Research Article - (2024) Volume 15, Issue 2
Complementary Medicine in Tuberculosis
Hamid Didi* and Made EkoAbstract
Vitamin A and Vitamin D play an important role in immunity. The purpose of this study is to analyze whether vitamin D3 and retinyl palmitate could induce the effectiveness of 2nd line Anti-Tuberculosis (TB) drugs. We majorly focus on cell death process. C3HeB/FeJ mice were infected with Multidrug-Resistant (MDR) strain Mycobacterium tuberculosis and grouped randomly. The 1st group was euthanized to evaluate TB germs grown in the lungs. 2nd group is received no therapy. The 3rd group was given 2nd line anti-TB drugs. The 4th was given retinyl palmitate along with 2nd line drugs. 5th group was given 2nd line drugs with D3. 6th group was given a combination of 2nd line drugs, retinyl palmitate and D3. Immunohistochemistry demonstrated the quantitative measurement of nuclear receptor expression of Vitamin D (VDR) and Vitamin A (Retinoic Acid Receptor Gamma 2) (RARγ2), apoptosis Caspase-3 (CASP3) marker, autophagy markers Cathelin-Related Antimicrobial Peptide (CRAMP) and Microtubule-Associated Protein 1 Light Chain 3B (MAP1LC3B), marker of necrosis namely, Receptor Interacting threonine Kinase 3 (RIPK3) and interstitial collagenase Matrix Metalloproteinase 1 (MMP1). TB germs in lung were counted in Colony Forming Units (CFU). Partial Least Square Structural Equation Modeling (PLS-SEM) with SmartPLS 3.2.6 software was used to analyze structural model within variable. Vitamin D3 plays an important role in increasing autophagy of infected cells and MMP1. Both vitamin D3 and retinyl palmitate played a role in increasing Casp3 expression and reducing CFU. The combination of D3 and retinyl palmitate reduced cell necrosis which was characterized by a decrease in RIPK3. Our study proves that the combination of D3 and retinyl palmitate supplementation on the 2nd line anti-TB drugs reduces cell necrosis directly.
sushihouse kolstads americanpridefasteners trueforge hotelposeidon youngswoodfinishing arditomason thebestofreno eeclongisland doktervanhecke famsales adla bogatylaw prefplastics kirbycontractinginc unlsp boban dottoressaromolimonica thebestofsanfrancisco vernix digitaldocuments tristatepropertybrokers sotrafib cemcorpny agenziaimmobiliarebuti joesitalianfoodmarket corpoguardiedicitta icsatc antoniniassicurazioni besel strappedincarseatsafety drevobeton blessingconstructionny centre-endorphine qmbtunisia elearning 357 mpress rrappliance ciaautomazioni antikva stubbanvel tacticalpublicrelations mpulshnk paulyboybrand lordshoes ijd-procom thebestrestaurants atkpalvelut ceat leonfukspc pilotexamssa ashgrovecabins universalshielding thebestofcharlotte johnjmazurinc rollnroaster thecabinetwarehouse pocketchangeduo thebestofmilwaukee resan sooli vwe centralwindowcleaning reinforcedplasticslab bugbustersofli ciandrigiardini arfada centrumeigenwijs thebestofmemphis maisonpearly islipll dcgraphicsinc agetranquille relisandroth thebestofcharleston traiteur-wn safi-ingenierie justmyvoice harrishardware louisbarbatolandscaping brightstartoursworld rga-insurance calacatajumper ezantia mrcheapocds van4holiday cigap richtour fagyhatar sicc weshopmall ventovuori sobatrapcapbon unityrubberllc thebestofsaltlakecity ircinc sescoindustries connply storen-servicesenter psnry greatneckcollision ironfitendurance tuscanycountryhouse sansovinocalcio bbradydesign samsam vdtarification springersoil thebestofdayton milantechnology labradoodlesoflongisland daliamohamed theconsultantpowerhouse gunnbrush kruunuosk shulmanproduce balcosupply conso-med federalnetworks bellmoreglass alwaysaffordableconcrete jayteeinsurance carolina-cabins cmorfinance stretchritepackaging dishaairwaysenterprise biocontrol distribio peterivill lcc inspekta jvidesigns royalroseinc calabriapizza fadhila prato-pronto-effetto arbemachine olsonelectricnj tkbl tuttifotokft alpernmd ggsupplywholesale almaxcorporation sotuflex ecustomgutter studenipotok metal-lineconcept rettsodontologi nutecsystems aclotbeach gjonnes-bygg cavalierinternational mnemos southfloridashuttles ajchemicalsupply rachelsfireisland concepto nesponge ultimatestylesofamerica techniquesmadeeasydrivingschool stevesmeatsfreeport eastwest gritbrush plussplan biosens viltkam wikaya paintballconcept justmyvoice securecarkeysupply justmyvoice allcountylegal catt plugandcharge ttandlcontracting justmyvoice islandboatlettering gms-tunisie sportsiena bloeiop touchofclasscollision kotekservice ittoscana pallongislandlacrosse industrialfinishings painoutband rakvag-batforening konemies rrfamilychiropractic infienile shbcgroup footpharmacydirect scuolaguidaprato ans-nettoyage autoskola saafa autolaky1 fixcars longislandelitelandscaping rayscan palaconstruction studio44 crealhome viniferi gavinburke ciprianigiardini davidpokorny osteriailcapodaglio thebestoffairfax centroorafofaccioli ateliervb bbdps thebestoffresno prodigus stratpak umisushirestaurant sotim suldalrenovasjon amer-equip k-kleven jerryspridepotatoes neuroky psicologozampoli jjslandscaping thebestofoklahomacity responsivesales paratie-antiallagamento-shop hearproof cfat ruspinameubles planetbioplastics lynbrook-plumber fcdf-ye brechanparkett valley-stream-plumber diagnosismaker aquavaria jedit garageennour heimdalbygg thebestoflittlerock raybomarine bayshorepaper gmstowing potatura-abbattimento-piante thebestoflouisville michaelalbert thebestofannarbor unitypavers mtnfueloil durub-mudiya lesgensdere dormerking thusney italianvistatravel maiemad gtiuniformcleaning suddenimpactli levituuli sourisalavie mayoiltank mschwartzfeather rands arieslimousines orthoticworld longislandcocktailhours waterjet allislandpaving dukediagnostic klfgoteborg yanezviaggi apruk decogato springeroilltd semapsolar irisgioiellicomprooro msedpsoftware fourcmanagement holemans 7consulting medinet evertile robertwitcomblandscape ceramics adrobotengineering volt-energy huisjacobs chimneyserviceboston eastmainstdental gcbt rememberingbriank elligiardiniespurghi paulslandscaping luisrestorations mgstunisie fixcarsny crcdd hotelprincipessalucca turvahallinta ralphjr thales irrigazione-giardini spantecsystems biomedic fgt-trading sols-egypt spectrumlaboratoriesinc prosecurite spongewarehouse afsainc dellafrancadevelopmentgroup leragazzedifirenze ferrettiwatches dovreentreprenor digitalhvac mostlymica fleurs-velghe autoscuolalebadie interlockingrubbertiles meadowcreekhoa bcn corpjetsupport jrkitchensflooring arteletti
Keywords
Cell death process, D3, Retinyl palmitate, MDR-TB, Caspase-3
Introduction
The efficacy of 2nd Anti-Tuberculosis (TB) drugs is very low. The success rate of Multidrug-Resistant Tuberculosis (MDR-TB) therapy in Indonesia is 51% (WHO, 2016). Literature shows that many of TB patients are having deficiencies of vitamin A and D (Srinivasan A, et al., 2013). The biological effects of the two vitamins are closely related. Vitamin D Receptors (VDR) can form heterodimers with Retinoid X Receptor (RXR) or Retinoic Acid Receptor (RAR) (Schräder M, et al., 1994; Koszewski NJ, et al., 2010; Syal K, et al., 2015). The heterodimer binds to the Vitamin D Response Element (VDRE) and initiates the transcription of the target gene. More than 60 genes have VDRE sequence. These genes are classified into 6 bioresponses-
• Bone metabolism
• Mineral homeostasis
• Detoxification
• Cell cycle (proliferation, differentiation, migration and cell death)
• Immune system
• Amino acids, fats and carbohydrates metabolism (Chengprapakorn W, 2016).
This study aims to analyze how the combination of D3 and retinyl palmitate could improve the effectiveness of the 2nd line anti-TB drugs. Analysis was done by observing the infected cells death pathway i.e., necrosis and apoptosis, or autophagy. Necrosis is a form of death that can release bacteria to infect new cells. Apoptosis is a double-edged knife that kills host cells and bacteria that infect it, while autophagy facilitates bacterial death through the formation of autophagolisosomes. Increased effectiveness of therapy was evidenced by a decrease in the necrosis, and increase in the apoptotic and autophagy pathway. The necrosis pathway was observed with Receptor Interacting Protein Kinase 3 (RIPK3) marker, apoptosis was observed with Caspase3 marker (CASP3), autophagy was measured with Cathelicidin Related Antimicrobial Peptide (CRAMP) and Light Chain 3B (LC3B) markers. The supplementation goal decreased MTR-TB Colony Forming Units (CFU), and reducing collagenase Matrix Metalloproteinase-1 (MMP-1).
Material and Methods
Animals and experimental procedures
Animal care and use were approved by the Institutional Animal Care and Use Committee of Faculty of Veterinary Medicine, Airlangga University, Indonesia (393-KE). 48 C3HeB/FeJ mice aged 5-8 weeks were infected with MDR strain Mycobacterium tuberculosis (100 μl) through intratracheal instillation (105 CFU/ml) and divided randomly into 6 groups of 8 animals. The 1st Group (G1) was euthanized 2 weeks after infection to see if pulmonary TB had occurred. 2nd Group (G2) was considered as control group which did not receive any therapy. 3rd Group (G3) was treated with the 2nd line anti-TB drugs as recommended by the Ministry of Health of the Republic of Indonesia after 2 weeks of infection. Similarly 4th Group (G4) was given retinyl palmitate and the 2nd line drugs. 5th Group (G5) was given 2nd line drugs and D3 while 6th Group (G6) was given the 2nd line drugs, retinyl palmitate and D3.
Kanamycin (Sigma K1876) was injected in 150 mg/kg Body Weight (BW) once daily on 5 days each week. Pyrazinamide (Sigma P7136) 150 mg/kg BW, levofloxacin (Sigma 28266) 200 mg/kg BW, ethionamide (Sigma E6005-5G) 50 mg/kg BW, cycloserine (Sigma 30020-1G) 300 mg/kg BW, retinyl palmitate (Sigma-Aldrich R1512) 16 IU/g BW and vitamin D3 (Dvion Drops, Merck) 1.25 IU/g BW were given through esophageal cannula 20 gauge once daily on 7 days, each week. After 6 months of therapy, the left lung was processed for immunohistochemistry, and the right lung was used to count bacterial viability.
Primary antibodies used include-anti-RAR2 (ab188000, abcam), anti-VDR antibodies (ab3508, abcam), anti-cathelicidin antibody (ab64892 abcam), anti-LC3B antibody (ab63817, abcam), anti-MMP1 antibody (ab137332, abcam), anti-CASP3 (P17) (PA1961-1, BosterBio), and anti-RIPK3(PA2242, BosterBio). Five visual fields were analyzed by 2 independent investigators using the Olympus BX51 light microscope 400X magnification. The brown precipitate indicated the presence of the target antigen.
Statistical analysis
Data was processed with Statistical Package of Social Sciences (SPSS) version 20. Confidence intervals are calculated at a confidence level of 95%. The structural model between variables was analyzed by Partial Least Square Structural Equation Modeling (PLS-SEM) SmartPLS 3.2.6.
Results and Discussion
Association of nuclear receptor expression
VDR and RARγ2 form heterodimers to start transcription, then it might be interesting to analyze whether VDR expression can be increased by administration of retinyl palmitate or otherwise the expression of RARγ2 can be increased by administration of D3. The post-Hoc Tukey HSD test concluded that the administration of retinyl palmitate 16 units/g BW of mice per day did not increase VDR expression (p=0.993). Vice versa, the Tukey HSD test concluded that the administration of D3 1.25 IU/g BW of mice per day did not increase the expression of RARγ2 (p=0.991). Each pathway is specifically activated by each ligand (Figures 1 and 2).
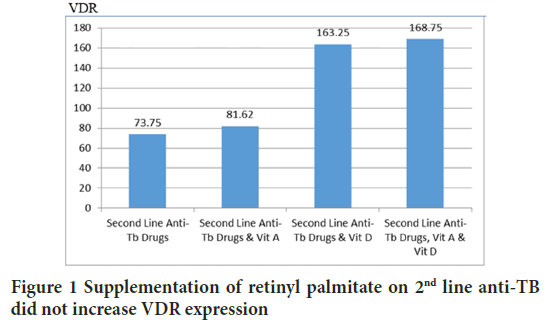
Figure 1: Supplementation of retinyl palmitate on 2nd line anti-TB did not increase VDR expression
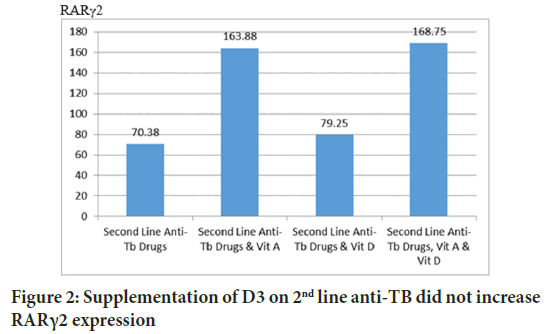
Figure 2: Supplementation of D3 on 2nd line anti-TB did not increase RARγ2 expression
Role of vitamin D3 in autophagy and decreasing MMP1
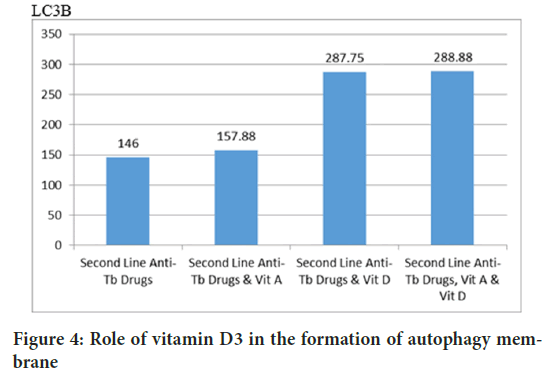
The Tukey HSD test concluded that D3 is absolutely essential in cathelicidin production (p=0.000), whereas retinyl palmitate did not increase CRAMP (p=0.860) (Figure 3). Autophagy was also analyzed by measuring LC3B-insoluble autophagosome membrane. The Mann-Whitney test concluded that cholecalciferol had a role in increasing autophagy membrane formation (p=0.001), retinyl palmitate did not increase LC3B (p=0.834) (Figures 4 and 5).
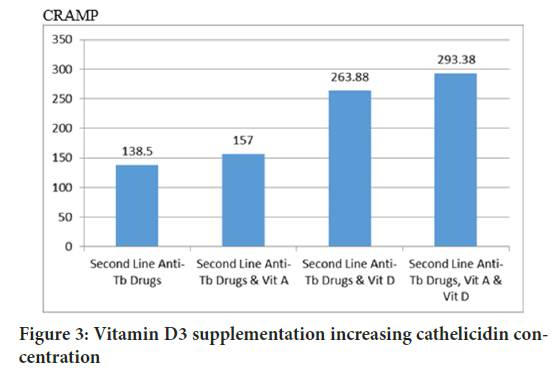
Figure 3: Vitamin D3 supplementation increasing cathelicidin concentration
Figure 4: Role of vitamin D3 in the formation of autophagy membrane
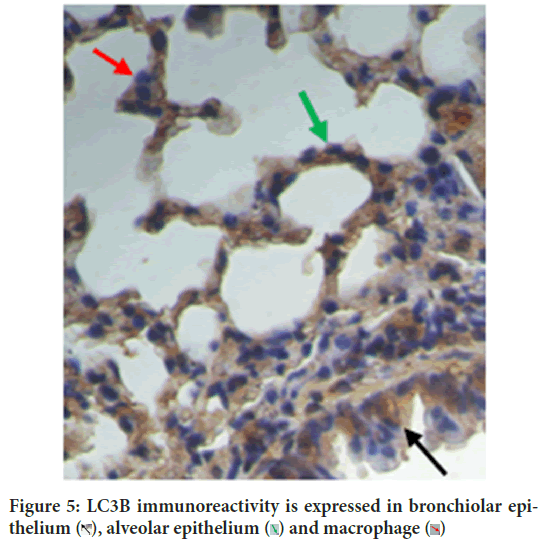
Figure 5: LC3B immunoreactivity is expressed in bronchiolar epithelium 
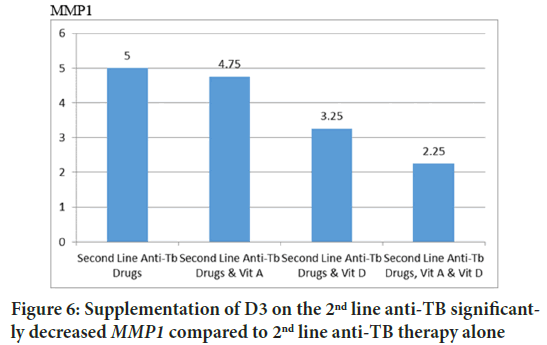
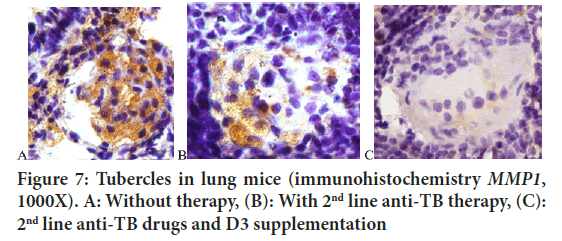
Some studies use pulmonary collagenase enzymes as markers of the effectiveness of therapy. MMP1 will decrease along with the success of therapy (Ugarte-Gil CA, et al., 2013). This study concluded that D3 supplementation significantly reduced MMP1 compared to 2nd line anti-TB therapy alone (Figures 6 and 7).
Figure 6: Supplementation of D3 on the 2nd line anti-TB significantly decreased MMP1 compared to 2nd line anti-TB therapy alone
Figure 7: Tubercles in lung mice (immunohistochemistry MMP1, 1000X). A: Without therapy, (B): With 2nd line anti-TB therapy, (C): 2nd line anti-TB drugs and D3 supplementation
Role of vitamin D3 and retinyl palmitate in apoptosis induction and bacterial suppression
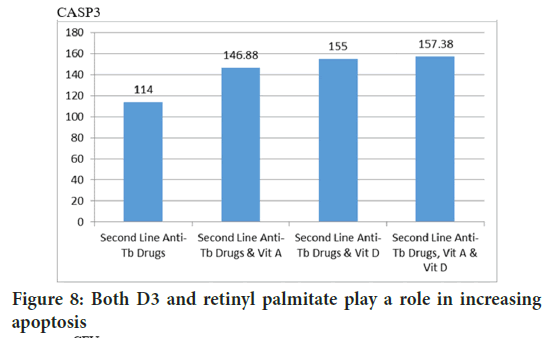
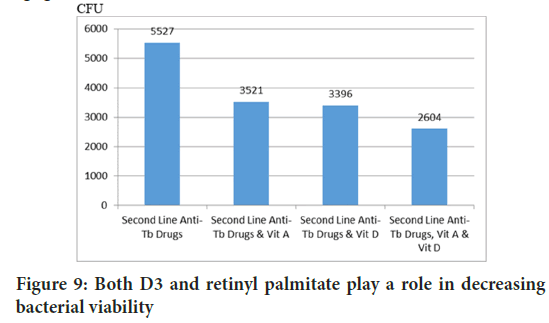
Apoptosis is the mechanism of infected cells to eliminate bacteria. The Mann-Whitney test concluded that both D3 and retinyl palmitate contribute to increase CASP3 (Figure 8) (p=0.035 and p=0.027). This is supported by the calculation of bacterial viability in lungs, both D3 and retinyl palmitate play a role in reducing CFU (p=0.000 and p=0.000) (Figure 9).
Figure 8: Both D3 and retinyl palmitate play a role in increasing apoptosis
Figure 9: Both D3 and retinyl palmitate play a role in decreasing bacterial viability
Association of vitamin D3 in combination with retinyl palmitate in necrotic cell death
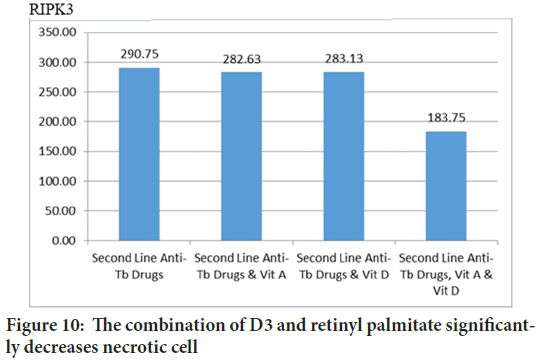
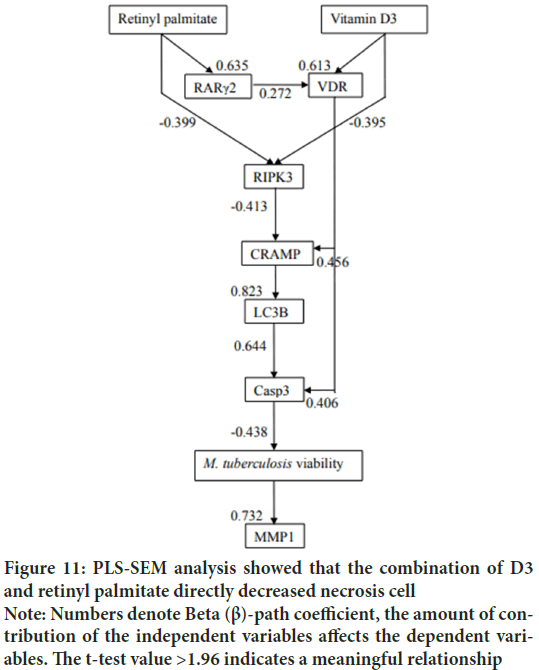
In previous study we showed that vitamin D3 reduce necrosis indirectly through increasing apoptosis or autophagy (Wahyunitisari MR, et al., 2017). This time, PLS-SEM proves that the combination of D3 and retinyl palmitate directly decreases RIPK3 (Figures 10and 11).
Figure 10: The combination of D3 and retinyl palmitate significantly decreases necrotic cell
Figure 11: PLS-SEM analysis showed that the combination of D3 and retinyl palmitate directly decreased necrosis cell Note: Numbers denote Beta (β)-path coefficient, the amount of contribution of the independent variables affects the dependent variables. The t-test value >1.96 indicates a meaningful relationship
TB and malnutrition have complex relationships. Although vitamin D can be produced by epithelial cells, lymphocytes, and antigen presenting cells, supplementation of vitamin D for active TB is still needed because of the high requirement (Wang Q, et al., 2018). Vitamin D nuclear receptors were first discovered in intestine (Brumbaugh PF, et al., 1974), then began to be found in other tissues including lung epithelial cells (Colston K, et al., 1982), and alveolar lymphocyte (Biyoudi-Vouenze R, et al., 1991).
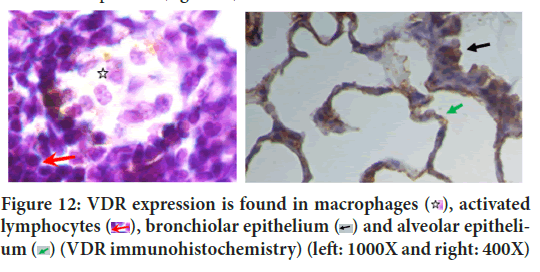
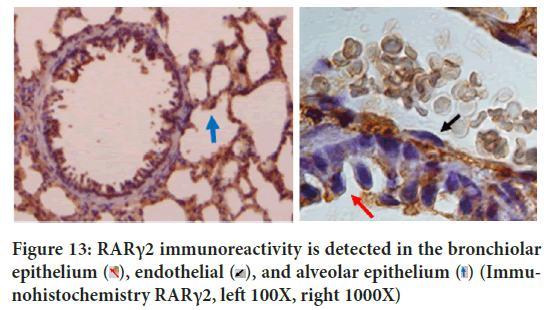
Although this study proved that VDR and RARγ2 were only induced by each ligand, PLS-SEM analysis proved that in TB pathogenesis, RARγ2 had an effect on VDR activities with path coefficient 0.272 (Figure 11). SEM not only evaluates the measurement but also asses the structural model. Structural model has been able to estimate the relationships among latent variable and model disturbances. VDR is a chromosomal protein detected in macrophages, activated lymphocytes, bronchioles epithelium and alveolar epithelium (Figure 12). RAR is proven to be able to bind to DNA enhancer sequence VDRE (Schüle R, et al., 1990). RAR can form heterodimers with VDR (Schräder M, et al., 1993). In lung tissue, RARγ2 immunoreactivity is detected in the bronchiolar epithelium, endothelial, and alveolar epithelial (Figure 13).
Figure 12: VDR expression is found in macrophages  , activated
lymphocytes
, activated
lymphocytes  , bronchiolar epithelium
, bronchiolar epithelium  and alveolar epithelium
and alveolar epithelium  (VDR immunohistochemistry) (left: 1000X and right: 400X)
(VDR immunohistochemistry) (left: 1000X and right: 400X)
Figure 13: RARγ2 immunoreactivity is detected in the bronchiolar
epithelium 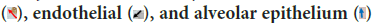 (Immunohistochemistry RARγ2, left 100X, right 1000X)
(Immunohistochemistry RARγ2, left 100X, right 1000X)
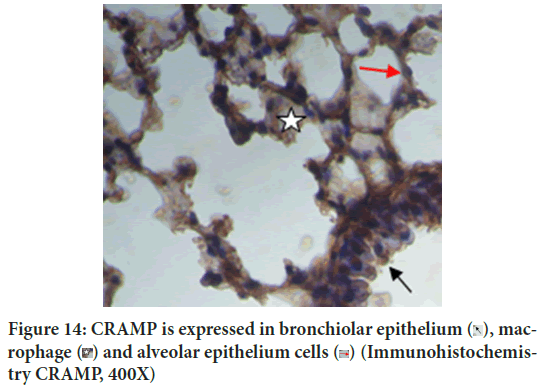
Autophagy is self-eating process. The two autophagy markers analyzed in this study were LC3B autophagy membrane precursor and antimicrobial CRAMP. CRAMP is expressed in bronchiolar epithelium, macrophage and alveolar epithelium cells (Figure 14). This study concluded that vitamin D3 plays an important role in autophagy, which is consistent with the literature. Vitamin D3 induces antimicrobial CRAMP through VDR (Liu PT, et al., 2009; Dhawan P, et al., 2015). Supplementation of retinyl palmitate does not improve CRAMP. The anti-mycobacterial activity of vitamin A was concluded through autophagosome acidification (Rajawat Y, et al., 2011; Wheelwright M, et al., 2014).
Figure 14: CRAMP is expressed in bronchiolar epithelium  , macrophage
, macrophage 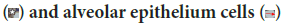 (Immunohistochemistry CRAMP, 400X)
(Immunohistochemistry CRAMP, 400X)
Vitamin D3 increases the expression of LC3B, this complete the data that vitamin D also increases the expression of ATG16L1 (Sun J, 2016), ATG5 and Beclin-1 (Yuk JM, et al., 2009; Campbell GR and Spector SA, 2011). Vitamin D supplementation is useful for immunocompromised patients, such as TB with diabetes mellitus (Kota SK, et al., 2011; Lopez-Lopez N, et al., 2014), and TB/HIV co-infection (Campbell GR and Spector SA, 2012). Vitamin D does not inhibit Th1 cell differentiation (Rode AK, et al., 2017). There is no literature that links RARγ with autophagy membrane formation. Vitamin A nuclear receptor that associated with LC3 is RARα (He W, et al., 2014).
As a general rule, host inflammatory mediators have been associated with tissue destruction. This study concluded that D3-VDR reduced lung damage through decreasing MMP1, this completes data from previous studies that concluded vitamin D3 reduced MMP7 and MMP9 expression and increased TIMP1 (Anand SP and Selvaraj P, 2009). Vitamin D3 is alveolar type-II cell growth factor (Edelson JD, et al., 1994). Vitamin D3 reduces TB immunopathology (Gemelli C, et al., 2013; Dauletbaev N, et al., 2015; Reeme AE, et al., 2016), and prevents pulmonary fibrosis (Ramirez AM , et al., 2010; Li F, et al., 2015). PLS-SEM analysis shows retinyl palmitate 16 IU/g BW decreases MMP1 expression through intermediate variables (Figure 11). The literature shows that RARγ agonist work depending on its concentration (Kimura K, et al., 2017). It is necessary to find the right concentration of supplementation so that retinyl palmitate can decreases MMP1 expression directly.
Apoptosis is a form of programmed cell death without causing an inflammatory reaction. Apoptosis is known to be an innate anti-mycobacterial mechanism in the presence of efferocytosis. According to the literature, this study proves that the retinyl palmitate-RARγ pathway induces M. tuberculosisinfected cell apoptosis (Szondy Z, et al., 1997). It has also been noted that vitamin A increases apoptosis receptor Tumor Necrosis Factor Receptor Superfamily member 6 (TNFRSF6) and apoptosis regulator gene Reticulon 3 (RTN3) (Balmer JE and Blomhoff R, 2002). The D3-VDR pathway has also been shown to increase M. tuberculosis infected cell apoptosis. In addition to the genomic pathway, D3 also increases apoptosis by increasing intracellular Ca2+ concentrations that lead to mitochondrial membrane potential change and cytochrome C release (Mehto S, et al., 2015).
Vitamin D3 is significantly associated with decreased viability of M. tuberculosis (Bloom BR and Modlin RL, 2016). In addition to direct antimycobacterial action (Greenstein RJ, et al., 2012), vitamin D reduces bacterial viability through increased macrophage activity (Abe E, et al., 1984), hepcidin expression inhibition which results in decreased intracellular iron concentrations. M. tuberculosis requires iron for its growth (Szymczak I and Pawliczak R, 2016). Vitamin A inhibits M. tuberculosis growth directly (Greenstein RJ, et al., 2012; Crowle AJ and Ross EJ, 1989), and also activating lymphocyte T and macrophages (Yamada H, et al., 2007). Necrosis is a form of premature death due to excessive oxidative stress. Necrosis and necroptosis have the same subcellular process, only the onset of necrosis is faster (Berghe TV, et al., 2010). Necrotic death of infection cells is a major contributor to lung injury. RIPK3 as a marker of necrosis, is known to play a role in NF-κB activation and inflammasome which are closely related to oxidative stress (Moriwaki K and Chan FM, 2017; Ramya D, et al., 2012; Wang Z, et al., 2016; Bohn T, 2017; Teixeira TM, et al., 2017). PLS-SEM analysis concluded that the combination of D3 and retinyl palmitate supplementation decreased RIPK3 expression directly.
Conclusion
This is supported by a literature stating that vitamin A and vitamin D increase the factors that contribute to an endogenous antioxidant activity such as Nuclear Factor like 2 (NRF2), Superoxide Dismutase 2 (SOD2), Glutathione Peroxidase (GPX), Nicotinamide adenine dinucleotide phosphate hydrogen Quinone Oxidoreductase 11 (NQO1), and Heme Oxygenase 1 (HO1). If vitamin D supplementation alone decreases cell necrosis through intermediate variables, this study proves that a combination of vitamin A and vitamin D supplementation can reduce cell necrosis directly. Proper supplementation can improve the effectiveness of TB therapy.
References
- Global tuberculosis report. World Health Organization (WHO). 2016.
- Srinivasan A, Syal K, Banerjee D, Hota D, Gupta D, Kaul D, et al. Low plasma levels of cholecalciferol and 13-cis-retinoic acid in tuberculosis: Implications in host-based chemotherapy. Nutrition. 2013; 29(10): 1245-1251.
[Crossref] [Google Scholar] [Pubmed]
- Schräder M, Müller KM, Becker-André M, Carlberg C. Response element selectivity for heterodimerization of vitamin D receptors with retinoic acid and retinoid X receptors. J Mol Endocrinol. 1994; 12(3): 327-39.
[Crossref] [Google Scholar] [Pubmed]
- Koszewski NJ, Herberth J, Malluche HH. Retinoic acid receptor gamma 2 interactions with vitamin D response elements. J Steroid Biochem Mol Biol. 2010; 120(4-5): 200-207.
[Crossref] [Google Scholar] [Pubmed]
- Syal K, Srinivasan A, Banerjee D. VDR, RXR, coronin-1 and interferonγ levels in PBMCs of type-2 diabetes patients: Molecular link between diabetes and tuberculosis. Indian J Clin Biochem. 2015; 30: 323-328.
[Crossref] [Google Scholar] [Pubmed]
- Chengprapakorn W. Toll like receptor 2 and Toll like receptor 4 activation and vitamin D effects in mouse osteoblasts. University of California. 2016.
- Ugarte-Gil CA, Elkington P, Gilman RH, Coronel J, Tezera LB, Bernabe-Ortiz A, et al. Induced sputum MMP-1,-3 and -8 concentrations during treatment of tuberculosis. PloS One. 2013; 8(4): e61333.
[Crossref] [Google Scholar] [Pubmed]
- Wahyunitisari MR, Mertaniasih NM, Amin M, Artama WT, Koendhori EB. Vitamin D, cell death pathways, and tuberculosis. Int J Mycobacteriol. 2017; 6(4): 349-355.
[Crossref] [Google Scholar] [PubMed]
- Wang Q, Liu Y, Ma Y, Han L, Dou M, Zou Y, et al. Severe hypovitaminosis D in active tuberculosis patients and its predictors. Clin Nutr. 2018; 37(3): 1034-1040.
[Crossref] [Google Scholar] [Pubmed]
- Brumbaugh PF, Haussler MR. 1α, 25-Dihydroxycholecalciferol receptors in intestine: I. association of 1α, 25-dihydroxycholecalciferol with intestinal mucosa chromatin. J Biol Chem. 1974; 249(4): 1251-1257.
[Crossref] [Google Scholar] [Pubmed]
- Colston K, Colston MJ, Fieldsteel AH, Feldman D. 1, 25-dihydroxyvitamin D3 receptors in human epithelial cancer cell lines. Cancer Res. 1982;42(3):856-859.
[Google Scholar] [Pubmed]
- Biyoudi-Vouenze R, Cadranel J, Valeyre D, Milleron B, Hance AJ, Soler P. Expression of 1, 25 (OH) 2D3 receptors on alveolar lymphocytes from patients with pulmonary granulomatous diseases. Am Rev Respir Dis. 1991; 143(6): 1376-1380.
[Crossref] [Google Scholar] [Pubmed]
- Schüle R, Umesono K, Mangelsdorf DJ, Bolado J, Pike JW, Evans RM. Jun-Fos and receptors for vitamins A and D recognize a common response element in the human osteocalcin gene. Cell. 1990; 61(3): 497-504.
[Crossref] [Google Scholar] [Pubmed]
- Schräder M, Bendik I, Becker-Andre M, Carlberg C. Interaction between retinoic acid and vitamin D signaling pathways. J Biol Chem. 1993;268(24):17830-17836.
[Crossref] [Google Scholar] [Pubmed]
- Liu PT, Schenk M, Walker VP, Dempsey PW, Kanchanapoomi M, Wheelwright M, et al. Convergence of IL-1β and VDR activation pathways in human TLR2/1-induced antimicrobial responses. PloS One. 2009; 4(6): e5810.
[Crossref] [Google Scholar] [Pubmed]
- Dhawan P, Wei R, Sun C, Gombart AF, Koeffler HP, Diamond G, et al. C/EBPα and the vitamin D receptor cooperate in the regulation of cathelicidin in lung epithelial cells. J Cell Physiol. 2015; 230(2): 464-472.
[Crossref] [Google Scholar] [Pubmed]
- Rajawat Y, Hilioti Z, Bossis I. Retinoic acid induces autophagosome maturation through redistribution of the cation-independent mannose-6-phosphate receptor. Antioxid Redox Signal. 2011;14(11):2165-77.
[Crossref] [Google Scholar] [Pubmed]
- Wheelwright M, Kim EW, Inkeles MS, de Leon A, Pellegrini M, Krutzik SR, et al. All-trans retinoic acid-triggered antimicrobial activity against Mycobacterium tuberculosisis dependent on NPC2. J Immunol. 2014; 192(5): 2280-2290.
[Crossref] [Google Scholar] [Pubmed]
- Sun J. VDR/vitamin D receptor regulates autophagic activity through ATG16L1. Autophagy. 2016; 12(6): 1057-1058.
[Crossref] [Google Scholar] [Pubmed]
- Yuk JM, Shin DM, Lee HM, Yang CS, Jin HS, Kim KK, et al. Vitamin D3 induces autophagy in human monocytes/macrophages via cathelicidin. Cell Host Microbe. 2009; 6(3): 231-243.
[Crossref] [Google Scholar] [Pubmed]
- Campbell GR, Spector SA. Hormonally active vitamin D3 (1α, 25-dihydroxycholecalciferol) triggers autophagy in human macrophages that inhibits HIV-1 infection. J Biol Chem. 2011; 286(21): 18890-18902.
[Crossref] [Google Scholar] [Pubmed]
- Kota SK, Jammula S, Kota SK, Tripathy PR, Panda S, Modi KD. Effect of vitamin D supplementation in type 2 diabetes patients with pulmonary tuberculosis. Diabetes Metab Syndr. 2011; 5(2): 85-89.
[Crossref] [Google Scholar] [Pubmed]
- Lopez-Lopez N, Gonzalez-Curiel I, Castaneda-Delgado J, Montoya-Rosales A, Gandara-Jasso B, Enciso-Moreno JA, et al. Vitamin D supplementation promotes macrophages' anti-mycobacterial activity in type 2 diabetes mellitus patients with low vitamin D receptor expression. Microbes Infect. 2014; 16(9): 755-761.
[Crossref] [Google Scholar] [Pubmed]
- Campbell GR, Spector SA. Autophagy induction by vitamin D inhibits both Mycobacterium tuberculosis and human immunodeficiency virus type 1. Autophagy. 2012; 8(10): 1523-1525.
[Crossref] [Google Scholar] [Pubmed]
- Rode AK, Kongsbak M, Hansen MM, Lopez DV, Levring TB, Woetmann A, et al. Vitamin D counteracts Mycobacterium tuberculosis-induced cathelicidin downregulation in dendritic cells and allows Th1 differentiation and IFNγ secretion. Front Immunol. 2017; 8: 253262.
[Crossref] [Google Scholar] [Pubmed]
- He W, Hu CX, Hou JK, Fan L, Xu YW, Liu MH, et al. Microtubule-associated protein 1 light chain 3 interacts with and contributes to growth inhibiting effect of PML. PLoS One. 2014; 9(11): e113089.
[Crossref] [Google Scholar] [Pubmed]
- Anand SP, Selvaraj P. Effect of 1, 25 dihydroxyvitamin D3 on matrix metalloproteinases MMP-7, MMP-9 and the inhibitor TIMP1 in pulmonary tuberculosis. Clin Immunol. 2009; 133(1): 126-131.
[Crossref] [Google Scholar] [Pubmed]
- Edelson JD, Chan S, Jassal D, Post M, Tanswell AK. Vitamin D stimulates DNA synthesis in alveolar type-II cells. Biochim Biophys Acta. 1994; 1221(2): 159-166.
[Crossref] [Google Scholar] [Pubmed]
- Gemelli C, Martello A, Montanari M, Marani TZ, Salsi V, Zappavigna V, et al. The Orosomucoid 1 protein is involved in the vitamin D-mediated macrophage de-activation process. Exp Cell Res. 2013; 319(20): 3201-3213.
[Crossref] [Google Scholar] [Pubmed]
- Dauletbaev N, Herscovitch K, Das M, Chen H, Bernier J, Matouk E, et al. Down‐regulation of IL‐8 by high‐dose vitamin D is specific to hyperinflammatory macrophages and involves mechanisms beyond up‐regulation of DUSP1. Br J Pharmacol. 2015; 172(19): 4757-4771.
[Crossref] [Google Scholar] [Pubmed]
- Reeme AE, Robinson RT. Dietary vitamin D3 suppresses pulmonary immunopathology associated with late-stage tuberculosis in C3HeB/FeJ mice. J Immunol. 2016; 196(3): 1293-1304.
[Crossref] [Google Scholar] [Pubmed]
- Ramirez AM, Wongtrakool C, Welch T, Steinmeyer A, Zügel U, Roman J. Vitamin D inhibition of pro-fibrotic effects of transforming growth factor β1 in lung fibroblasts and epithelial cells. J Steroid Biochem Mol Biol. 2010; 118(3): 142-150.
[Crossref] [Google Scholar] [Pubmed]
- Li F, Zhang A, Shi Y, Ma Y, Du Y. 1α, 25-Dihydroxyvitamin D3 prevents the differentiation of human lung fibroblasts via microRNA-27b targeting the vitamin D receptor. Int J Mol Med. 2015; 36(4): 967-974.
[Crossref] [Google Scholar] [Pubmed]
- Kimura K, Zhou H, Orita T, Kobayashi M, Nishida T, Sonoda KH. Suppression by an RAR-γ agonist of collagen degradation mediated by corneal fibroblasts. Invest Ophthalmol Vis Sci. 2017; 58(4): 2250-2257.
[Crossref] [Google Scholar] [Pubmed]
- Szondy Z, Reichert U, Bernardon JM, Michel S, Tóth R, Ancian P, et al. Induction of apoptosis by retinoids and retinoic acid receptor γ-selective compounds in mouse thymocytes through a novel apoptosis pathway. Mol Pharmacol. 1997; 51(6): 972-982.
[Crossref] [Google Scholar] [Pubmed]
- Balmer JE, Blomhoff R. Gene expression regulation by retinoic acid. J Lipid Res. 2002; 43(11): 1773-1808.
[Crossref] [Google Scholar] [Pubmed]
- Mehto S, Antony C, Khan N, Arya R, Selvakumar A, Tiwari BK, et al. Mycobacterium tuberculosis and human immunodeficiency virus type 1 cooperatively modulate macrophage apoptosis via toll like receptor 2 and calcium homeostasis. PLoS One. 2015; 10(7): e0131767.
[Crossref] [Google Scholar] [Pubmed]
- Bloom BR, Modlin RL. Mechanisms of defense against intracellular pathogens mediated by human macrophages. Microbiol Spectr. 2016; 4(3): 10-128.
[Crossref] [Google Scholar] [Pubmed]
- Greenstein RJ, Su L, Brown ST. Vitamins A and D inhibit the growth of mycobacteria in radiometric culture. PloS One. 2012; 7(1): e29631.
[Crossref] [Google Scholar] [Pubmed]
- Abe E, Shiina YO, Miyaura C, Tanaka H, Hayashi T, Kanegasaki S, et al. Activation and fusion induced by 1 alpha, 25-dihydroxyvitamin D3 and their relation in alveolar macrophages. Proc Natl Acad Sci USA. 1984; 81(22): 7112-7116.
[Crossref] [Google Scholar] [Pubmed]
- Szymczak I, Pawliczak R. The active metabolite of vitamin D3 as a potential immunomodulator. Scand J Immunol. 2016; 83(2): 83-91.
[Crossref] [Google Scholar] [Pubmed]
- Crowle AJ, Ross EJ. Inhibition by retinoic acid of multiplication of virulent tubercle bacilli in cultured human macrophages. Infect Immun. 1989; 57(3): 840-844.
[Crossref] [Google Scholar] [Pubmed]
- Yamada H, Mizuno S, Ross AC, Sugawara I. Retinoic acid therapy attenuates the severity of tuberculosis while altering lymphocyte and macrophage numbers and cytokine expression in rats infected with Mycobacterium tuberculosis. J Nutr. 2007; 137(12): 2696-2700.
[Crossref] [Google Scholar] [Pubmed]
- Berghe TV, Vanlangenakker N, Parthoens E, Deckers W, Devos M, Festjens N, et al. Necroptosis, necrosis and secondary necrosis converge on similar cellular disintegration features. Cell Death Differ. 2010; 17(6): 922-930.
[Crossref] [Google Scholar] [Pubmed]
- Moriwaki K, Chan FM. The inflammatory signal adaptor RIPK3: Functions beyond necroptosis. Int Rev Cell Mol Biol. 2017; 328: 253-275.
[Crossref] [Google Scholar] [Pubmed]
- Ramya D, Siddikuzzaman, Manjamalai A, Berlin Grace VM. Chemoprotective effect of All-Trans Retinoic Acid (ATRA) on oxidative stress and lung metastasis induced by benzo (a) pyrene. Immunopharmacol Immunotoxicol. 2012; 34(2): 317-325.
[Crossref] [Google Scholar] [Pubmed]
- Wang Z, Zhang H, Sun X, Ren L. The protective role of vitamin D3 in a murine model of asthma via the suppression of TGF-β/Smad signaling and activation of the Nrf2/HO-1 pathway. Mol Med Rep. 2016; 14(3): 2389-2396.
[Crossref] [Google Scholar] [Pubmed]
- Bohn T. Bioactivity of carotenoids-chasms of knowledge. Int J Vitam Nutr Res. 2017;87(1-2):5-9.
[Crossref] [Google Scholar] [Pubmed]
- Teixeira TM, da Costa DC, Resende AC, Soulage CO, Bezerra FF, Daleprane JB. Activation of Nrf2-antioxidant signaling by 1, 25-dihydroxycholecalciferol prevents leptin-induced oxidative stress and inflammation in human endothelial cells. J Nutr. 2017; 147(4): 506-513.
[Crossref] [Google Scholar] [Pubmed]
Author Info
Hamid Didi* and Made EkoCitation: Didi H: Complementary Medicine in Tuberculosis
Received: 31-Jan-2024 Accepted: 15-Feb-2024 Published: 22-Feb-2024, DOI: 10.31858/0975-8453.15.2.74-80
Copyright: This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
ARTICLE TOOLS
- Dental Development between Assisted Reproductive Therapy (Art) and Natural Conceived Children: A Comparative Pilot Study Norzaiti Mohd Kenali, Naimah Hasanah Mohd Fathil, Norbasyirah Bohari, Ahmad Faisal Ismail, Roszaman Ramli SRP. 2020; 11(1): 01-06 » doi: 10.5530/srp.2020.1.01
- Psychometric properties of the World Health Organization Quality of life instrument, short form: Validity in the Vietnamese healthcare context Trung Quang Vo*, Bao Tran Thuy Tran, Ngan Thuy Nguyen, Tram ThiHuyen Nguyen, Thuy Phan Chung Tran SRP. 2020; 11(1): 14-22 » doi: 10.5530/srp.2019.1.3
- A Review of Pharmacoeconomics: the key to “Healthcare for All” Hasamnis AA, Patil SS, Shaik Imam, Narendiran K SRP. 2019; 10(1): s40-s42 » doi: 10.5530/srp.2019.1s.21
- Deuterium Depleted Water as an Adjuvant in Treatment of Cancer Anton Syroeshkin, Olga Levitskaya, Elena Uspenskaya, Tatiana Pleteneva, Daria Romaykina, Daria Ermakova SRP. 2019; 10(1): 112-117 » doi: 10.5530/srp.2019.1.19
- Dental Development between Assisted Reproductive Therapy (Art) and Natural Conceived Children: A Comparative Pilot Study Norzaiti Mohd Kenali, Naimah Hasanah Mohd Fathil, Norbasyirah Bohari, Ahmad Faisal Ismail, Roszaman Ramli SRP. 2020; 11(1): 01-06 » doi: 10.5530/srp.2020.1.01
- Manilkara zapota (L.) Royen Fruit Peel: A Phytochemical and Pharmacological Review Karle Pravin P, Dhawale Shashikant C SRP. 2019; 10(1): 11-14 » doi: 0.5530/srp.2019.1.2
- Pharmacognostic and Phytopharmacological Overview on Bombax ceiba Pankaj Haribhau Chaudhary, Mukund Ganeshrao Tawar SRP. 2019; 10(1): 20-25 » doi: 10.5530/srp.2019.1.4
- A Review of Pharmacoeconomics: the key to “Healthcare for All” Hasamnis AA, Patil SS, Shaik Imam, Narendiran K SRP. 2019; 10(1): s40-s42 » doi: 10.5530/srp.2019.1s.21
- A Prospective Review on Phyto-Pharmacological Aspects of Andrographis paniculata Govindraj Akilandeswari, Arumugam Vijaya Anand, Palanisamy Sampathkumar, Puthamohan Vinayaga Moorthi, Basavaraju Preethi SRP. 2019; 10(1): 15-19 » doi: 10.5530/srp.2019.1.3