Research Article - (2024) Volume 15, Issue 5
Design and Evaluation of Forskolin-Loaded Liposomes
Balaram Ghosh, Manas Chakraborty* and Rabindranath PalAbstract
The goal of this study is to design liposome carrier system of forskolin for the treatment of muscle relaxant that is capable of delivering the encapsulated drug over a prolonged period of time by overcoming the limitations of conventional dosage forms. Foskolin is partially water-soluble drug but has low permeability. The main objective is to improve the permeability thereby improving the bioavailability. It is prepared by thin film hydration method, using materials like non-ionic surfactants (cholesterol, soylecithin) and solvents such as chloroform and ethanol. The Fourier Transform Infrared Spectroscopy (FTIR) results revealed that there is no interaction between forskolin and excipients; all the formulations showed better encapsulation efficacy. Scanning Electron Microscopy (SEM) analysis revealed the surface morphology of forskolin-loaded liposome while dissolution studies showed prolonged drug release. On comprising all formulations, F3 showed sustained release of 98.44% up to 12 h. This may be due to the longest saturated alkyl chain and shows the highest entrapment
Keywords
Bioavailability, Forskolin, Liposomes, Sustained release, Encapsulation
Introduction
Liposomes are known as non-ionic surfactant vesicles which are microscopic lamellar structures formed on admixture of a non-ionic surfactant, cholesterol and dicetyl phosphate with subsequent hydration with aqueous media (Chen S, et al., 2019; Sankhyan A and Pawar PK, 2013). Liposomes are capable of entrapping a variety of drugs and found as an alternative delivery system, with similar physical properties and are comparatively inexpensive with other delivery systems.
In the current years, transferring drug molecules to the desired site in the biological systems has become a very precise and sophisticated area of pharmaceutical research. The role of the drug delivery system is not only limited to a drug package just meant for administration and convenience but also to bring a required improvement in pharmacological efficacy and safety by carrying the drug molecules to the required site in the most convenient manner. Drug delivery system using colloidal particulate carriers like liposome has distinct merits over conventional dosage form as the colloidal particulate can act as drug reservoirs. Among different nanovesicular carriers, liposomes are selected as a carrier of choice because of its dominance over others carrier with regard to stability and cost effectiveness (Stulzer HK, et al., 2008; Shalini M, et al., 2016). Conventional drug delivery systems face some significant challenges, such as unfavorable pharmacokinetics and undesirable side effects. Nanocarriers have been extensively investigated in the past decades to overcome the challenges associated with conventional drug delivery systems, due to the advantages such as-
• Facilitate targeted drug delivery to the diseased site
• Enhance absorption as surface area increases and hence increase bioavailability
• Improve pharmacokinetics and bio distribution of active agents
• Increase retention in biological systems and extend the efficacy of drugs
Forskolin exerts 75% bioavailability but in the presence of food the bioavailability reduces to 30%-50% due to its relative short half-life. The main aim and objective of this study was to design a prolonged release Forskolin formulation which would benefit the patients by reducing dose frequency, increased patient compliance, effective treatment, reduction in plasma concentration fluctuations, and reduced side effects.
Materials and Methods
Materials
Forskolin was obtained as a gift sample form Emami Pvt. Ltd., Kolkata. Soyabean and cholesterol purchased from lobe chemical, Mumbai. Chloroform and methanol of analytical reagent were used.
Preparation of forskolin-loaded liposomes
Forskolin-loaded liposomes were developed by thin film hydration method in a rotary evaporator. Accurately weighed amounts of non-ionic surfactants (span 20, 40, 60 and 80) and cholesterol were transferred in a round bottom flask of the rotary evaporator. The chloroform and methanol (4:1) solution was added to the flask and evaporated at 50°C for 15-20 min under vacuum. The formed film is then hydrated with phosphate buffer pH 7.4 containing drug and vortexes at room temperature for 20 min which forms milky white suspension. The resultant dispersion was then cooled in an ice bath and sonicated for 3-5 min. Then, the resultant liposomes were stored at refrigerator (4°C-6°C). Various formulations were prepared (Table 1).
| Composition | Quantities | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| F1 | F2 | F3 | F4 | F5 | F6 | F7 | F8 | F9 | F10 | ||
| Forskolin | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | |
| Soy lecithin | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | |
| Cholesterol | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | |
| Span 20 | 1 | - | - | - | 0.5 | 0.5 | 0.5 | - | - | - | |
| Span 40 | - | 1 | - | - | 0.5 | - | - | 0.5 | 0.5 | - | |
| Span 60 | - | - | 1 | - | - | 0.5 | - | 0.5 | - | 0.5 | |
| Span 80 | - | - | - | 1 | - | - | 0.5 | - | 0.5 | 0.5 | |
| Ethanol (ml) | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | |
| Chloroform (ml) | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 | |
| Phosphate buffer (pH 7.4) | 25 | 25 | 25 | 25 | 25 | 25 | 25 | 25 | 25 | 25 | |
Table 1: Composition of liposome containing forskolin
Evaluation of liposomes
%practical yield: Percentage practical yield is calculated to know efficiency of any method, thus its help in selection of appropriate method of formulation. Liposome were collected and weighed to determine Practical Yield (PY) from the following equation. The results are shown in Table 2.
| Formulation code | % yield |
|---|---|
| F1 | 77.6 |
| F2 | 79.2 |
| F3 | 94.2 |
| F4 | 83.6 |
| F5 | 84.4 |
| F6 | 87.2 |
| F7 | 85.2 |
| F8 | 92.4 |
| F9 | 86.8 |
| F10 | 87.6 |
Table 2: % practical yield of liposomes
%practical yield=practical yield/theoretical yield × 100
%drug content: Liposome equivalent to 50 mg of forskolin was taken and dissolved in distilled water for the extraction of encapsulated drug with regularly shaking and kept undisturbed for 24 h for complete extraction. The extract was filtered and diluted serially with phosphate buffer pH 7.4 and the absorbance was measured at 210 nm and the drug content was calculated from the calibration curve, as shown in Table 3.
| Formulation code | % drug content ± SD |
|---|---|
| F1 | 86.84 ± 0.14 |
| F2 | 88.62 ± 0.24 |
| F3 | 97.18 ± 0.37 |
| F4 | 89.99 ± 0.28 |
| F5 | 91.93 ± 0.37 |
| F6 | 92.25 ± 0.48 |
| F7 | 95.96 ± 0.14 |
| F8 | 96.37 ± 0.24 |
| F9 | 93.46 ± 0.24 |
| F10 | 94.59 ± 0.14 |
Table 3: % drug content of liposome (n=3)
%drug content=test absorbance/standard absorbance × 100
In vitro drug release: The in vitro drug release was studied using United States Pharmacopeia (USP) dissolution apparatus (Karthick K and Kumaran KS, 2016; Gupta A, et al., 2007). Liposome equivalent to 50 mg of forskolin were placed in the dialysis membrane tubes. The tubes were immersed in dissolution vessel containing phosphate buffer pH 7.4 maintained at 37°C ± 0.5°C. Samples were withdrawn at periodic time intervals and replaced with equal amount of buffer to maintain sink condition. The samples were analyzed by Ultraviolent (UV)/visible spectrophotometer (Table 4).
| Time (h) | Pure drug | % cumulative drug release (meant ± SD) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| F1 | F2 | F3 | F4 | F5 | F6 | F7 | F8 | F9 | F10 | ||
| 0.5 | 27.99 ± 0.14 | 16.13 ± 0.10 | 15.16 ± 0.06 | 10.47 ± 0.05 | 14.16 ± 0.10 | 17.03 ± 0.05 | 17.41 ± 0.05 | 10.53 ± 0.05 | 10.63 ± 0.22 | 12.75 ± 0.09 | 13.16 ± 0.05 |
| 1 | 48.74 ± 0.14 | 27.97 ± 0.05 | 26.22 ± 0.05 | 20.72 ± 0.09 | 25.34 ± 0.05 | 25.34 ± 0.05 | 28.84 ± 0.05 | 21.06 ± 0.14 | 20.81 ± 0.25 | 23.06 ± 0.10 | 24.88 ± 0.11 |
| 2 | 70.86 ± 0.16 | 70.86 ± 0.16 | 44.31 ± 0.05 | 37.16 ± 0.05 | 41.78 ± 0.05 | 47.59 ± 0.05 | 48.53 ± 0.05 | 37.16 ± 0.05 | 37.34 ± 0.30 | 39.63 ± 0.05 | 10.63 ± 0.22 |
| 3 | 97.40 ± 0.94 | 72.91 ± 010 | 57.16 ± 0.06 | 44.72 ± 0.09 | 54.97 ± 0.11 | 60.72 ± 0.05 | 62.69 ± 0.11 | 45.81 ± 0.11 | 51.13 ± 0.05 | 47.69 ± 0.11 | 49.87 ± 0.19 |
| 4 | - | 90.06 ± 0.05 | 70.72 ± 0.05 | 55.34 ± 0.05 | 72.72 ± 0.05 | 70.94 ± 0.14 | 73.81 ± 0.05 | 55.91 ± 0.11 | 61.53 ± 0.05 | 61.53 ± 0.05 | 60.69 ± 0.11 |
| 5 | - | 96.49 ± 0.03 | 89.41 ± 0.11 | 64.06 ± 0.22 | 82.66 ± 0.05 | 85.09 ± 0.05 | 89.88 ± 0.11 | 66.06 ± 0.05 | 72.72 ± 0.20 | 69.69 ± 0.05 | 72.75 ± 0.19 |
| 6 | - | - | 72.75 ± 0.19 | 72.75 ± 0.19 | 72.75 ± 0.19 | 72.75 ± 0.19 | 98.31 ± 0.19 | 76.16 ± 0.05 | 82.91 ± 0.05 | 80.50 ± 0.11 | 80.50 ± 0.11 |
| 7 | - | - | - | - | - | 82.81 ± 0.06 | 94.78 ± 0.10 | 94.69 ± 0.16 | 91.75 ± 0.11 | 96.75 ± 0.49 | 97.32 ± 0.82 |
| 8 | - | - | - | - | - | - | - | - | - | 91.81 ± 0.05 | 97.53 ± 0.05 |
| 9 | - | - | - | - | - | - | - | - | - | - | 98.44 ± 0.10 |
Table 4: % comparison of dissolution data of liposomes containing forskolin (n=3)
Drug release kinetics: The release data obtained from various formulations were studied further for fitness of data in different kinetic models such as zero order, first order, Higuchi equation, Korsmeyer-Peppas and Hixson-Crowell release models as presented in Tables 5 and 6.
| Kinetic models | F1 | F2 | F3 | F4 | F5 | F6 | F7 | F8 | F9 | F10 |
|---|---|---|---|---|---|---|---|---|---|---|
| Zero order | ||||||||||
| r | 0.998 | 0.994 | 0.997 | 0.993 | 0.995 | 0.994 | 0.997 | 0.997 | 0.995 | 0.993 |
| k | 23.87 | 22.15 | 18.58 | 20.89 | 23.79 | 24.26 | 18.58 | 18.67 | 19.81 | 20.87 |
| First order | ||||||||||
| r | 1 | 0.999 | 1 | 0.999 | 1 | 0.999 | 0.999 | 1 | 0.999 | 0.999 |
| k | 0.667 | 0.557 | 0.307 | 0.382 | 0.531 | 0.6 | 0.326 | 0.358 | 0.379 | 0.415 |
| Higuchi model | ||||||||||
| r | 0.99 | 0.991 | 0.995 | 0.993 | 0.993 | 0.993 | 0.991 | 0.99 | 0.994 | 0.995 |
| k | 45.39 | 40.52 | 30.5 | 38.06 | 39.68 | 40.86 | 33.32 | 34.57 | 34.35 | 35.4 |
| Korsemeyer-Peppas model | ||||||||||
| r | 0.998 | 0.999 | 0.996 | 0.999 | 0.998 | 0.998 | 0.998 | 0.999 | 0.997 | 0.996 |
| k | 0.857 | 0.74 | 0.711 | 0.745 | 0.71 | 0.707 | 0.715 | 0.816 | 0.68 | 0.668 |
| Hixson Crowell model | ||||||||||
| r | 0.999 | 0.998 | 0.999 | 0.997 | 0.999 | 0.998 | 0.999 | 0.999 | 0.998 | 0.998 |
| k | 0.639 | 0.52 | 0.333 | 0.41 | 0.498 | 0.538 | 0.334 | 0.334 | 0.365 | 0.389 |
| T50 | 2.09 | 2.44 | 3.5 | 2.62 | 2.18 | 2.1 | 3.41 | 2.92 | 3.23 | 3.01 |
| T90 | 4 | 5.07 | 9.6 | 6.13 | 5.39 | 5.01 | 7.49 | 7.6 | 7.17 | 6.6 |
Table 5: Correlation coefficient (r) and release rate constant (k) values of forskolin- loaded liposomes
| Time (h) | Zero order % cumulative drug release | First order time | First order log % cumulative drug remaining | Higuchi square root of time | Higuchi % cumulative drug release | Korsemeyer-Peppas log time | Korsemeyer-Peppas log % cumulative drug release | Hixson-Crowell time | Hixson-Crowell cub root of % drug remaining |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 20.72 | 1 | 1.899 | 1 | 20.72 | 0 | 1.316 | 1 | 4.295 |
| 2 | 37.16 | 2 | 1.798 | 1.414 | 37.16 | 0.301 | 1.57 | 2 | 3.975 |
| 4 | 44.72 | 3 | 1.742 | 1.7325 | 44.72 | 0.477 | 1.65 | 3 | 3.809 |
| 4 | 55.34 | 4 | 1.649 | 2 | 55.34 | 0.602 | 1.743 | 4 | 3.547 |
| 5 | 64.06 | 5 | 1.555 | 2.236 | 64.06 | 0.698 | 1.806 | 5 | 3.3 |
| 6 | 73.63 | 6 | 1.421 | 2.449 | 73.63 | 0.778 | 1.867 | 6 | 2.976 |
| 8 | 82.81 | 8 | 1.235 | 2.828 | 82.81 | 0.903 | 1.918 | 8 | 2.58 |
| 10 | 91.81 | 10 | 0.913 | 3.162 | 91.81 | 1 | 1.962 | 10 | 2.015 |
| 12 | 98.44 | 12 | 0.193 | 3.464 | 98.44 | 1 | 1.993 | - | - |
Table 6: Kinetic data for the optimized formulation
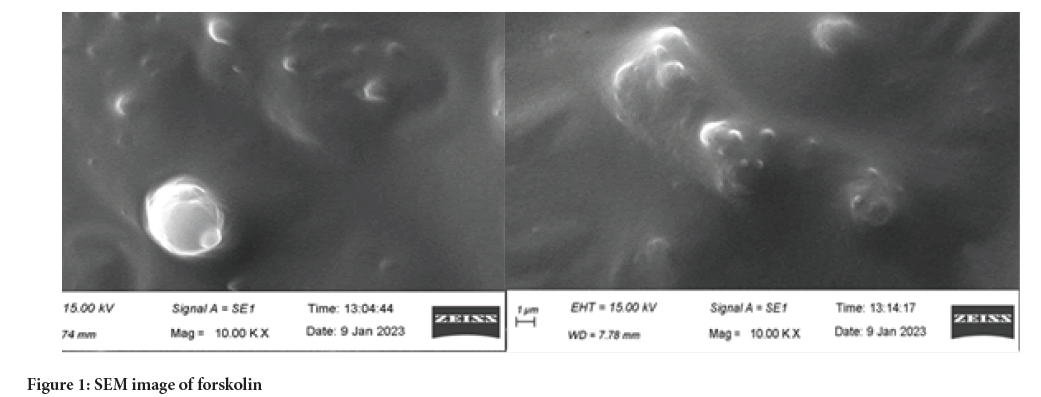
Scanning electron microscopy
Scanning: Electron microscopy of the liposome was performed to examine the particle size and surface morphology. Liposomes were mounted on metal stubs and the stub was then coated with conductive gold with sputter coater attached to the equipment. The pictures were taken using a scanning electron microscope (Figure 1).
Figure 1: SEM image of forskolin
Fourier Transform Infrared Spectroscopy (FTIR) studies: The chance of drug-excipients (cholesterol and non-ionic surfactants) interactions was investigated by FTIR spectrum studies. The FTIR spectrum of pure drug (forskolin) and combination of drug with excipients were recorded using FTIR spectrophotometer. The spectrum was scanned in the wavelength region of (4000-400) cm-1. The results are shown in Table 7.
| Functional group | Characteristic peaks (cm-1) | Observed peaks of forskolin (cm-1) | Observed peaks (optimized formulation) (cm-1) |
|---|---|---|---|
| C-S (Stretching) | 600-800 | 672.35 | 670.65 |
| C-N (Stretching) | 1020-1250 | 1193.64 | 1193.33 |
| C-H (Bending) | 1450-1470 | 1647.12 | 1467.45 |
| N H (Bending) | 1580-1650 | 1581.9 | 1582.41 |
| C=O (Stretching) | 1690-1760 | 1740.9 | 1740.86 |
| S-H (Stretching) | ≈2550 | 2561.28 | 2561.61 |
Table 7: FT-IR interpretation of pure drug and optimized formulation
Stability studies: Stability studies for the optimized formulation were carried out at elevated temperature 45°C ± 2°C with Relative Humidity (RH) 75% for a period of 3 months according to the International Council for Harmonization (ICH). At definite time periods, the samples from each batch were taken and evaluated for drug release (Table 8).
| Time (h) | % cumulative drug release ( mean ± S.D) | |||
|---|---|---|---|---|
| Initial | 1st month | 2nd month | 3rd month | |
| 0.5 | 10.47 ± 0.05 | 10.47 ± 0.14 | 10.34 ± 0.24 | 10.28 ± 0.24 |
| 1 | 20.72 ± 0.09 | 20.72 ± 0.16 | 20.66 ± 0.24 | 20.53 ± 0.16 |
| 2 | 44.72 ± 0.09 | 37.13 ± 0.28 | 36.94 ± 0.43 | 36.84 ± 0.28 |
| 3 | 44.72 ± 0.09 | 44.31 ± 0.27 | 44.03 ± 0.30 | 43.88 ± 0.41 |
| 4 | 55.34 ± 0.05 | 55.06 ± 0.87 | 54.75 ± 0.90 | 54.34 ± 0.87 |
| 5 | 64.06 ± 0.22 | 63.78 ± 1.09 | 63.47 ± 1.15 | 62.75 ± 1.13 |
| 6 | 73.63 ± 0.05 | 73.56 ± 1.55 | 73.25 ± 1.63 | 72.22 ± 1.28 |
| 7 | 82.81 ± 0.06 | 82.53 ± 0.87 | 82.06 ± 1.03 | 81.78 ± 0.91 |
| 8 | 91 ± 0.05 | 91.78 ± 0.84 | 91.50 ± 0.50 | 91.16 ± 0.55 |
| 9 | 98.44 ± 0.10 | 98.06 ± 1.44 | 97.75 ± 1.11 | 97.13 ± 1.14 |
Table 8: Dissolution data of optimized formulation
Results and Discussion
%practical yield
From the results, it was observed that there was no significant loss of the drug and excipients during the preparation of liposomes by thin film hydration method.
%drug content
The solutions were analyzed for drug content spectrophotometrically at 210 nm. The drug content was calculated by estimating the amount of drug in liposomes. Results shown in Table 3 revealed that there was no significant loss of the drug during the preparation and all the formulations exhibited fairly uniform drug content.
In vitro drug release
The formulated niosomes were subjected to in vitro drug release studies using phosphate buffer pH 7.4 as a dissolution medium (Ruckmani K and Sankar V, 2010; Jadon PS, et al., 2009; Bansal, et al., 2013; Patel KK, et al., 2012; Qumbar M, et al., 2017). The amount of forskolin released was estimated spectrophotometrically at 210 nm. The free drug was released 97.40% within 3 h. F1 showed release of 96.49% within 5 h, F2 showed release of 97.50% within 6 h, F3 showed release of 98.44% within 12 h, F4 showed release of 94.78% within 8 h, F5 showed release of 97.56% within 6 h, F6 showed release of 98.31% within 6 h, F7 showed release of 94.69% within 8 h, F8 showed release of 97.53% within 10 h, F9 showed release of 96.75% within 8 h and F10 showed release of 97.32% within 8 h. These results showed that forskolin-loaded liposomes have shown sustained release when compared to pure drug. This is because the drug is released slowly for a prolonged period of time in liposomal formulation (Kumar YA and Setty CM, 2017; Sunilkumar MR, et al., 2016; Meenakshi M and Elangoo K, 2016). The difference in the drug release was attributed to the structure of surfactant. As known, non-ionic surfactants such as span 20, 40 and 60, have the same head group and different alkyl chain. Among these surfactants, only span 80 have an unsaturated alkyl chain. The introduction of double bonds made the chains bend. This means that the adjacent molecular cannot be tight when they form the membrane of liposomes. These cause the membrane to be more permeable, which possibly made the lowest entrapment efficiency of the span 80 formulation. However, of the other three kinds of non-ionic surfactants, span 60 has the longest saturated alkyl chain and show the highest entrapment. The encapsulation efficiency shows the trend C16 (span 60)>C14 (span 40)>C12 (span 20). It proposes that the length of alkyl chain is a crucial factor of permeability and high entrapment. Out of all 10 formulation, F3 consists of span 60 was considered as optimized formulation.
The time point to dissolve 50% of drug is t50 which was found to be 2.09 h for F12. 44 h for F2, 3.50 h for F3 2.62 h for F4, 5.39 h for F5, 5.01 h for F6, 7.49 h for F7, 7.60 h for F8, 7.17 h for F9 and 6.60 h for F10 formulations. The drug release data were fitted in different kinetic equations and “r” values are shown table. The drug release patterns from the liposome have found to be followed the first-order kinetic model predominantly followed by Hixson-Crowell’s cube root model as well. This release patterns are evident with the correlation coefficient “r” values. Zero order, first order, Higuchi, Korsemeyer-Peppas and Hixson-Crowell data were drawn for the optimized formulation to depict the release kinetics of the drug.
Scanning Electron Microscopy (SEM) analysis
Scanning: Electron microscopy was used to characterize the surface morphology. The particle size of forskolin was found to be 74 mm whereas particle size of forskolin-loaded liposome was found to be 7.78 mm. The particle size of forskolin was size reduced 10 times on liposomal formulation.
Pure forskolin showed principal absorption peaks at 672.35 cm-1 (C-S stretching), 1193.64 cm-1 (C-N stretching), 1467.12 cm-1 (C-H bending), 1581.90 cm-1 (N-H bending), 1740.90 cm-1 (C=O stretching), and 2561.28 cm-1 (S-H stretching). The identical peaks of C-S stretching, C-N stretching, C-H bending, N-H bending, C=O stretching, and S-H stretching vibrations were also noticed in the spectra of drug-loaded liposome. FT-IR spectra revealed that there was no interaction between the drug and the polymers used for liposome preparation.
Stability studies: A stability study for the optimized formulation was carried out at elevated temperature (45 ± 2°C) and RH of 75% ± 5% for a period of 3 months as per ICH guidelines. Samples were withdrawn at definite time periods (1st month, 2nd month and 3rd month) and dissolution studies were performed. The results from dissolution studies indicated that there was no significant difference of drug release. Stability studies indicated that optimized formulation F3 was stable.
Conclusion
The forskolin-loaded liposomes were prepared by thin film hydration method. Cholesterol and non-ionic surfactants were used for the preparation of liposome. Span 20, 40, 60 and 80 were used as non-ionic surfactants. The prepared forskolin-loaded liposomes were evaluated for %yield, %drug content, FTIR, SEM analysis, and stability studies. The in vitro release studies were performed. Good results were obtained for %yield, %drug content, and in vitro studies. FT-IR spectra revealed that there was no interaction between the drug and the polymers used for liposome preparation. F3 formulation containing cholesterol and span 60 exhibited the release of 98.44% up to 12 h. On comprising all formulations, F3 showed sustained release up to 12 h. This may be due to longest saturated alkyl chain of span 60 and shows the highest entrapment. From this study, it is concluded that forkolin-loaded liposome have shown sustained release when compared to pure drug.
References
- Chen S, Hanning S, Falconer J, Locke M, Wen J. Recent advances in non-ionic surfactant vesicles (niosomes): Fabrication, characterization, pharmaceutical and cosmetic applications. Eur J Pharm Biopharm. 2019; 144: 18-39.
[Crossref] [Google Scholar] [PubMed]
- Sankhyan A, Pawar PK. Metformin loaded non-ionic surfactant vesicles: Optimization of formulation, effect of process variables and characterization. Daru. 2013; 21: 1-8.
[Crossref] [Google Scholar] [PubMed]
- Stulzer HK, Silva SMA, Fernandes D, Assreuy J. Development of controlled release captopril granules coated with ethylcellulose and methylcellulose by fluid bed dryer. Drug Deliv. 2008; 15(1): 11-18.
[Crossref] [Google Scholar] [PubMed]
- Shalini M, Ali MH, Lakshmi PK. Formulation and evaluation of elastic niosomes of eletriptan hydrobromide. Int. J Pharm Sci Res. 2016; 7(4): 1679.
- Karthick K, Kumaran KS. Formulation and evaluation of niosomes co-loaded with 5-fluorouracil and leucovorin: Characterization and in vitro release study. Int J Res Pharm Nano Sci. 2016; 5: 239-250.
- Gupta A, Prajapati SK, Balamurugan M, Singh M, Bhatia D. Design and development of a proniosomal transdermal drug delivery system for captopril. Trop J Pharm Res. 2007; 6(2): 687-693.
- Ruckmani K, Sankar V. Formulation and optimization of zidovudine niosomes. Aaps Pharmscitech. 2010; 11: 1119-1127.
[Crossref] [Google Scholar] [PubMed]
- Jadon PS, Gajbhiye V, Jadon RS, Gajbhiye KR, Ganesh N. Enhanced oral bioavailability of griseofulvin via niosomes. AAPS PharmSciTech. 2009; 10: 1186-1192.
[Crossref] [Google Scholar] [PubMed]
- Bansal S, Aggarwal G, Chandel P, Harikumar SL. Design and development of cefdinir niosomes for oral delivery. J Pharm Bioallied Sci. 2013;5(4):318-325.
[Crossref] [Google Scholar] [PubMed]
- Patel KK, Kumar P, Thakkar HP. Formulation of niosomal gel for enhanced transdermal lopinavir delivery and its comparative evaluation with ethosomal gel. AAPS PharmSciTech. 2012; 13: 1502-1510.
[Crossref] [Google Scholar] [PubMed]
- Qumbar M, Imam SS, Ali J, Ahmad J, Ali A. Formulation and optimization of lacidipine loaded niosomal gel for transdermal delivery: In- vitro characterization and in-vivo activity. Biomed Pharmacother. 2017;93:255-266.
[Crossref] [Google Scholar] [PubMed]
- Kumar YA, Setty CM. Preparation and evaluation of maltodextrin based proniosomes containing capecitabine. Int J Res Dev Pharm Life Sci. 2017; 6(7): 2856-2861.
- Sunilkumar MR, Nesalin J, Mani TT. Development and evaluation of niosomes containing ketoconazole. World J Pharm Pharm Sci. 2016; 5(2): 1318-1327.
- Meenakshi M, Elangoo K. Reparation and evaluation of lamivudine proniosomes for HIV infection. World J Pharm Pharm Sci. 2016; 5: 1002-1023.
Author Info
Balaram Ghosh, Manas Chakraborty* and Rabindranath PalCitation: Ghosh B: Design and Evaluation of Forskolin-Loaded Liposomes
Received: 02-May-2024 Accepted: 14-May-2024 Published: 25-May-2024, DOI: 10.31858/0975-8453.15.5.183-188
Copyright: This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
ARTICLE TOOLS
- Dental Development between Assisted Reproductive Therapy (Art) and Natural Conceived Children: A Comparative Pilot Study Norzaiti Mohd Kenali, Naimah Hasanah Mohd Fathil, Norbasyirah Bohari, Ahmad Faisal Ismail, Roszaman Ramli SRP. 2020; 11(1): 01-06 » doi: 10.5530/srp.2020.1.01
- Psychometric properties of the World Health Organization Quality of life instrument, short form: Validity in the Vietnamese healthcare context Trung Quang Vo*, Bao Tran Thuy Tran, Ngan Thuy Nguyen, Tram ThiHuyen Nguyen, Thuy Phan Chung Tran SRP. 2020; 11(1): 14-22 » doi: 10.5530/srp.2019.1.3
- A Review of Pharmacoeconomics: the key to “Healthcare for All” Hasamnis AA, Patil SS, Shaik Imam, Narendiran K SRP. 2019; 10(1): s40-s42 » doi: 10.5530/srp.2019.1s.21
- Deuterium Depleted Water as an Adjuvant in Treatment of Cancer Anton Syroeshkin, Olga Levitskaya, Elena Uspenskaya, Tatiana Pleteneva, Daria Romaykina, Daria Ermakova SRP. 2019; 10(1): 112-117 » doi: 10.5530/srp.2019.1.19
- Dental Development between Assisted Reproductive Therapy (Art) and Natural Conceived Children: A Comparative Pilot Study Norzaiti Mohd Kenali, Naimah Hasanah Mohd Fathil, Norbasyirah Bohari, Ahmad Faisal Ismail, Roszaman Ramli SRP. 2020; 11(1): 01-06 » doi: 10.5530/srp.2020.1.01
- Manilkara zapota (L.) Royen Fruit Peel: A Phytochemical and Pharmacological Review Karle Pravin P, Dhawale Shashikant C SRP. 2019; 10(1): 11-14 » doi: 0.5530/srp.2019.1.2
- Pharmacognostic and Phytopharmacological Overview on Bombax ceiba Pankaj Haribhau Chaudhary, Mukund Ganeshrao Tawar SRP. 2019; 10(1): 20-25 » doi: 10.5530/srp.2019.1.4
- A Review of Pharmacoeconomics: the key to “Healthcare for All” Hasamnis AA, Patil SS, Shaik Imam, Narendiran K SRP. 2019; 10(1): s40-s42 » doi: 10.5530/srp.2019.1s.21
- A Prospective Review on Phyto-Pharmacological Aspects of Andrographis paniculata Govindraj Akilandeswari, Arumugam Vijaya Anand, Palanisamy Sampathkumar, Puthamohan Vinayaga Moorthi, Basavaraju Preethi SRP. 2019; 10(1): 15-19 » doi: 10.5530/srp.2019.1.3