Short Communication - (2024) Volume 15, Issue 1
Computational Analysis of Density Functional Theory (DFT method), Thermodynamic Investigations and Molecular Docking Studies on 1-(2'-Thiophen)-3- (2,3,5-trichlorophenyl)-2-propen-1-one
Hacer Gümüş1* and Cengiz İpek2Abstract
In this study, quantum chemical calculations of 1-(2’-Thiophen)-3-(2,3,5-trichlorophenyl)-2-propen-1-one have been performed using Gaussian 09 program. Theoretical computational analysis data of the molecule in the ground state have been calculated using HSEH1PBE (The exchange part of the screened Coulomb potential of Heyd, Scuseria, and Ernzerhof) and Becke’s 3-Parameter (B3LYP) hybrid functional using B exchange and, Lee-Yang-Par (LYP) correlation levels of Density Functional Method (DFT) with the 6-311++G(d,p) basis set. The effect of temperature on the 1-(2´-Thiophen)-3-(2,3,5-trichlorophenyl)-2-propen-1-one and its thermodynamic parameters entropy, enthalpy, and heat capacity have been analyzed. Mulliken, Natural Bond Orbital (NBO) charges and Atomic Polar Tensor (APT) charges of the investigated molecule have also been calculated. In addition, the molecular frontier orbital energies Highest Occupied Molecular Orbital (HOMO), HOMO-1, Lowest Unoccupied Molecular Orbital (LUMO) and LUMO+1) of the 1-(2´-Thiophen)-3-(2,3,5-trichlorophenyl)-2-propen-1-one have been calculated. Finally, Molecular Docking (MD) study has been carried out with the help of AutoDock computational program.
Keywords
1-(2´-Thiophen)-3-(2,3,5-trichlorophenyl)-2-propen-1-one, DFT, APT charge, Molecular docking
Introduction
Thiophene based materials, due to the richness of thiophene chemistry and the general stability of its compounds, have found applications in fields ranging from antistatic coatings to polymer electronics (Manjunath HR, et al., 2011; Huang W, et al., 1998). In medicinal chemistry, sulfur-containing heterocyclic is well known for its therapeutic applications. Thiophene-containing compounds are also widely used in electroluminescent polymer, electronic and optoelectronic devices, and modern drug design (Blumstengel S, et al., 1999; Batista RM, et al., 2009; Hosmane RS and Liebman JF, 1991). Compounds containing thiophene nucleus possess a broad range of biological activities such as anti-inflammatory, analgesic, antifungal, ocular hypertensive activities, and antimicrobial activities (Lin JW, Dudek LP, 1980; Jen KY, et al., 1986; Hu X and Xu L, 2000).
About the Study
Computational details
The molecular simulation of 1-(2´-Thiophen)-3-(2,3,5-trichlorophenyl)-2-propen-1-one molecule was performed with Gaussian 09W program package (Frisch MJ, et al., 2009) and the output files were visualized by means of the GaussianView 5 software (Dennington R, et al., 2009). All theoretical data of 1-(2´-Thiophen)-3-(2,3,5-trichlorophenyl)-2-propen-1-one molecule were calculated using B3LYP (Becke’s three parameter hybrid functional using the LYP correlation functional) (Becke AD, 1992; Lee C, et al., 1988) and HSEH1PBE (HeydScuseria-Ernzerh of functional) (Heyd J and Scuseria GE, 2004; Heyd J, Scuseria GE, 2004; Heyd J, et al., 2005; Heyd J, et al., 2003) levels with 6-311++G(d,p) basis set (Frisch MJ, et al., 1984).Geometric structure
Optimized geometrical structure of 1-(2´-Thiophen)-3-(2,3,5- trichlorophenyl)-2-propen-1-one molecule was shown in Figure 1.
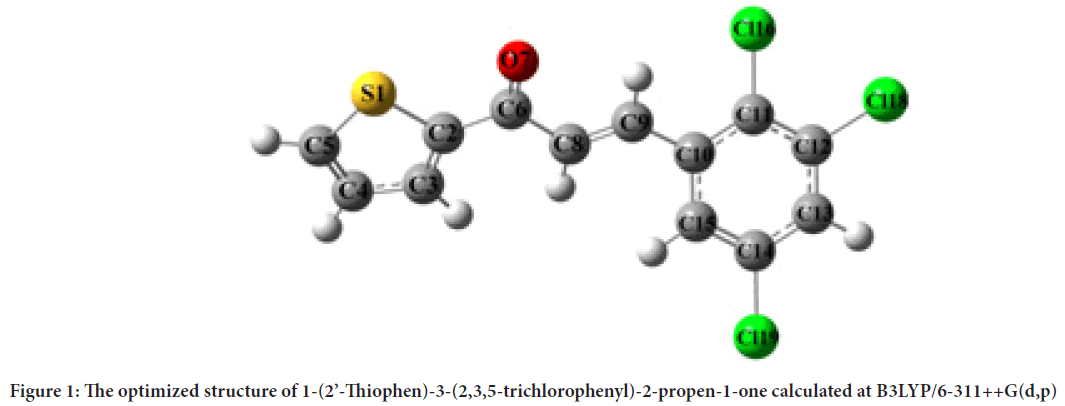
Figure 1: The optimized structure of 1-(2’-Thiophen)-3-(2,3,5-trichlorophenyl)-2-propen-1-one calculated at B3LYP/6-311++G(d,p)
Thermodynamic properties
The thermodynamic values of the 1-(2´-Thiophen)-3-(2,3,5-trichlorophenyl)-2-propen-1-one molecule was calculated at DFT/B3LYP and DFT/HSEH1PBE methods. The calculated thermodynamic parameters were presented as shown in Table 1.
| Thermal energy, E (Kcal/mol) | B3LYP/6-311++G (d,p) | HSEH1PBE/6-311++G (d,p) |
|---|---|---|
| Rotational | 0.889 | 0.889 |
| Translational | 0.889 | 0.889 |
| Vibrational | 109.807 | 110.72 |
| Total | 111.584 | 112.498 |
| Heat capacity, Cv (cal/mol K) | ||
| Rotational | 2.981 | 2.981 |
| Translational | 2.981 | 2.981 |
| Vibrational | 54.395 | 53.917 |
| Total | 60.356 | 59.879 |
| Entropy, S (cal/mol K) | ||
| Rotational | 35.331 | 35.299 |
| Translational | 43.147 | 43.147 |
| Vibrational | 59.322 | 58.899 |
| Total | 137.8 | 137.345 |
| Rotational constants (GHz) | ||
| A | 0.48472 | 0.49041 |
| B | 0.11713 | 0.11839 |
| C | 0.09612 | 0.0971 |
| Rotational temperature (Kelvin) | ||
| A | 0.02326 | 0.02354 |
| B | 0.00562 | 0.00568 |
| C | 0.00461 | 0.00466 |
| Thermal properties (Hartree/particle) | ||
| Zero-point correction | 0.161242 | 0.162813 |
| Thermal correction to energy | 0.177821 | 0.179276 |
| Thermal correction to enthalpy | 0.178765 | 0.18022 |
| Thermal correction to Gibbs free energy | 0.113292 | 0.114963 |
| Sum of electronic and zero-point Energies | -2353.67 | -2352.47 |
| Sum of electronic and thermal energies | -2353.65 | -2352.45 |
| Sum of electronic and thermal free energies | -2353.72 | -2352.52 |
| Zero point vibrational energy (kcal/mol) | 101.1809 | 102.1668 |
Table 1: Thermodynamic parameters of 1-(2’-Thiophen)-3-(2,3,5-trichlorophenyl)-2-propen-1-one
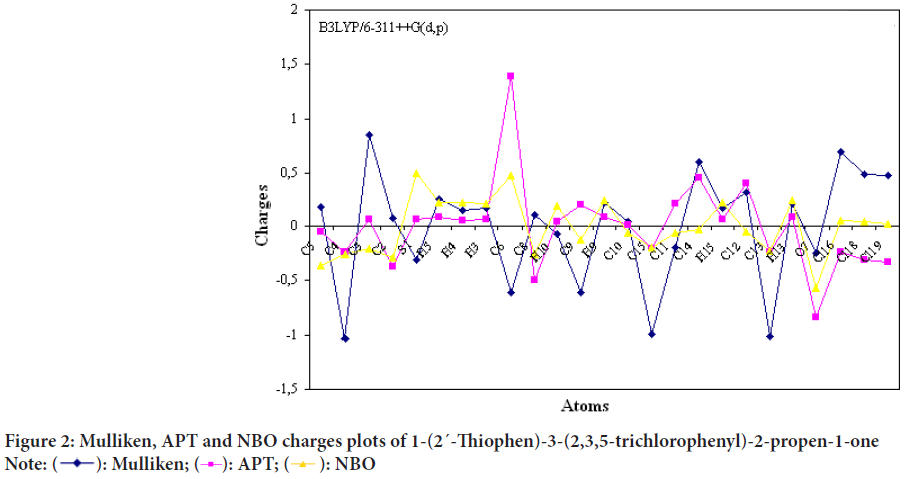
Mulliken, APT, NBO charge analysis
Mulliken, APT and NBO charges of 1-(2´-Thiophen)-3-(2,3,5-trichlorophenyl)-2-propen-1-one molecule were calculated and results were given in Figure 2 and Table 2.
| Atom | Mulliken | APT | NBO | |||
|---|---|---|---|---|---|---|
| B3LYP | HSEH1PBE | B3LYP | HSEH1PBE | B3LYP | HSEH1PBE | |
| C5 | 0.182877 | 0.242625 | -0.0512 | -0.06046 | -0.35915 | -0.37059 |
| C4 | -1.02756 | -1.2941 | -0.23192 | -0.23498 | -0.26104 | -0.26769 |
| C3 | 0.853826 | 1.084759 | 0.067185 | 0.060301 | -0.19995 | -0.2062 |
| C2 | 0.078725 | 0.04221 | -0.37674 | -0.38242 | -0.28708 | -0.29755 |
| S1 | -0.30987 | -0.44474 | 0.069065 | 0.074749 | 0.49527 | 0.50474 |
| H5 | 0.253474 | 0.302216 | 0.088389 | 0.094965 | 0.22764 | 0.23554 |
| H4 | 0.155224 | 0.195729 | 0.057511 | 0.063034 | 0.22129 | 0.22823 |
| H3 | 0.168715 | 0.222099 | 0.071079 | 0.076065 | 0.21709 | 0.22409 |
| C6 | -0.61573 | -0.69056 | 1.382795 | 1.390493 | 0.4781 | 0.47821 |
| C8 | 0.113801 | 0.137899 | -0.49717 | -0.50789 | -0.25304 | -0.26317 |
| H10 | -0.07143 | -0.07955 | 0.045291 | 0.050518 | 0.19546 | 0.20248 |
| C9 | -0.60694 | -0.66571 | 0.206979 | 0.205659 | -0.11967 | -0.1259 |
| H9 | 0.22852 | 0.271274 | 0.087625 | 0.09289 | 0.24091 | 0.24828 |
| C10 | 0.049648 | 0.08434 | 0.013585 | 0.011463 | -0.06042 | -0.06498 |
| C15 | -0.98813 | -1.104 | -0.20286 | -0.20434 | -0.19811 | -0.20342 |
| C11 | -0.18953 | -0.31847 | 0.211441 | 0.205244 | -0.05607 | -0.0634 |
| C14 | 0.598797 | 0.530977 | 0.455038 | 0.44591 | -0.02654 | -0.03697 |
| H15 | 0.175482 | 0.214328 | 0.066657 | 0.069803 | 0.22553 | 0.2322 |
| C12 | 0.321991 | 0.361641 | 0.402394 | 0.403663 | -0.0519 | -0.06075 |
| C13 | -1.00449 | -1.04518 | -0.24013 | -0.23866 | -0.225 | -0.23029 |
| H13 | 0.217082 | 0.259692 | 0.088641 | 0.092138 | 0.23997 | 0.24648 |
| O7 | -0.24489 | -0.22566 | -0.83519 | -0.83888 | -0.56578 | -0.5613 |
| Cl16 | 0.690923 | 0.785682 | -0.23989 | -0.23626 | 0.05408 | 0.06401 |
| Cl18 | 0.490234 | 0.561975 | -0.30383 | -0.30188 | 0.04816 | 0.05759 |
| Cl19 | 0.479251 | 0.570533 | -0.33476 | -0.33111 | 0.02024 | 0.03037 |
Table 2: The Mulliken, APT and NBO charges of 1-(2’-Thiophen)-3-(2,3,5-trichlorophenyl)-2-propen-1-one
Figure 2: Mulliken, APT and NBO charges plots of 1-(2´-Thiophen)-3-(2,3,5-trichlorophenyl)-2-propen-1-one
Electronic properties
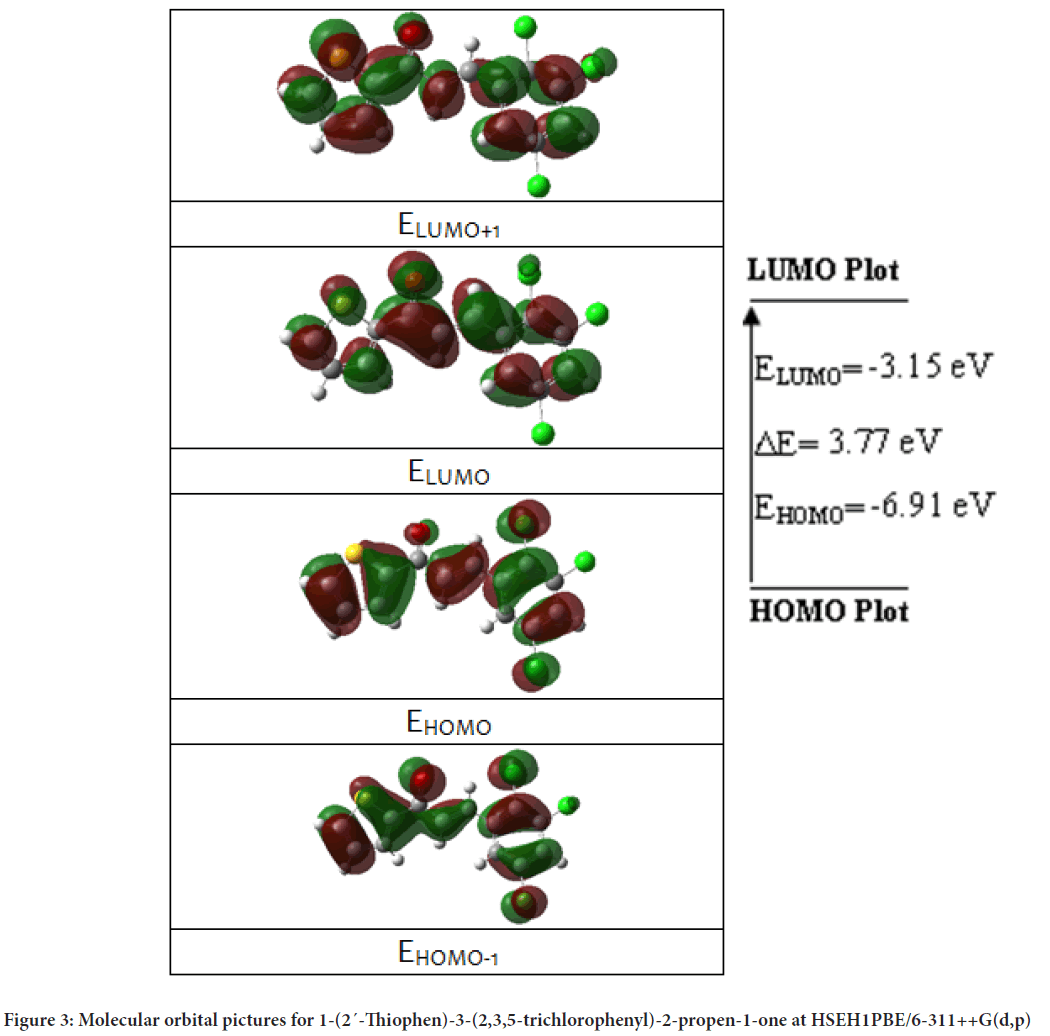
The HOMO and LUMO are very important parameters in the electronic studies by quantum chemical calculations. The total energy, HOMO and LUMO energies, the energy gap (ΔE), the ionization potential (I), the electron affinity (A), the absolute electronegativity (c), the absolute hardness (h) and softness (S) for 1-(2´-Thiophen)-3-(2,3,5-trichlorophenyl)-2-propen-1-one were calculated at B3LYP and HSEH1PBE levels in the 6-311++G(d,p) basis set, and the results were presented in Table 3. The frontier orbital picture was depicted in Figure 3 with the 3D plots for the gas phase, and the positive and negative phases are represented in red and green color, respectively.
| Electronic properties | B3LYP /6-311++G(d,p) | HSEH1PBE/6-311++G(d,p) |
|---|---|---|
| EHOMO (eV) | -7.10196 | -6.91447 |
| ELUMO (eV) | -2.95382 | -3.14593 |
| ΔE=ELUMO-EHOMO (eV) | 4.148136 | 3.768535 |
| I (eV) | 7.101955 | 6.914468 |
| A (eV) | 2.953819 | 3.145933 |
| c (eV) | 5.027887 | -5.0302 |
| h (eV) | 2.074068 | -1.88427 |
| S (eV-1) | 0.070403 | -0.07231 |
| ETotal (a.u) | -2353.83 | -2352.63 |
Table 3: Fragment Molecular Orbital (FMOs), energies and calculated physico-chemical properties
Figure 3: Molecular orbital pictures for 1-(2´-Thiophen)-3-(2,3,5-trichlorophenyl)-2-propen-1-one at HSEH1PBE/6-311++G(d,p)
Molecular docking
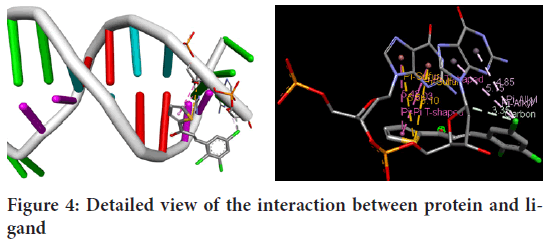
1-(2´-Thiophen)-3-(2,3,5-trichlorophenyl)-2-propen-1-one molecule docking has been examined in active sites of the selected protein. The 1Z8V protein exhibits the min binding energy of -4.95 kcal/mol, intermolecular energy of -5.4 kcal/mol, and inhibition constant of 234.9 micromolar (uM). The deviation between the ligand-protein has been analyzed, where the Root Mean Square Deviation (RMSD) value has been calculated as 2.63 for 1-(2´-Thiophen)-3-(2,3,5-trichlorophenyl)-2-propen-1-one. The molecular interaction diagrams of target protease Protein Data Bank (PDB) 1Z8V and ligand (1-(2´-Thiophen)-3-(2,3,5-trichlorophenyl)-2-propen-1-one) were shown in Figure 4.
Figure 4: Detailed view of the interaction between protein and ligand
Conclusion
The geometry of 1-(2´-Thiophen)-3-(2,3,5-trichlorophenyl)-2-propen-1-one molecule was optimized in different levels with DFT/B3LYP and DFT/ HSEH1PBE method using 6-311++G(d,p) basis set. The correlations between the statistical thermo-dynamics and temperature are also obtained. It is seen that the heat capacities, entropies and enthalpies increase with the increasing temperature owing to the intensities of the molecular vibrations increase with increasing temperature. Frontier molecular orbitals, energies and energy gap between HOMO and LUMO were calculated. Additionally, interaction between 1-(2´-Thiophen)-3-(2,3,5-trichlorophenyl)-2-pro-pen-1-one and PDB 1Z8V protein has been docked.
References
- Manjunath HR, Kumar PR, Naveen S, Ravindrachary V, Sridhar MA, Prasad JS, et al. Growth, characterization, crystal and molecular structure studies of 1-(2′-thiophen)-3-(2, 3, 5-trichlorophenyl)-2-propen-1-one. J Cryst Growth. 2011; 327(1): 161-166.
- Huang W, Yu WL, Meng H, Pei J, Li SF. New series of blue-light-emitting polymers constituted of 3-alkylthiophenes and 1, 4-di (1, 3, 4-oxadiazolyl) phenylene. Chem Mater. 1998; 10(11): 3340-3345.
- Blumstengel S, Sokolik I, Dorsinville R, Voloschenko D, He M, Lavrentovich O, et al. Photo-, and electroluminescence studies of 2, 5-bis [2′-(4 ″-(6-hexoxy benzyl))-1′-ethenyl]-3, 4-dibutyl thiophenes. Synth Met. 1999; 99(1): 85-90.
- Batista RM, Costa SP, Belsley M, Raposo MM. Synthesis and optical properties of novel, thermally stable phenanthrolines bearing an arylthienyl-imidazo conjugation pathway. Dyes Pigm. 2009; 80(3): 329-336.
- Hosmane RS, Liebman JF. Aromaticity of heterocycles: Experimental realization of dewar-breslow definition of aromaticity. Tetrahedron Lett. 1991; 32(32): 3949-3952.
- Lin JW, Dudek LP. Synthesis and properties of poly (2, 5‐thienylene). J Polym Sci Polym Chem Ed. 1980; 18(9): 2869-2873.
- Jen KY, Miller GG, Elsenbaumer RL. Highly conducting, soluble, and environmentally-stable poly (3-alkylthiophenes). J Chem Soc Chem Commun. 1986; 17: 1346-1347.
- Hu X, Xu L. Structure and properties of 3-alkoxy substituted polythiophene synthesized at low temperature. Polymer. 2000; 41(26): 9147-9154.
- Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, et al. Gaussian 09, Revision A.1. Gaussian, Inc., Wallingford CT. 2009
- Dennington R, Keith T, Millam J. GaussView, version 5. 2009.
- Becke AD. Density‐functional thermochemistry. I. The effect of the exchange‐only gradient correction. J Chem Phys. 1992; 96(3): 2155-2160.
- Lee C, Yang W, Parr RG. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys Rev B Condens Matter. 1988; 37(2): 785.
[Crossref] [Google Scholar] [Pubmed]
- Heyd J, Scuseria GE. Efficient hybrid density functional calculations in solids: Assessment of the Heyd-Scuseria-Ernzerhof screened Coulomb hybrid functional. The J Chem Phys. 2004; 121(3): 1187-1192.
[Crossref] [Google Scholar] [Pubmed]
- Heyd J, Scuseria GE. Assessment and validation of a screened Coulomb hybrid density functional. J Chem Phys. 2004; 120(16): 7274-7280.
[Crossref] [Google Scholar] [Pubmed]
- Heyd J, Peralta JE, Scuseriaa GE, Martin RL. Proof copy 301539JCP. J Chem Phys. 2005; 123: 1.
- Heyd J, Scuseria GE, Ernzerhof M. Hybrid functionals based on a screened Coulomb potential. J Chem Phys. 2003; 118(18): 8207-8215.
- Frisch MJ, Pople JA, Binkley JS. Self‐consistent molecular orbital methods 25. Supplementary functions for Gaussian basis sets. J Chem Phys. 1984; 80(7): 3265-3569.
Author Info
Hacer Gümüş1* and Cengiz İpek22Department of Civil Engineering, Istanbul Medeniyet University, Istanbul, Turkey
Citation: Gümüş H: Computational Analysis of Density Functional Theory (DFT method), Thermodynamic Investigations and Molecular Docking Studies on 1-(2’-Thiophen)-3-(2,3,5-trichlorophenyl)-2-propen-1-one
Received: 11-Dec-2023 Accepted: 25-Dec-2023 Published: 01-Jan-2024, DOI: 10.31858/0975-8453.15.1.24-28
Copyright: This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
ARTICLE TOOLS
- Dental Development between Assisted Reproductive Therapy (Art) and Natural Conceived Children: A Comparative Pilot Study Norzaiti Mohd Kenali, Naimah Hasanah Mohd Fathil, Norbasyirah Bohari, Ahmad Faisal Ismail, Roszaman Ramli SRP. 2020; 11(1): 01-06 » doi: 10.5530/srp.2020.1.01
- Psychometric properties of the World Health Organization Quality of life instrument, short form: Validity in the Vietnamese healthcare context Trung Quang Vo*, Bao Tran Thuy Tran, Ngan Thuy Nguyen, Tram ThiHuyen Nguyen, Thuy Phan Chung Tran SRP. 2020; 11(1): 14-22 » doi: 10.5530/srp.2019.1.3
- A Review of Pharmacoeconomics: the key to “Healthcare for All” Hasamnis AA, Patil SS, Shaik Imam, Narendiran K SRP. 2019; 10(1): s40-s42 » doi: 10.5530/srp.2019.1s.21
- Deuterium Depleted Water as an Adjuvant in Treatment of Cancer Anton Syroeshkin, Olga Levitskaya, Elena Uspenskaya, Tatiana Pleteneva, Daria Romaykina, Daria Ermakova SRP. 2019; 10(1): 112-117 » doi: 10.5530/srp.2019.1.19
- Dental Development between Assisted Reproductive Therapy (Art) and Natural Conceived Children: A Comparative Pilot Study Norzaiti Mohd Kenali, Naimah Hasanah Mohd Fathil, Norbasyirah Bohari, Ahmad Faisal Ismail, Roszaman Ramli SRP. 2020; 11(1): 01-06 » doi: 10.5530/srp.2020.1.01
- Manilkara zapota (L.) Royen Fruit Peel: A Phytochemical and Pharmacological Review Karle Pravin P, Dhawale Shashikant C SRP. 2019; 10(1): 11-14 » doi: 0.5530/srp.2019.1.2
- Pharmacognostic and Phytopharmacological Overview on Bombax ceiba Pankaj Haribhau Chaudhary, Mukund Ganeshrao Tawar SRP. 2019; 10(1): 20-25 » doi: 10.5530/srp.2019.1.4
- A Review of Pharmacoeconomics: the key to “Healthcare for All” Hasamnis AA, Patil SS, Shaik Imam, Narendiran K SRP. 2019; 10(1): s40-s42 » doi: 10.5530/srp.2019.1s.21
- A Prospective Review on Phyto-Pharmacological Aspects of Andrographis paniculata Govindraj Akilandeswari, Arumugam Vijaya Anand, Palanisamy Sampathkumar, Puthamohan Vinayaga Moorthi, Basavaraju Preethi SRP. 2019; 10(1): 15-19 » doi: 10.5530/srp.2019.1.3