Review Article - (2023) Volume 14, Issue 3
Different Types of Vaccines against Novel Coronavirus SARS-CoV-2
An Zhu*Abstract
The (Coronavirus Disease 2019) COVID-19 outbreak has caused a public health emergency globally. The SARS-CoV2 that causes COVID-19 belongs to the beta strain of the coronavirus family. It is genetically like MERS-CoV and SARS-CoV. Until now, COVID-19 had infected over 190,000,000 people. The people with the infection usually suffer acute respiratory distress syndrome and cytokine storm in the elder and other populations with chronic diseases, death commonly occurs. As the number of infections is increasing dramatically, and there is still no effective therapy for this disease, to prevent the further outbreak, developing an effective SARS-CoV-2 vaccine is urgently demanded. Currently, several institutions and companies have achieved great success in developing the SARS-CoV-2 vaccine. There have adenovirus vector vaccines, mRNA vaccines, and inactivated virus vaccines available in the market. However, during the fermentation of the pandemic, many variants of SARSCoV-2 occurred, for example, the Delta and Delta plus strain which may have the ability to make the immune escape and dramatically decrease the effectiveness of the vaccines. This literature review will summarize several types of candidates of COVID-19 vaccines in four aspects, i.e., nucleic acid, inactivated virus, subunit protein, and viral vector, to promote further investigation and advertising of the vaccines.
Keywords
COVID-19, SARS-CoV-2, Vaccine
Introduction
In December 2019, the Coronavirus disease 2019 (COVID-19) was first detected in China. C. The people infected with this virus will have fever, cough, and muscle ache, sometimes with difficulty in-breath (CDC, 2021). Moreover, it will cause death, especially in the elders and kids (CDC, 2021). This virus can infect people through exposure to the respiratory fluid which contains it (CDC, 2019). COVID-19 has been declared as a pandemic by WHO on March 11, 2020 (Habas K, et al., 2020). Compared with the SARS and COVID-19 has higher contagiously (Wiersinga WJ, et al., 2020). Some research indicates that in the conjunctiva, the replication rate of SARS-CoV was weaker than SARS-CoV-2 and the same situation occur in the bronchus (Hui KP, et al., 2020). Until July 2021, 194,825,130 (Worldometer. 2021) people had been infected by COVID-19, and 4,175,08 of them died. Furthermore, the outbreak of COVID-19 also causes severe economic setbacks in countries around the world. The current literature suggests that people who live in the situation of the COVID-19 pandemic may have a high burden of mental health problems such as depression, anxiety disorders (Hossain MM, et al., 2020). Currently, there have no specific therapy or medication available to treat COVID-19. All the managements are based on supportive therapy to prevent the failure of the respiration system. With no effective treatment, vaccination can be an effective way to prevent further outbreaks. Vaccination is one of the most effective ways to prevent infectious diseases in the world. It plays a critical role in eradicating smallpox and restricting some diseases such as polio, measles, and tetanus, saving many lives (Han HJ, et al., 2016). The first vaccine in the world was produced in 1796 by English physician Edward Jenner (Memish ZA, et al., 2013). With the development of biotechnology and understanding the virus, more and more vaccines are manufactured against infectious diseases. In this 2019 COVID-19 pandemic, many different types of vaccines are produced against the disease. In general, there are four types of vaccines available now: Inactive vaccines, nucleic acid vaccines, protein subunit vaccines, and viral vaccines. Some of them have already reached the market for the public. This article aims to briefly introduce the mechanism of these vaccines and briefly discuss their strength and weakness.
Literature Review
SARS-CoV-2 virus
Coronavirus family: SARS-CoV-2 stands for the Severe Acute Respiratory Syndrome Coronavirus 2 (WHO, 2019). SARS-CoV-2 shares a similar structure as the other coronavirus family members, with crown-like thorns on their surface. In addition, they include spike protein, membrane protein, envelope and nucleocapsid protein, and viral RNA (Kaur N, et al., 2021) (Figure 1).
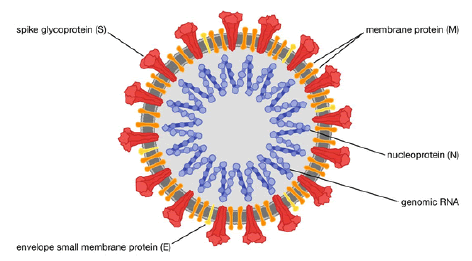
Figure 1: Structure of the SARS-CoV2 (Rogers K, 2021)
There have more than 30 types of coronavirus in the world (Masters PS, 2006) which are divided into four distinct genera (alpha, beta, gamma, and delta) (de Groot RJ, et al., 2013) Outsides that alpha-CoVs and beta-CoVs circulate in mammalian hosts and gamma-CoVs and delta-CoVs mainly infect birds (Wertheim JO, et al., 2013). Seven strains of the virus are found to be circulating in the human population, including two alpha coronavirus (HCoV-229E; HCoV-NL63), five beta strains (HCoV-OC43; HCoV-HKU1; MERS-CoV; SARS-CoV, and novel SARS-CoV-2) (Kaur N, et al., 2021). In these seven strains, the MERS-CoV and SARS-CoV have cause serious issues globally before. For example, in February 2003, the SARS-CoV was first reported in Asia and causes a global pandemic of SARS (CDC, 2021). In that pandemic, 8422 people got infected, and 919 died, approximately 11% (CDC, 2021). Furthermore, in 2012, the MERS-CoV is reported in Saudi Arabia, which can cause MERS (CDC, 2019). Until 2019, WHO got 2982 reported cases, and 852 of the patients died; the death rate is approximately 34%. Usually, the bat SARS-CoV will not infect people; however, as coronavirus are RNA-based, progress called RNA recombination is frequently in progress. The RNA combination is a way that the RNA virus uses to enhance its diversity (Worldometer, 2021). The RNA recombination usually occurs within the RNA virus at regions containing S genes and the upstream of ORF8. The S genes encode the spike proteins with the receptor-binding domains, and the ORF8 encodes an accessory protein (Worldometer, 2021; Hossain MM, et al., 2020). Therefore, some scientists suggest that the emergence of SARS-CoV2 may result from the frequent RNA recombination and tremendous genetic diversity of coronavirus (CDC, 2021). SARS-CoV2 is genetically close to SARS-CoV; they share 83% similarity (Memish ZA, et al., 2021). Additionally, the proteomic similarity analyses show that over 95% homology is funded between most proteins in these two strains of SARS-CoVs, i.e., RdRp and 3CLpro protease (CDC, 2021). The S proteins in the SARS-CoV2 also share high similarities with the SARS-CoV, i.e., an S protein domain, 76% of sequences (CDC, 2021; Memish ZA, et al., 2013). ORF8 and ORF10 are the two unique proteins that show no homology between the SARS-CoV and SARS-CoV2, not only with the SARS-CoV, but these proteins are also genetically different from any other CoV strains (WHO, 2019).
Origin
Recent studies and databases show that all human CoVs are originated from animals (Forni D, et al., 2017). So far, much research indicates that the bats may be the natural hosts of alpha and beta CoVs, as close phylogenetic relationships are found between them (Woo PC, et al., 2012). However, the bats were unlikely to be the direct source of these diseases, as many patients have no history of contact with bats (Han HJ, et al., 2016). Therefore, an intermediate host is needed to transmit the disease. For example, in the study of MERS-CoV that scientists find that MERS-CoV may originate from bats,but camels are the direct source of MERS-CoV, which transmit the disease to the human (Memish ZA, et al., 2013; AlBarrak AM, et al., 2012; Price MS, et al., 2014). In addition, according to Su S, et al., 2016, the genome of SARS-CoV2 shows a 96.2% homology to RaTG13, a bat SARS-related coronavirus collected in Yunnan province, China, which indicates that this disease also originates for bats. However, the intermediate host of transmitting the SARS-CoV-2 is still unknown.
Mutation
As the pandemic progressing, genetic variants of SARS-CoV2 have been found and reported around the world. Outsides those variants the B.1.1.7 (Alpha), B.1.351 (Beta), B.1.617.2 (Delta), and P.1 (Gamma), variants are considered as a variant of concern, as they may lead to an increase in transmissibility, more server disease (Rogers K, 2021). The emerging and circulating of the variants around the world have changeling the existing public health systems. The variants may cause the failure of diagnosis, vaccine effectiveness reduction, existing therapies, and higher outbreak (Rogers K, 2021; Wertheim JO, et al., 2016). The virus invades the human body by interacting between the spike protein receptor-binding domain and human receptor ACE2 (Armstrong J, et al., 1984). Usually, ACE2 is highly expressed in the lung, ileum, Colon, kidney esophagus, myocardium, bladder, and oral mucosa, making infection easy to occur at these tissues (De Groot RJ, et al., 2013).
Discussion
Vaccine
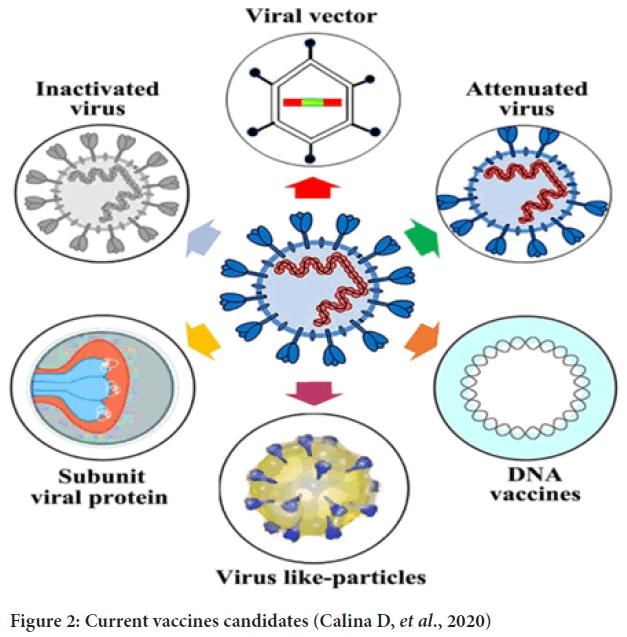
Currently, there has no effective clinical treatment for the COIVD-19. Therefore, vaccination seems to be the most effective way to prevent the further outbreak of the disease. There are four categories of vaccines in clinical trials (Gavi, 2020) and the markets: Inactivated, protein subunit, nucleic acid, and viral vector. Until 26/01/2021, the COVID-19 vaccines candidates in clinical phase I-III sort by types are 15, 13,20,15, respectively (WHO, 2023) (Figure 2).
Figure 2: Current vaccines candidates (Calina D, et al., 2020)
Inactivate vaccine
In the long history of vaccine development, the inactivated vaccine has been practiced many times and gained massive success. They have been used to prevent various infectious diseases caused by the virus, such as influenza virus and poliovirus (Drosten C, et al., 2023; Price MS, et al., 2014). Inactive vaccines use the whole virus with the genetic destroyed, so replication will not occur in the receptors’ body but can still trigger an immune response. The inactivated vaccines are produced by growing the bacterium or virus in culture media and then inactivating it with heat or chemicals, usually formalin (Woo PC, et al., 2012). As the most mature vaccine system, the inactivated vaccine is used against the COVID-19. Moreover, currently, the inactivated vaccine is widely used in China. Several companies have already launched their inactivated vaccine, such as SINOVAC, BEIJING INSTITUTE OF BIOLOGICAL COMPANY (https://www.bjbpi.com/).
Advantage and concern of inactivated vaccine: The inactivated vaccines have the following benefits. Primarily, its preparation is traditional and classical, which is mature and reliable (Woo PC, et al., 2012). Furthermore, compared with other technical routes, inactivated vaccines have mature R and D platforms, stable production processes, controllable quality standards, sound protection effects, and are easy to produce on a large scale. Moreover inactivated vaccines can increase human antibodies and reduce the chance of contracting new coronavirus, suitable for the elderly or children, people with low body immunity, and weak constitution. Inactivated vaccines are easy to store (Armstrong J, et al., 1984). Although the inactivated vaccines are well-practiced in recent years, they also have some limitations. The first is that an inactivated vaccine always requires multiple doses to achieve the desire concentration of antibodies. Usually, the protective response will not develop until the receiver gets their second or third dose (Gao Q, et al., 2020). In some situations that some inactivated vaccines may require periodic supplemental doses to increase or “boost” antibody titers (CDC, 2021).
Protein subunit vaccine
Protein subunit vaccine is another candidate against COVID-19. During the investigating process, which protein will be chosen to put in the vaccine is critical. The proteins need to satisfy at least two criteria that will not cause the disease and will be able to trigger the immune response. Among various kinds of proteins, the S protein becomes the most popular target, as it will not cause the COVID-19 and will trigger the Antigen-Presenting cell (Armstrong J, et al., 2014). When the APC recognizes the S protein of the virus, it will present the marker peptide of S protein to the T-help cell to further stimulate the Killer T-Cell and the memorization of the B cell. Several institutions have initiated programs on the SARS-CoV-2 subunit vaccine that uses the S protein as antigens. In this COVID-19 pandemic, many companies put their efforts into investigating the protein subunit vaccines containing refined proteins from viral or bacterial pathogens against the disease, such as Novavax, Anhui Zhifei Longcom Biopharmaceutical, Kentucky Bioprocessing, and Sanofi/GlaxoSmithKline (Challener CA, 2021). For instance, Novavax uses Novavax’s recombinant nanoparticle technology to generate antigen derived from the S protein and uses Novavax’s patented saponin-based Matrix-MTM to act as the adjuvant to stimulate the immune response high level of neutralizing antibodies. NVX-CoV2373 was created using Novavax’s recombinant nanoparticle technology to generate antigen derived from the coronavirus spike (S) protein and adjuvanted with Novavax’s patented saponin-based Matrix-MTM to enhance the immune response and stimulate high levels of neutralizing antibodies (WHO, 2023). In two pivotal phase 3 trials, NVX-CoV2373 demonstrated the efficacy of 96.4% in the UK against the original virus strain, and for the B.1.1.7 (Alpha) variant, 86.3% efficacy was found (WHO, 2023). The vaccine manufactures by the Anhui Zhifei Longcom Biopharmaceutical is also based on the S protein; the vaccine was approved for registration in Uzbekistan on March 1, 2021 and is the first recombinant protein vaccine to be approved for use in the world. The vaccine was also approved for emergency use in China on March 10, 2021. The ZF2001 vaccine was based on the dimer concept of the receptor-binding region (RBD) of the previous MERS coronavirus spike-in protein (S). The neo-coronavirus RBD was designed as a dimer (RBD-dimer) antigen by tandem repeats, which successfully preserved the vaccine’s efficacy and resulted in higher neutralizing antibody titers in mice after immunization the monomeric immunization effect (Calina D, et al., 2020). After two doses of vaccine in the clinical trials, 76% of people can produce neutralizing antibodies. Neutralizing antibodies were produced in 97% of individuals after three doses of vaccine. The geometric mean titer (GMT) of antibodies reached 102.5, exceeding the serum neutralizing antibody level (GMT, 51) in 89 newly recovered patients (Calina D, et al., 2020).
Advantage and concern of protein subunit vaccine: A significant advantage of protein subunit protein is that it uses part of the pathogen, so it will not be able to transmit the disease (Drosten C, et al., 2013). In addition to this, subunit vaccines are less prone to generate side effects at the inoculation site. Furthermore, due to stable conditions and definite pathogen fragments, subunit vaccines achieve reliable production (Armstrong J, et al., 1984). However, this type of vaccine still has some limitations. Primarily, the antigens used to excite an immune response may lack pathogen-associated molecular patterns, common to a pathogen class. The absence of this structure may weaken the immune response, as the immune cell can read the structure and recognized it as a danger signal. Also, because the antigens cannot infect the cells, only the antibody-mediated immune system can be triggered, which means it needs to use the adjuvant to boost the immune response. Moreover, sometimes the booster doses may be required (Gavi, 2021).
Viral vector
Adenovirus vectored vaccine: Adenovirus vector vaccine is made by a recombinant adenovirus that expresses protective antigen genes by recombinantly inserting the protective antigen genes into the adenovirus genome (Tatsis N and Ertl HC, 2004). To produce an adenoviruses vector, scientists need to knock off the E1, E2 regions of the viruses to attenuate the virulence of the virus. As the E1 region are mainly responsible for the reproduction of viruses (Gao Q, et al., 2020). The gene expression products of the E2 region can be divided into E2A, and E2B, where E2A is the DNA Binding Protein (DBP) and E2B has two main products, the terminal protein precursor (pTP) and the viral DNA polymerase (pol), respectively. The three proteins interact with at least three intracellular factors to initiate adenovirus DNA replication and the transcription and translation processes of late viral genes (https://www.bjbpi.com/). In this COVID-19 pandemic, an adenovirus vector vaccine is also used against the disease. The revised adenoviruses are designed to enter the cell in the human body and produce some harmless parts SARS-CoVs (usually use the Spike proteins as transgenes) with the production line of the cells (Tatsis N and Ertl HC, 2004). The cells in the human infected by these vectors will display the spike protein of SARS-CoVs on their surfaces. Then these cells will alter the immune system and trigger it to produce antibodies against the SARS-CoV-2 (Gavi, 2021). So, why use adenovirus as a vector of the vaccine against COVID-19? Adenovirus has been used as a vaccine vector for a long time and has several successful examples. Currently, adenovirusbased vaccines are used against many pathogens such as Mycobacterium tuberculosis, Human Immunodeficiency Virus (HIV), and Plasmodium falciparum (CDC, 2021). The adenoviruses work excellent for delivering the desired antigens to the mammalian hosts, as they can induce both innate and adaptive immune (Gao Q, et al., 2020). Figure 3 shows the interaction between adenovirus and the innate and adaptive immune environment. Until now, several vaccines against COVID-19 have already reached the public, such as Johnson and Johnson, AstraZeneca, CanSino Biological Inc./Beijing Institute of Biotechnology.
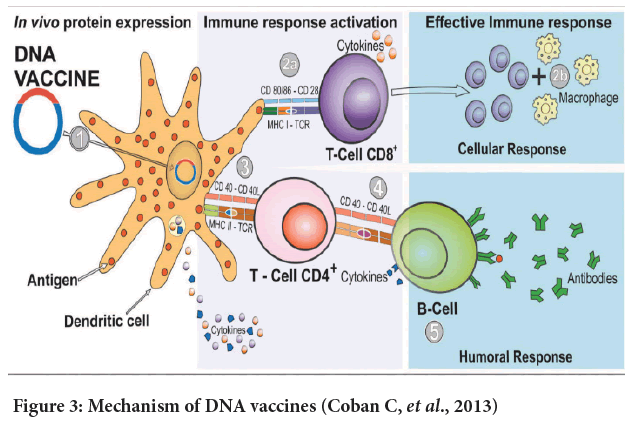
Figure 3: Mechanism of DNA vaccines (Coban C, et al., 2013)
Advantage and concern of adenovirus vectored vaccines: The main advantage of the adenoviruses vector vaccines is that they induce immune response to further trigger the adaptive immune response by expressing the immunogen in the environment of heterologous viral infection (WHO, 2023). Another advantage of these types of vaccines is it has been practiced in an extended period its technique is quite mature. For instance, since the 1950s, the live Ad types 4 (Ad4) and 7 (Ad7) have been used to prevent severe respiratory illness in the US (CDC, 2021). The concern of this type of vaccine is as the Ad family is frequently used as the carrier virus of the vaccine. In a significant portion of the human population, people have pre-existing immunity against adenovirus, so the pre-existing immunity of Adenoviruses (i.e., Ad5) may be high. And it may influence T-cell immune response post-vaccination. For example, in phase II clinical trial done in Wuhan, the present rate of neutralizing antibodies to adenovirus type 5 was 52% (Zhang S, et al., 2013). Because of this, the immunization effect of the elderly participants in the trial was significantly poor, indicating that vector immunization is likely to affect the vaccination effect. The presence of neutralizing antibodies to adenovirus type 5 reached 75% in some areas of China, 40% in the United States, and up to 80%-90% in sub-Saharan Africa and West Africa (Fausther-Bovendo H and Kobinger GP, 2014). Thus, the effect of vector immunization on CanSino Biological Inc./Beijing Institute of Biotechnology may be significant. Furthermore, a reduction of NAbs response may occur with the increasing of ages (Challener CA, 2021). So, to overcome pre-existing immunity, scientists need to use rare adenovirus or viruses from other species. For example, the vaccine of AstraZeneca uses the adenoviruses from chimpanzees (Custers J, et al., 2021).
Nucleic acid
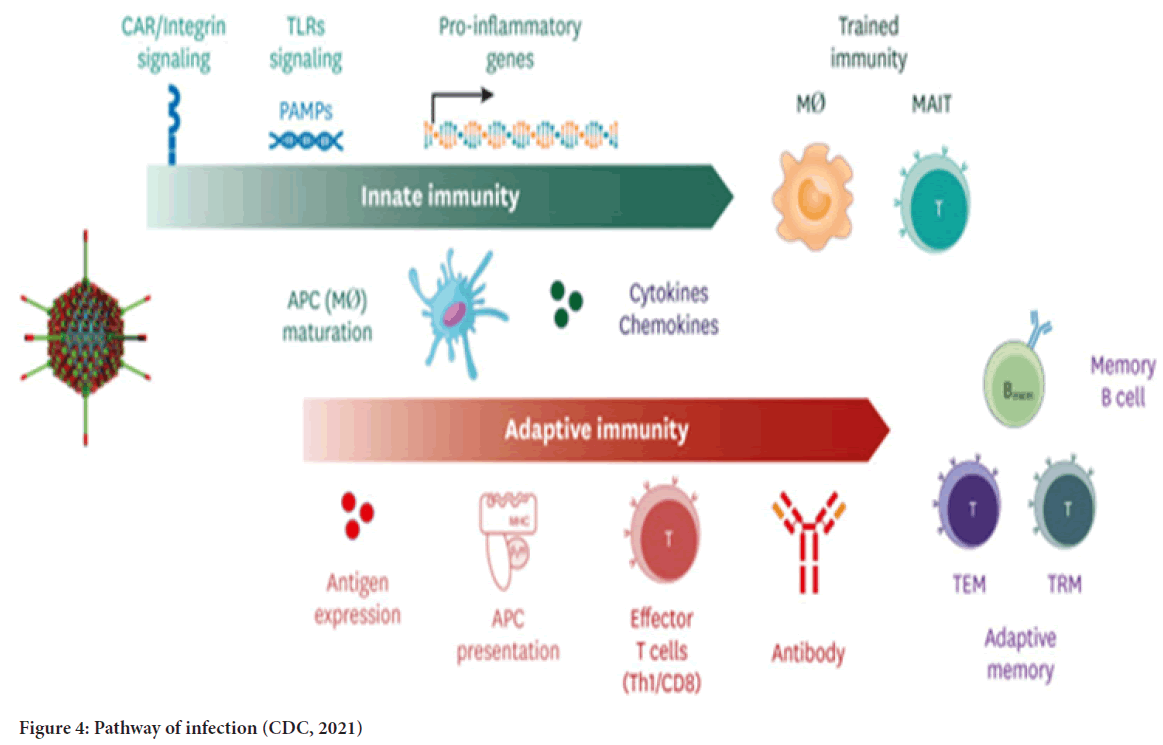
DNA vaccine: The concept of DNA vaccines is first tested in the 1990s by injecting DNA molecules expressing chloramphenicol acetyltransferase, luciferase, and beta-galactosidase into mouse skeletal muscles. The results show the feasibility of DNA vaccines. Furthermore, up to two months after the injection, the expression of the reporter genes could still be detected (Wolff JA, et al., 1990; Silveira MM, et al., 2021; Li L and Petrovsky N, 2016). Figure 3 shows a mechanism of the DNA vaccine (Coban C, et al., 2013). A DNA vaccine consists of a plasmid produced in bacteria. This plasmid encodes the protein of interest (an antigen) in the presence of a mammalian promoter (Wolff JA, et al., 1990; Silveira MM, et al., 2021). When it reaches the cell nucleus, the mammalian promoter present in the vector structure will be activated, then triggering the transcription of the gene of interest through the host’s cellular machinery (Silveira MM, et al., 2021). Once the encoded protein is expressed in the host’s cells, the vaccine antigen can then be presented to Antigen-Presenting Cells (APCs). The figure is a dendritic cell through the Major Histocompatibility Complex (MHC) pathways and is presented to activate naïve T cells (Silveira MM, et al., 2021). Process 2a shows that CD8+ T cell immunity is predominantly activated by endogenously expressed antigens presented on MHC class I molecules (Silveira MM, et al., 2021; Duerr GD, et al., 2021). After the activation steps, the cytokines will be released under the stimulation of active CD8+ T cells. The releasing of the cytokines will inhibit viral replication and increase the expression of MHC I molecules (Coban C, et al., 2013). After that, the macrophages are activated to support cell-mediated immune responses (Duerr GD, et al., 2020). Step 3 shows that CD4+ T helper cell activation is triggered through MHC class II from APC. So, once the vaccine proteins are secreted, these targets are recognized by B cell receptors in naïve B cells, which also use MHC-II to get activated (Coban C, et al., 2013, Duerr GD, et al., 2020; Silveira MM, et al., 2021; Ingolotti M, et al., 2010). In step 5, different classes of antibodies will be produced by activated B cells to protect against the disease (Silveira MM, et al., 2021) (Figure 4). During this COVID-19 pandemic, the DNA vaccine is also a candidate against the disease. However, the DNA vaccine still has not reached the market yet. So, what makes the DNA vaccine a candidate? The reason can be that the DNA vaccines can induce a broad immune response without any risk of being associated with getting the COVID-19. The DNA vaccine can stimulate both cellular and humoral immunity. Its vector can be constructed to encoding different antigens in a single vaccine (Silveira MM, et al., 2021; Xu Y, et al., 2014). Furthermore, DNA vaccines can be highly stable, requiring less refrigeration (Ingolotti M, et al., 2010).
Figure 4: Pathway of infection (CDC, 2021)
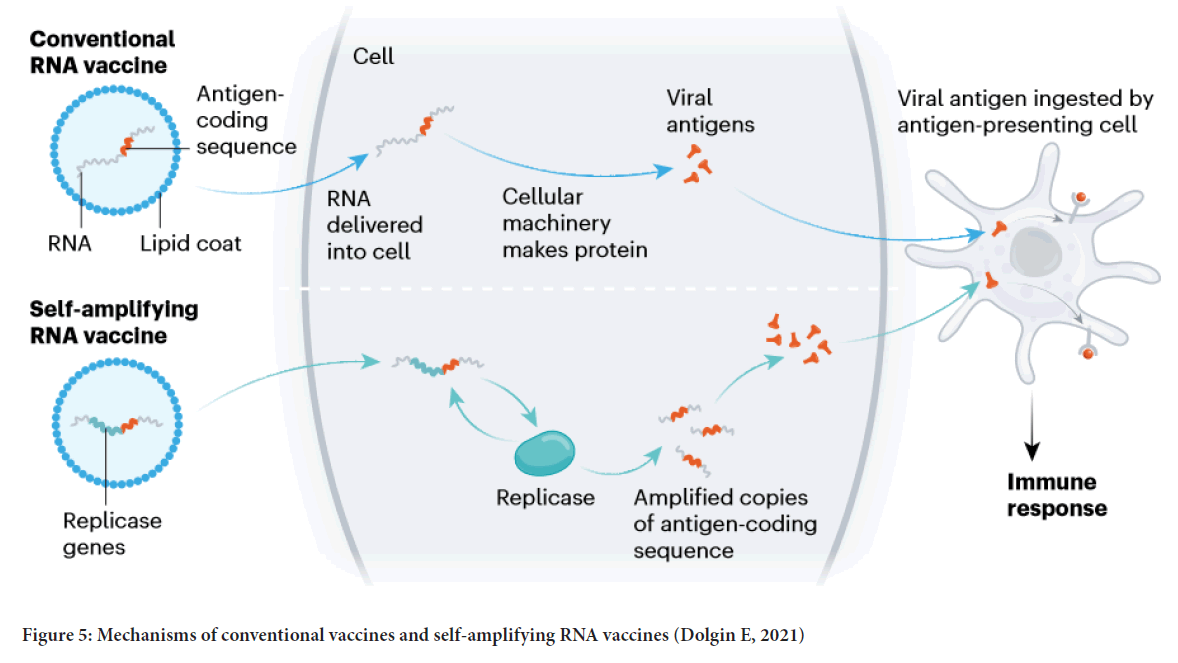
RNA vaccine: The concept of RNA vaccines has been around for nearly thirty years (Dolgin E, 2021). The researchers in France first used RNA encoding an influenza antigen in mice (Martinon F, et al., 1993). The response occurred at that time; however, the lipid delivery system they used is too toxic to use in humans. Thus, it took a decade for the scientist to looking at RNA-interference therapeutics and discovered the LNP technologies that would make today’s COVID-19 vaccines possible (Dolgin E, 2021). The mRNA-based vaccines comprise mRNA that encodes a protein antigen as RNA is relatively unstable, so novel vaccine designs were developed to improve its stability and protein translation efficiency to enhanced immune response (Silveira MM, et al., 2021). The first mRNA vaccine to be designed against COVID-19 is mRNA-1273, designed by Moderna Inc. Cambridge, MA, USA. From vaccine design to human trials, this vaccine only took 63 days (Silveira MM, et al., 2021). In mRNA-1273, the RNA of a full-length, prefusion stabilized S protein of SARS-CoV-2 was encapsulated in a Lipid Nanoparticle (LNP) (Shih HI, et al., 2020; Chakraborty R and Parvez S, 2020; Zhang C, et al., 2019). Data showed that mRNA-1273 could induce both potent neutralizing antibody and CD8 T cell responses (Corbett KS, et al., 2020; Koirala A, et al., 2020). Figure 5 shows two types of RNA vaccines’ working mechanisms.
Figure 5: Mechanisms of conventional vaccines and self-amplifying RNA vaccines (Dolgin E, 2021)
The first one is the conventional RNA vaccine that RNA consists of the antigen coding sequence encapsulated in LNP. Once the RNA is delivered into the cell, the cellular machinery will start making proteins and forming the viral antigen. The vial antigen will be ingested by the APC and then trigger the immune responses. The other type of vaccine is called the self-amplifying RNA vaccine. This vaccine encodes a replicase gene with the antigen coding sequence into the RNA vector compared with the conventional one. Once the RNA is delivered into the cell, a replicase will be made by the cellular machinery. Furthermore, the replicates will produce more amplified copies of the antigen-coding sequence (Silveira MM, et al., 2021).
Advantage and concern of RNA vaccine: The RNA-based vaccine’s great advantage is the short time required from the design to clinical trials. Therefore, once a new mutation occurs, the companies can design, test, and release their vaccines as soon as possible (Silveira MM, et al., 2021). Effective mRNA vaccines by Moderna and BioNTech have proven their ability against COVID-19. However, there are still have some problems related to the RNA vaccine that need to be improved. The first is that the distribution of vaccines currently requires a costly cold chain to maintain the integrity of the RNA; the vaccines need to be stored at -70°C (both the Pfizer-BioNTech and Moderna) (Dolgin E, 2021). There is another challenge that the RNA vaccines have generally required a double dose to be effective. Big data shows that many people who get the first shot probably will not get the second (Dolgin E, 2021).
Conclusion
The outbreak of the COVID-19 causes a dramatic loss of lives, human resources, and economics. Recently, there had several kinds of vaccines available in the market, such as the mRNA vaccine (i.e., Pfizer, Moderna), adenovirus vector vaccine (i.e., Jahnson and Jahnson, AstraZeneca, CanSino Biological Inc./Beijing Institute of Biotechnology), and inactivated virus vaccines (i.e., CanSino Biological Inc./Beijing Institute of Biotechnology). However, there have some problems related to the existing vaccines. Many pharmaceuticals and intuitions are still on their way to producing and testing new vaccines. The most outstanding problem is the appearance of the variants. SARS-CoV-2 is an RNA virus, its mutation rate is high, and the mutation of the virus will weaken the effectiveness of the vaccines. According to the CDC, at least 5 new variants are already occurring now, and some of them may cause an even more serious pandemic. For example, in many countries, people are infected by the Delta variant of COVID-19 after vaccination. As the COVID-19 vaccines are just launched recently, maybe with the increasing maturity and diversity of COVID-19 vaccines, these problems will be solved in the future. Although the COVID-19 vaccines are available now, there are still some issues against the fight with SARS-CoV-2, such as the willingness of public to get COVID-19 vaccines. The unwellness of vaccination is a global issue related to vaccine safety these years, not only in this case. For the public health department to solve this issue still has a long way to go.
References
- Human coronavirus types. Centers for Disease Control and Prevention (CDC). 2021.
- Severe Acute Respiratory Syndrome (SARS). Centers for Disease Control and Prevention (CDC). 2021.
- Middle East Respiratory Syndrome (MERS). Centers for Disease Control and Prevention (CDC). 2019.
- Habas K, Nganwuchu C, Shahzad F, Gopalan R, Haque M, Rahman S, et al. Resolution of coronavirus disease 2019 (COVID-19). Expert Rev Anti Infect Ther. 2020; 18(12): 1201-1211.
[Crossref] [Google Scholar] [Pubmed]
- Wiersinga WJ, Rhodes A, Cheng AC, Peacock SJ, Prescott HC. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): A review. JAMA. 2020; 324(8): 782-793.
[Crossref] [Google Scholar] [Pubmed]
- Hui KP, Cheung MC, Perera RA, Ng KC, Bui CH, Ho JC, et al. Tropism, replication competence, and innate immune responses of the coronavirus SARS-CoV-2 in human respiratory tract and conjunctiva: An analysis in ex-vivo and in-vitro cultures. Lancet Respir Med. 2020; 8(7): 687-695.
[Crossref] [Google Scholar] [Pubmed]
- Human coronavirus types
- Hossain MM, Tasnim S, Sultana A, Faizah F, Mazumder H, Zou L, et al. Epidemiology of mental health problems in COVID-19: A review. F1000Res. 2020; 9.
[Crossref] [Google Scholar] [Pubmed]
- Han HJ, Yu H, Yu XJ. Evidence for zoonotic origins of middle east respiratory syndrome coronavirus. J Gen Virol. 2016; 97(Pt 2): 274.
[Crossref] [Google Scholar] [Pubmed]
- Memish ZA, Mishra N, Olival KJ, Fagbo SF, Kapoor V, Epstein JH, et al. Middle east respiratory syndrome coronavirus in bats, Saudi Arabia. Emerg Infect Dis. 2013; 19(11): 1819.
[Crossref] [Google Scholar] [Pubmed]
- Naming the coronavirus disease (COVID-19) and the virus that causes it. World Health Organization (WHO). 2019.
- Kaur N, Singh R, Dar Z, Bijarnia RK, Dhingra N, Kaur T. Genetic comparison among various coronavirus strains for the identification of potential vaccine targets of SARS-CoV2. Infect Genet Evol. 2021; 89: 104490.
[Crossref] [Google Scholar] [Pubmed]
- Rogers K. Coronavirus encyclopedia. Britannica. 2021.
- Masters PS. The molecular biology of coronaviruses. Adv Virus Res. 2006; 66: 193-292.
[Crossref] [Google Scholar] [Pubmed]
- de Groot RJ, Baker SC, Baric RS, Brown CS, Drosten C, Enjuanes L, et al. Commentary: Middle East Respiratory Syndrome Coronavirus (MERS-Cov): Announcement of the coronavirus study group. J Virol. 2013; 87(14): 7790-7792.
[Crossref] [Google Scholar] [Pubmed]
- Wertheim JO, Chu DK, Peiris JS, Kosakovsky Pond SL, Poon LL. A case for the ancient origin of coronaviruses. J Virol. 2013; 87(12): 7039-7045.
[Crossref] [Google Scholar] [Pubmed]
- Forni D, Cagliani R, Clerici M, Sironi M. Molecular evolution of human coronavirus genomes. Trends Microbiol. 2017; 25(1): 35-48.
[Crossref] [Google Scholar] [Pubmed]
- Woo PC, Lau SK, Lam CS, Lau CC, Tsang AK, Lau JH, et al. Discovery of seven novel mammalian and avian coronaviruses in the genus deltacoronavirus supports bat coronaviruses as the gene source of alphacoronavirus and betacoronavirus and avian coronaviruses as the gene source of gammacoronavirus and deltacoronavirus. J Virol. 2012; 86(7): 3995-4008.
[Crossref] [Google Scholar] [Pubmed]
- AlBarrak AM, Stephens GM, Hewson R, Memish ZA. Recovery from severe novel coronavirus infection. Saudi Med J. 2012; 33(12): 1265-1269.
[Google Scholar] [Pubmed]
- Price MS, Miazgowicz KL, Munster VJ. The emergence of the middle east respiratory syndrome coronavirus. Pathog Dis. 2014; 71(2): 121-136.
[Crossref] [Google Scholar] [Pubmed]
- Su S, Wong G, Shi W, Liu J, Lai AC, Zhou J, et al. Epidemiology, genetic recombination, and pathogenesis of coronaviruses. Trends Microbiol. 2016; 24(6): 490-502.
[Crossref] [Google Scholar] [Pubmed]
- Armstrong J, Niemann H, Smeekens S, Rottier P, Warren G. Sequence and topology of a model intracellular membrane protein, E1 glycoprotein, from a coronavirus. Nature. 1984; 308(5961): 751-752.
[Crossref] [Google Scholar] [Pubmed]
- There are four types of COVID-19 vaccines: Here’s how they work. Gavi, the vaccine alliance. 2020.
- COVID-19 vaccine tracker and landscape. World Health Organization (WHO). 2023.
- Drosten C, Seilmaier M, Corman VM, Hartmann W, Scheible G, Sack S, et al. Clinical features and virological analysis of a case of middle east respiratory syndrome coronavirus infection. Lancet Infect Dis. 2013; 13(9): 745-751.
[Crossref] [Google Scholar] [Pubmed]
- Calina D, Docea AO, Petrakis D, Egorov AM, Ishmukhametov AA, Gabibov AG, et al. Towards effective COVID‑19 vaccines: Updates, perspectives and challenges. Int J Mol Med. 2020; 46(1): 3-16.
[Crossref] [Google Scholar] [Pubmed]
- Gao Q, Bao L, Mao H, Wang L, Xu K, Yang M, et al. Development of an inactivated vaccine candidate for SARS-CoV-2. Science. 2020; 369(6499): 77-81.
[Crossref] [Google Scholar] [Pubmed]
- Epidemiology of VPDs: Principles of vaccination. Centers for Disease Control and Prevention (CDC). 2021.
- Challener CA. Subunit vaccines and the fight against COVID-19 advances in technology are accelerating the development and manufacture of this established class of vaccines. BioPharm Int. 2021; 34(8): 23-25.
- What are whole virus vaccines and how could they be used against COVID-19? Gavi, the vaccine alliance. 2021.
- Tatsis N, Ertl HC. Adenoviruses as vaccine vectors. Mol Ther. 2004; 10(4): 616-629.
[Crossref] [Google Scholar] [Pubmed]
- Zhang S, Huang W, Zhou X, Zhao Q, Wang Q, Jia B. Seroprevalence of neutralizing antibodies to human adenoviruses type‐5 and type‐26 and chimpanzee adenovirus type‐68 in healthy Chinese adults. J Med Virol. 2013; 85(6): 1077-1084.
[Crossref] [Google Scholar] [Pubmed]
- Fausther-Bovendo H, Kobinger GP. Pre-existing immunity against Ad vectors: Humoral, cellular, and innate response, what's important? Hum Vaccin Immunother. 2014; 10(10): 2875-2884.
[Crossref] [Google Scholar] [Pubmed]
- Custers J, Kim D, Leyssen M, Gurwith M, Tomaka F, Robertson J, et al. Vaccines based on replication incompetent Ad26 viral vectors: Standardized template with key considerations for a risk/benefit assessment. Vaccine. 2021; 39(22): 3081-3101.
[Crossref] [Google Scholar] [Pubmed]
- Wolff JA, Malone RW, Williams P, Chong W, Acsadi G, Jani A, et al. Direct gene transfer into mouse muscle in vivo. Science. 1990; 247(4949): 1465-1468.
[Crossref] [Google Scholar] [Pubmed]
- Silveira MM, Moreira GM, Mendonça M. DNA vaccines against COVID-19: Perspectives and challenges. Life Sci. 2021; 267: 118919.
[Crossref] [Google Scholar] [Pubmed]
- Li L, Petrovsky N. Molecular mechanisms for enhanced DNA vaccine immunogenicity. Expert Rev Vaccines. 2016; 15(3): 313-329.
[Crossref] [Google Scholar] [Pubmed]
- Coban C, Kobiyama K, Jounai N, Tozuka M, Ishii KJ. DNA vaccines: A simple DNA sensing matter? Hum Vaccin Immunother. 2013; 9(10): 2216-2221. [Crossref]
[Google Scholar] [Pubmed]
- Silveira MM, Oliveira TL, Schuch RA, McBride AJ, Dellagostin OA, Hartwig DD. DNA vaccines against leptospirosis: A literature review. Vaccine. 2017; 35(42): 5559-5567.
[Crossref] [Google Scholar] [Pubmed]
- Duerr GD, Heine A, Hamiko M, Zimmer S, Luetkens JA, Nattermann J, et al. Parameters predicting COVID-19-induced myocardial injury and mortality. Life Sci. 2020; 260: 118400.
[Crossref] [Google Scholar] [Pubmed]
- Ingolotti M, Kawalekar O, Shedlock DJ, Muthumani K, Weiner DB. DNA vaccines for targeting bacterial infections. Expert Rev Vaccines. 2010; 9(7): 747-763.
[Crossref] [Google Scholar] [Pubmed]
- Xu Y, Yuen PW, Lam JK. Intranasal DNA vaccine for protection against respiratory infectious diseases: The delivery perspectives. Pharmaceutics. 2014; 6(3): 378-415.
[Crossref] [Google Scholar] [Pubmed]
- Dolgin E. How COVID unlocked the power of RNA vaccines. Nature. 2021; 589(7841): 189-192.
[Crossref] [Google Scholar] [Pubmed]
- Martinon F, Krishnan S, Lenzen G, Magné R, Gomard E, Guillet JG, et al. Induction of virus‐specific cytotoxic T lymphocytes in vivo by liposome‐entrapped mRNA. Eur J Immunol. 1993; 23(7): 1719-1722.
[Crossref] [Google Scholar] [Pubmed]
- Shih HI, Wu CJ, Tu YF, Chi CY. Fighting COVID-19: A quick review of diagnoses, therapies, and vaccines. Biomed J. 2020; 43(4): 341-354.
[Crossref] [Google Scholar] [Pubmed]
- Chakraborty R, Parvez S. COVID-19: An overview of the current pharmacological interventions, vaccines, and clinical trials. Biochem Pharmacol. 2020; 180: 114184.
[Crossref] [Google Scholar] [Pubmed]
- Zhang C, Maruggi G, Shan H, Li J. Advances in mRNA vaccines for infectious diseases. Front Immunol. 2019: 594.
[Crossref] [Google Scholar] [Pubmed]
- Corbett KS, Edwards DK, Leist SR, Abiona OM, Boyoglu-Barnum S, Gillespie RA, et al. SARS-CoV-2 mRNA vaccine design enabled by prototype pathogen preparedness. Nature. 2020; 586(7830): 567-571.
[Crossref] [Google Scholar] [Pubmed]
- Koirala A, Joo YJ, Khatami A, Chiu C, Britton PN. Vaccines for COVID-19: The current state of play. Paediatr Respir Rev. 2020; 35: 43-49.
[Crossref] [Google Scholar] [Pubmed]
Author Info
An Zhu*Citation: Zhu A: Different Types of Vaccines against Novel Coronavirus SARS-CoV-2
Received: 15-Feb-2023 Accepted: 10-Mar-2023 Published: 17-Mar-2023, DOI: 10.31858/0975-8453.14.3.183-189
Copyright: This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
ARTICLE TOOLS
- Dental Development between Assisted Reproductive Therapy (Art) and Natural Conceived Children: A Comparative Pilot Study Norzaiti Mohd Kenali, Naimah Hasanah Mohd Fathil, Norbasyirah Bohari, Ahmad Faisal Ismail, Roszaman Ramli SRP. 2020; 11(1): 01-06 » doi: 10.5530/srp.2020.1.01
- Psychometric properties of the World Health Organization Quality of life instrument, short form: Validity in the Vietnamese healthcare context Trung Quang Vo*, Bao Tran Thuy Tran, Ngan Thuy Nguyen, Tram ThiHuyen Nguyen, Thuy Phan Chung Tran SRP. 2020; 11(1): 14-22 » doi: 10.5530/srp.2019.1.3
- A Review of Pharmacoeconomics: the key to “Healthcare for All” Hasamnis AA, Patil SS, Shaik Imam, Narendiran K SRP. 2019; 10(1): s40-s42 » doi: 10.5530/srp.2019.1s.21
- Deuterium Depleted Water as an Adjuvant in Treatment of Cancer Anton Syroeshkin, Olga Levitskaya, Elena Uspenskaya, Tatiana Pleteneva, Daria Romaykina, Daria Ermakova SRP. 2019; 10(1): 112-117 » doi: 10.5530/srp.2019.1.19
- Dental Development between Assisted Reproductive Therapy (Art) and Natural Conceived Children: A Comparative Pilot Study Norzaiti Mohd Kenali, Naimah Hasanah Mohd Fathil, Norbasyirah Bohari, Ahmad Faisal Ismail, Roszaman Ramli SRP. 2020; 11(1): 01-06 » doi: 10.5530/srp.2020.1.01
- Manilkara zapota (L.) Royen Fruit Peel: A Phytochemical and Pharmacological Review Karle Pravin P, Dhawale Shashikant C SRP. 2019; 10(1): 11-14 » doi: 0.5530/srp.2019.1.2
- Pharmacognostic and Phytopharmacological Overview on Bombax ceiba Pankaj Haribhau Chaudhary, Mukund Ganeshrao Tawar SRP. 2019; 10(1): 20-25 » doi: 10.5530/srp.2019.1.4
- A Review of Pharmacoeconomics: the key to “Healthcare for All” Hasamnis AA, Patil SS, Shaik Imam, Narendiran K SRP. 2019; 10(1): s40-s42 » doi: 10.5530/srp.2019.1s.21
- A Prospective Review on Phyto-Pharmacological Aspects of Andrographis paniculata Govindraj Akilandeswari, Arumugam Vijaya Anand, Palanisamy Sampathkumar, Puthamohan Vinayaga Moorthi, Basavaraju Preethi SRP. 2019; 10(1): 15-19 » doi: 10.5530/srp.2019.1.3