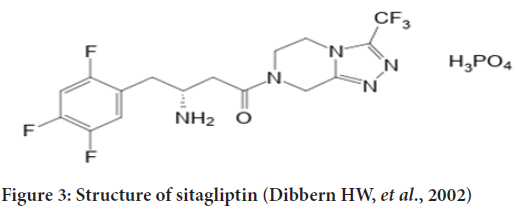
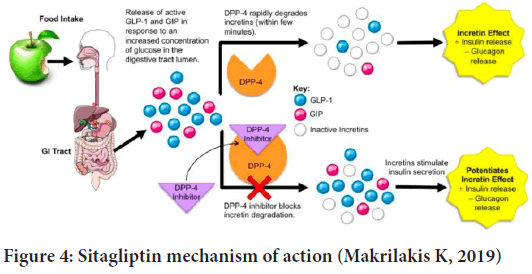
The gliptin family of diabetes medicines includes sitagliptins. DPP-4, an enzyme that degrades and inactivates Glucagon-Like Peptide-1 (GLP-1), is inhibited by this drug (GLP-1) (Figure 3). Sitagliptin-induced increases in GLP-1 lead to enhanced glucose tolerance and higher postprandial insulin secretion. Because of this and the fact that patients don’t gain weight while on the medication, sitagliptin has become more popular as a second-line treatment for those with type 2 diabetes (Bennett RG, 2018). Chemically, Sitagliptin (2R)-4-Oxo-4-(3-(trifluoromethyl)-5,6-dihydro(1,2,4)triazolo(4,3-a)pyrazin-7(8H)-yl)-1-(2,4,5-trifluorophenyl) Butan-2-amine exhibits a significant degree of DPP-4 selectivity. DDP enzymes do not form a bond with each other (DPP-8 and DPP-9). It has been licensed in the United States and Europe for the treatment of type 2 diabetes and is registered as Januvia ® (Merck Pharmaceuticals, Whitehouse Station, NJ, USA) (Gallwitz B, 2007). The dissolving method was used to study sitagliptin’s in vitro drug release characteristics (USP 1, 100 rpm at pH 6.8 and pH 5.5). The immediate-release version of sitagliptin dissolving in phosphate buffer pH 6.8 and acetate buffer pH 5.5 exhibited around 91% to 95% of solubility. The breakdown of Januvia® and FDC appeared to be thorough and slow, despite the differences in salt content. However, there were certain variances in the breakdown process. The dissolution profiles of the reference and test formulations were compared using the similarity factor (f2) to see if the differences in dissolution had any effect. (Boddu R, et al., 2021).The enzyme amylase is responsible for breaking down starch into oligosaccharides in the small intestine so that glucose can be produced. Glucoamylase and other membrane-bound enzymes such as maltase, isomaltase, isomaltase, and sucrase then continue the breakdown process. The-glucosidase enzyme can be inhibited pharmacologically to modify carbohydrate digestion and absorption. They must be taken before meals because of their competitive character, which means they must be taken in the beginning (Aschenbrenner DS and Venable SJ, 2012) (Figure 4).
Figure 3: Structure of sitagliptin (Dibbern HW, et al., 2002)
Figure 4: Sitagliptin mechanism of action (Makrilakis K, 2019)
Repaglinide
Type 2 diabetes treatment with a BCS class II medication. There is a pH-dependent dissolving profile for repaglinide, according to Preformulation tests, with the medication being more soluble in water at a higher pH. Because this treatment is needed to control postprandial glucose levels, the drug should disperse quickly in the stomach (where the pH of the contents can be low). Due of the drug’s instability in basic solutions, it is also hydrolyzed at high pH. For the manufacture of a repaglinide powder formulation that dissolves quickly but is chemically stable, alkalizing agents and nonpolymeric surfactants could be added to the mix (Purvis T, et al., 2007) (Table 1).
| Drug | Glibenclamide | Metformin | Sitagliptin |
|---|---|---|---|
| pH 1.2 | 1.3 mg/ml | 5.5 mg/ml | 10 mg/ml |
| pH 5.5 | 15.5 mg/ml | 8 mg/ml | 3.4 mg/ml |
| pH 6.8 | 10.5 mg/ml | 13.3 mg/ml | 5.56 mg/ml |
Table 1: Drugs saturation solubility in different pH
Discussion
Determination of λmax
Glibenclamide: The 100 g/ml prepared solution A UV spectrophotometer was used to scan a stock solution of glibenclamide in three different pH and determine the absorbance of the drug. These standard curves were created by making various concentrations of glibenclamide from stock solution at concentrations of 10 micrograms per milliliter, 20 microgram per milliliter, 40 microgram per milliliter, and 80 micrograms per milliliter. At a wavelength of max 300 nm, spectroscopy was used to examine the prepared samples. Absorbance measurements were plotted against sample concentration. UV spectrophotometer scans at 200-400 nm for a solution containing 100 g/ml of glibenclamide at different pH gave same peak. The findings are consistent with those reported in the literature (Bischoff H, 1994).
Metformin: The maximum concentration of metformin was determined by scanning the stock solution with a UV spectrophotometer at three different pH levels. Metformin stock solution 10 mg/ml, 20 mg/ml, 40 mg/ml, and 80 mg/ml were prepared from stock solution in order to obtain glibenclamide standard curves at pH 1.2, 5.5, and 6.8. The samples were prepared using spectrophotometric analysis at a wavelength of 234 nm. Plots were made of the absorbance measurements for each sample and the corresponding concentration. Glibenclamide at pH 1.2, 5.5, and 6.8 all had the same peak in a UV spectrophotometer scan at 200-400 nm. The results are consistent with those previously reported (Patil SS and Bonde CG, 2009).
Sitagliptin phosphate: The λmax of sitagliptin was determined by scanning the prepared solution with a UV spectrophotometer at wavelengths ranging from 300-700 nm in three pH. Alkaline potassium permanganate used as an oxidizing agent to establish a calibration curve in previous work. The sitagliptin concentration is measured through 15-minute kinetic studies of its oxidation at room temperature was found to be 267 nm in wavelength. When the concentrations of sitagliptin were 4, 5, 10, 15, and 20 g/ml, the absorbance concentration plot was rectilinear. In practice, the method worked well for determining dosage forms of drugs. According to the reference methods, the results were in close agreement. UV spectrophotometer scans at 300-700 nm for solutions containing 100 g/ml of glibenclamide at all pH values gave the same peak. The outcomes are in line with what was previously reported (Ajithdas A and Nancy K, 2000).
In-vitro determination of pH-solubility profile
Using a water bath shaker at 37°C, the solubility of glibenclamide, metformin, and sitagliptin at pH (1.2,5.5, and 6.8) was tested. Small vials were filled with buffer and 10 ml of medication were poured into each of them to ensure a complete saturation solution. At 37°C, the shaker was filled with flasks of phosphate buffer. Using a UV scan set to a certain λmax value for each drug, the solubility of the samples was evaluated (Patil SS and Bonde CG, 2009).
In-vitro drug dissolution
This formulation’s in-vitro release testing was carried out using the USP drug dissolving equipment II (paddle type). Three different pH levels were tested in the dissolution flask, which contained 900 ml of dissolving media. (pH 1.1) to (pH 5.5) is the range of phosphorus. Every 10 minutes, 10 ml samples were obtained and replaced with the equal amount of new buffer (1.2, 5.5, and 6.8). Glibenclamide was classified as a very barely soluble drug; at pH 6.8, it’s just marginally solubilized, approximately 20 milligrams per milliliter.
Classification of biopharmaceuticals: In water and acid, glibenclamide (pKa=5.3) is essentially insoluble, yet it is very permeable (class 2) (BCS). As the pH approached the pKa-point, solubility declined, while ionization increased in this higher pH environment (Caroline D and Clifford JB, 2007). This drug’s pH ranges from a sparingly soluble in 10 milliliters at pH 1.2 to an almost completely non-soluble in 10 milliliters (pH 5.5) at pH 6.8 due to its weak base and its very slight solubility at pH 1.2, 70 milliliters (pH 5.5) at pH 5.8, and very slight soluble at pH 6.8. (4 mg in 10 ml) (Holt PR, et al., 1996). At pH 4.5 and 25°C, the solubility of sitagliptin phosphate monohydrate is 69.5 mg/ml. The solubility of sitagliptin phosphate monohydrate in 0.01 M HCl and 0.10 M sodium citrate is 68.1 mg/ml and 66.1 mg/ml, respectively. For 0.10 M sodium carbonate solutions, the solubility of sitagliptin free base is 42.2 mg/ml. Depending on pH, sitagliptin’s octanol/water distribution coefficient (Ko/w) is affected. At pH 5.0, pH 7.0, and pH 9.0, the Ko/w is -1.08, -0.03, and 1.11. 1.8 has also been reported as a partition coefficient in the scientific literature as well. The distribution and partition coefficients are in general agreement with the pKa of sitagliptin (7.7) (Charoo NA, et al., 2022). The product’s in-vivo performance could not have been predicted without the data obtained from the in-vitro release. What can be absorbed depends on the amount of medicine that is released into the bloodstream. Study of drug release pattern from dosage form utilizing USP dissolving device I (Butler J, et al., 2019). Drug dissolution is greatly affected by each drug’s physiochemical properties as pH changes throughout the G.I.T. In contrast to glibenclamide, metformin and sitagliptin, which have higher pKa values than glibenclamide, released more in the acidic stomach (pH 1.2). Several studies have shown that the stomach is the primary or even the only site of absorption for the drug glib enclamide (Brockmeier D, et al., 1985). When a drug is injected into the stomach, blood levels rise in an s-shape. This shows that a transport mechanism is at play. There are two plausible explanations for this phenomenon-dissolution because glibenclamide dissolves only at pH levels that are neutral to slightly alkaline or delayed transit time to the intestinal site of absorption. As a result, the mean residence time shows no discernible change in the amount or rate at which a drug is absorbed (Sultana S, 2019). Lower gastrointestinal tract absorption is poor. When using an incorrect pH value of 5.3 instead of the more accurate 6.3-6.8 depending on the method, this hypothesis is flawed (Brockmeier D, 1985). To the contrary of what they claim, absorption rates are slower in the colon than in the upper gastrointestinal tract, but the same amount is absorbed from both. As colon absorption is slower, it’s possible that the amount of time a drug spends in contact with an absorbing surface can affect how much of it gets absorbed (Ikegami H, et al., 1986). Glibenclamide dissolution was found to be slowed or even halted by foods, antacids like aluminum hydroxide and aluminum trisilicate, calcium carbonate, magaldrate, and simethicone, as well as magnesium oxide, magnesium trisilicate, and sodium bicarbonate, according to literature reviews (Arayne MS, et al., 2004).
Conclusion
To summarize, we may deduce that glibenclamide, metformin and sitagliptin dissolve and absorb at different pH levels. According to the physicochemical features of each medicine, the optimal pH for each is the one that results in the highest release. It has been established that taking glibenclamide with antacid or meal alters mechanism of absorption of the drug from G.I.T because antacids interfere with the pH of the dissolving medium in the G.I.T. so this can be used to modify the dissolution and oral absorption of antidiabetic drugs to give high extent and release according to the physiochemical properties of the antidiabetic drugs and the onset of action needed from oral amination of drugs.
Acknowledgement
All authors are grateful for the members of the College of Pharmacy of Baghdad University in Iraq.
References
- American diabetes association. Standards of medical care in diabetes. Diabetes care. 2013; 36(Suppl 1): S11-S61.
[Crossref] [Google scholar] [Pubmed]
- Ramachandran A, Ma WRC, Snehalatha C. Diabetes in Asia. Lancet. 2010; 375(9712): 408-418.
[Crossref] [Google scholar] [Pubmed]
- Charoo NA, Abdallah DB, Bakheit AA, Haque KU, Hassan HA, Abrahamsson B, et al. Biowaiver monograph for immediate-release solid oral dosage forms: Sitagliptin phosphate monohydrate. J pharma Sci. 2022; 111(1): 2-13.
[Crossref] [Google scholar] [Pubmed]
- Daneman D. Type 1 diabetes. The Lancet. 2006; 367(9513): 847-858.
[Crossref] [Google scholar] [Pubmed]
- DiMeglio LA. Molina EC, Oram RA. Type 1 diabetes. The Lancet. 2018; 391 (10138): 2449-2462.
[Crossref] [Google scholar] [Pubmed]
- DeFronzo RA, Ferrannini E, Groop L, Henry RR, Herman WH, Holst JJ, et al. Type 2 diabetes mellitus. Nat Rev Dis Primers. 2015; 1(1): 1-22.
[Crossref] [Google scholar] [Pubmed]
- Olokoba AB, Obateru OA, Olokoba LB. Type 2 diabetes mellitus: A review of current trends. Oman Med J. 2012; 27(4): 269.
[Crossref] [Google scholar] [Pubmed]
- Jovanovic L, Pettitt DJ. Gestational diabetes mellitus. Jama. 2001; 286(20): 2516-2518.
[Crossref] [Google scholar] [Pubmed]
- Emerging risk factors collaboration, Sarwar N, Gao P, Seshasai KSR, Gobin R, Kaptoge S. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: A collaborative meta-analysis of 102 prospective studies. The Lancet. (2001); 375(9733): 2215-22.
[Crossref] [Google scholar] [Pubmed]
- Kjos SL, Buchanan TA. Gestational diabetes mellitus. New Eng J Med. 1999; 341(23): 1749-1756.
[Crossref] [Google scholar] [Pubmed]
- Caspersen CJ, Thomas GD, Boseman LA, Beckles GL, Albright AL. Aging, diabetes, and the public health system in the United States. Am J Public Health. 2012; 102(8): 1482-1497.
[Crossref] [Google scholar] [Pubmed]
- Rother KI. Diabetes treatment-bridging the divide. N Engl J Med. 2007; 356(15): 1499.
[Crossref] [Google scholar] [Pubmed]
- Peñalver JJM, Timón IM, Collantes CS, Del Gómez FJC. Update on the treatment of type 2 diabetes mellitus. World J Diabetes. 2016; 7(17): 354-935.
[Crossref] [Google scholar] [Pubmed]
- Coppola A, Sasso L, Bagnasco A, Giustina A, Gazzaruso C. The role of patient education in the prevention and management of type 2 diabetes: An overview. Endocrine. 2016; 53(1): 18-27.
[Crossref] [Google scholar] [Pubmed]
- Luzi L, Pozza G. Glibenclamide: An old drug with a novel mechanism of action? Acta diabetol. 1997; 34(4): 239-244.
[Crossref] [Google scholar] [Pubmed]
- Gianotto EA, Arantes RP, Lara-Filho MJ, Filho ACC, Nery FMM. Dissolution test for glibenclamide tablets. Química Nova. 2007; 30: 1218-1221.
- Adrogué HJ. Glucose homeostasis and the kidney. Kidney int. 1992; 42(5): 1266-1282.
[Crossref] [Google scholar] [Pubmed]
- Ahad HA, Meghana M, Navya K, Mallika B, Aaslesha A, Srilatha V, et al. Designing and evaluation of Glibenclamide Azadirachta indica mucilage based controlled release matrix tablets. Der Pharmacia Lettre. 2010; 2(1): 117-1121.
- Hu LD, Liu Y, Tang X, Zhang Q. Preparation and in vitro/in vivo evaluation of sustained-release metformin hydrochloride pellets. Eur J Pharm Biopharm. 2006; 64(2): 185-192.
[Crossref] [Google scholar] [Pubmed]
- Dibbern HW, Müller RM, Wirbitzki E. UV and IR spectra of pharmaceutical substances and IR spectra of pharmaceutical and cosmetic excipients. ECV Editio Cantor Verlag, CD-ROM. 2002.
- Bennett RG. Sitagliptin. Ref Mod Biomed Sci. 2018.
- Gallwitz B. Review of sitagliptin phosphate: A novel treatment for type 2 diabetes. Vasc Health Risk Manag. 2007; 3(2): 203.
[Crossref] [Google scholar] [Pubmed]
- Boddu R, Vadla HC, Prathap VR, Kothamasu U, Rallabandi BC, Gannu R. Development of an in vitro-in vivo correlation for sitagliptin and metformin prolonged-release tablet formulations. Turk J Pharm Sci. 2021; 18(2): 233.
[Crossref] [Google scholar] [Pubmed]
- Aschenbrenner DS, Venable SJ. Drug therapy in nursing. Lippincott Williams and Wilkins; 2009.
- Makrilakis K. The role of DPP-4 inhibitors in the treatment algorithm of type 2 diabetes mellitus: When to select, what to expect. Int J Environ Res Public Health. 2019; 16(15): 2720.
[Crossref] [Google scholar] [Pubmed]
- Purvis T, Mattucci ME, Crisp MT, Johnston KP, Williams RO. Rapidly dissolving repaglinide powders produced by the ultra-rapid freezing process. AAPS PharmSciTech. 2007; 8(3): E52-60.
[Crossref] [Google scholar] [Pubmed]
- Bischoff H. Pharmacology of α‐glucosidase inhibition. Eur J Clin Invest. 1994; 24(S3): 3-10.
[Crossref] [Google scholar] [Pubmed]
- Patil SS, Bonde CG. Development and validation of analytical method for simultaneous estimation of glibenclamide and metformin HCl in bulk and tablets using UV visible spectroscopy. Int J Chemtech Res. 2009; 1(4): 905-909.
- Ajithdas A, Nancy K. Simultaneous estimation of metformin hydrochloride and glipizidin solid dosage forms by ultraviolet spectrophotometry. Indian Drugs. 2000; 37(11): 533-536.
- Caroline D, Clifford JB. Metformin, the comprehensive pharmacology reference. 2007.
- Holt PR, Atillasoy E, Lindenbaum J, Ho SB, Lupton JR, McMahon D, et al. Effects of sitagliptin on fecal nutrients, colonic pH, and short-chain fatty acids and rectal proliferative indices. Metabolism. 1996; 45(9): 1179-1187.
- Butler J, Hens B, Vertzoni M, Brouwers J, Berben P, Dressman J, et al. In vitro models for the prediction of in vivo performance of oral dosage forms: Recent progress from partnership through the IMI OrBiTo collaboration. Eur J Pharm Biopharm. 2019; 136: 70-83.
[Crossref] [Google scholar] [Pubmed]
- Brockmeier D, Grigoleit HG, Leonhardt H. Absorption of glibenclamide from different sites of the gastro-intestinal tract. Eur J Clin Pharmacol. 1985; 29(2): 193-197.
[Crossref] [Google scholar] [Pubmed]
- Sultana S. Degradation kinetic studies of non-pharmacopeial drug products and determination of their forced degradants and impurities (Doctoral dissertation, University of Dhaka). 2019.
- Ikegami H, Shima K, Tanaka A, Tahara Y, Hirota M, Kumahara Y. Interindividual variation in the absorption of glibenclamide in man. Eur J Endocrinol. 1986; 111(4): 528-532.
[Crossref] [Google scholar] [Pubmed]
- Arayne MS, Sultana N, Kamran Zaman RM. In vitro availability of glibenclamide in presence of antacids. Pak J Pharm Sci. 2004; 17(2): 41-56.
[Google scholar] [Pubmed]
Author Info
Omar S Salih*, Mowafaq M Ghareeb and Mais Fadhel MohammedCitation: Salih OS: Comparison In-vitro Release and pH Effect among Different Oral Antidiabetic Drugs: A Review
Received: 02-May-2022 Accepted: 27-May-2022 Published: 03-Jun-2022, DOI: 10.31858/0975-8453.13.6.366-370
Copyright: This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
ARTICLE TOOLS
- Dental Development between Assisted Reproductive Therapy (Art) and Natural Conceived Children: A Comparative Pilot Study Norzaiti Mohd Kenali, Naimah Hasanah Mohd Fathil, Norbasyirah Bohari, Ahmad Faisal Ismail, Roszaman Ramli SRP. 2020; 11(1): 01-06 » doi: 10.5530/srp.2020.1.01
- Psychometric properties of the World Health Organization Quality of life instrument, short form: Validity in the Vietnamese healthcare context Trung Quang Vo*, Bao Tran Thuy Tran, Ngan Thuy Nguyen, Tram ThiHuyen Nguyen, Thuy Phan Chung Tran SRP. 2020; 11(1): 14-22 » doi: 10.5530/srp.2019.1.3
- A Review of Pharmacoeconomics: the key to “Healthcare for All” Hasamnis AA, Patil SS, Shaik Imam, Narendiran K SRP. 2019; 10(1): s40-s42 » doi: 10.5530/srp.2019.1s.21
- Deuterium Depleted Water as an Adjuvant in Treatment of Cancer Anton Syroeshkin, Olga Levitskaya, Elena Uspenskaya, Tatiana Pleteneva, Daria Romaykina, Daria Ermakova SRP. 2019; 10(1): 112-117 » doi: 10.5530/srp.2019.1.19
- Dental Development between Assisted Reproductive Therapy (Art) and Natural Conceived Children: A Comparative Pilot Study Norzaiti Mohd Kenali, Naimah Hasanah Mohd Fathil, Norbasyirah Bohari, Ahmad Faisal Ismail, Roszaman Ramli SRP. 2020; 11(1): 01-06 » doi: 10.5530/srp.2020.1.01
- Manilkara zapota (L.) Royen Fruit Peel: A Phytochemical and Pharmacological Review Karle Pravin P, Dhawale Shashikant C SRP. 2019; 10(1): 11-14 » doi: 0.5530/srp.2019.1.2
- Pharmacognostic and Phytopharmacological Overview on Bombax ceiba Pankaj Haribhau Chaudhary, Mukund Ganeshrao Tawar SRP. 2019; 10(1): 20-25 » doi: 10.5530/srp.2019.1.4
- A Review of Pharmacoeconomics: the key to “Healthcare for All” Hasamnis AA, Patil SS, Shaik Imam, Narendiran K SRP. 2019; 10(1): s40-s42 » doi: 10.5530/srp.2019.1s.21
- A Prospective Review on Phyto-Pharmacological Aspects of Andrographis paniculata Govindraj Akilandeswari, Arumugam Vijaya Anand, Palanisamy Sampathkumar, Puthamohan Vinayaga Moorthi, Basavaraju Preethi SRP. 2019; 10(1): 15-19 » doi: 10.5530/srp.2019.1.3