Research Article - (2022) Volume 13, Issue 12
Determination of Immunostimulatory Efficacy of Benzimidazole Derivatives Against Leishmania infantum
Kübra Kelleci1,2*Abstract
Leishmaniasis is a parasitic disease affecting more than 12 million people worldwide. Although the use of chemotherapeutic drugs is among the current treatment methods, it is emphasized that the limitations of the drugs, their disadvantages such as insufficient safety and efficacy, the disease being endemic, and the need to develop drugs for this disease. In this study, in vitro anti-leishmanial activities of two benzimidazole derivatives (B1 and B2) were tested against L. infantum promastigote and amastigote for the first time. In our study, the anti-leishmanial effects of B1 and B2 on both amastigote and promastigote forms of Leishmania infantum were investigated using J774 macrophage cells. It was found that the tested compounds were not toxic to the host cell. In addition, when compared to the drug miltefosine, which is used worldwide in the treatment of leishmaniasis, it has been determined that it has a serious inhibitory effect by reducing the proliferation of promastigotes and the metabolic activities of amastigotes, and it acts at lower concentrations than miltefosine. We found that benzimidazolium derivatives (B1 and B2) used for the first time in this study were more effective on both forms of L. infantum. The obtained results showed that benzimidazolium derivatives have high anti-leishmanial potential against L. infantum, which is the cause of Visceral Leishmaniasis (VL), which is known to be the deadly form of leishmaniasis. It has been shown that the obtained results will help the development of safe, stable, and effective anti- leishmanial drug formulations against VL.
Keywords
Benzimidazolium, Leishmania infantum, Visceral leishmaniasis, Treatment, Anti-leishmanial, Macrophage cells
Introduction
Leishmaniasis, a parasitic disease caused by protozoa of the genus Leishmania, still affects more than 12 million people in 98 endemic countries, according to World Health Organization (WHO) data. Among its different clinical forms, VL causes fatal systemic diseases if left untreated (Abongomera C, et al, 2020). Symptoms of VL include anemia, weight loss, hepatomegaly, fever, and splenomegaly. An estimated 300,000 new cases and more than 20,000 deaths occur each year worldwide. Most of the cases are in developing countries, weak immune systems, wars, climate changes, immigration, and most importantly, resistance to drugs are the factors that increase the incidence of the disease (Desjeux P, 2001). VL is caused by Leishmania infantum in Central Asia, the Middle East, the Mediterranean Basin, and Leishmania donovaniin Asia and Africa (Tabrez S, et al., 2021).
Although there is no official vaccine for the prevention of leishmaniasis, treatment methods are generally based on the use of antineoplastic drugs. Besides the use of antineoplastic drugs, liposomal amphotericin B, pentavalent antimonials, a combination of paromomycin, pentamidine, and miltefosine, and orally administered “azoles” (ketoconazole, itraconazole, fluconazole) are the main strategies in the treatment of leishmaniasis. Current treatments limit the use of drugs because of their disadvantages such as poor and variable efficacy, high toxicity, high cost, and the emergence of drug-resistant parasites. These reasons encourage the urgent investigation and optimization of new drug alternatives with new mechanisms of action.
When the literature is examined, benzimidazolium is a heterocyclic organic compound consisting of benzene and imidazole, which can interact with a wide variety of biological targets due to an electron-rich heterocyclic system and the presence of two hetero-nitrogen atoms (Tahlan S, et al., 2019). Benzimidazole and its derivatives are antimicrobial (Marinescu M, 2021; Aragón-Muriel A, et al, 2021; Marinescu M, 2019) antifungal (Karaburun AÇ, et al., 2019), antihistamine (Hadole DC, et al., 2018), antineoplastic (Cheong JE, et al., 2018). It is known to show different biological and pharmacological properties such as anti-inflammatory (Gaba M, et al., 2014), anti-Alzheimer’s (Chaves S, et al., 2018), anti-diabetic (Naim MJ, et al., 2018; Aboul-Enein and El Rashedy, 2015).
When the literature is examined, it is seen that benzimidazole derivatives are used as an antiparasitic drug against protozoan infections by inhibiting the polymerization of tubulin, which is known to play a key role in the growth and differentiation (Valdez J, et al., 2002) of protozoa (Croft SL, 1997; Werbovetz K, et al., 1999; Jayanarayan KG and Dey CS, 2004). It is known that α, β-and γ-tubulins are essential for microtubule formation (Katiyar SK, et al., 1994; Wang YT, et al., 2019). Benzimidazoles prevent tubulin formation by binding to microtubules and disrupting their polymerization (Wang YT et al., 2019; Wu K, et al., 2022). Disruptions in the tubulin structure prevent the division of parasite cells by blocking the cell cycle in the G2/M phase, and may disrupt the cellular microtubule network by inducing apoptosis (Son DS, et al., 2020). It is seen that there are a limited number of studies in the literature investigating the antileishmanial effects of benzimidazoles against all these anti-parasitic effects. The most common parasite in research is L. major, and it is known to be active against L. donovani, L. infantum, and L. mexicana, respectively.
When the literature was examined, we could not find any study examining the anti-leishmanial activities of the benzimidazolium compounds (B1 and B2), which are the subject of our study, against L. infantum, which was a VL agent before. Therefore, the anti-leishmanial effects of benzimidazolium compounds B1 and B2 investigated in this study were evaluated by us in vitro against amastigote and promastigotes of L. infantum, which is a visceral leishmaniasis agent. The antileishmanial effect and cytotoxicity of B1 and B2 components against the drug miltefosine (Dorlo TP, et al., 2012), which is known to be widely used in the treatment of Leishmaniasis, were investigated comparatively.
Materials and Methods
RPMI-1640, Fetal Bovine Serum (FBS), Dimethylsulfoxide (DMSO), Trypsin-EDTA, gentamicin, filter paper (Hoffmann), 0.45 and 0.22 µm filters were all purchased from Sigma-Aldrich. All aqueous solutions were prepared with milli-Q water. An inverted microscope Olympus CKX 41 was used to observe morphological changes.
Preparation of benzimidazolium derivatives
The benzimidazolium derivatives (B1 and B2) that were the subject of the study were commercially available in powder form with 98% purity. Benzimidazolium components were dissolved in 200 µl of methanol and benzimidazolium solutions of various concentrations were prepared using RPMI-1640 culture medium and stored at 4°C for later use (Figures 1 and 2).
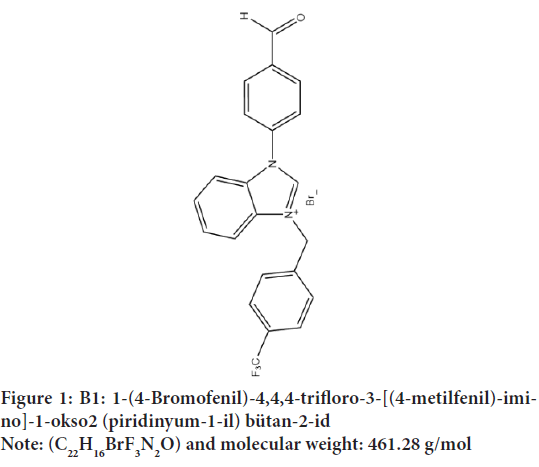
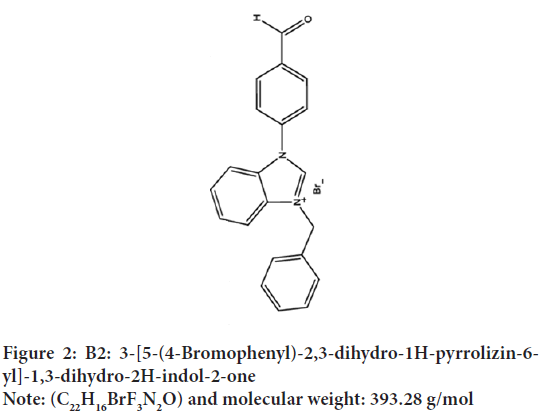
Figure 1: B1: 1-(4-Bromofenil)-4,4,4-trifloro-3-[(4-metilfenil)-imino]-
1-okso2 (piridinyum-1-il) bütan-2-id
Note: (C22H16BrF3N2O) and molecular weight: 461.28 g/mol
Figure 2: B2: 3-[5-(4-Bromophenyl)-2,3-dihydro-1H-pyrrolizin-6-yl]-1,3-dihydro-2H-indol-2-one
Note: (C22H16BrF3N2O) and molecular weight: 393.28 g/mol
Leishmania parasite and J774 macrophage cell culture
L. infantum promastigotes were cultured in flasks in RPMI-1640 medium supplemented with L-glutamine, antibiotic (Gentamycin), and 10% fetal bovine serum at 27°C. The culture was passaged after 3-4 days of incubation by monitoring the proliferation of promastigotes daily using an inverted microscope.
J774 Murine macrophage cells to be used for cytotoxicity experiments and amastigote-macrophage culture, 1 M HEPES, 10% Fetal Bovine Serum (FBS), RPMI medium containing antibiotics (Gentamycin), 5% CO2 in 37 °C ambient conditions were cultured in T25 culture flasks. Macrophage cells were passaged when they reached 80%-90% confluency and cell proliferation was monitored daily with an inverted microscope.
Determination of cytotoxic concentrations
To determine non-toxic concentrations of benzimidazolium components in J774 macrophage cells, 5 × 104 J774 macrophage cells and L. infantumparasites as hosts were seeded into each well of a 96-well microplate and incubated at 37°C for 24 hours. After adhesion of macrophages, B1 and B2 compounds, which were homogenized by dissolving in DMSO/H2O (10%) and sterilized by a 0.20-μm membrane filter, were obtained in 8 different concentrations (0.8 µg/ml, 1.6 µg/ml, 3.2 µg/ml, 6.4 µg/ml, 12.8 µg/ ml, 25.6 µg/ml, 50 µg/ml, 100 µg/ml) were diluted and added to the wells and incubated for another 24 hours. After 48 hours of incubation, an MTT assay was performed on macrophage cells. Miltefosine was used as the reference drug in the study. 10 µl of MTT solution with a final concentration of 10 mg/ml in PBS was transferred to each well of the microplate and cells were incubated at 37°C for 4 hours. Then, 100 µl of DMSO was added to each well. Absorbance was measured at 570 nm using an ELISA reader. All studies were performed in 3 replicates. Half maximum cytotoxicity concentration (C C50) was determined.
Determination of the antileishmanial effects of B1 and B2 on L. infantum promastigotes
To determine the antileishmanial effects of benzimidazolium derivatives (B1 and B2) on L. infantum promastigotes, 1 × 106/ml L. infantum promastigotes in log phase were transferred to 2 ml Eppendorf tubes and incubated overnight at 27°C. L. infantum promastigotes taken simultaneously into a different eppendorf tube were exposed to non-toxic concentrations of B1 and B2 for 3 days. To determine the antileishmanial activity, promastigote counts were determined with the Thoma slide at certain hours (24, 48 and 96 hrs) during the incubation period of all samples tested. Thus, the antileishmanial activities of benzimidazolium derivatives on the proliferation of parasites were determined and compared.
In vitro infection of J774 macrophage cells with L. infantum promastigotes
To detect the antileishmanial effects of benzimidazolium components on L. infantum amastigotes, J774 murine macrophage cells were infected with
L. infantum promastigotes. 5 × 104 macrophages and promastigotes were seeded into microplates at a 1:10 ratio of 200 µL/well and incubated for 24 hours at 33°C, 5% CO2. The incubation temperature was increased to 37°C for 24 hours. Cells were washed 3 times with PBS to remove non infective free promastigotes. Benzimidazole compounds ranging from 100 to 0.8 µg/mL were diluted to form 8 different concentrations and 200 µL/well was added to the microplates. The culture medium was incubated for 4 hours at 37°C to enable the transformation of promastigotes into intracellular amastigotes. All assays were performed in triplicate. In these assays, miltefosine, which is used in the treatment of L. infantum, was used as the reference drug.
Statistical analysis
The data obtained in the study were calculated as mean ± standard deviation. For statistical analysis, parametric tests (unpaired sample t-test, analysis of variance, and Mann-Whitney U test) were used using the “SPSS 16.0 for Windows” program. The significance level was accepted as p<0.05.
Results and Discussion
Firstly, the cytotoxic effects of benzimidazolium salts (B1 and B2), on the J774 macrophage cell line were investigated. In our study, it was determined that B1 and B2 did not show significant cytotoxicity at low concentrations (0.8 μg/mL). It has been observed that B1 and B2 have an inhibitory effect in direct proportion to increasing concentrations. However, B2 was less toxic than B1 at all tested concentrations (p˂0.05).
To investigate the survival percentages of L. infantum promastigotes, the MTT cellular viability assay was performed after 24 hours of incubation. It shows that benzimidazolium compounds are not very toxic to macrophage cells compared to leishmania parasites. The activity of compounds B1 and B2 was evaluated against L. infantum promastigotes. The results are shown in Table 1. It has been determined that B1 and B2 compounds are more active than Miltefosine used as a reference drug. Benzimidazolium derivatives have been found to have superior inhibitory effects on the growth of parasites. For both compounds B1 and B2, cell viability decreased significantly due to the increase in concentration 24 hours after inhibition. In addition, it has been determined that the tested benzimidazolium derivatives are less toxic than miltefosine, which is used as an antileishmanial drug. It was found to be much more effective than miltefosine (I C50 value 30.12 μM+(-1.36), with an I C50 value of 3.42 μM+(-1.2) for B1 and 2.23 μM+(-1.34) for B2. In the light of these data, we can say that the B2 component is less toxic than B1. B1 and B2 benzimidazolium derivatives have been found to have an inhibitory effect on the metabolic activity and proliferation of L. infantum promastigotes. According to the results of our study, it can be said that miltefosine (30.12μM) exhibits very high values against L. infantum promastigotes compared to the I C50 values detected in the literature (15.0 μM; 16.7 μM; 19.6 μM) (Plano D, et al., 2011; Wulsten IF, et al., 2017). Our results show us that B2 is much more effective than B1 and miltefosine on L. infantum amastigotes. Compared to miltefosine, B2 appears to be approximately 13.5 times more effective against L. infantum, while B1 is approximately 9 times more effective. The selectivity indexes (SI) of B1 and B2 are between 82 and 239. It can be said that the tested compounds showed promising antileishmanial activity against L. infantum.
The cytotoxic effects of B1 and B2 were tested using J774 cells. The data obtained are presented in Table 1. It has been shown to exhibit less toxicity for J774 macrophages than drugs currently used in the treatment of leishmaniasis, such as miltefosine. The C C50 value for miltefosine, which was used as the reference drug, was found to be 270.12 µM. B1(280.97 µM) and B2 (534.91 µM) appeared to respond more strongly to the reference drug miltefosine. It is known that compounds B1 and B2 show a much higher Selectivity Index (SI) than miltefosine used as a reference drug. The selectivity index is obtained by the ratio of the C C50 value to the I C50 value. Compounds B1 and B2 were found to have higher SI values against L. infantum promastigotes compared to miltefosine. It was determined that B2 was 26.77 times more selective than miltefosine, and B1 was 9.17 times more selective.
| Benzimidazolium derivatives | I C50 (µM)+SD | J774 C C50 (µM)+SD | SI |
| B1 | 3.42+(-1.2) | 280.97+(-0.17) | 82.155 |
| B2 | 2.23+(-1.34) | 534.91+(-0.13) | 239.86 |
| Miltefosin | 30.12+(-11.3) | 270.12 | 8.96 |
Note: *I C50: Concentration that provides a 50% reduction in L. infantum parasites; C C50: Concentration with 50% reduction in J774 macrophage cells; SD: Standard Deviation from three replicates; SI: Selectivity Index, (C C50/I C50)
Table 1: In vitro data on antileishmanial activity and cytotoxicity of benzimidazole derivatives (B1 and B2) against L. infantum promastigotes on the J774 macrophage cell line
When Table 2is examined, Bl and B2 I C50 values on L.infantum amastigotes were observed to be 0.37 µM and 0.18 µM, respectively. Compared to miltefosine (1.04 µM), these data appear to be quite valuable. B1 and B2 were found to have SI values of 759.37 and 2971.72 against L. infantum amastigote. B2 was found to be 11.44 times more selective than miltefosine.
| Benzimidazolium derivatives | I C50 (µM)+(-SD) | SI |
|---|---|---|
| B1 | 0,37+(-0.01) | 759.37 |
| B2 | 0,18+(-0.07) | 2971.72 |
| Miltefosin | 1,04+(-0.06) | 259.73 |
Note: *I C50: Concentration that provides a 50% reduction in L. infantum parasites; C C50: Concentration with 50% reduction in J774 macrophage cells; SD: Standard Deviation from three replicates; SI: Selectivity Index, (C C50/I C50)
Table 2: In vitro data on antileishmanial activities of benzimidazole derivatives (B1 and B2) against L. infantum amastigotes
It should be noted that B2 showed the highest selectivity against L. infantum promastigotes (239.86) and amastigotes (2971.72). Among the formulations investigated, B2 was found to be more effective against both forms of Leishmania parasites (amastigote-promastigote), as they significantly abolished the parasites’ important biological properties, proliferation, and metabolic activities compared to B1 and miltefosine. It is possible to say that the data obtained from this study is supported by the literature. Besides, as mentioned earlier, there are very limited studies investigating the biological activities of benzimidazolium derivatives in the literature, and cytotoxic analysis of the benzimidazolium derivatives used in our study in the J774 macrophage cell line has not been performed before. In line with this information, we can clearly state that this study is the first to examine the cytotoxic behavior of benzimidazolium derivatives B1 and B2. Compound B1 at all concentrations tested showed important inhibitory effects on the metabolic activity and growth of L. infantum amastigotes and promastigotes compared to B2 and miltefosine. Since miltefosine shows much higher toxicity to macrophage cells than B1 and B2, B2, in particular, turned out to be quite safe for eradicating L. infantum parasites. Of course, when compared with the literature, it is possible to say that possible differences in I C50 and C C50 values are related to different factors such as parasite strain, growth medium, and the method used for I C50 and C C50 determination.
Although leishmaniasis is an endemic disease, affecting more than 12 million people, it is a parasitic disease that is not well known and there is no effective vaccine and treatment method.
In this study, the activities of two different benzimidazolium derivatives against L infantum, which is the causative agent of visceral leishmaniasis, were tested. The results of the study revealed that benzimidazolium derivatives have a high potential to be used in the treatment of leishmaniasis and opened a new avenue for further research. It has been determined that benzimidazolium derivatives that we have examined within the scope of this study, especially B2, have significant antileishmanial activity against L. infantum when compared with miltefosine used in the treatment of leishmaniasis. Our results also prove the interaction between benzimidazoles and tubulin, as mentioned before.
Conclusion
As a result of these findings, more detailed studies are needed for potential antileishmanial therapy using benzimidazole derivatives for the treatment of visceral leishmaniasis. To examine the in vivo antileishmanial performance of B2, which is a benzimidazolium derivative, we suggest that experimental studies be carried out on animals as soon as possible. On the other hand, we think that B2 should be investigated in combination with traditional antileishmanial drugs and antileishmanial activities can be increased by increasing synergistic effects with combination therapy. We also hope that the promising results obtained in this study will motivate researchers to synthesize new benzimidazolium compounds. Thus, we believe that new drug formulations that can eliminate leishmaniasis-related infections in endemic areas will be developed.
References
- Abongomera C, van Henten S, Vogt F, Buyze J, Verdonck K, van Griensven J. Prognostic factors for mortality among patients with visceral leishmaniasis in East Africa: Systematic review and meta-analysis. PLoS Negl Trop Dis. 2020; 14(5): e0008319.
[Crossref] [Google scholar] [Pubmed]
- Desjeux P. The increase in risk factors for leishmaniasis worldwide. Trans R Soc Trop Med Hyg. 2001; 95(3): 239-243.
[Crossref] [Google scholar] [Pubmed]
- Tabrez S, Rahman F, Ali R, Muhammad F, Alshehri BM, Alaidarous MA, et al. Repurposing of FDA‐approved drugs as inhibitors of sterol C‐24 methyltransferase of Leishmania donovani to fight against leishmaniasis. Drug Dev Res. 2021; 82(8): 1154-1161.
[Crossref] [Google scholar] [Pubmed]
- Tahlan S, Kumar S, Kakkar S, Narasimhan B. Benzimidazole scaffolds as promising antiproliferative agents: A review. BMC chem. 2019; 13(1): 1-6.
[Crossref] [Google scholar] [Pubmed]
- Marinescu M. Synthesis of antimicrobial benzimidazole–pyrazole compounds and their biological activities. Antibiotics. 2021; 10(8): 1002.
[Crossref] [Google scholar] [Pubmed]
- Aragón-Muriel A, Liscano Y, Upegui Y, Robledo SM, Ramírez-Apan MT, Morales-Morales D, et al. In vitro evaluation of the potential pharmacological activity and molecular targets of new benzimidazole-based schiff base metal complexes. Antibiotics. 2021; 10(6): 728.
[Crossref] [Google scholar] [Pubmed]
- Marinescu M. Chemistry and applications of benzimidazole and its derivatives. BoD-Books on Demand. 2019.
- Karaburun AÇ, Kaya Çavuşoğlu B, Acar Çevik U, Osmaniye D, Sağlık BN, Levent S, et al. Synthesis and antifungal potential of some novel benzimidazole-1, 3, 4-oxadiazole compounds. Molecules. 2019; 24(1): 191.
[Crossref] [Google scholar] [Pubmed]
- Hadole CD, Rajput JD, Bendre RS. Concise on some biologically important 2-substituted benzimidazole derivatives. Org Chem Curr Res. 2018; 7(3): 1-9.
- Cheong JE, Zaffagni M, Chung I, Xu Y, Wang Y, Jernigan FE, et al. Synthesis and anticancer activity of novel water soluble benzimidazole carbamates. Eur J Med Chem. 2018; 144: 372-385.
[Crossref] [Google scholar] [Pubmed]
- Gaba M, Singh S, Mohan C. Benzimidazole: An emerging scaffold for analgesic and anti-inflammatory agents. Eur J Med Chem. 2014;76: 494-505.
[Crossref] [Google scholar] [Pubmed]
- Chaves S, Hiremathad A, Tomás D, Keri RS, Piemontese L, Santos MA. Exploring the chelating capacity of 2-hydroxyphenyl-benzimidazole based hybrids with multi-target ability as anti-Alzheimer's agents. New J Chem. 2018; 42(20): 16503-16515.
- Naim MJ, Alam O, Alam MJ, Shaquiquzzaman M, Alam MM, Naidu VG. Synthesis, docking, in vitro and in vivo antidiabetic activity of pyrazole‐based 2, 4‐thiazolidinedione derivatives as PPAR‐γ modulators. Archiv der Pharmazie. 2018; 351(3-4): 1700223.
[Crossref] [Google scholar] [Pubmed]
- Aboul-Enein HY, El Rashedy AA. Benzimidazole derivatives as antidiabetic agents. Med Chem. 2015; 5: 318-325.
- Valdez J, Cedillo R, Hernández-Campos A, Yepez L, Hernández-Luis F, Navarrete-Vazquez G, et al. Synthesis and antiparasitic activity of 1H-benzimidazole derivatives. Bioorg Med Chem Lett. 2002; 12(16): 2221-2224.
[Crossref] [Google scholar] [Pubmed]
- Croft SL. The current status of antiparasite chemotherapy. Parasitology. 1997; 114(7): 3-15.
[Crossref] [Google scholar] [Pubmed]
- Werbovetz K, Brendle J, Sackett D. Purification, characterization, and drug susceptibility of tubulin from Leishmania. Molecular and biochemical parasitology. 1999; 98(1): 53-65.
[Crossref] [Google scholar] [Pubmed]
- Jayanarayan KG, Dey CS. Altered expression, polymerisation and cellular distribution of α-/β-tubulins and apoptosis-like cell death in arsenite resistant Leishmania donovani promastigotes. Int J Parasitol. 2004; 34(8): 915-925.
[Crossref] [Google scholar] [Pubmed]
- Katiyar SK, Gordon VR, McLaughlin GL, Edlind TD. Antiprotozoal activities of benzimidazoles and correlations with beta-tubulin sequence. Antimicrob Agents Chemother. 1994; 38(9): 2086-2090.
[Crossref] [Google scholar] [Pubmed]
- Wang YT, Shi TQ, Zhu HL, Liu CH. Synthesis, biological evaluation and molecular docking of benzimidazole grafted benzsulfamide-containing pyrazole ring derivatives as novel tubulin polymerization inhibitors. Bioorg Med Chem. 2019; 27(3): 502-515.
[Crossref] [Google scholar] [Pubmed]
- Wu K, Peng X, Chen M, Li Y, Tang G, Peng J, et al. Recent progress of research on anti‐tumor agents using benzimidazole as the structure unit. Chem Biol Drug Des. 2022; 99(5): 736-757.
[Crossref] [Google scholar] [Pubmed]
- Son DS, Lee ES, Adunyah SE. The antitumor potentials of benzimidazole anthelmintics as repurposing drugs. Immune netw. 2020; 20(4).
[Crossref] [Google scholar] [Pubmed]
- Dorlo TP, Balasegaram M, Beijnen JH, de Vries PJ. Miltefosine: A review of its pharmacology and therapeutic efficacy in the treatment of leishmaniasis. J Antimicrob Chemother. 2012; 67(11): 2576-2597.
[Crossref] [Google scholar] [Pubmed]
- Plano D, Baquedano Y, Moreno-Mateos D, Font M, Jiménez-Ruiz A, Palop JA, et al. Selenocyanates and diselenides: A new class of potent antileishmanial agents. Eur J Med Chem. 2011; 46(8): 3315-3323.
[Crossref] [Google scholar] [Pubmed]
- Wulsten IF, Costa-Silva TA, Mesquita JT, Lima ML, Galuppo MK, Taniwaki NN, et al. Investigation of the anti-Leishmania (Leishmania) infantum activity of some natural sesquiterpene lactones. Molecules. 2017; 22(5): 685.
[Crossref] [Google scholar] [Pubmed]
Author Info
Kübra Kelleci1,2*2Department of Bioengineering, Yıldız Technical University, Istanbul, Turkey
Citation: Kelleci K: Determination of Immunostimulatory Efficacy of Benzimidazole Derivatives against Leishmania infantum
Received: 01-Nov-2022 Accepted: 25-Nov-2022 Published: 02-Dec-2022, DOI: 10.31858/0975-8453.13.12.826-830
Copyright: This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
ARTICLE TOOLS
- Dental Development between Assisted Reproductive Therapy (Art) and Natural Conceived Children: A Comparative Pilot Study Norzaiti Mohd Kenali, Naimah Hasanah Mohd Fathil, Norbasyirah Bohari, Ahmad Faisal Ismail, Roszaman Ramli SRP. 2020; 11(1): 01-06 » doi: 10.5530/srp.2020.1.01
- Psychometric properties of the World Health Organization Quality of life instrument, short form: Validity in the Vietnamese healthcare context Trung Quang Vo*, Bao Tran Thuy Tran, Ngan Thuy Nguyen, Tram ThiHuyen Nguyen, Thuy Phan Chung Tran SRP. 2020; 11(1): 14-22 » doi: 10.5530/srp.2019.1.3
- A Review of Pharmacoeconomics: the key to “Healthcare for All” Hasamnis AA, Patil SS, Shaik Imam, Narendiran K SRP. 2019; 10(1): s40-s42 » doi: 10.5530/srp.2019.1s.21
- Deuterium Depleted Water as an Adjuvant in Treatment of Cancer Anton Syroeshkin, Olga Levitskaya, Elena Uspenskaya, Tatiana Pleteneva, Daria Romaykina, Daria Ermakova SRP. 2019; 10(1): 112-117 » doi: 10.5530/srp.2019.1.19
- Dental Development between Assisted Reproductive Therapy (Art) and Natural Conceived Children: A Comparative Pilot Study Norzaiti Mohd Kenali, Naimah Hasanah Mohd Fathil, Norbasyirah Bohari, Ahmad Faisal Ismail, Roszaman Ramli SRP. 2020; 11(1): 01-06 » doi: 10.5530/srp.2020.1.01
- Manilkara zapota (L.) Royen Fruit Peel: A Phytochemical and Pharmacological Review Karle Pravin P, Dhawale Shashikant C SRP. 2019; 10(1): 11-14 » doi: 0.5530/srp.2019.1.2
- Pharmacognostic and Phytopharmacological Overview on Bombax ceiba Pankaj Haribhau Chaudhary, Mukund Ganeshrao Tawar SRP. 2019; 10(1): 20-25 » doi: 10.5530/srp.2019.1.4
- A Review of Pharmacoeconomics: the key to “Healthcare for All” Hasamnis AA, Patil SS, Shaik Imam, Narendiran K SRP. 2019; 10(1): s40-s42 » doi: 10.5530/srp.2019.1s.21
- A Prospective Review on Phyto-Pharmacological Aspects of Andrographis paniculata Govindraj Akilandeswari, Arumugam Vijaya Anand, Palanisamy Sampathkumar, Puthamohan Vinayaga Moorthi, Basavaraju Preethi SRP. 2019; 10(1): 15-19 » doi: 10.5530/srp.2019.1.3