Case Report - (2023) Volume 14, Issue 7
Concomitant BRAF V600E and NRAS Q61R Mutations in the Same Thyroid Nodule: A Case Report
Brogna Marianna1*, Francesca Collina1, Simona Losito1, Eduardo Clery2, Angela Montone1, Michele Del Sesto1 and Gerardo Ferrara1Abstract
Background: Papillary Thyroid Cancer (PTC) is the most common type of well differentiated endocrine malignancy. Generally thyroid nodules with multiple oncogenic mutations are uncommon with an occurrence which may be related to more aggressive biological behavior of tumors. Rearranged in Transformation/ Papillary Thyroid Carcinomas (RET/PTC) rearrangement, RAS and BRAF mutations are considered to be mutually exclusive in PTC. Concomitant RET/PTC, RAS, or BRAF mutations have been documented, although the impact of these mutations for tumor growth and survival is debated.
Case presentation: Here, we present a rare case of woman 46 years old with a neck mass and thyroid nodule classified as Toll-Like Receptor 5 (TIR5) on cytological examination. We found contemporary BRAF p.(Val600Glu) (p. (V600E); c. 1799T>A) and NRAS p. (Gln61Arg) (p. (Q61R); c.182A>G) mutations in morphologically different areas within the same lobe (the right one). Two lesions show different morphology. The mutated BRAF lesion showed morphological characteristics compatible with classic papillary carcinoma while the mutant NRAS lesion shows morphological features compatible with follicular variant papillary carcinoma.
Results and conclusion: To the best of our knowledge, this is the first time that such mutations, which are normally mutually exclusive, have been detected at the same time. The findings of synchronous mutations are rare occurrence, suggesting for Intratumoral Heterogeneity (ITH) even in PTC. Patients with multiple mutations have a clinical worse prognosis, generally characterized by an aggressive thyroid cancer, which may influence the surgical treatment, chemotherapy, and BRAF V600E mutation-targeting therapy.
Keywords
Papillary thyroid cancer, Concomitant mutations, Intratumoral heterogeneity, Prognostic markers, Cytology
Introduction
Thyroid cancer is the most common malignancy of the endocrine system (Al Hamad AM, et al., 2019). More than 95% of thyroid carcinomas originate from the follicular cells of the thyroid, while only a minority (~3%) originate from C-parafollicular cells, leading to the onset of medullary thyroid carcinomas (Abdullah MI, et al., 2019; Henderson YC, et al., 2009).
Differentiated carcinomas have been identified into 3 histological subtypes-
• Papillary Carcinoma (PTC) (80%-85%)
• Follicular Carcinoma (FTC) (10%-15%)
• Hürtle Cell Carcinoma (HCC) (3%-5%) whose development and prognosis is similar to that of follicular carcinoma
Despite most of PTCs and FTCs has a good prognosis with a 5-year survival rate of more than 90%, a low fraction might become more aggressive over time (Xing M, et al., 2014; Henderson YC, et al., 2009). PTC is a solid tumor that normally occurs inside the thyroid. Histologically, it is characterized by the presence of papillae, epithelial cells arranged around a fibrovascular stem (Abdullah MI, et al., 2019). Despite most of PTCs are well differentiated with a low rate of local invasion, recurrences, or metastases (regional or distant), there are several tumor variants, with distinct pathological, and molecular features. Because of their aggressive behavior, the latest American Thyroid Association (ATA) guidelines have classified these pathological subtypes as having an intermediate risk of recurrence (Sak SD, 2015; Coca-Pelaz A, et al., 2020).
The main variants of PTC with prognostical implications are the Follicular Variant PTC (FVPTC), Diffuse Sclerosing Variant (DSV), Tall Cell Variant (TCV), Columnar Cell Variant (CCV), Cribriform Variant (CV), Hurtle Cell Variant (HCV), and Hobnail Variant (HV). Their evaluation is usually histological due to the uncommon morphological features (Coca-Pelaz A, et al., 2020). The main molecular alterations are the same as in traditional PTC, with the exception of the hobnail variant, which has changes in the Telomerase Reverse Transcriptase (TERT) promoter (44.4%), Phosphatidylinositol-4,5-Bisphosphate 3-Kinase Catalytic subunit Alpha (PIK3CA) (28.8%), Catenin Beta 1 (CTNNB1) (16.7%), estimated Glomerular Filtration Rate (eGFR) (11.1%), Serine/Threonine Kinase 1 (AKT1) (5.5%), and Neurogenic locus notch homolog protein 1 (NOTCH1) (5.5%) and HCV, whose oncocytic features are linked to mitochondrial DNA mutations (Sak SD, 2015). Alterations in BRAF gene have been referred as absent in the Criobriforme variant and uncommon in DSV, where ALK gene rearrangement was recently identified (Sak SD, 2015; CocaPelaz A, et al., 2020).
The FVPTC is a well-circumscribed or encapsulated tumor with architecture that might be mistaken for follicular adenoma or follicular carcinoma, has also been widely characterized (Rivera M, et al., 2010). It is known that mutations in the RAS oncogene has been linked to encapsulated/well-circumscribed tumors, whereas invasive FVPTC has been related to both BRAF and RAS mutations (Rivera M, et al., 2010).
The gold standard approach for the diagnosis of PTC is Fine Needle Aspiration and Cytology (FNAC) which classifies thyroid biopsies as suspicious or malignant based on their cytological outcome (Bagga PK and Mahajan NC, 2010). Although FNAC can reveal papillary structures, preoperative diagnosis is primarily based on the detection of typical nuclear features such as “Orphan Annie” nuclei (clear intranuclear pseudoinclusions) and nuclear grooves (folds in the nuclear membrane) (Shrestha RT, et al., 2015). PTC is confirmed by the presence of psammoma bodies, calcium salt deposits, in a cervical lymph node (Abdullah MI, et al., 2019; Krishnamurthy A and Vaidhyanathan A, 2011). The cytological examination allows the diagnosis of cancer in most cases. However, due to limited sampling or a lack of well-expressed PTC markers, several nodules later confirmed as malignant are indeed classified as indeterminate by cytology (Bagga PK and Mahajan NC, 2010). As a result, the use of molecular test is required in order to have a better understanding of the disease progression (Colombo C, et al., 2019). The most prevalent genetic mutation in papillary thyroid cancer is BRAF V600E, as well as the less common RAS mutations, or RET (RET/ PTC traslocations) or NTRK rearrangements, which play a key role in the PTC pathogenesis (Al Hamad AM, et al., 2019; Zou M, et al., 2014; Henderson YC, et al., 2009; Wang YL, et al., 2008; Ren H, et al., 2020; Li XO, et al., 2014). Genetic changes in RET/PTC, RAS, and BRAF are thought to be mutually exclusive and their overlapping has been debated for a long time. However, concurrent mutations in PTC have recently been reported and regarded as proof of existence of Intratumoral Heterogeneity (ITH) also in PTC (Zou M, et al., 2014; Henderson YC, et al., 2009; Chmielik E, et al., 2018).
Case Presentation
A 46-year-old, female patient was admitted to our hospital in May 2020 with a neck lump that grew in size over the year. Previous history of thyroid disease in the family was not reported and laboratory test did not detect thyroid disorders such as hyperthyroidism and hypothyroidism. The right lobe presented in section, macroscopically, present at the upper/middle III a nodule of 1.5 cm and a further nodule of 4 mm at the III medium.
Fine needle aspiration from the right thyroid lobe was interpreted as TIR5, sign of aggressiveness and high level of tumor malignancy. Therefore the patient underwent to total thyroidectomy with central neck dissection.
For the right lobe, the surgical pathology examination revealed a size of 5 × 3 × 2 cm; for the left lobe, 4 × 3 × 1 cm. In addition, two nodules, one 1.5 cm in diameter and the other 4 mm, have been identified in the right lobe. Although both lesions were classified as PTC, morphological variances were detected (Figure 1).
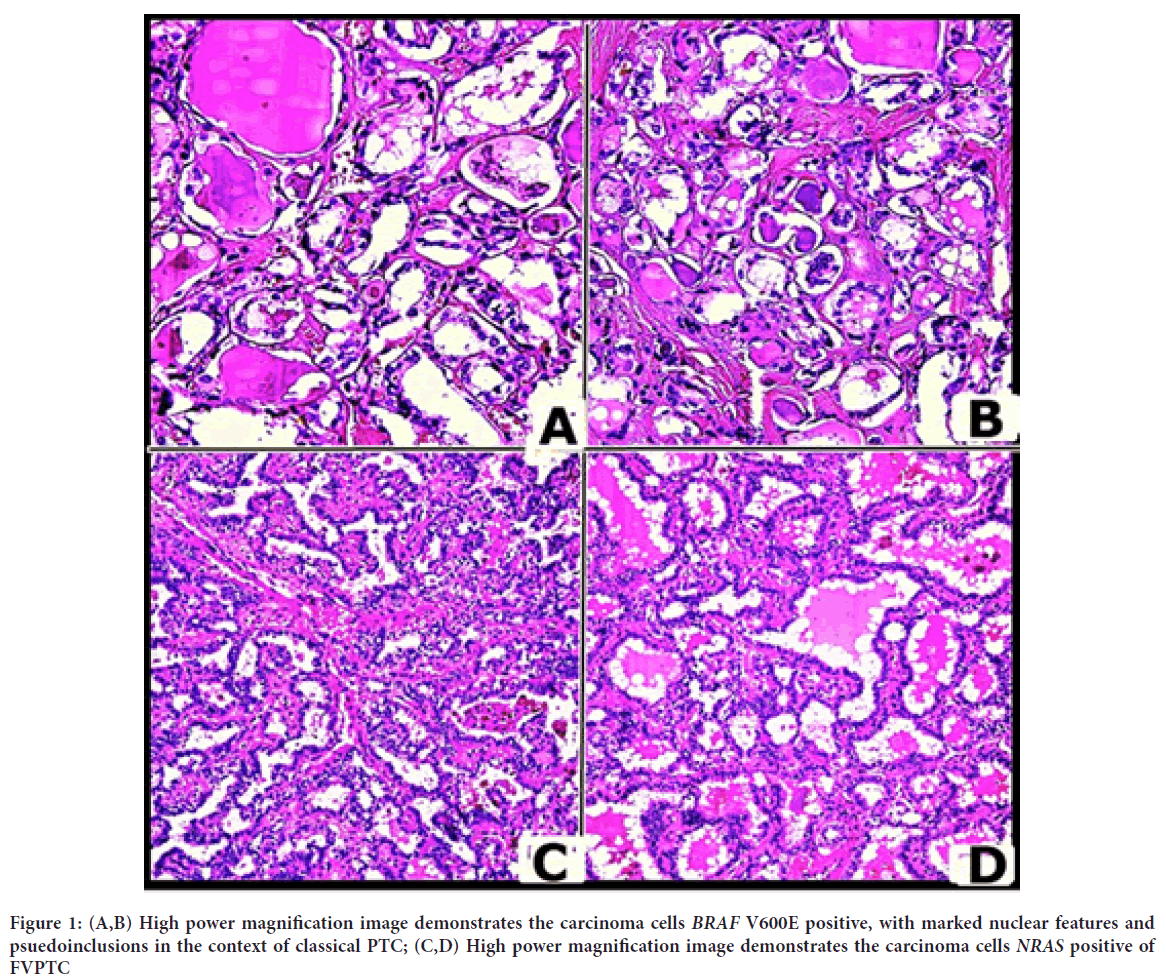
Figure 1: (A,B) High power magnification image demonstrates the carcinoma cells BRAF V600E positive, with marked nuclear features and psuedoinclusions in the context of classical PTC; (C,D) High power magnification image demonstrates the carcinoma cells NRAS positive of FVPTC
The largest lump had a classic papillary histology with well-developed nuclear features of PTC and inflammatory cells mixed in typical nuclear features such as orphan Annie nuclei (clear intranuclear pseudoinclusions) and nuclear grooves (folds in the nuclear membrane) suggestive of PTC have been described. The diagnosis has been confirmed by the presence of psammoma bodies (calcium salt deposits) in a cervical lymph node. Instead, microscopic examination of 4 mm nodule revealed morphological features of a variant follicular PTC, FVPTC, extracapsular type.
Formalin Fixed Paraffin Embedded tissue (FFPE) sample from surgical resection, was used for DNA extraction and each sample underwent molecular analysis to determine subclonality with regard to BRAF and RAS mutational status. The slides were reviewed by 2 expert pathologists; areas tumor displaying distinct histological pattern were separately micro-dissected to ensure high tumor tissue content and a minimum of 10% tumor purity cells was required for sample processing. DNA and RNA were extracted using respectively Qiagen QIAMP DNA FFPE KIT and RNeasy FFPE Kit according to manifacturer instructions and sample concentration was evaluated with nanodrop and Qubit.
BRAF and RAS mutational status was investigated by RealTime PCR using kit Thyroid Cancer Mutation Detection Kit (THDNA-RT64, Entrogen) intended for the detection of BRAF, KRAS, NRAS and HRAS somatic mutation in human genomic DNA. RET/PTC, RET/PTC2, RET/PTC3, PAX8/ PPARY translocation analysis on RNA were investigated by Easy Pgx Thyroid Fusion kit and the one-step real-time PCR as amplification method. Neither area was confirmed rearranged in terms of gene fusions.
Results and Discussion
The results highlighted BRAF p.(Val600Glu) for the 1.5 cm nodule while NRAS p. (Gln61Arg) for the 4 mm nodule. We enforced our findings because of several clinical cases with concurrent mutations in PTC which are referred in literature (Costa AM, et al., 2008; Zou M, et al., 2014, Xing M, et al., 2014).
Genetic changes in the RAS gene, as well as RET/PTC fusion or TERT1 promoter alterations, were simultaneously observed in PTC BRAF V600E (Costa AM, et al., 2008). However, to the best of our knowledge, this is the first time that the BRAF V600E and NRAS Q61R mutations, which are normally mutually exclusive, have been detected at the same time. Papillary thyroid carcinoma is an indolent tumor with a low death rate (Shrestha RT, et al., 2015). Several studies have identified two types of genetic alterations in thyroid cancer-
Point mutations in BRAF, KRAS, NRAS or HRAS, and chromosomal translocations involving RET/PTC1, RET/PTC3, or PAX8/PPARY (Xing M, et al., 2014; Ieni A, et al., 2021). The detection of these genetic markers allows a definitive diagnosis of malignant tumor that is distinct from thyroid nodules, which is considered to be benign. Furthermore, these gene markers may provide important prognostic value for patients with various subtypes.
All genetic alterations are assumed to be mutually exclusive and their overlapping has been debated for a long time. However, concurrent mutations in PTC have been discovered and regarded as a rare occurrence (Zou M, et al., 2014). RAS mutation and RET/PTC1 fusion co-expression was reported by Di Cristofaro et al. in 1/24 follicular variant PTC (FVPTC) and BRAF mutation RET/PTC3 fusion were found in 1/26 of classic PTC patients (CPTC) (Di Cristofaro J, et al., 2006).
Henderson et al. reported 5/54 PTC patients with coexisting BRAF mutation RET/PTC fusion where the authors referred a correlation between mutation status and clinicopathological variables since patients with dual mutation were older and had more advanced tumor (80% in T4) than those with BRAF V600E mutation only (27% in T4) (Henderson YC, et al., 2009).
Xing et al. referred that BRAF V600E and TERT promoter mutations cooperatively identify an aggressive papillary thyroid cancer with the worst clinicopathological outcome (Xing M, et al., 2014).
In 8/15 (53%) subclonal or nonclonal PTC, Zhu et al. confirmed RET/PTC rearrangement and RAS or BRAF mutations, but none in clonal PTC. According to the authors concomitant mutations were considered to occur more frequently in advanced stages of disease, and long-term follow-up showed that patients with contemporary mutations had a poor response to treatment and a reduced disease-free survival rate (Zou M, et al., 2014).
Costa et al. observed concomitant BRAF V600E and KRASG13D+G12S mutations in 4/35 PTC (Costa AM, et al., 2008) focusing on the assumption that BRAF alone isn’t a predictor of poor outcome. Nevertheless, when combined with other genetic alterations, it identifies a subset of PTC with higher risk of recurrence and decreased survival. In agreement with this, we have reported the first case of two distinct oncogenic driver mutations, respectively BRAF V600E and NRAS Q61R within the same thyroid nodule; this could enforce the evidence of a wide variety of biological behaviors in PTCs, ranging from the most indolent (well differentiated type) to the most aggressive malignancy. Only a few molecular markers linked to an increased risk of death are currently available, and their effectiveness in preoperative risk stratification and therapeutical planning remains unknown. As a result, a proper molecular characterization may be a useful tool for personalizing the initial surgical strategy, the follow up and ultimately to apply new therapies. Clinical trials using BRAF inhibitors for advanced thyroid cancer have revealed conflicting outcomes (Savvides P, et al., 2013; Ho AL and Sherman E, 2011). In the context of a RAS mutation, recent investigations have shown that BRAF inhibitors might paradoxically boost Mitogen-Activated Protein Kinase (MAPK) activation (Heidorn SJ, et al., 2010). As a result, patients who have both a BRAF and a RAS mutation may not be candidates for BRAF inhibitors treatment since the reactivation of MAPK is involved in the resistance mechanism (Lo RS, 2012). Moreover, the occurrence of concomitant mutations enforced the evidence of Intratumoral Heterogeneity (ITH) even in PTC. ITH refers to subclonal genetic variability within a tumor in contrast to the concept of a tumor as a clonal and homogeneous swarm (Finkel A, et al., 2016; Guerra A, et al., 2014). It is caused by genetic instability and the accumulation of genetic changes, both key factors in the growth of a tumor from an early stage to a more aggressive cancer.
The existence of ITH in PTC, its extension and biological impact is debated. However, several studies have shown that it is not a minor event in PTC, but a key factor for therapeutic failure and poor prognosis (Chmielik E, et al., 2018; Fugazzola L, et al., 2020). Nowadays, in the era of personalized medicine, the discovery that some tumors are heterogeneous in terms of individual mutations has significant therapeutic implications as well as translational value (Chmielik E, et al., 2018; Ieni A, et al., 2021). Tumors with a specific molecular alteration in only a minority of neoplastic cells are likely to have low sensitivity to targeted therapies; as a consequence the finding of this internal tumor-like complexity, was the starting point for the use of a drug combination to restrict tumor growth. As a result, this report supports the assumption that synchronous mutations in PTC are linked to more aggressive tumor behavior, which could affect surgical procedure selection as well as post-surgery care. It also shows the potential impact of molecular testing/screening in selecting patients for more aggressive treatment. However the benefits of such an approach have yet to be confirmed. Anyway, it would be useful to increase the understanding of ITH in order to properly advise therapy and improve survival of patients.
Conclusion
The identification of specific genomic alterations drivers for several malignancies has enabled the development of customized therapies with promising response rates. Unfortunately, most cancers are caused by a complex interaction of genetic, transcriptomic, and proteomic alterations, as well as anomalies in the tumor microenvironment and immune system.
The recent development of new technologies such as second generation sequencing, Next Generation Sequencing (NGS), and the numerous advances in the field of genomics have allowed a continuous and further evolution of “precision oncology”. While recognizing the value of morphological and histological data, the new paradigm of “mutational oncology”, has opened the era of genomic profiling tests, this would improve the selection of the anticancer drug based on the “driver” mutation and agnostic approval, namely the therapeutic indication regardless of the tumor site.
Due to the high levels of tumor heterogeneity and individual genomic complexity, customized drug combination is a key factor in therapeutic management optimization. This implies a patient-centered cancer therapy approach in order to get the correct drug(s) to the right patients at the right time and, as a result, overcome resistance mechanisms.
Declarations
All authors have read, edited, and contributed to the content of this manuscript. This work has not been previously published and has not been considered for publication elsewhere.
Ethical Approval
All authors certify that the study was performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards
Consent to Participate
The participant has consented to the submission of the case study to the journal.
References
- Al Hamad AM, Albisher HM, Al Saeed WR, Almumtin AT, Allabbad FM, A Shawarby M. BRAF gene mutations in synchronous papillary thyroid carcinoma and Langerhans cell histiocytosis co-existing in the thyroid gland: A case report and literature review. BMC Cancer. 2019; 19: 1-6.
[Crossref] [Google Scholar] [Pubmed]
- Abdullah MI, Junit SM, Ng KL, Jayapalan JJ, Karikalan B, Hashim OH. Papillary thyroid cancer: Genetic alterations and molecular biomarker investigations. Int J Med Sci. 2019; 16(3): 450.
[Crossref] [Google Scholar] [Pubmed]
- Henderson YC, Shellenberger TD, Williams MD, El-Naggar AK, Fredrick MJ, Cieply KM, et al. High rate of BRAF and RET/PTC dual mutations associated with recurrent papillary thyroid carcinoma. Clin Cancer Res. 2009; 15(2): 485-491.
[Crossref] [Google Scholar] [Pubmed]
- Xing M, Liu R, Liu X, Murugan AK, Zhu G, Zeiger MA, et al. BRAF V600E and TERT promoter mutations cooperatively identify the most aggressive papillary thyroid cancer with highest recurrence. J Clin Oncol. 2014; 32(25): 2718.
[Crossref] [Google Scholar] [Pubmed]
- Sak SD. Variants of papillary thyroid carcinoma: Multiple faces of a familiar tumor. Turk Patoloji Derg. 2015; 31(Suppl 1): 34-47.
[Crossref] [Google Scholar] [Pubmed]
- Coca-Pelaz A, Shah JP, Hernandez-Prera JC, Ghossein RA, Rodrigo JP, Hartl DM, et al. Papillary thyroid cancer-Aggressive variants and impact on management: A narrative review. Adv Ther. 2020; 37: 3112-3128.
[Crossref] [Google Scholar] [Pubmed]
- Rivera M, Ricarte-Filho J, Knauf J, Shaha A, Tuttle M, Fagin JA, et al. Molecular genotyping of papillary thyroid carcinoma follicular variant according to its histological subtypes (encapsulated vs. infiltrative) reveals distinct BRAF and RAS mutation patterns. Mod Pathol. 2010; 23(9): 1191-1200.
[Crossref] [Google Scholar] [Pubmed]
- Bagga PK, Mahajan NC. Fine needle aspiration cytology of thyroid swellings: How useful and accurate is it? Indian J Cancer. 2010; 47(4): 437-442.
[Crossref] [Google Scholar] [Pubmed]
- Shrestha RT, Karunamurthy A, Amin K, Nikiforov YE, Caramori ML. Multiple mutations detected preoperatively may predict aggressive behavior of papillary thyroid cancer and guide management-a case report. Thyroid. 2015; 25(12): 1375-1378.
[Crossref] [Google Scholar] [Pubmed]
- Krishnamurthy A, Vaidhyanathan A. Axillary lymph node metastasis in papillary thyroid carcinoma: Report of a case and review of the literature. J Cancer Res Ther. 2011; 7(2): 220-222.
[Crossref] [Google Scholar] [Pubmed]
- Colombo C, Muzza M, Proverbio MC, Tosi D, Soranna D, Pesenti C, et al. Impact of mutation density and heterogeneity on papillary thyroid cancer clinical features and remission probability. Thyroid. 2019; 29(2): 237-251.
[Crossref] [Google Scholar] [Pubmed]
- Zou M, Baitei EY, Alzahrani AS, BinHumaid FS, Alkhafaji D, Al-Rijjal RA, et al. Concomitant RAS, RET/PTC, or BRAF mutations in advanced stage of papillary thyroid carcinoma. Thyroid. 2014; 24(8): 1256-1266.
[Crossref] [Google Scholar] [Pubmed]
- Wang YL, Wang JC, Wu Y, Zhang L, Huang CP, Shen Q, et al. Incidentally simultaneous occurrence of RET/PTC, H4-PTEN and BRAF mutation in papillary thyroid carcinoma. Cancer Lett. 2008; 263(1): 44-52.
[Crossref] [Google Scholar] [Pubmed]
- Ren H, Ke N, Tan C, Wang X, Cao W, Liu X. Unusual metastasis of papillary thyroid cancer to the pancreas, liver, and diaphragm: A case report with review of literature. BMC Surg. 2020; 20(1): 1-4.
[Crossref] [Google Scholar] [Pubmed]
- Li XO, Li ZP, Wang P, Li CL, Wu JH, Zhang JZ, et al. Pancreatic metastasis of papillary thyroid carcinoma: A case report with review of the literature. Int J Clin Exp Pathol. 2014; 7(2): 819.
[Google Scholar] [Pubmed]
- Chmielik E, Rusinek D, Oczko-Wojciechowska M, Jarzab M, Krajewska J, Czarniecka A, et al. Heterogeneity of thyroid cancer. Pathobiology. 2018; 85(1-2): 117-129.
[Crossref] [Google Scholar] [Pubmed]
- Costa AM, Herrero A, Fresno MF, Heymann J, Alvarez JA, Cameselle‐Teijeiro J, et al. BRAF mutation associated with other genetic events identifies a subset of aggressive papillary thyroid carcinoma. Clin Endocrinol. 2008; 68(4): 618-634.
[Crossref] [Google Scholar] [Pubmed]
- Ieni A, Vita R, Pizzimenti C, Benvenga S, Tuccari G. Intratumoral heterogeneity in differentiated thyroid tumors: An intriguing reappraisal in the era of personalized medicine. J Pers Med. 2021; 11(5): 333.
[Crossref] [Google Scholar] [Pubmed]
- Di Cristofaro J, Marcy M, Vasko V, Sebag F, Fakhry N, Wynford-Thomas D, et al. Molecular genetic study comparing follicular variant versus classic papillary thyroid carcinomas: Association of N-ras mutation in codon 61 with follicular variant. Hum Pathol. 2006; 37(7): 824-830.
[Crossref] [Google Scholar] [Pubmed]
- Savvides P, Nagaiah G, Lavertu P, Fu P, Wright JJ, Chapman R, et al. Phase II trial of sorafenib in patients with advanced anaplastic carcinoma of the thyroid. Thyroid. 2013; 23(5): 600-604.
[Crossref] [Google Scholar] [Pubmed]
- Ho AL, Sherman E. Clinical development of kinase inhibitors for the treatment of differentiated thyroid cancer. Clin Adv Hematol Oncol. 2011; 9(1): 32-41.
[Google Scholar] [Pubmed]
- Heidorn SJ, Milagre C, Whittaker S, Nourry A, Niculescu-Duvas I, Dhomen N, Hussain J, et al. Kinase-dead BRAF and oncogenic RAS cooperate to drive tumor progression through CRAF. Cell. 2010; 140(2): 209-221.
[Crossref] [Google Scholar] [Pubmed]
- Lo RS. Receptor tyrosine kinases in cancer escape from BRAF inhibitors. Cell Res. 2012; 22(6): 945-947.
[Crossref] [Google Scholar] [Pubmed]
- Finkel A, Liba L, Simon E, Bick T, Prinz E, Sabo E, et al. Subclonality for BRAF mutation in papillary thyroid carcinoma is associated with earlier disease stage. J Clin Endocrinol Metab. 2016; 101(4): 1407-1413.
[Crossref] [Google Scholar] [Pubmed]
- Guerra A, Zeppa P, Bifulco M, Vitale M. Concomitant BRAF V600E mutation and RET/PTC rearrangement is a frequent occurrence in papillary thyroid carcinoma. Thyroid. 2014; 24(2): 254-259.
[Crossref] [Google Scholar] [Pubmed]
- Fugazzola L, Muzza M, Pogliaghi G, Vitale M. Intratumoral genetic heterogeneity in papillary thyroid cancer: Occurrence and clinical significance. Cancers. 2020; 12(2): 383.
[Crossref] [Google Scholar] [Pubmed]
Author Info
Brogna Marianna1*, Francesca Collina1, Simona Losito1, Eduardo Clery2, Angela Montone1, Michele Del Sesto1 and Gerardo Ferrara12Department of Public Health, University of Naples Federico II, Naples, Italy
Citation: Marianna B: Concomitant BRAF V600E and NRAS Q61R Mutations in the Same Thyroid Nodule: A Case Study
Received: 12-Jun-2023 Accepted: 07-Jul-2023 Published: 14-Jul-2023, DOI: 10.31858/0975-8453.14.7.447-451
Copyright: This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
ARTICLE TOOLS
- Dental Development between Assisted Reproductive Therapy (Art) and Natural Conceived Children: A Comparative Pilot Study Norzaiti Mohd Kenali, Naimah Hasanah Mohd Fathil, Norbasyirah Bohari, Ahmad Faisal Ismail, Roszaman Ramli SRP. 2020; 11(1): 01-06 » doi: 10.5530/srp.2020.1.01
- Psychometric properties of the World Health Organization Quality of life instrument, short form: Validity in the Vietnamese healthcare context Trung Quang Vo*, Bao Tran Thuy Tran, Ngan Thuy Nguyen, Tram ThiHuyen Nguyen, Thuy Phan Chung Tran SRP. 2020; 11(1): 14-22 » doi: 10.5530/srp.2019.1.3
- A Review of Pharmacoeconomics: the key to “Healthcare for All” Hasamnis AA, Patil SS, Shaik Imam, Narendiran K SRP. 2019; 10(1): s40-s42 » doi: 10.5530/srp.2019.1s.21
- Deuterium Depleted Water as an Adjuvant in Treatment of Cancer Anton Syroeshkin, Olga Levitskaya, Elena Uspenskaya, Tatiana Pleteneva, Daria Romaykina, Daria Ermakova SRP. 2019; 10(1): 112-117 » doi: 10.5530/srp.2019.1.19
- Dental Development between Assisted Reproductive Therapy (Art) and Natural Conceived Children: A Comparative Pilot Study Norzaiti Mohd Kenali, Naimah Hasanah Mohd Fathil, Norbasyirah Bohari, Ahmad Faisal Ismail, Roszaman Ramli SRP. 2020; 11(1): 01-06 » doi: 10.5530/srp.2020.1.01
- Manilkara zapota (L.) Royen Fruit Peel: A Phytochemical and Pharmacological Review Karle Pravin P, Dhawale Shashikant C SRP. 2019; 10(1): 11-14 » doi: 0.5530/srp.2019.1.2
- Pharmacognostic and Phytopharmacological Overview on Bombax ceiba Pankaj Haribhau Chaudhary, Mukund Ganeshrao Tawar SRP. 2019; 10(1): 20-25 » doi: 10.5530/srp.2019.1.4
- A Review of Pharmacoeconomics: the key to “Healthcare for All” Hasamnis AA, Patil SS, Shaik Imam, Narendiran K SRP. 2019; 10(1): s40-s42 » doi: 10.5530/srp.2019.1s.21
- A Prospective Review on Phyto-Pharmacological Aspects of Andrographis paniculata Govindraj Akilandeswari, Arumugam Vijaya Anand, Palanisamy Sampathkumar, Puthamohan Vinayaga Moorthi, Basavaraju Preethi SRP. 2019; 10(1): 15-19 » doi: 10.5530/srp.2019.1.3






