Review Article - (2022) Volume 13, Issue 10
Current Applications of Chitosan Nanoparticles
Prasad R Deshmukh1*, Abhilash Joshi1, Chaitanya Vikhar1, SS Khdabadi2 and Mukund Tawar1Abstract
Nanoparticles are currently becoming one of the important parts for various segments. Much more process/application becomes effective or more economical and time saving because of nanoparticles. Polymers with distinct characteristics are widely used but nature origin polymer has its own important properties that make it a choice as polymer for nanoparticles. Many methods were successfully evaluated to prepare chitosan nanoparticles for loading various compounds including ionotropic gelation, micelles and emulsification methods. In this article we have reviewed the current aspects made-up from chitosan nanoparticles. Chitosan nanoparticles having application to improve bioavailability, controlled released of loaded drug also increases cellular uptake and targeting to cancer cells, stabilization of proteins and enhanced the affectivity of anti-microbial agents. In agriculture chitosan nanoparticles are used for herbicides, insecticide and pesticide loading to improve the cultivation of crops and used in foods packaging. Chitosan nanoparticles are also having wide application as an implant and therapeutics agent.
https://blogum.blogaaja.fi/
https://blogum-1.jimdosite.com/
https://blogummm.edublogs.org/
https://blogummm.websites.co.in/
https://blogum18.wordpress.com/
https://benim-blogum.jigsy.com/
https://fuiegs-symbeaurds-build.yolasite.com/
https://blogum-03.webselfsite.net/
https://blogummm.mystrikingly.com/
https://blogum.splashthat.com/
https://blogum3.webnode.com.tr/
https://blogum.odoo.com/
https://blogum.creatorlink.net/
https://whiteseotr1-s-site.thinkific.com/enrollments
https://blogum.estranky.cz/
https://653ba4fbb538c.site123.me/
https://blogum12m.blogspot.com/
https://blogum.hashnode.dev/
https://whiteseoturkey1.wixsite.com/blogum
https://sites.google.com/view/blogummm/
https://codepen.io/blogum
https://blogumm.livejournal.com/
https://wakelet.com/@blogum82816
https://www.homify.com/users/9538383/blogum
https://lessons.drawspace.com/profile/323613/blogum
https://my.desktopnexus.com/blogum/
https://writeupcafe.com/profile/BLOGUM/
https://www.pearltrees.com/blogum
https://www.easyfie.com/blogum
https://pharmahub.org/members/27615/profile
https://www.zupyak.com/u/blogum/posts
https://www.metroflog.co/blogum
https://www.fuzia.com/fz/blogum-blogum
https://tr.pinterest.com/blogum12/
https://my.getjealous.com/blogum
https://micro.blog/blogum
https://www.tumblr.com/blogummm
https://hub.docker.com/u/blogum
https://fire.blogfree.net/?act=Profile&MID=1342323
https://blogum.pixnet.net/blog
https://www.threadless.com/@blogum/activity
https://blogum.neocities.org/
https://blogum12.amebaownd.com/
https://teletype.in/@blogum
https://ubl.xml.org/users/blogum
https://educatorpages.com/site/blogum/
https://blogum.onlc.fr/
Keywords
Chitosan, Nanoparticles, Pharmaceutical application, Agriculture, Implants
Introduction
Design, production and application of material molding at the scale of atomic, molecular and macromolecular to convert into nano size were defined nanotechnology. In large, used full application of nanotechnology makes its best acceptable systems in multiple material developments. Nanoparticles are a type of colloidal system comprising particles with a size range from 10 to 1000 nm in diameter (Figure 1). Nanoparticles exhibit size-related physiochemical properties that make significantly differ from those observed in fine particles or bulk materials (Buzea C, et al., 2007). Nanoparticles are used for many more purposed including improvement of physical and chemical stability, improvement in enhanced efficiency, improvement of shelf life of the food products, in packaging materials, bioavailability of drug by improving aqueous solubility, increasing resistance time in the biological system, increasing half-life, increasing specificity and targeting drug to specific location in the body also in agriculture to improve and increased nutrients levels, productivity and protection against several insect pest and microbial diseases.
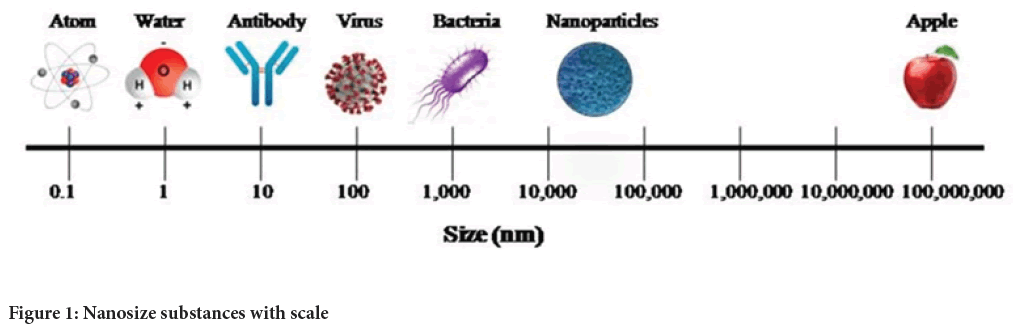
Figure 1: Nanosize substances with scale
Polysaccharide material chitosan was obtained by extensive deacetylation by hydrolysis under alkali condition at high temperature of chitin, originated from crustacean shells and also present in numerous natural resources viz. In the cell walls of fungi, in the exoskeletons of arthropods mostly in crustaceans (e.g,. crabs, lobsters and shrimps), insects, radulae of molluscs and in beaks and internal shells of cephalopods (e.g. octopus and squid). Chitosan is a cationic linear copolymer made up of a random distribution of β (1→4) linked 2-amino-2-deoxy-D-glucose (D-glucosamine) and 2-acetamido-2-deoxy-D-glucose (N-acetyl-D-glucosamine) units (Figure 2). Chitosan is insoluble in water, organic solvents, and aqueous bases and it is soluble in acids such as acetic, nitric, hydrochloric, perchloric and phosphoric (Reddy N and Yang Y, 2015). Chemical properties of linear polyamines, reactive amino groups and reactive hydroxyl group available, chelates transitional metal ions. Biological properties of biocompatible, binding to mammalian and microbial cells aggressively, regeneration effect on connective gum tissue, accelerates the formation of osteoblasts responsible for bone formation, hemostatic, fungi static, spermicidal, antitumor, anticholesteremic, accelerates bone formation (Dutta PK, et al., 2004). Chitosan is considered one of the most valuable polymers for biomedical and pharmaceutical applications and can be used in the development of nanoparticles, microspheres, hydrogels, films, and fibers (Kumar MN, 2000).
Figure 2: Structure of chitosan
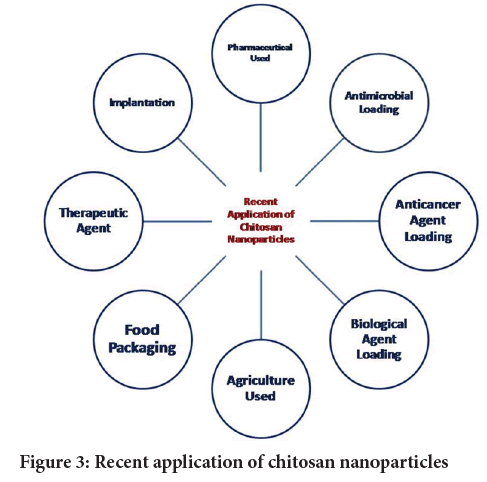
Chitosan widely used as a material for nanotechnology of encapsulate material because of natural availability and its physiochemical properties. Various methods were adopted to prepare nanosize material from chitosan including ionic gelation/polyelectrolyte complexation, emulsion droplet coalescence, emulsion solvent diffusion, reverse micellisation, desolvation, modified ionic gelation with radical polymerization, emulsification cross-inking, nanoprecipitation, spray-drying, cross linkers by aldehyde, tripolyphosphate, genipin, carboxylic groups (Divya K and Jisha MS, 2018). Chitosan nanoparticles are successfully applied for various purposes in chemical, pharmaceuticals, antimicrobials, food, agricultures and therapy. Significant difference in the activity of encapsulated material in chitosan nanoparticles was observed as compared the same non-capsulated material. This review covers recent applications of nano technology using chitosan as a polymeric material for encapsulation and improvement of efficiency in pharmaceutical drugs, antimicrobial agents, anti-cancer agents, biological materials like antigen, proteins, RNA loading, pesticides, herbicides and nutrition used in agriculture also for the improvement of efficiency of food packaging materials, therapeutics purposed and as implanting materials (Figure 3).
Figure 3: Recent application of chitosan nanoparticles
Literature Review
Current applications of chitosan nanoparticles
Nanoparticles are able to improve the effectiveness of loaded particles which makes its important in various applications. Nature origin materials like chitosan used as polymeric material for nanoparticles resulted into synergetic effect. Following are the discussion on the current research work carried out on chitosan nanoparticles in various areas.
Pharmaceutical applications: Polymeric materials used for the purposed of nanoparticles play an important role in drug loading, release, bioavailability and ultimately therapeutic effect of loaded drugs. Chitosan, because of its unique properties was evaluated the most compatible and acceptable material for nanoparticles. Chitosan nanoparticles help to improve therapeutics properties of loaded drug like release, permeability and stability. Antihistaminic agent Olopatadine hydrochloride was incorporated into chitosan nanoparticles using spray drying method. Particle size analyses showed that the nanoparticles were within the nanometer range (119 ± 9 nm to 227 ± 18 nm) with a relatively homogenous size distribution (0.453 ± 0.078 to 0.643 ± 0.124 Poly Dispersity Index (PDI) data) with zeta potentials within the range of +27.8 ± 0.3 mV to +35.5 ± 1.2 mV. High encapsulation efficacies up to 73.33% ± 0.25% were detected by Ultra-High Performance Liquid Chromatography (U-HPLC) method. In vivo corneal residence time of nanoparticles studies on sheep’s revealed that the ocular residence time was enhanced up to 24 h with single instillation (Güven UM and Başaran E, 2021). Epinephrine-entrapped in chitosan nanopar ticles were electrosprayed on a base pad and covered by a gelatin nanofiber layer was provided an ultimate interface to induce red blood cell absorption and aggregation, resulting in augmented blood coagulation. Nanoparticles were proved for safe and effective hemostatic agents and provide a new approach for fast and safe hemorrhage control (Atashgahi M, et al., 2021). Propranolol loaded chitosan nanoparticles were prepared by double emulsion technique, as an effective alternative for the treatment of infantile hemangioma. The in vivo skin deposition in rats showed an accumulation of propranolol on the lecithin/chitosan nanocarrier by 1.56-1.91 fold compared to the drug solution. Fluorescence across the skin by confocal laser scanning microscopy also proved the permeation by nanoparticles (Khalil RM, et al., 2021).
Chitosan along with alginate complex was used for coating for improve the photostability, sustained releases and bio-accessibility of resveratrol. Chitosan was used to coat zein nanoparticles of resveratrol obtained by the liquid-liquid dispersion method. In the in vitro release assay evaluated chitosan coating improved the nanoparticles protection against premature resveratrol release in simulated gastrointestinal fluids and gave biphasic and prolonged resveratrol release. Chitosan played an essential role in the adsorption of mucin on the nanoparticles surface, demonstrating its mucoadhesive properties (Khan MA, et al., 2019). Lithium loaded nanoparticles prepared by ionic gelation technique using chitosan was improved therapeutic efficacy for the treatment of bipolar disorders. Moreover controlled lithium releases from nanoparticles overcome the pulmonary toxicity due to the direct use of lithium carbonate. 1.3 times increase in cell proliferation by lithium carbonate loaded chitosan nanoparticles was observed in Pheochromocytoma (PC12) cells (Narayan S, 2018).
Loading antimicrobials agents: Used of antimicrobials was restricted due to various parameters including resistance, cellular uptake by microorganisms and physiochemical nature of the antimicrobial agent. Chitosan nanoparticle proven to enhance the therapeutics efficiency of loaded antimicrobial agents due to promotion permeability of the microbial, high surface area-to-volume ratio and alternation of physiochemical properties. Chitosan with poly (D, L-lactide-co-glycolide nanoparticele were used for enhanced antimicrobial activity of natural antimicrobial Trans-cinnamaldehyde and generated a delivery system with pH-sensitivity for controlled release (Pola CC, et al., 2019). Chitosan nanoparticles were used for incorporating the antifungal miconazole nitrate for the treatment of vulvovaginal candidiasis. Nanoparticles had an increase in miconazole nitrate’s therapeutic efficacy than commercial cream formulation (Amaral AC, et al., 2019). Chitosan was evaluated as a carrier of betamethasone and tetracycline, with sodium citrate as the cross-linking agent (Taghizadeh MT, et al., 2019). Higher encapsulation efficiency and particle stable was achieved in antifungal Origanum vulgare essential oil loaded in chitosan nanoparticles using electrospraying technique. Dynamic light scattering technique evaluated the stability of nanoparticles; the spectrophotometry study evaluated the encapsulation efficiency in the range of 70.1% and 79.6%. Agar dilution method was carried out for fungistatic activity (Yilmaz MT, et al., 2019). Controlled release ciprofloxacin loaded chitosan-based nanoparticles were synthesized by ionic crosslinking method showing the highest antibacterial activity against Escherichia coli, Bacillus thuringiensis, Methicillin-Resistant Staphylococcus aureus (MRSA) and Pseudomonas aeruginosa with Minimum inhibitory concentration (MIC) varying from 0.0043 to 0.01 μg/ml and from 0.07 to 0.14 μg/ml (Marei N, et al., 2019). Nanoparticles of tetrazole derivatives of chitosan by ionic gelation with cross-linking sodium tripolyphosphate showed improved catalytic properties and antibacterial activity against S. aureus and E. coli than tetrazole-chitosan polymers (Kritchenkov AS, et al., 2019). Quaternary pyridinium compounds attached to chitosan nanoparticles showed significant effect on the Gram-positive bacteria. DPPH (2,2-diphenyl-1-picryl-hydrazyl-hydrate) radical scavenging assay shows mild improvement in antioxidant activity (Omidi S and Kakanejadifard A, 2019). Spiramycin-loaded chitosan nanoparticles were prepared for the treatment of human toxoplasmosis. Nanoparticles showed a maximum survival time of more than 200 days with no mortality on the sacrifice day (8th) was observed in mice. Spiramycin-loaded nanoparticles showed the highest significant percent reduction of tachyzoites (about 90% reduction) in liver, spleen, and brain compared to the other used drugs denoting successful bypass of Blood Brain Barrier (BBB). Light microscopy of the treated peritoneal tachyzoites showed sluggish tachyzoite movement while the nanoparticles caused loss of their movement. Scanning Electron Microscopy (SEM) of the treated tachyzoites were more mutilated and some of them appeared rupturing (Hagras NA, et al., 2019). Chitosan nanoparticles were prepared by ionic gelation method using cross-linking agent sodium tripolyphosphate, used in the treatment of ocular infection by encapsulate antibacterial agent levofloxacin. Reduction of corneal clearance, naso-lachrymal drainage as well as higher retention of levofloxacin with higher antibacterial activity against P. aeruginos, and S. aureus was achieved by chitosan nanoparticles (Imam SS, et al., 2018). In dentistry, chitosan nanoparticles showed an inhibitory effect on the growth of bacteria and pathogenic fungi analyzed by cultured in suspensions (Mousavi SM, et al., 2018).
Loading anticancer agents: Drug solubility and permeability decide the therapeutic value by bioavailability. Nanosize polymeric material succeeds to overcome low bioavailability by improving the solubility and permeability of the loaded drug. Chitosan nanoparticles can able to cross the tight junctions between cells and promote permeation and hydrophilic properties help for solubility. Morever adverse effects on normal cell restricted the use of anti-cancer drugs, loading in chitosan nanoparticles selectively achieved targeted delivery to specific organs that improvement in therapeutics efficiency. Cytarabine loaded chitosan nanoparticles were used for targeted delivery in breast. Folates conjugated to chitosan using carbodiimide helps to target adeno carcinoma cell lines by making use of the over-expressed folate receptors on the surface of Michigan Cancer Foundation-7 (MCF-7) (Geethakumari D, et al., 2022). Essential oils of greater celandine were loaded in chitosan nanoparticles using the emulsion-ionic gelation method with 69.1% encapsulation efficiency. Implying that encapsulation chitosan become an efficient technique for improving their anticancer activity against MCF-7 cell line (Hesami S, et al., 2022). Enhancement of therapeutic efficacy and reduced side effects of doxorubicin was gained by chitosan nanoparticles prepared by using Supercritical-fluid Assisted Atomization introduced by a Hydrodynamic Cavitation Mixer (SAA-HCM) from aqueous solution. Nanoparticles showed strongly pH-responsive drug release behavior by nanoparticles in the media with pH of 4.5, 6.5 and 7.4 respectively (Peng HH, et al., 2019). For treatment with Doxorubicin (DOX)-resistant cancer cells Lecithin/Chitosan nanoparticles were used to load doxorubicin and Piperine P-gp inhibitors. Nanoparticle was showed prolonged drug release by Fickian-diffusion mechanism (Alkholief M, 2019). Chitosan-tripolyphosphate nanoparticles using ionotropic gelation method with decorated chlorin e6 chromophores were used to prepare photocontrolled doxorubicin delivery system for cancer treatment. High photo stability, singlet oxygen generation and pH controlled release nanoparticles show significant antiproliferative activity against MCF-7 breast cancer cells after irradiation at near infrared ranges (Bhatta A, et al., 2019). Carboxymethyl Chitosan was used to prepare nanoparticles by pre-grafted N-(3-Aminopropyl)-imidazole then surface-modified with perfluorobutyric anhydride. Nanoparticles showing pH-triggered drug release, higher bioavailability and superior antitumor efficiency by enhancing cellular uptake and improving cytotoxicity in different tumor cells without targeting recognition between host and ligands (Cheng X, et al., 2019). Chitosan was used with fucoidan nanoparticles to load methotrexate for topical delivery. Nanoparticles had lower cytotoxicity than free methotrexate, in fibroblasts and human keratinocytes. Safe, exerts an anti-inflammatory effect and increase skin permeation thus can potentially be used for methotrexate nanoparticles for topical delivery (Barbosa AI, et al., 2019). Chitosan along with N-deoxycholic acid glycol nanoparticles were used as a carrier for docetaxel, Gastric Cancer peptide-polyethylene glycol (GX1-PEG)-deoxycholic acid conjugate was used for targete ligand for gastric cancer. Angiogenesis marker peptide GX1 efficiently enhanced the cellular uptake of nanoparticles. Nanoparticles showed stronger cytotoxicity against co-cultured gastric cancer cells and human umbilical vein endothelial cells (Zhang E, et al., 2019). Chitosan with alginate with doxorubicin loaded nanoparticles by electrostatic complexation in water-in-oil (w/o) emulsion process. The uptake and efficacy was successfully evaluated by a murine breast cancer cell line, 4T1, with comparable 72 h IC50 values of the nanoparticle solution (0.15 μg/mL) and free DOX (0.13 μg/mL), spherical morphology and uniformity (Rosch JG, et al., 2019). Chitosan with alginate nanoparticles were also used to encapsulate curcumin diethyl diglutarate for oral delivery was prepared by o/w emulsification and ionotropic gelation method. Nanoparticle of curcumin diethyl diglutarate in Chitosan/alginate showed better stability under UV irradiation and thermal exposure, storage stability, digestive stability, in vitro digestibility, bioaccessibility and in vitro uptake in Caco-2 cells than also in vitro release profile showed sustained-release manner and best fit with the Korsmeyer-Peppas kinetic model, indicating the Fickian diffusion mechanism compare to free curcumin diethyl diglutarate (Sorasitthiyanukarn FN, et al., 2019).
Cell-biotinylated curcumin loaded chitosan nanoparticles hybrid vector was constructed using mesenchymal stem cells. Nanoparticles showed no effect on their viability and homing properties. Biotin-avidin binding lasted over 48 h, which could be sufficient for cell-directed tumor tropic delivery. Cell-nanoparticle hybrid vector could prove beneficial in pulmonary melanoma metastasis therapy by in vitro and in vivo anti-tumor (Xu M, et al., 2019). Succinate conjugated chitosan with D-α-tocopherol polyethylene glycol 1000 was used to load Docetaxel. To enhanced cellular uptake and cytotoxicity with a promising bioadhesion property. In vivo pharmacokinetic studies showed 2.33, and 2.82-fold enhancement in relative bioavailability (Mehata AK, et al., 2019).
Cell-biotinylated curcumin loaded chitosan nanoparticles hybrid vector was constructed using mesenchymal stem cells. Nanoparticles showed no effect on their viability and homing properties. Biotin-avidin binding lasted over 48 h, which could be sufficient for cell-directed tumor tropic delivery. Cell-nanoparticle hybrid vector could prove beneficial in pulmonary melanoma metastasis therapy by in vitro and in vivo anti-tumor (Xu M, et al., 2019). Succinate conjugated chitosan with D-α-tocopherol polyethylene glycol 1000 was used to load Docetaxel. To enhanced cellular uptake and cytotoxicity with a promising bioadhesion property. In vivo pharmacokinetic studies showed 2.33, and 2.82-fold enhancement in relative bioavailability (Mehata AK, et al., 2019).
Loading biological agents: Encapsulation of protein contained in polymeric nano size devices raised instability problems. Colloidal characteristics like particle size, PDI, and zeta potential are mainly concerned with the stability of nanoparticles. Natural origin along with compatibility and degradation in biological systems and providing excellent stability to nano systems, chitosan has become choice for encapsulation of pretentious compounds like amino acid, albumin, enzymes, antigen, mRNA, and hormones. Chitosan and ascorbate chitosan nanoparticles with load enzymes were produced by dissolution in acetic acid, formed spherical with average particle sizes of 44 ± 8.4 nm and 87 ± 13.6 nm, respectively. Were taken up by the cells and showed dose-dependent cytotoxicity. Chitosan-glucoamylase nanoparticles were synthesized by dripping granulation method followed by ionic cross-linking with sodium tripolyphosphate. Nanosize optimized the reaction conditions, pH durance, loading capacity and storage stability of the immobilized enzyme (Wang D and Jiang W, 2019). siRNA-containing chitosan nanoparticles were improved colloidal stability and exhibited low toxicity with efficient cell uptake, explored by confocal microscopy of rhodamine labeled vectors (Martins GO, et al., 2019). Bovine serum albumin in chitosan tripolyphosphate nanoparticles were effectively internalized by the cells. Fluorescence microscopy and gel electrophoresis showed that the highest cellular uptake takes place of protein (Stie MB, et al., 2019). LSC chimeric proteins encapsulated in chitosan nanoparticles were evaluated as a protective effect against the toxins produced by Enterotoxigenic Escherichia coli (ETEC), Enterohemorrhagic Escherichia coli (EHEC), and Vibrio cholera bacteria. Morever was able to induce systemic and mucosal immune responses by generating a useful titer of IgG and IgA by antibody titer by Enzyme-Linked Immunosorbent Assays (ELISA) and test of the toxins on the immunized mouse (Marandi BH, et al., 2019). Ionotropic gelification was used for entrapment of TistH, a peptide identified in the venom gland of the Tityus stigmurus scorpion in chitosan nanoparticles for improved antifungal efficacy against vulvovaginal candidiasis of C. albicans. Nanoparticles showed peptide loading greater than 96.5%, stable for 8 weeks and were able to induce the desired slow in vitro peptide release. Cytotoxicity assay proved biocompatibility of nanoparticles (Torres-Rego M, et al., 2019). Antigens loaded on the nanoparticles of curdlan sulfate/O-(2-hydroxyl) propyl-3-trimethyl ammonium chitosan chloride was evaluated for immuno stimulatory activity. It observed that nanoparticles were improving the activation of antigen-presenting cells, up-regulating the production of inflammatory factors and cytokines, induce cross-presentation, simultaneously activating type I interferon-related genes and also activated the Phosphoinositide 3-Kinase/ Protein kinase B (PI3K/AKT) and Mitogen-Activated Protein Kinase (MAPK) pathways and significantly promoted Interleukin-2 (IL-2) transcription to induce the proliferation of lymphocytes (Zhang S, et al., 2019). Amine functionalized chitosan was derived to synthesize using N-(2-hydroxyethyl) ethylenediamine used for loaded green fluorescent protein circular plasmid DNA in nanoparticles, which were prepared by ionic gelation method. Resulted nanoparticles were nontoxic, had high transfection efficiency in human embryonic kidney and primary ovine fibroblast cell lines, and would be used as an efficient gene carrier system (Gök MK, et al., 2019). Chitosan nanoparticles were successfully evaluated for entrapment of egg white derived peptides for improvement of its bioavailability. Peptides influenced the zeta potential as the the peptide charged groups were in different locations relative to the nanoparticles surfaces. Fourier Transform Infrared Spectroscopy (FTIR) study showed that peptides interacted with chitosan through strong hydrogen bonds and electrostatic interactions (Du Z, et al., 2019).
Chitosan nanoparticles were used to introduce anti-Human Immunodeficiency Virus (HIV) Small interfering RNA (siRNA) into two mammalian cell lines, macrophage Ralph And William’s cell line (RAW) 264.7 and HEK293 (Human Embryonic Kidney Cells) for gene therapy. Nanoparticles with the combination of chitosan with both carboxymethyl dextran and polyethylenimine significantly improved cell viability and siRNA delivery. Nanoparticles noticeably increased siRNA delivery efficiency with no significant cytotoxicity or apoptosis-inducing effects compared to the control cells and significantly reduced the RNA and protein expression of HIV-1 tat in both stable cells (Mobarakeh VI, et al., 2019).
Nanoparticles using chitosan derivatives: Thiolated trimethyl chitosan, methylated 4-N,N dimethyl aminobenzyl N,O carboxymethyl chitosan and thiolated trimethyl aminobenzyl chitosan were prepared for in vitro DNA transfection efficiency. All nanoparticles showed DNA condensing ability, negligible cytotoxicity, and methylated 4-N,N dimethyl aminobenzyl N,O carboxymethyl chitosan was the most effective vehicle for gene delivery in HEK-293T cells and thiolated trimethyl aminobenzyl chitosan exhibited the highest transfection efficiency by cell line study (Rahmani S, et al., 2019). For improving the properties of chitosan gelatin scaffolds in tissue engineering applications of fibroblast growth factor and bovine serum albumin loaded chitosan nanoparticles by the ionic gelation method introduced into chitosan gelatin scaffolds. Structural characterization and biological assays showed that nanoparticles significantly affected the physical properties of the scaffold and could provide a sustained release of growth factors to enhance the proliferation of fibroblast cells significantly and enhance their biological properties (Azizian S, et al., 2018). Ovalbumin as a model antigen was firstly encapsulated by cyclodextrin, either β-cyclodextrin or carboxymethyl-hydroxypropyl-β-cyclodextrin and formed inclusion complexes by a precipitation/coacervation method and then loaded in chitosan nanoparticles. Nanoparticles were improved ovalbumin loading efficiency and showed low initial release at pH 1.2 for 2 h less than 3.0% and delayed release which was below to 30% at pH 6.8 for further 72 h. Ovalbumin nanoparticles increasesing the ovalbumin-specific sIgA levels in the jejunum by 3.6-fold and 1.9-fold that of ovalbumin solution and also enhance the efficacy for inducing intestinal mucosal immune response (He M, et al., 2019). Chitosan nanoparticles containing B subunit of Chlora toxin immunogen of V. cholera, evaluated for used to improve the immune response and it may be used as a carrier for vaccine delivery. Nanoparticles were studied on BALB/c mice in three groups, including oral, oral-injection, and injection groups. Serum and fecal IgA and IgG were evaluated by ELISA test. 17.5 kDa recombinant CtxB was confirmed by Sodium Dodecyl-Sulfate Polyacrylamide Gel Electrophoresis (SDS-PAGE) and western blotting. Nanoparticles prescription showed 1/102400 IgG endpoint titers for injection group and 1/1600, 1/6400 for oral, oral-injection groups, respectively, and Serum and fecal IgA endpoint titers showed above 1/160 in all groups. Immunized mice were able to neutralize Ctx toxin by ileal loop test (Tabrizi NM, et al., 2018). Chitosan nanoparticles are evaluated in vitro by using multiple spectroscopic methods, thermodynamic analysis, Transmission Electron Microscope (TEM) images, and modeling for transporting testosterone. The loading efficacy of testosterone nanocarrier was 40%-55% and increased as chitosan size increased (Chanphai P and Tajmir-Riahi HA, 2017).
Applications in agriculture: Used of chemicals in agriculture is common practice for improving growth by preventing the crop from external microbial attack, enhancing metabolism. Nutrients, insecticides, pesticides, and herbicides were successfully delivered by encapsulating in chitosan nanoparticles. Encapsulation results were as controlled released with stability, biocompatibility that enhanced the crop quality and efficiency. Compatibility with encapsulated materials and easy encapsulation methods, non-toxicity character of chitosan make it the best material for agriculture. Chitosan nanoparticles loaded with clove oil showed the controlled release for 56 days with superior performance against Aspergillus niger, isolated from spoiled pomegranate, compared with free oil (Hasheminejad N, et al., 2019). Used of Cuminum cyminum essential oil loaded chitosan nanoparticles on the shelf life of button mushroom showed effective in maintaining color, firmness, and overall acceptability and inhibiting the investigated bacteria and mold and yeast growth, which resulted significantly higher Superoxide Dismutase (SOD) and Ascorbate Peroxidase (APX) activity, antioxidant capacity and total phenolic content and lower Polyphenol Oxidase (PPO) activity throughout the storage period (Karimirad R, et al., 2019). Spinosad and permethrin loaded chitosan nanoparticles showed to be more effective with a lasting residual effect compared to the free agrochemicals in toxicity study on Drosophila melanogaster at several concentrations (10, 50, 100 μg/mL). Evaluated by survivability, climbing, and larval crawling assays (Sharma A, et al., 2019). Chitosan nanoparticles induced upregulation of Pathogenesis-Related (PR)-protein and antioxidant genes which play a significant role for successful biocontrol of wilt disease caused by Fusarium andiyazi. The expression pattern of Pathogenesis-Related (PR) proteins genes such as PR-1, PR-2 (β-1,3-glucanase), PR-8 (chitinase), and PR-10 showed that 5.0 mg/ml concentration of chitosan nanoparticles produced maximum inhibition of radial mycelial growth also recorded pregulating the expression of β-1,3-glucanase, chitinase, PR-1 and PR-10 genes and transcript profile of SOD (Chun SC and Chandrasekaran M, 2019). S-nitrosoglutathione encapsulated into chitosan nanoparticles would be more effective in attenuating the effects of water deficit on sugarcane plants compared to the supplying of S-nitrosoglutathione in its free form. Results showed higher photosynthetic rates under water, the deficitroot/shoot ratio was also increased; delayed release of NO improves the drought tolerance of sugarcane plants that useful for plant metabolism and increasing biomass allocation to root system (Silveira NM, et al., 2019). Zinc loaded chitosan Nanoparticles were evaluated via seed priming and foliar application in maize plants in zinc deficient and/or alkaline soil conditions. Used of nanoparticles resulted strong in vitro antifungal and seedling growth promoter activities, exhibited significant disease control through strengthening of plant innate immunity by elevating antioxidant and defense enzymes, balancing of reactive oxygen species and enhancing lignin accumulation, significantly controlled Curvularia leaf spot disease, increased grain yield from 20.5% to 39.8% and enriched the grain with zinc micronutrient from 41.27 to 62.21 μg/g dw (Choudhary RC, et al., 2019). Nanoparticles of chitosan showed positive effect on seed germination and seedling growth of wheat at a lower concentration than chitosan due to higher adsorption on the surface of wheat seeds showed by studies of energy-dispersive spectroscopy and confocal laser scanning microscopy. Chitosan nanoparticles had a growth promoting effect at a lower concentration (5 μg/mL) compared with Chitosan (50 μg/mL). Moreover induced the auxin-related gene expression, accelerated Indole-3-Acetic Acid (IAA) biosynthesis and transport, and reduced IAA oxidase activity, resulting in the increase of IAA concentration in wheat shoots and roots (Li R, et al., 2019). It also evaluated as a germination elicitor of Oryza sativa L. Treatment of chitosan nanoparticles in the concentration of 1 mg/ ml for 120 min gave the highest growth rates. No toxicity was found by Environmental Protection Agency (EPA) guidelines (Divya K, et al., 2019). A Chitosan thymol nanoparticle prepared by ionic gelation was added in quinoa protein/chitosan edible films that improve the performance on the extension of postharvest life of blueberries and tomato cherries. Film with chitosan nanopartile was more effective in reducing water vapour permeability, and also potential application as an antimicrobial (Medina E, et al., 2019). Fabricated chitosan-based silver nanoparticles are onto linen fabric. Colouring, antibacterial, and radical scavenging activity of chitosan-silver nanoparticles could be transferred to the linen fabric surface. Chitosan favours coating and stabilization of silver ions also synergistically with silver nanoparticles and also exhibited strong antibacterial against E. coli and S. aureus and antioxidant effects investigated photometrically by DPPH assay onto linen surface (Butola BS, 2019). Chitosan was used to prepare nanoparticles with botanical pesticide PONNEEM® using cross-linking agents Glutaraldehyde (GLA) and Tripolyphosphate (TPP) were observed as a great promise in H. armigera management. Both types of nanoparticles showed antifeedant activity and larvicidal activity against H. armigera resulted in significantly lowering the weights of H. armigera pupae (Paulraj MG, et al., 2017).
Using for food packaging: Polymeric chitosan can become an important alternative for food packaging because of acting as a mechanical barrier and reduces water evaporation, prevented from microbial contamination, compatible with packed material, and easily degradable in eco systems. In addition, chitosan nanoparticles may be used as a filler material for biodegradable plastic matrixes which are in need of improvement in terms of mechanical and barrier properties. To deal with the serious environmental pollution resulting from plastic packaging materials, biodegradable films using chitosan are gaining considerable increase gradually. Bio-based multilayer films incorporated with carboxymethyl chitosan-ZnO nanoparticles synthesized by direct precipitation method and multilayer films with chitosan film as the outer layer and sodium alginate film as the inner layer were prepared by solution casting method. Films overcome the weakness and showing enhanced tensile strength, and to better water vapor resistance, exhibited distinctive antibacterial activity against S. aureus and E. coli and evaluated as a promising material for food packaging (Wang H, et al., 2019). Chitosan/silver nanoparticles was prepared with controllable size by cross-linking agent concentration and evaluated that the small size chitosan/silver nanoparticles are promising candidate in the field of antibacterial and fruit preservation applications because of strong bacteriostasis and fresh-keeping function of specific surface area and more encapsulated silver content (Liang J, et al., 2019). Films with nanoparticles were applied as an internal coating to a Polyethylene Terephthalate (PET) lowering the weight loss store foods (Medina E, et al., 2019).
Self-assembled monodisperse chitosan nanoparticles with a particle size of 90 nm and zeta potential of 30.15 mV in solutions of 0.1% low molecular weight chitosan at pH 4.6 and 3:1 (chitosan: Tripolyphosphate Polyanion (TPP)) mass ratio was evaluated as a packaging materials with antimicrobial property (Sullivan DJ, et al., 2018). The film was a composite of ZnO-chitosan nanoparticles and incorporated them into the modified starch matrix. Films showed suppression in Gram-positive S. aureus than Gram-negative E. coli. chitosan nanoparticles in the film help to improve a substantial reduction of water vapor permeability from 51.0% to 43.7% accompanied with an increase of tensile strength from 4.11 to 12.79 MPa (p<0.05) (Hu X, et al., 2019).
Therapeutic applications: Chitosan chemically was a sugar and fibrous compound. That is also widely used for the treatment of high pressure, high cholesterol, with reduce fat and as a blood clotting in wound healing. Nanosize, molecular weight and chemical derivatization of chitosan was able to improve the therapeutic activity of chitosan.
Chitosan nanoparticles were prepared using ball-milling technique and were evaluated as natural compounds in human breast cancer treatment. 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide assay asserts the significant inhibitory action of both chitosan and its nanoparticles on the proliferation of human breast cancer cell in vitro. Chitosan nanoparticles had more antiproliferative effects on MD Anderson-Metastatic Breast (MDA-MB) 231 and SK-BR-3 cell lines than its corresponding chitosan (Taher FA, et al., 2019). Nanoparticles were made from poly (lactid-co-glycolide) acid and chitosan for targeted delivery. Receptor-mediated endocytosis showed monocyte-derived DC internalized chitosan-poly (lactid-co-glycolide) acid nanoparticles more efficiently than poly (lactid-co-glycolide) acid nanoparticles, presumably because of receptor-mediated endocytosis. Chitosan nanoparticles were delivered mostly to endosomal compartments, whereas poly (lactid-co-glycolide) acid nanoparticles primarily ended up in lysosomes resulting enhanced delivery to the endosomal compartments of antigen presenting cells (Durán V, et al., 2019). Chitosan nanoparticles were used as a supplement to Nile tilapia (Oreochromis niloticus) resulting favorable growth promoting and feed utilization effects by enhancing the activities of digestive enzymes, inhibition of the growth of intestinal microbial populations and improving certain indicators of innate immunity (Abd El-Naby FS, et al., 2019). Chitosan nanoparticles were synthesized by the ionic gelation process, using tripolyphosphate as a crosslinking agent for the treatment of oral candidiasis. Nanoparticles was evaluated by C. albicans time-kill assay showed 25%-50% inhibition of C. albicans which resulted lower C. albicans viability over 24 h in comparison with nystatin and chitosan (de Carvalho FG, et al., 2019). Chitosan nanoparticles were evaluated a promising candidate for in vitro cytotoxicity against HeLa cells in chitosan-quinoline nanoparticles prepared by oil-in-water nanoemulsion technique. Nanoparticles were nanorod shape, monolithic structure, drug loading capacity, encapsulation efficiency, and great pH-sensitive release behavior (Amjadi S, et al., 2019). Chitosan nanoparticles embedded with diclofenac sodium was prepared by ionic gelation method showed antibacterial activity against gram-positive Staphylococcus aureus and Bacillus subtilis by agar diffusion and broth dilution methods (Alqahtani FY, et al., 2019).
Implants: Chitosan had limited function for bone regeneration due to its low mechanical robustness and non-osteogenic inductivity. Hybridized chitosan with TiO2 nanoparticles sponges were used for bone tissue engineering by improving its bone regeneration capability. Degradation test showed a significant effect of TiO2 nanoparticles addition in retaining its integrity. Dentin Matrix Protein 1 (DMP1) and Osteocalcin (OCN) gene up regulation in TiO2 treated group indicated bone regeneration. Cytotoxicity analysis showed the biocompatible of sponge (Ikono R, et al., 2019). Chitosan nanoparticles loaded with sinapic acid which is a plant-derived phenolic compound known for its multiple biological properties, incorporated into polycaprolactone fibers via an electro spinning method. A critical-sized rat calvarial bone defect model system identified that the inclusion of sinapic acid into polycaprolactone/chitosan nanoparticles fibers, accelerated bone formation by activating the Transforming growth factor (TGF)-β1/Bone Morphogenetic Protein (BMP)/Smads/Runx2 signaling pathway, might have therapeutic benefits in bone regeneration (Balagangadharan K, et al., 2019). Two-step coating process i.e. anodizing and consequently coating with chitosan-heparin nanoparticles with nitinol could promote both endothelial cell compatibility and blood compatibility to the nitinol surface which might be appropriate for coronary stent application. This resulted in a significant reduction in nickel release, while promoting human umbilical vein endothelial cells attachment, spreading, and proliferation. Furthermore, this two-step coating could significantly contribute to the reduction of blood coagulation by releasing heparin in a controlled manner (Mohammadi F, et al., 2019). A biodegradable dressing containing chitosan nanoparticles were prepared by ionic gelation method and then assembled into the porous chitosan dressing by lyophilization. Evaluated as efficient for removing necrotic tissues and accelerating the hemostasis activity and become useful for efficient and rapid wound healing by in vitro cellular investigations with human dermal fibroblasts and human thrombin-antithrombin based in vitro ELISA assay (Biranje SS, et al., 2019).
Discussion and Conclusion
Chitosan emerged as a potential polymeric material for various applications because of their remarkable properties. Chitosan not only loads a delivered compound but also provides physiochemical stability which results in improvement in effectively. Pharmaceutical drug deliveries are especially for anticancer, anti-microbial and protein need special requirements to get therapeutics response. Moreover for the application of herbs and plants with enhance cultivation chitosan nanoparticles were raised as a major tool for loading herbicides, pesticides, and insecticides. Same observations of nanoparticles were also found in food packaging, and implants. Chitosan has itself therapeutics property and converted to nano sized, definitely assist to improve property. Thus, chitosan becomes a choice of polymeric material for nanoparticles that overcome many problems and improved the affectivity of loaded compounds.
Declarations
Acknowledgements
We thank PR Pote College of Pharmacy, Amravati for this article.
Availability of data and materials
All data generated or analyzed during this study are included in this published article and available from the corresponding author on reasonable request.
References
- Buzea C, Pacheco II, Robbie K. Nanomaterials and nanoparticles: Sources and toxicity. Biointerphases. 2007; 2(4): 17-71.
[Crossref] [Google Scholar] [Pubmed]
- Reddy N, Yang Y. Introduction to Chitin, Chitosan, and Alginate Fibers. Innovative Biofibers from Renewable Resources. 2015: 93-94.
- Dutta PK, Dutta J, Tripathi VS. Chitin and chitosan: Chemistry, properties and applications. J Sci Ind Res. 2004; 63: 20-31.
- Kumar MN. A review of chitin and chitosan applications. React Funct Polym. 2000; 46(1): 1-27.
- Divya K, Jisha MS. Chitosan nanoparticles preparation and applications. Environ Chem Lett. 2018; 16(1): 101-112.
- Güven UM, Başaran E. In vitro-in vivo evaluation of olopatadine incorporated chitosan nanoparticles for the treatment of ocular allergy. J Drug Deliv Sci Technol. 2021; 64: 102518.
- Atashgahi M, Ghaemi B, Valizadeh A, Moshiri A, Nekoofar MH, Amani A. Epinephrine-entrapped chitosan nanoparticles covered by gelatin nanofibers: A bi-layer nano-biomaterial for rapid hemostasis. Int J Pharm. 2021; 608: 121074.
[Crossref] [Google Scholar] [Pubmed]
- Khalil RM, el Arini SK, AbouSamra MM, Zaki HS, El-Gazaerly ON, Elbary AA. Development of lecithin/chitosan nanoparticles for promoting topical delivery of propranolol hydrochloride: Design, optimization and in-vivo evaluation. J Pharm Sci. 2021; 110(3): 1337-1348.
[Crossref] [Google Scholar] [Pubmed]
- Khan MA, Yue C, Fang Z, Hu S, Cheng H, Bakry AM, et al. Alginate/chitosan-coated zein nanoparticles for the delivery of resveratrol. J Food Eng. 2019; 258: 45-53.
- Narayan S. Lithium entrapped chitosan nanoparticles to reduce toxicity and increase cellular uptake of lithium. Environ Toxicol Pharmacol. 2018; 61: 79-86.
[Crossref] [Google Scholar] [Pubmed]
- Pola CC, Moraes AR, Medeiros EA, Teófilo RF, Soares NF, Gomes CL. Development and optimization of pH-responsive PLGA-chitosan nanoparticles for triggered release of antimicrobials. Food Chem. 2019; 295: 671-679.
[Crossref] [Google Scholar] [Pubmed]
- Amaral AC, Saavedra PH, Souza AC, de Melo MT, Tedesco AC, Morais PC, et al. Miconazole loaded chitosan-based nanoparticles for local treatment of vulvovaginal candidiasis fungal infections. Colloids Surf B Biointerfaces. 2019; 174: 409-415.
[Crossref] [Google Scholar] [Pubmed]
- Taghizadeh MT, Ashassi-Sorkhabi H, Afkari R, Kazempour A. Cross-linked chitosan in nano and bead scales as drug carriers for betamethasone and tetracycline. Int J Biol Macromol. 2019; 131: 581-588.
[Crossref] [Google Scholar] [Pubmed]
- Yilmaz MT, Yilmaz A, Akman PK, Bozkurt F, Dertli E, Basahel A, et al. Electrospraying method for fabrication of essential oil loaded-chitosan nanoparticle delivery systems characterized by molecular, thermal, morphological and antifungal properties. Innov Food Sci Emerg Technol. 2019; 52: 166-178.
- Marei N, Elwahy AH, Salah TA, El Sherif Y, Abd El-Samie E. Enhanced antibacterial activity of Egyptian local insects' chitosan-based nanoparticles loaded with ciprofloxacin-HCl. Int J Biol Macromol. 2019; 126: 262-272.
[Crossref] [Google Scholar] [Pubmed]
- Kritchenkov AS, Egorov AR, Krytchankou IS, Dubashynskaya NV, Volkova OV, Shakola TV, et al. Synthesis of novel 1H-tetrazole derivatives of chitosan via metal-catalyzed 1, 3-dipolar cycloaddition. Catalytic and antibacterial properties of [3-(1H-tetrazole-5-yl) ethyl] chitosan and its nanoparticles. Int J Biol Macromol. 2019; 132: 340-350.
[Crossref] [Google Scholar] [Pubmed]
- Omidi S, Kakanejadifard A. Modification of chitosan and chitosan nanoparticle by long chain pyridinium compounds: Synthesis, characterization, antibacterial, and antioxidant activities. Carbohydr Polym. 2019; 208: 477-485.
[Crossref] [Google Scholar] [Pubmed]
- Hagras NA, Allam AF, Farag HF, Osman MM, Shalaby TI, Mogahed NM, et al. Successful treatment of acute experimental toxoplasmosis by spiramycin-loaded chitosan nanoparticles. Exp Parasitol. 2019; 204: 107717.
[Crossref] [Google Scholar] [Pubmed]
- Imam SS, Bukhari SN, Ahmad J, Ali A. Formulation and optimization of levofloxacin loaded chitosan nanoparticle for ocular delivery: In vitro characterization, ocular tolerance and antibacterial activity. Int J Biol Macromol. 2018; 108: 650-659.
[Crossref] [Google Scholar] [Pubmed]
- Mousavi SM, Hashemi SA, Ghasemi Y, Atapour A, Amani AM, Savar Dashtaki A, et al. Green synthesis of silver nanoparticles toward bio and medical applications: Review study. Artif Cells Nanomed Biotechnol. 2018; 46(3): 855-872.
[Crossref] [Google Scholar] [Pubmed]
- Geethakumari D, Sathyabhama AB, Sathyan KR, Mohandas D, Somasekharan JV, Puthiyedathu ST. Folate functionalized chitosan nanoparticles as targeted delivery systems for improved anticancer efficiency of cytarabine in MCF-7 human breast cancer cell lines. Int J Biol Macromol. 2022; 199: 150-161.
[Crossref] [Google Scholar] [Pubmed]
- Hesami S, Safi S, Larijani K, Badi HN, Abdossi V, Hadidi M. Synthesis and characterization of chitosan nanoparticles loaded with greater celandine (Chelidonium majus L.) essential oil as an anticancer agent on MCF-7 cell line. Int J Biol Macromol. 2022; 194: 974-981.
[Crossref] [Google Scholar] [Pubmed]
- Peng HH, Hong DX, Guan YX, Yao SJ. Preparation of pH-responsive DOX-loaded chitosan nanoparticles using supercritical assisted atomization with an enhanced mixer. Int J Pharm. 2019; 558: 82-90.
[Crossref] [Google Scholar] [Pubmed]
- Alkholief M. Optimization of Lecithin-Chitosan nanoparticles for simultaneous encapsulation of doxorubicin and piperine. J Drug Deliv Sci Technol. 2019; 52: 204-214.
- Bhatta A, Krishnamoorthy G, Marimuthu N, Dihingia A, Manna P, Biswal HT, et al. Chlorin e6 decorated doxorubicin encapsulated chitosan nanoparticles for photo-controlled cancer drug delivery. Int J Biol Macromol. 2019; 136: 951-961.
[Crossref] [Google Scholar] [Pubmed]
- Cheng X, Zeng X, Zheng Y, Wang X, Tang R. Surface-fluorinated and pH-sensitive carboxymethyl chitosan nanoparticles to overcome biological barriers for improved drug delivery in vivo. Carbohydr Polym. 2019; 208: 59-69.
[Crossref] [Google Scholar] [Pubmed]
- Barbosa AI, Lima SA, Reis S. Development of methotrexate loaded fucoidan/chitosan nanoparticles with anti-inflammatory potential and enhanced skin permeation. Int J Biol Macromol. 2019; 124: 1115-1122.
[Crossref] [Google Scholar] [Pubmed]
- Zhang E, Xing R, Liu S, Li K, Qin Y, Yu H, et al. Vascular targeted chitosan-derived nanoparticles as docetaxel carriers for gastric cancer therapy. Int J Biol Macromol. 2019; 126: 662-672.
[Crossref] [Google Scholar] [Pubmed]
- Rosch JG, Winter H, DuRoss AN, Sahay G, Sun C. Inverse-micelle synthesis of doxorubicin-loaded alginate/chitosan nanoparticles and in vitro assessment of breast cancer cytotoxicity. Colloid Interface Sci Commun. 2019; 28: 69-74.
[Crossref] [Google Scholar] [Pubmed]
- Sorasitthiyanukarn FN, Bhuket PR, Muangnoi C, Rojsitthisak P, Rojsitthisak P. Chitosan/alginate nanoparticles as a promising carrier of novel curcumin diethyl diglutarate. Int J Biol Macromol. 2019; 131: 1125-1136.
[Crossref] [Google Scholar] [Pubmed]
- Xu M, Asghar S, Dai S, Wang Y, Feng S, Jin L, et al. Mesenchymal stem cells-curcumin loaded chitosan nanoparticles hybrid vectors for tumor-tropic therapy. Int J Biol Macromol. 2019; 134: 1002-1012.
[Crossref] [Google Scholar] [Pubmed]
- Mehata AK, Bharti S, Singh P, Viswanadh MK, Kumari L, Agrawal P, et al. Trastuzumab decorated TPGS-g-chitosan nanoparticles for targeted breast cancer therapy. Colloids Surf B Biointerfaces. 2019; 173: 366-377.
[Crossref] [Google Scholar] [Pubmed]
- Miranda-Calderón JE, Macías-Rosales L, Gracia-Mora I, Ruiz-Azuara L, Faustino-Vega A, Gracia-Mora J, et al. Effect of casiopein III-ia loaded into chitosan nanoparticles on tumor growth inhibition. J Drug Deliv Sci Technol. 2018; 48: 1-8.
- Gomathi T, Sudha PN, Florence JA, Venkatesan J, Anil S. Fabrication of letrozole formulation using chitosan nanoparticles through ionic gelation method. Int J Biol Macromol. 2017; 104: 1820-1832.
[Crossref] [Google Scholar] [Pubmed]
- Wang D, Jiang W. Preparation of chitosan-based nanoparticles for enzyme immobilization. Int J Biol Macromol. 2019; 126: 1125-1132.
[Crossref] [Google Scholar] [Pubmed]
- Martins GO, Petronio MS, Lima AM, Junior AM, de Oliveira Tiera VA, de Freitas Calmon M, et al. Amphipathic chitosans improve the physicochemical properties of siRNA-chitosan nanoparticles at physiological conditions. Carbohydr Polym. 2019; 216: 332-342.
[Crossref] [Google Scholar] [Pubmed]
- Stie MB, Thoke HS, Issinger OG, Hochscherf J, Guerra B, Olsen LF. Delivery of proteins encapsulated in chitosan-tripolyphosphate nanoparticles to human skin melanoma cells. Colloids Surf B Biointerfaces. 2019; 174: 216-223.
[Crossref] [Google Scholar] [Pubmed]
- Marandi BH, Zolfaghari MR, Kazemi R, Motamedi MJ, Amani J. Immunization against Vibrio cholerae, ETEC, and EHEC with chitosan nanoparticle containing LSC chimeric protein. Microb Pathog. 2019; 134: 103600.
[Crossref] [Google Scholar] [Pubmed]
- Torres-Rego M, Glaucia-Silva F, Soares KS, de Souza LB, Damasceno IZ, dos Santos-Silva E, et al. Biodegradable cross-linked chitosan nanoparticles improve anti-Candida and anti-biofilm activity of TistH, a peptide identified in the venom gland of the Tityus stigmurus scorpion. Mater Sci Eng C. 2019; 103: 109830.
[Crossref] [Google Scholar] [Pubmed]
- Zhang S, Jiang H, Huang S, Li P, Wang F. Curdlan sulfate/O-linked quaternized chitosan nanoparticles acting as potential adjuvants promote multiple arms of immune responses. Carbohydr Polym. 2019; 213: 100-111.
[Crossref] [Google Scholar] [Pubmed]
- Gök MK, Demir K, Cevher E, Özgümüş S, Pabuccuoğlu S. Effect of the linear aliphatic amine functionalization on in vitro transfection efficiency of chitosan nanoparticles. Carbohydr Polym. 2019; 207: 580-587.
[Crossref] [Google Scholar] [Pubmed]
- Du Z, Liu J, Zhang T, Yu Y, Zhang Y, Zhai J, et al. A study on the preparation of chitosan-tripolyphosphate nanoparticles and its entrapment mechanism for egg white derived peptides. Food Chem. 2019; 286: 530-536.
[Crossref] [Google Scholar] [Pubmed]
- Mobarakeh VI, Modarressi MH, Rahimi P, Bolhassani A, Arefian E, Atyabi F, et al. Optimization of chitosan nanoparticles as an anti-HIV siRNA delivery vehicle. Int J Biol Macromol. 2019; 129: 305-315.
[Crossref] [Google Scholar] [Pubmed]
- Rahmani S, Hakimi S, Esmaeily A, Samadi FY, Mortazavian E, Nazari M, et al. Novel chitosan based nanoparticles as gene delivery systems to cancerous and noncancerous cells. Int J Pharm. 2019; 560: 306-314.
[Crossref] [Google Scholar] [Pubmed]
- Azizian S, Hadjizadeh A, Niknejad H. Chitosan-gelatin porous scaffold incorporated with Chitosan nanoparticles for growth factor delivery in tissue engineering. Carbohydr Polym. 2018; 202: 315-322.
[Crossref] [Google Scholar] [Pubmed]
- He M, Zhong C, Hu H, Jin Y, Chen Y, Lou K, et al. Cyclodextrin/chitosan nanoparticles for oral ovalbumin delivery: Preparation, characterization and intestinal mucosal immunity in mice. Asian J Pharm Sci. 2019; 14(2): 193-203.
[Crossref] [Google Scholar] [Pubmed]
- Tabrizi NM, Amani J, Ebrahimzadeh M, Nazarian S, Kazemi R, Almasian P. Preparation and evaluation of chitosan nanoparticles containing CtxB antigen against Vibrio cholera. Microb Pathog. 2018; 124: 170-177.
[Crossref] [Google Scholar] [Pubmed]
- Chanphai P, Tajmir-Riahi HA. Encapsulation of testosterone by chitosan nanoparticles. Int J Biol Macromol. 2017; 98: 535-541.
[Crossref] [Google Scholar] [Pubmed]
- Hasheminejad N, Khodaiyan F, Safari M. Improving the antifungal activity of clove essential oil encapsulated by chitosan nanoparticles. Food Chem. 2019; 275: 113-122.
[Crossref] [Google Scholar] [Pubmed]
- Karimirad R, Behnamian M, Dezhsetan S. Application of chitosan nanoparticles containing Cuminum cyminum oil as a delivery system for shelf life extension of Agaricus bisporus. Lwt. 2019; 106: 218-228.
- Sharma A, Sood K, Kaur J, Khatri M. Agrochemical loaded biocompatible chitosan nanoparticles for insect pest management. Biocatal Agric Biotechnol. 2019; 18: 101079.
- Chun SC, Chandrasekaran M. Chitosan and chitosan nanoparticles induced expression of pathogenesis-related proteins genes enhances biotic stress tolerance in tomato. Int J Biol Macromol. 2019; 125: 948-954.
[Crossref] [Google Scholar] [Pubmed]
- Silveira NM, Seabra AB, Marcos FC, Pelegrino MT, Machado EC, Ribeiro RV. Encapsulation of S-nitrosoglutathione into chitosan nanoparticles improves drought tolerance of sugarcane plants. Nitric Oxide. 2019; 84: 38-44.
[Crossref] [Google Scholar] [Pubmed]
- Choudhary RC, Kumaraswamy RV, Kumari S, Sharma SS, Pal A, Raliya R, et al. Zinc encapsulated chitosan nanoparticle to promote maize crop yield. Int J Biol Macromol. 2019; 127: 126-135.
[Crossref] [Google Scholar] [Pubmed]
- Li R, He J, Xie H, Wang W, Bose SK, Sun Y, et al. Effects of chitosan nanoparticles on seed germination and seedling growth of wheat (Triticum aestivum L.). Int J Biol Macromol. 2019; 126: 91-100.
[Crossref] [Google Scholar] [Pubmed]
- Divya K, Vijayan S, Nair SJ, Jisha MS. Optimization of chitosan nanoparticle synthesis and its potential application as germination elicitor of Oryza sativa L. Int J Biol Macromol. 2019; 124: 1053-1059.
[Crossref] [Google Scholar] [Pubmed]
- Medina E, Caro N, Abugoch L, Gamboa A, Díaz-Dosque M, Tapia C. Chitosan thymol nanoparticles improve the antimicrobial effect and the water vapour barrier of chitosan-quinoa protein films. J Food Eng. 2019; 240: 191-198.
- Butola BS. Recent advances in chitosan polysaccharide and its derivatives in antimicrobial modification of textile materials. Int J Biol Macromol. 2019; 121: 905-912.
[Crossref] [Google Scholar] [Pubmed]
- Paulraj MG, Ignacimuthu S, Gandhi MR, Shajahan A, Ganesan P, Packiam SM, et al. Comparative studies of tripolyphosphate and glutaraldehyde cross-linked chitosan-botanical pesticide nanoparticles and their agricultural applications. Int J Biol Macromol. 2017; 104: 1813-1819.
[Crossref] [Google Scholar] [Pubmed]
- Wang H, Gong X, Miao Y, Guo X, Liu C, Fan YY, et al. Preparation and characterization of multilayer films composed of chitosan, sodium alginate and carboxymethyl chitosan-ZnO nanoparticles. Food Chem. 2019; 283: 397-403.
[Crossref] [Google Scholar] [Pubmed]
- Liang J, Wang J, Li S, Xu L, Wang R, Chen R, et al. The size-controllable preparation of chitosan/silver nanoparticle composite microsphere and its antimicrobial performance. Carbohydr Polym. 2019; 220: 22-29.
[Crossref] [Google Scholar] [Pubmed]
- Sullivan DJ, Cruz-Romero M, Collins T, Cummins E, Kerry JP, Morris MA. Synthesis of monodisperse chitosan nanoparticles. Food Hydrocoll. 2018; 83: 355-364.
- Hu X, Jia X, Zhi C, Jin Z, Miao M. Improving the properties of starch-based antimicrobial composite films using ZnO-chitosan nanoparticles. Carbohydr Polym. 2019; 210: 204-209.
- Taher FA, Ibrahim SA, Abd El-Aziz A, Abou El-Nour MF, El-Sheikh MA, El-Husseiny N, et al. Anti-proliferative effect of chitosan nanoparticles (extracted from crayfish Procambarus clarkii, Crustacea: Cambaridae) against MDA-MB-231 and SK-BR-3 human breast cancer cell lines. Int J Biol Macromol. 2019; 126: 478-487.
[Crossref] [Google Scholar] [Pubmed]
- Durán V, Yasar H, Becker J, Thiyagarajan D, Loretz B, Kalinke U, et al. Preferential uptake of chitosan-coated PLGA nanoparticles by primary human antigen presenting cells. Nanomedicine. 2019; 21: 102073.
[Crossref] [Google Scholar] [Pubmed]
- Abd El-Naby FS, Naiel MA, Al-Sagheer AA, Negm SS. Dietary chitosan nanoparticles enhance the growth, production performance, and immunity in Oreochromis niloticus. Aquaculture. 2019; 501: 82-89.
- de Carvalho FG, Magalhaes TC, Teixeira NM, Gondim BL, Carlo HL, dos Santos RL, et al. Synthesis and characterization of TPP/chitosan nanoparticles: Colloidal mechanism of reaction and antifungal effect on C. albicans biofilm formation. Mater Sci Eng C. 2019; 104: 109885.
[Crossref] [Google Scholar] [Pubmed]
- Amjadi S, Emaminia S, Davudian SH, Pourmohammad S, Hamishehkar H, Roufegarinejad L. Preparation and characterization of gelatin-based nanocomposite containing chitosan nanofiber and ZnO nanoparticles. Carbohydr Polym. 2019; 216: 376-384.
[Crossref] [Google Scholar] [Pubmed]
- Alqahtani FY, Aleanizy FS, el Tahir E, Alquadeib BT, Alsarra IA, Alanazi JS, et al. Preparation, characterization, and antibacterial activity of diclofenac-loaded chitosan nanoparticles. Saudi Pharm J. 2019; 27(1): 82-87.
[Crossref] [Google Scholar] [Pubmed]
- Ikono R, Li N, Pratama NH, Vibriani A, Yuniarni DR, Luthfansyah M, et al. Enhanced bone regeneration capability of chitosan sponge coated with TiO2 nanoparticles. Biotechnol Rep. 2019; 24: 350.
[Crossref] [Google Scholar] [Pubmed]
- Balagangadharan K, Trivedi R, Vairamani M, Selvamurugan N. Sinapic acid-loaded chitosan nanoparticles in polycaprolactone electrospun fibers for bone regeneration in vitro and in vivo. Carbohydr Polym. 2019; 216: 1-6.
[Crossref] [Google Scholar] [Pubmed]
- Mohammadi F, Golafshan N, Kharaziha M, Ashrafi A. Chitosan-heparin nanoparticle coating on anodized NiTi for improvement of blood compatibility and biocompatibility. Int J Biol Macromol. 2019; 127: 159-168.
[Crossref] [Google Scholar] [Pubmed]
- Biranje SS, Madiwale PV, Patankar KC, Chhabra R, Dandekar-Jain P, Adivarekar RV. Hemostasis and anti-necrotic activity of wound-healing dressing containing chitosan nanoparticles. Int J Biol Macromol. 2019; 121: 936-946.
[Crossref] [Google Scholar] [Pubmed]
Author Info
Prasad R Deshmukh1*, Abhilash Joshi1, Chaitanya Vikhar1, SS Khdabadi2 and Mukund Tawar12Department of Pharmacognosy, Government College of Pharmacy, Maharashtra, India
Citation: Deshmukh PR: Current Applications of Chitosan Nanoparticles
Received: 15-Sep-2022 Accepted: 10-Oct-2022 Published: 17-Oct-2022, DOI: 10.31858/0975-8453.13.10.685-693
Copyright: This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
ARTICLE TOOLS
- Dental Development between Assisted Reproductive Therapy (Art) and Natural Conceived Children: A Comparative Pilot Study Norzaiti Mohd Kenali, Naimah Hasanah Mohd Fathil, Norbasyirah Bohari, Ahmad Faisal Ismail, Roszaman Ramli SRP. 2020; 11(1): 01-06 » doi: 10.5530/srp.2020.1.01
- Psychometric properties of the World Health Organization Quality of life instrument, short form: Validity in the Vietnamese healthcare context Trung Quang Vo*, Bao Tran Thuy Tran, Ngan Thuy Nguyen, Tram ThiHuyen Nguyen, Thuy Phan Chung Tran SRP. 2020; 11(1): 14-22 » doi: 10.5530/srp.2019.1.3
- A Review of Pharmacoeconomics: the key to “Healthcare for All” Hasamnis AA, Patil SS, Shaik Imam, Narendiran K SRP. 2019; 10(1): s40-s42 » doi: 10.5530/srp.2019.1s.21
- Deuterium Depleted Water as an Adjuvant in Treatment of Cancer Anton Syroeshkin, Olga Levitskaya, Elena Uspenskaya, Tatiana Pleteneva, Daria Romaykina, Daria Ermakova SRP. 2019; 10(1): 112-117 » doi: 10.5530/srp.2019.1.19
- Dental Development between Assisted Reproductive Therapy (Art) and Natural Conceived Children: A Comparative Pilot Study Norzaiti Mohd Kenali, Naimah Hasanah Mohd Fathil, Norbasyirah Bohari, Ahmad Faisal Ismail, Roszaman Ramli SRP. 2020; 11(1): 01-06 » doi: 10.5530/srp.2020.1.01
- Manilkara zapota (L.) Royen Fruit Peel: A Phytochemical and Pharmacological Review Karle Pravin P, Dhawale Shashikant C SRP. 2019; 10(1): 11-14 » doi: 0.5530/srp.2019.1.2
- Pharmacognostic and Phytopharmacological Overview on Bombax ceiba Pankaj Haribhau Chaudhary, Mukund Ganeshrao Tawar SRP. 2019; 10(1): 20-25 » doi: 10.5530/srp.2019.1.4
- A Review of Pharmacoeconomics: the key to “Healthcare for All” Hasamnis AA, Patil SS, Shaik Imam, Narendiran K SRP. 2019; 10(1): s40-s42 » doi: 10.5530/srp.2019.1s.21
- A Prospective Review on Phyto-Pharmacological Aspects of Andrographis paniculata Govindraj Akilandeswari, Arumugam Vijaya Anand, Palanisamy Sampathkumar, Puthamohan Vinayaga Moorthi, Basavaraju Preethi SRP. 2019; 10(1): 15-19 » doi: 10.5530/srp.2019.1.3