Research Article - (2023) Volume 14, Issue 4
Dolutegravir Based Therapy Showed CD4+ T Cell Count Recovery and Viral Load Suppression among Art Naive HIV Positive Individuals: A Longitudinal Evaluation
Teshager Gebremedhin1, Melak Ayenalem2, Mohammed Adem3, Demeke Geremew4, Yetemwork Aleka3* and Amare Kiflie3Abstract
Background: Recently, Dolutegravir (DTG)-based combined therapy, a more effective and safer first-line Antiretroviral Therapy (ART), has been recommended by the World Health Organization (WHO) for the treatment of Human Immunodeficiency Virus (HIV) since July 2018. However, its effectiveness in CD4+ T-cells count recovery and viral load suppression has not been studied yet in Ethiopia, where HIV is endemic. Therefore, we aimed to assess the effect of DTG-based therapy on CD4+ T-cell count and viral load count among HIV-positive patients in Ethiopia.
Methods: A longitudinal prospective cohort study was conducted from July 2020-February 2021. 109 HIV-positive individuals who are ART naive but plan to initiate DTG-based therapy were recruited. HIV viral Ribonucleic Acid (RNA) copies were determined using a CD4+ T-cell count and quantitative Polymerase Chain Reaction (PCR). To compute the difference in viral load and CD4+ T-cell counts between the baseline, 3rd, and 6th months, a Friedman test was used.
Results: The study included 109 HIV-positive people who had never received antiretroviral medication. Participants taking DTG-based treatment showed significantly decreasing median (IQR) values of viral load count (copies/mL) from 446,812 (237,649.5- 732,994.5) at baseline to 34 (23.5-46) at 3 months and 0.0 (0-19) at 6 months of treatment follow-up. Although the treatment increases the proportion of participants with HIV-1 RNA 50 copies/mL from 0 (0% at baseline) to 87 (79.8%) and 100 (91.7%) at the 3rd and 6th months of treatment, respectively. On the other hand, the CD4+ T-cell count increased significantly during treatment-median (IQR): 209 (81.5-417.5) versus 291 (132-522) versus 378 (181-632.5) cells/L at baseline, the 3rd and 6th months of the treatment follow- up period, respectively.
Conclusion: We found Dolutegravir-based therapy was a promising option with high virological suppression rates and CD4+ T-cell count recovery demonstrating a restoration of cellular immunity. More over viral load suppression rates were high after the initiation of the treatment.
Keywords
Human immunodeficiency virus, CD4+ T-cell count, Viral load
Abbreviations
3TC: Lamivudine; AIDS: Acquired Immune Deficiency Syndrome; ART: Antiretroviral Therapy; BD FACS: Becton Dickinson Fluorescence Activated Cell Sorting; BMI: Body Mass Index; CART: Combination Antiretroviral Therapy; CD: Cluster of Differentiation; DTG: Dolutegravir; EDTA: Ethylene Diamine Tetra-Acetic Acid: EFV: Efavirenz; FTC: Emtricitabine; HAART: Highly Active Antiretroviral Therapy; HIV: Human Immunodeficiency Virus; ML: Milliliter; PCR: Polymerase Chain Reaction; PMTCT: Prevention of Mother-To-Child Transmission; QS: Quantification standard; RNA: Ribonucleic Acid; SPU: Sample Processing Unit; TDF: Tenofovir Disoproxil Fumarate; UNAIDS: United Nations Program on HIV/AIDS; UoG: University of Gondar; WHO: World Health Organization
Introduction
Effectiveness, safety, and durability of the antiretroviral treatment regimen have always been important factors in Human Immunodeficiency (HIV) chronic care. Recent developments in antiretroviral therapy have demonstrated the development of more effective and secure regimens (Brehm TT, et al., 2019).
According to the most recent World Health Organization (WHO) guidelines, a combination of at least three antiretroviral therapies (CART) is required for the management of chronic Human Immunodeficiency Virus (HIV) (WHO, 2016). Two nucleoside reverse transcriptase inhibitors with a non-nucleoside reverse transcriptase inhibitor make up the initial line of ART that is recommended for adults. DTG+TDF+3TC based regimen is recommended by the World Health Organization as the first-line antiretroviral therapy for patients with HIV (WHO, 2016). These regimens outperform conventional treatment regimens in both treatment-naive and treatment-experienced individuals, including those who have previously failed raltegravir or elvitegravir (Llibre JM, et al., 2022).
In the United States and Europe, Dolutegravir-based antiretroviral therapy has been approved for first-line HIV treatment. Around 70 low-and middle-income countries planned to incorporate DTG into their national recommendations by the end of 2017, shifting to a DTG-based first-line regimen as recommended by WHO (WHO, 2016).
However, extensive analysis of several important populations, such as those with different genetic diversity, pregnant women, those with HIV-tuberculosis co-infection receiving rifampicin-based treatments, and patients from developing nations who had previously received treatment but were treatment-naive or drug-resistant to nucleoside reverse transcriptase inhibitors, was not included in the development programs for DTG-based regimens (Vitoria M, et al., 2018; Omondi FH, et al., 2021). The effectiveness and safety of DTG-based therapy have other limitations. Therefore, there is an urgent need for additional populations with relevance to public health and for supplementary investigations (Vitoria M, et al., 2016).
Despite the fact that different treatment efficacy studies have revealed that the drug regimen dolutegravir antiretroviral therapy promotes immune system recovery (Brehm TT, et al., 2019, Morón-López S, et al., 2019; Gillman J, et al., 2019), no longitudinal studies have been conducted in Ethiopia because viral strain types differ and the number of clients on secondline ART has increased in recent years. A pilot study, for example, found that DTG+3TC participants developed resistance mutations. While new DTG-based regimens must be tested for efficacy if health system factors related to the emergence of drug resistance has to be minimized (Ndashimye E, et al., 2020; Yang X, et al., 2020; de Miguel R, et al., 2020).
Early ART initiation once an HIV diagnosis is established plays a significant role in lowering mortality and morbidity due to HIV. Therefore, the goal of this study is to provide further light on the effectiveness of DTG- based regimens and their function in immunological recovery and viral load suppression, especially in Ethiopian settings. It will also serve as a starting point for scaling up the treatment in the future for the purposes of viral suppression and immunologic advantages.
Materials and Methods
Study area
The study was conducted at ART clinics of Gondar town health facilities. Currently, University of Gondar comprehensive specialized referral hospital, Teda, Azezo, Maraki, Gondar Health centers, Haset clinic, and Ibex Hospital who provide services related to ART.
Study design and population
A longitudinal prospective cohort study was performed from July 2020 to February 2021 at Gondar town health facilities of ART clinic with a continuing enrolment of ART naïve HIV positive individuals who initiated ART containing TDF+3TC (FTC)+DTG among ART naïve HIV positive individuals in Gondar town, North West Ethiopia. Socio-demographic data were collected using a structured questionnaire while clinical data was collected from patient record forms from all study participants.
Operational definitions
A good immunological response is >20% increase in CD4+ T-cells count from baseline during the first the 6th month of ART whereas Immunologic failure is defined as having a CD4+ T-cell count result <20% cells/µL at the end of the 6th month (Marziali M, et al., 2006).
Viral suppression is defined as proportion of patients achieving plasma viral load suppression <50 copies/mL within the 3rd and 6th months of DTG based regimen initiation. Virologic failure is defined as the proportion of patients achieving plasma viral load suppression >50 copies/mL within the 3rd and 6th months of DTG based regimen initiation (FDA, 2015).
Good ART adherence-equal to or greater than 95% adherence i.e., missing only 1 out of 30 doses or missing 2 from the 60 doses implies good adherence. Fair ART adherence-85%-94% adherence, i.e., missing 2-4 doses out of 30 doses or 4 to 9 doses from 60 doses. Poor ART adherence-less than 85% adherence, i.e., missing >5 doses out of 30 doses or >10 doses from 60 doses implies poor adherence (WHO, 2018). BMI levels <18.5, 18.5-24.9, 25.0-29.9, 30.0, and above are underweight, normal, overweight, and obese, respectively (Weir CB and Jan A, 2022).
Blood sample collection
For CD4+ T-cell and viral load counts, eight mL of venous blood was collected into two separate EDTA vacutainer tubes. On the same day as sample collection, whole blood and plasma samples were transported to the UOG comprehensive specialized hospital ART clinic. One tube containing 5 ml of whole blood was centrifuged at 1500 rpm for 10 minutes, and plasma was separated for HIV-1 viral load testing at the site of sample collection while the other tube remained at room temperature.
Flowcytometry and TaqMan nucleic acid amplification assay
A CD4+ T helper cell count was performed using the BD FACSPresto Near-Patient CD4 Counter System. A whole blood sample was stained using fluorochrome-conjugated antibodies (CD4 PE-Cy5, CD3 APC, and CD45RA APC dried antibody reagents), and when the specific antibodies bind to the surface antigens on the T lymphocytes during the incubation period, dedicated software identifies and counts the CD4+ T lymphocyte (BD Biosciences, 2016).
HIV-1 viral load was performed from a stored plasma sample following an appropriate thawing procedure using the COBAS Ampliprep/COBAS TaqMan nucleic acid amplification test for the quantification of HIV-1 RNA in plasma (Roche Diagnostics, 2023).
Data analysis and processing
The data was entered and cleared by EPI INFO 7, exported, and analyzed using GraphPad prism V.5.03 (GraphPad Software, USA) comparisons between baseline, 3rd, and 6th months of HIV viral load, and CD4+ T-cell counts were performed by using Friedman test. A continuous variable was expressed as median, Interquartile Range (IQR), and proportion or percentage. All p-values <0.05 were considered statistically significant.
Results
Socio-demographic and clinical characteristics
We followed 109 study subjects longitudinally. Fifty-six (51.4%) of them were male with a median age of 32 (IQR: 26.5-40) and 94 (86.2%) were living in urban. At baseline, 38 (34.9%) and 31 (28.4%) of study participants were in WHO clinical stage I and III, respectively. Besides, 86 (78.9%) and 84 (77.1%) of the study participants were having good ART adherence during the 3rd and 6th months of follow-up, respectively (Table 1).
| Variables | N (%) |
|---|---|
| Age, median (range) | 32 (18%-56) |
| Sex | |
| Male | 56 (51.4%) |
| Female | 53 (48.6%) |
| Residence | |
| Urban | 94 (86.2%) |
| Rural | 15 (13.8%) |
| WHO clinical stage | |
| Stage I | 38 (34.9%) |
| Stage II | 24 (22.0%) |
| Stage III | 31 (28.4 %) |
| Stage IV | 16 (14.7%) |
| 3-month ART adherences | |
| Good | 86 (78.9%) |
| Fair | 10 (9.2%) |
| Poor | 13 (11.9%) |
| 6-month ART adherences | |
| Good | 84 (77.1%) |
| Fair | 10 (9.2 %) |
| Poor | 15 (13.8%) |
| BMI categories | |
| <18.5 | 35 (32.1%) |
| 18.5-24.9 | 65 (59.6%) |
| 25-29.9 | 8 (7.3%) |
| >30 | 1 (0.9%) |
Table 1: Socio-demographic and clinical characteristics of the study participants attending ART clinics at Gondar, North West Ethiopia
Virological efficacy
Of 109 participants, the median baseline, 3rd, and 6th months of HIV viral load count was 446812 (IQR: 237650- 732995), 34 (IQR: 23.5-46), 0.0 (IQR: 0-19), copies/mL, respectively. At the 3rd and 6th months of follow-up, the proportion of participants with HIV-1 RNA <50 copies/mL were 87 (79.8%) and 100 (91.7%) of 109 participants, respectively.
Dolutegravir based therapy and viral load
There was significant viral suppression observed between baseline, 3rd, and 6th months. The median (IQR) viral RNA copies per ml were 446812 (IQR: 237650- 732995) at baseline while it becomes 34 (IQR: 23.5-46), and 0.0 (IQR: 0-19) at 3rd and 6 months of treatment follow-up periods, respectively (p<0.001) (Figure 1).
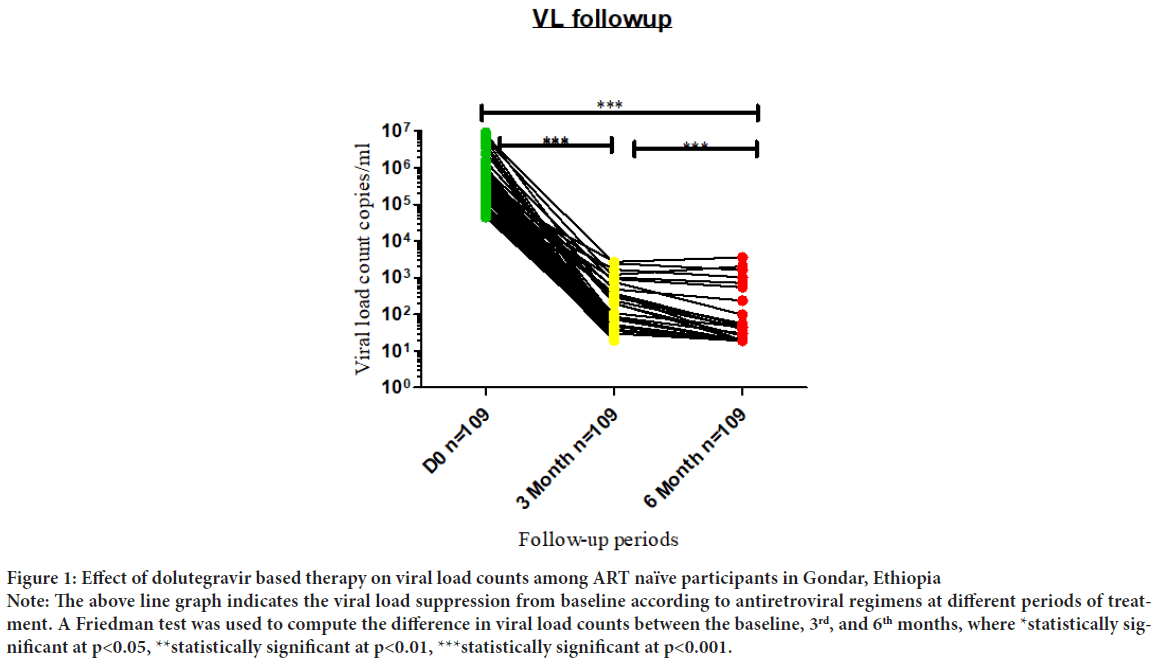
Figure 1: Effect of dolutegravir based therapy on viral load counts among ART naïve participants in Gondar, Ethiopia
Note: The above line graph indicates the viral load suppression from baseline according to antiretroviral regimens at different periods of treatment. A Friedman test was used to compute the difference in viral load counts between the baseline, 3rd, and 6th months, where *statistically significant at p<0.05, **statistically significant at p<0.01, ***statistically significant at p<0.001.
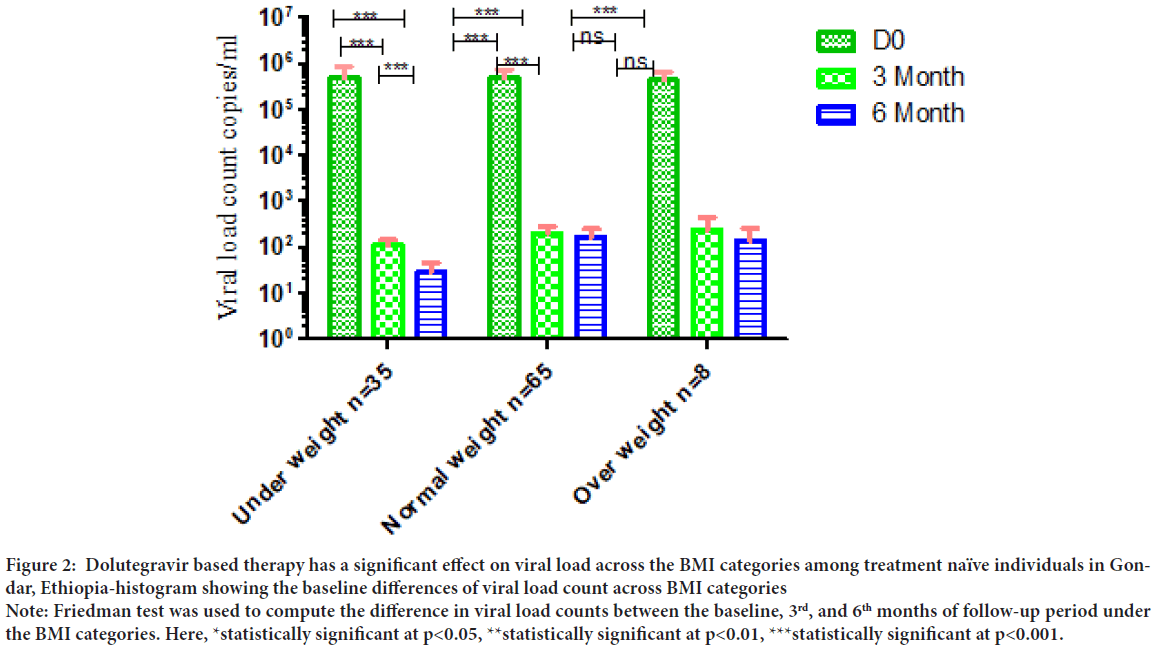
Dolutegravir based therapy has a significant effect on viral load count across the BMI categories during follow-up periods. There was no significant difference comparing the median viral load count across all the BMI categories under-weight, normal, overweight and obese individuals during baseline. However, we observed statically significant viral load suppression during follow-up periods (Between D0, 3rd and 6th months) compared across BMI categories (p<0.001) (Figure 2).
Figure 2: Dolutegravir based therapy has a significant effect on viral load across the BMI categories among treatment naïve individuals in Gondar, Ethiopia-histogram showing the baseline differences of viral load count across BMI categories
Note: Friedman test was used to compute the difference in viral load counts between the baseline, 3rd, and 6th months of follow-up period under
the BMI categories. Here, *statistically significant at p<0.05, **statistically significant at p<0.01, ***statistically significant at p<0.001.
Dolutegravir based therapy effect across WHO clinical stage
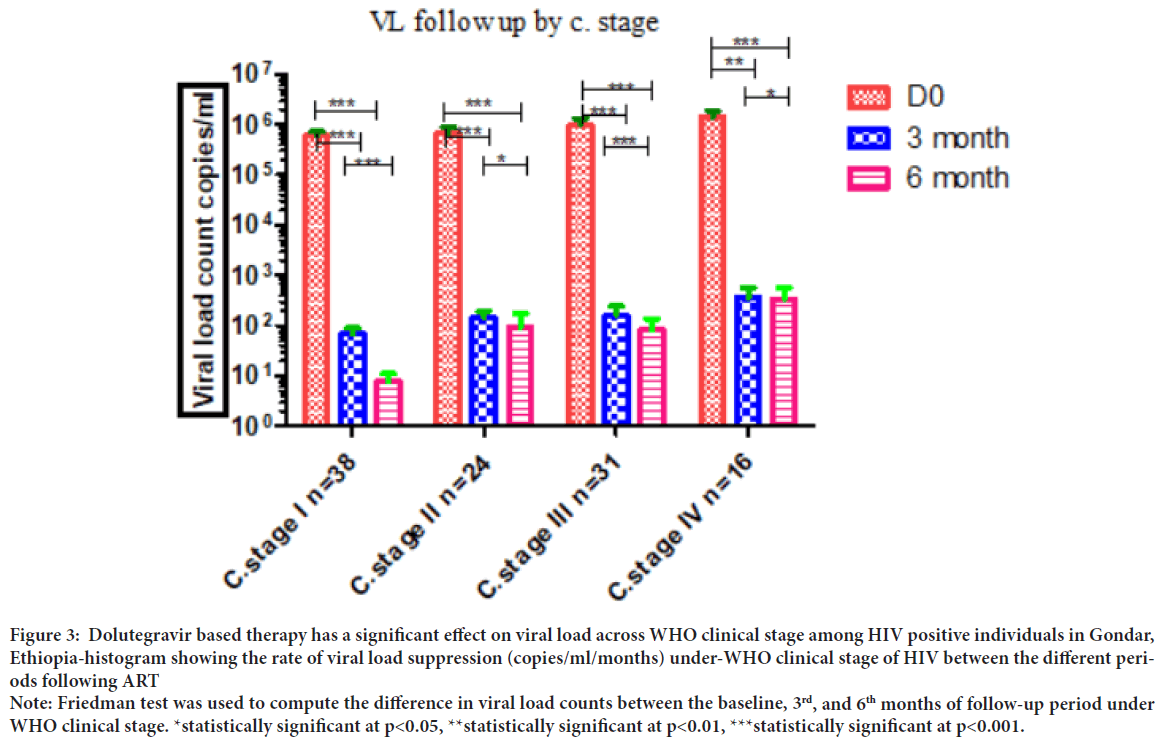
Comparing the viral load count between the baseline with 3rd, and 6th months under-WHO clinical stage I (p<0.001), stage II (p<0.001), and stage III (p<0.001) showed a significant difference. We also observed high virological suppression in the comparison of baseline with 3rd (p<0.01), and 6th (p<0.001) months of follow-up in those patients under WHO stage IV presented (Figure 3). Good ART adherence showed significant viral load suppression during treatment periods. Study participants who had good adherence had shown association with viral load suppression (p=0.000) while study participants with fair adherence did not show significant association with viral load counts (p=0.625) (Table 2).
| Independent variables | COR | 95% CI | p-value | |
|---|---|---|---|---|
| Three months adherence | Good | 0.073 | 0.020-0.272 | 0 |
| Fair | 0.625 | 0.118-3.316 | 0.581 | |
| Poor | 1 | - | - | |
| Six months adherence | Good | 0.016 | 0.002-0.149 | 0 |
| Fair | 0.333 | 0.051-2.177 | 0.333 | |
| Poor | 1 | - | - | |
Table 2: Association of viral load with adherence of study subjects attending ART clinics at Gondar, North West Ethiopia
Figure 3: Dolutegravir based therapy has a significant effect on viral load across WHO clinical stage among HIV positive individuals in Gondar,
Ethiopia-histogram showing the rate of viral load suppression (copies/ml/months) under-WHO clinical stage of HIV between the different periods following ART
Note: Friedman test was used to compute the difference in viral load counts between the baseline, 3rd, and 6th months of follow-up period under
WHO clinical stage. *statistically significant at p<0.05, **statistically significant at p<0.01, ***statistically significant at p<0.001.
CD4+ T-cell recovery
Of 109 participants, the median baseline, 3rd, and 6th months of CD4+ T-cell count were 209 (IQR: 81.5-417.5), 291 (IQR: 132-522), 378 (IQR: 181.-632.5), cells/µL, respectively. Among WHO clinical stage one, the median baseline, 3rd, and 6th months of CD4+ T-cell count was 370.5 (IQR: 229- 480.8), 501.5 (IQR: 317.8-634), 571 (IQR: 391.8-747.5), cells/ µL, re spectively.
DTG based therapy has effects on CD4+ T cell counts
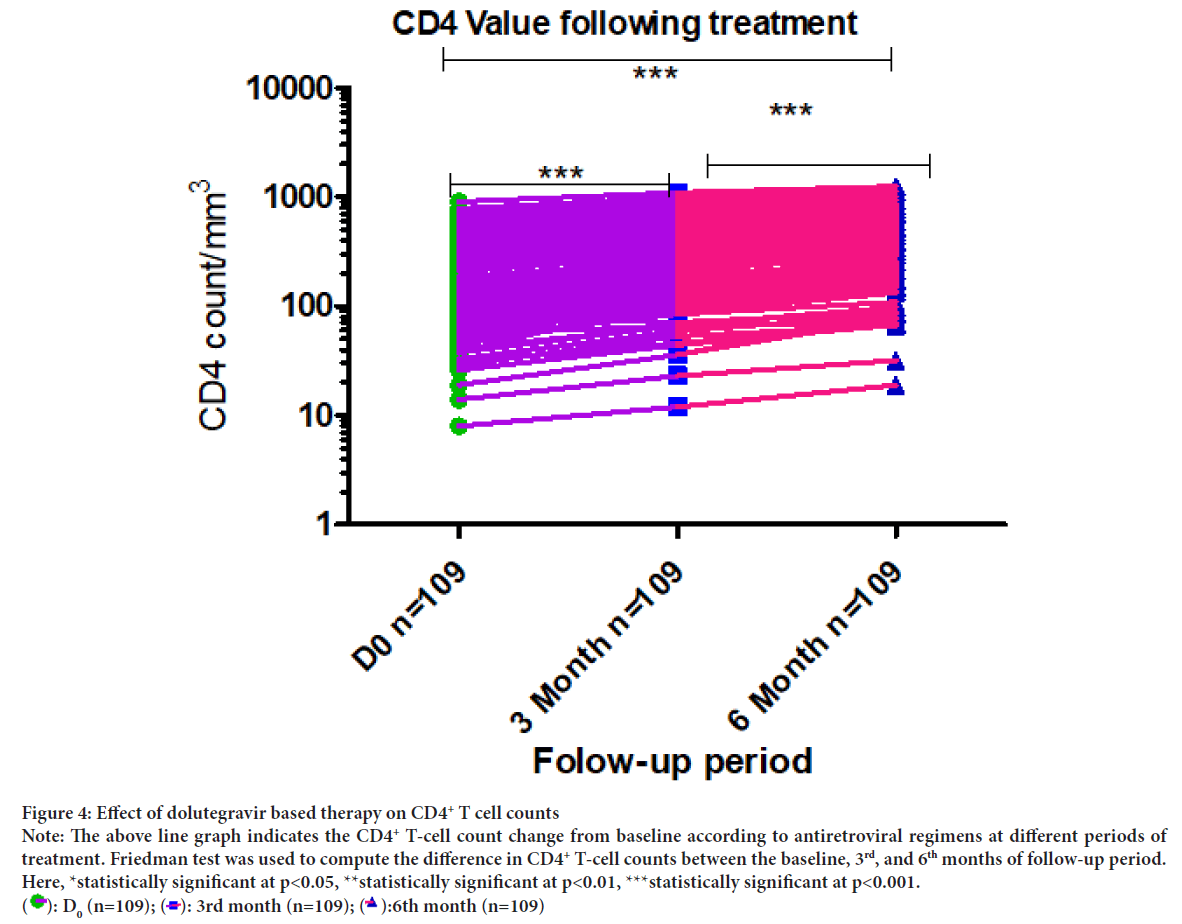
A good CD4+ T-cell counts recovery was observed between baseline and 3rd, and 6th months of follow up periods (p<0.001) (Figure 4).
Figure 4: Effect of dolutegravir based therapy on CD4+ T cell counts
Note: The above line graph indicates the CD4+ T-cell count change from baseline according to antiretroviral regimens at different periods of
treatment. Friedman test was used to compute the difference in CD4+ T-cell counts between the baseline, 3rd, and 6th months of follow-up period.
Here, *statistically significant at p<0.05, **statistically significant at p<0.01, ***statistically significant at p<0.001.  : D0 (n=109);
: D0 (n=109);  : 3rd month (n=109);
: 3rd month (n=109);  :6th month (n=109)
:6th month (n=109)
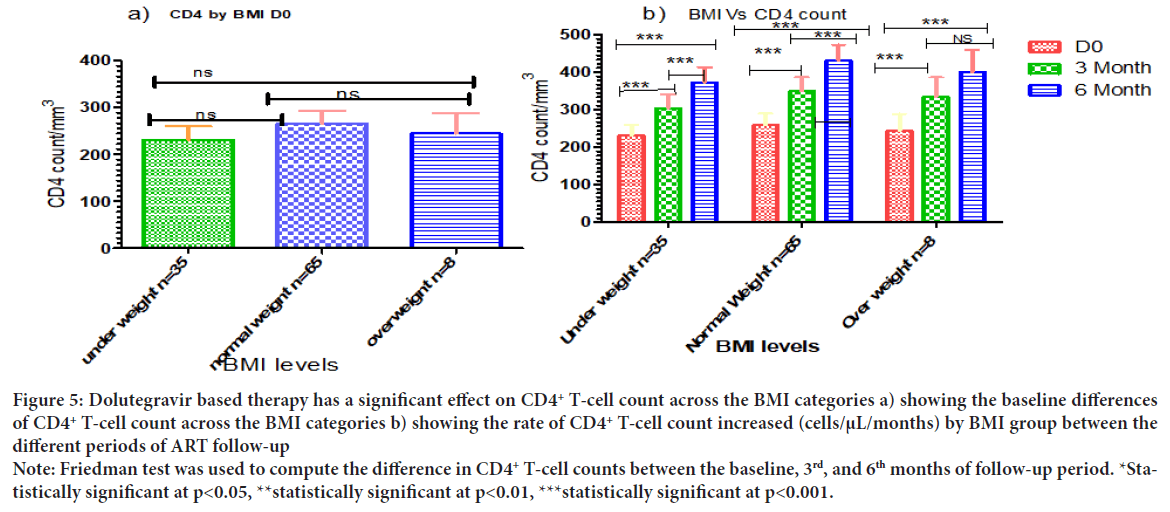
Dolutegravir based therapy has a significant effect on CD4+ T-cell count across the BMI categories. Similarly with viral load count, there was no significant association in CD4+ T-cell counts at base line comparing across all BMI categories (Figure 5a). In contrast, a good CD4+ T-cell counts recovery was observed between baseline and 6th months follow-up of CD4+ T-cell count across all the BMI categories (p<0.001) (Figure 5b).
Figure 5: Dolutegravir based therapy has a significant effect on CD4+ T-cell count across the BMI categories a) showing the baseline differences
of CD4+ T-cell count across the BMI categories b) showing the rate of CD4+ T-cell count increased (cells/μL/months) by BMI group between the
different periods of ART follow-up
Note: Friedman test was used to compute the difference in CD4+ T-cell counts between the baseline, 3rd, and 6th months of follow-up period. *Statistically significant at p<0.05, **statistically significant at p<0.01, ***statistically significant at p<0.001.
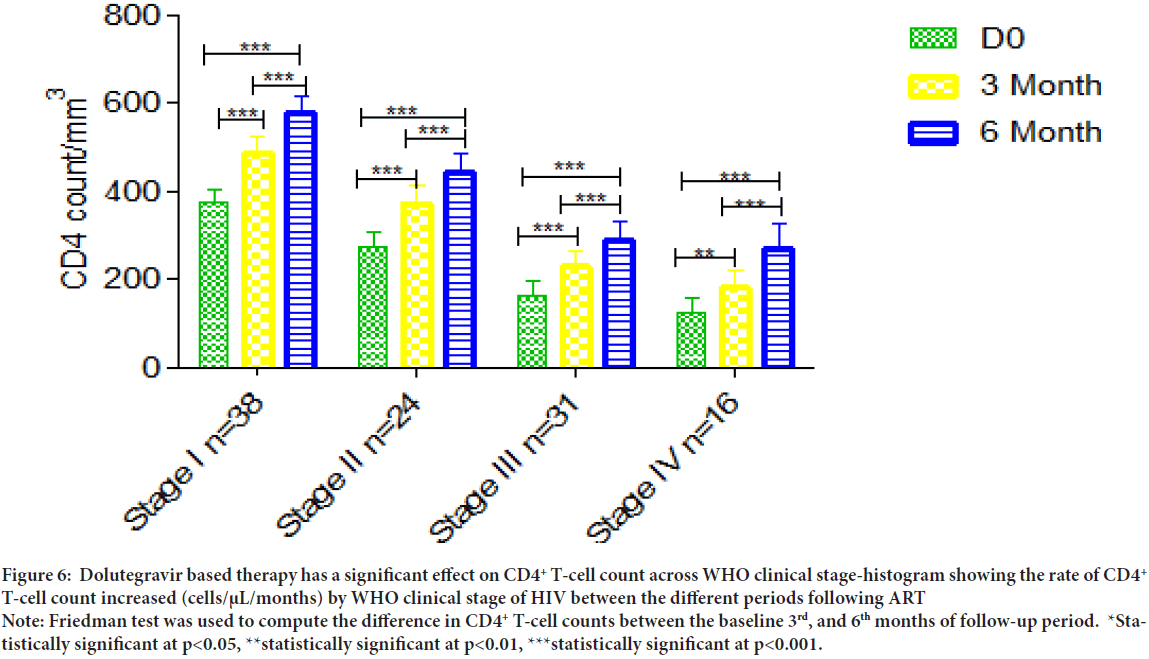
CD4+ T-cell count recovery across WHO clinical stage
A decent CD4+ T-cell counts changing was observed between 3rd and 6th months follow-up treatment of CD4+ T-cell count under-all WHO clinical stage (p<0.001) (Figure 6).
Figure 6: Dolutegravir based therapy has a significant effect on CD4+ T-cell count across WHO clinical stage-histogram showing the rate of CD4+ T-cell count increased (cells/μL/months) by WHO clinical stage of HIV between the different periods following ART
Note: Friedman test was used to compute the difference in CD4+ T-cell counts between the baseline 3rd, and 6th months of follow-up period. *Statistically significant at p<0.05, **statistically significant at p<0.01, ***statistically significant at p<0.001.
Discussion
The viral load count indicates the activity of HIV infection and hence viral load count is a useful blood test to tell us how much HIV is in the blood. Over time, the viral load increases as more and more viruses are produced, resulting in rapid progression to AIDS (WHO, 2018).
Dolutegravir based regimen therapy is an efficient, well-tolerated drug, and associated with decreasing viral RNA copies (Carbone A, et al., 2014). As revealed by previous studies (Brehm TT, et al., 2019; Gillman J, et al., 2019, Fantauzzi A and Mezzaroma I, 2014; de Vito A, et al., 2019; Correa A, et al., 2020), 91.7% individuals with plasma HIV RNA less than 50 copies/mL is increased sharply from baseline to 6th month. The proportion of participants achieving plasma HIV-1 RNA less than 50 copies/mL were 79.8% (95% CI: 71.6-87.2), and 91.7% (95% CI: 86.2-96.3) at 3rd and 6th
months, respectively. There was a statistically significant difference in viral load suppression between baseline and 3rd and 6th months of follow-up (p<0.001). These results highlight the tremendous accomplishments of the widespread implementation of the treatment, which has led to significant health benefits and an increase in life expectancy (Correa A, et al., 2020).
In contrast to the Italian investigation, which revealed that 81% of individuals had viral suppression of 50 copies/mL during the six months of follow-up, we discovered lower levels of viral RNA copies (de Vito A, et al., 2019). In a related study done in French, it was discovered that 3 months after starting a Dolutegravir-based treatment, 58% of participants had viral suppression that was fewer than 50 copies/mL (de Miguel R, et al., 2020). This discrepancy between findings may be caused by differences in geographic location or genetic make-up.
The DTG-based regimen showed a rise in CD4+ T-cell count in patients with significant viremia, demonstrating its efficacy in naive individuals. In addition, we demonstrated that the median CD4+ T-cell count at baseline was 209 (IQR: 81.5-417.5), cells/L, corresponding to a 28 cells/L monthly recovery rate. This was also comparable with a Brazilian survey (Correa A, et al., 2020).
During follow-ups at the third and sixth months of DTG-based regimen treatment, higher median CD4+ T-cell counts were seen in the current study. The median CD4+ T-cell count at baseline was 209 cells/L (IQR: 81.5- 417.5), and at the third and sixth months, it was 291 cells/L (IQR: 132-522) and 378 cells/L (IQR: 181-632.5), respectively (p=0.001). Comparably, a study done in Germany showed that, based on a median CD4+ T-cell count of 258, the obtained cells at the third and sixth months were 345 and 465 cells/L, respectively (Brehm TT, et al., 2019). Similar findings were found in a study on dolutegravir-based therapy carried out in the United States, which showed that the median (interquartile range) increase in CD4+ T-cell counts from baseline at six months was 167 (86, 275) cells/L (Taiwo BO, et al., 2018).
Furthermore, severe immune suppression may continue in some patients, particularly those who began ART with a very low CD4 cell count and who do not experience a significant improvement in their CD4 cell count after treatment (Teshome W and Assefa A, 2014). However, like the study conducted in Kenya (Ilovi CS, et al., 2011), the median CD4+ T-cell counts changing across all WHO clinical stages in our study clearly exhibited statistical significance (p=0.001).
A WHO recommendation states that sustained high levels of adherence are necessary to stop viral replication and reduce the risk of developing antiretroviral treatment resistance (WHO, 2016). Additionally, we found a significant association between viral load and ART adherence (p=0.000). Interestingly, we found that poor ART adherence had predictors of firstline ART treatment failure (p=0.000). Research conducted in Kenya and Ethiopia provide additional evidence in favor of this (Teshome W and Assefa A, 2014; Ilovi CS, et al., 2011).
Likewise, the absence of viral suppression along with immunologic, clinical, or other deterioration is a symptom of therapeutic failure (Vitoria M, et al., 2016). It is interesting to note that between baseline and the third and sixth months of follow-up, the viral load reduced statistically significantly across all WHO clinical stages. The studies carried out in Kenya and Ethiopia (Teshome W and Assefa A, 2014) offered additional proof of this (Ilovi CS, et al., 2011). Also, research undertaken in Switzerland and the Island revealed a correlation between the WHO clinical stage and treatment failure (Teshome W and Assefa A, 2014, Vitoria M, et al., 2016).
Low body mass, weight loss, and HIV disease progression are all independently risk factors for mortality, according to WHO guidelines. Therefore, nutritional supplements may be necessary for HIV-infected individuals who are malnourished in addition to ART to aid in their nutritional recovery (WHO, 2016). In our investigation, we discovered a statistically significant difference in viral load suppression between baseline, third, and sixth months of treatment with all BMI levels (p=0.001). Likewise, According to studies done in the United States, China, and Island, HIV-infected people who are overweight or obese had lower viral load levels than those who are normal weight or have low baseline BMI (Teshome W and Assefa A, 2014; Safren SA, 2014). Since there was only one obese individual in our study and Leptin may play a role in the mechanism connecting fat and overweight to immunological function in HIV-infected people, which may account for the difference. An adipocyte-derived hormone that affects body weight is leptin. Serum leptin levels have been discovered to be greater in overweight and obese people than in people of normal weight (Safren SA, 2014).
Conclusion
The findings of our study clearly demonstrate that for HIV-infected people who have never received treatment, Dolutegravir-based therapy is a viable treatment option with high rates of virological suppression and a median change in CD4+ T-cell count. Viral load suppression rates were high among on ART patients. We suggest that additional research including a large participant pool associated with various underline clinical condition and comparison analysis need to be conducted.
References
- Brehm TT, Franz M, Hüfner A, Hertling S, Schmiedel S, Degen O, et al. Safety and efficacy of elvitegravir, dolutegravir, and raltegravir in a real-world cohort of treatment-naïve and-experienced patients. Medicine. 2019; 98(32).
[Crossref] [Google Scholar] [Pubmed]
- Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: Recommendations for a public health approach. WHO. 2016.
- Llibre JM, Cahn PE, Lo J, Barber TJ, Mussini C, van Welzen BJ, et al. Changes in inflammatory and atherogenesis biomarkers with the 2-drug regimen dolutegravir plus lamivudine in antiretroviral therapy-experienced, virologically suppressed people with HIV-1: A systematic literature review. Open Forum Infect Dis. 2022; 9(4):ofac068.
[Crossref] [Google Scholar] [Pubmed]
- Vitoria M, Hill A, Ford N, Doherty M, Clayden P, Venter F, et al. The transition to dolutegravir and other new antiretrovirals in low-income and middle-income countries: What are the issues? AIDS. 2018; 32(12): 1551-1561.
[Crossref] [Google Scholar] [Pubmed]
- Omondi FH, Sudderuddin H, Shahid A, Kinloch NN, Jones BR, Miller RL, et al. HIV proviral burden, genetic diversity, and dynamics in viremic controllers who subsequently initiated suppressive antiretroviral therapy. MBio. 2021; 12(6): e02490-21.
[Crossref] [Google Scholar] [Pubmed]
- Vitoria M, Hill AM, Ford NP, Doherty M, Khoo SH, Pozniak AL. Choice of antiretroviral drugs for continued treatment scale‐up in a public health approach: What more do we need to know? J Int AIDS Soc. 2016; 19(1): 20504.
[Crossref] [Google Scholar] [Pubmed]
- Morón-López S, Navarro J, Jimenez M, Rutsaert S, Urrea V, Puertas MC, et al. Switching from a protease inhibitor-based regimen to a dolutegravir-based regimen: A randomized clinical trial to determine the effect on peripheral blood and ileum biopsies from antiretroviral therapy-suppressed human immunodeficiency virus-infected individuals. Clin Infect Dis. 2019; 69(8): 1320-1328.
[Crossref] [Google Scholar] [Pubmed]
- Gillman J, Janulis P, Gulick R, Wallis CL, Berzins B, Bedimo R, et al. Comparable viral decay with initial dolutegravir plus lamivudine versus dolutegravir-based triple therapy. J Antimicrob Chemother. 2019; 74(8): 2365-2369.
[Crossref] [Google Scholar] [Pubmed]
- Ndashimye E, Avino M, Olabode AS, Poon AF, Gibson RM, Li Y, et al. Accumulation of integrase strand transfer inhibitor resistance mutations confers high-level resistance to dolutegravir in non-B subtype HIV-1 strains from patients failing raltegravir in Uganda. J Antimicrob Chemother. 2020; 75(12): 3525-3533.
[Crossref] [Google Scholar] [Pubmed]
- Yang X, Su B, Zhang X, Liu Y, Wu H, Zhang T. Incomplete immune reconstitution in HIV/AIDS patients on antiretroviral therapy: Challenges of immunological non‐responders. J Leukoc Biol. 2020; 107(4): 597-612.
[Crossref] [Google Scholar] [Pubmed]
- de Miguel R, Rial-Crestelo D, Dominguez-Dominguez L, Montejano R, Esteban-Cantos A, Aranguren-Rivas P, et al. Dolutegravir plus lamivudine for maintenance of HIV viral suppression in adults with and without historical resistance to lamivudine: 48-week results of a non-randomized, pilot clinical trial (ART-PRO). EBioMedicine. 2020; 55: 102779.
[Crossref] [Google Scholar] [Pubmed]
- Marziali M, de Santis W, Carello R, Leti W, Esposito A, Isgrò A, et al. T-cell homeostasis alteration in HIV-1 infected subjects with low CD4 T-cell count despite undetectable virus load during HAART. AIDS. 2006; 20(16): 2033-2041.
[Crossref] [Google Scholar] [Pubmed]
- Guidance for industry human immunodeficiency virus-1 infection : Developing antiretroviral drugs for treatment guidance for industry human immunodeficiency virus-1 infection: Developing antiretroviral drugs for treatment. Food and drug Administration (FDA). 2015.
- National consolidated guidelines for comprehensive HIV prevention, care and treatment. WHO. 2018.
- Weir CB, Jan A. BMI classification percentile and cut off points. Statpearls. 2022.
- BD FACSPresto TM Cartridge: 100 tests-catalogue No. 657681. BD Biosciences. 2016.
- COBAS® AmpliPrep/COBAS® TaqMan® HIV-1 Test, v2.0. Roche Diagnostics. 2023.
- Carbone A, Lazzarin A, Castagna A. Dolutegravir: A new option for HIV treatment. Future Virol. 2014; 9(9): 801-810.
- Fantauzzi A, Mezzaroma I. Dolutegravir: Clinical efficacy and role in HIV therapy. Ther Adv Chronic Dis. 2014; 5(4): 164-177.
[Crossref] [Google Scholar] [Pubmed]
- de Vito A, Caruana G, Clark F, Nunnari G, Pellicanò G, Angioni G, et al. Efficacy, safety and tolerability of dolutegravir-based combination antiretroviral therapy in clinical practice in HIV-infected patients: Results from a multicenter study. Infect Dis Trop Med. 2019; 5: e565.
- Correa A, Monteiro P, Calixto F, Batista JD, de Alencar Ximenes RA, Montarroyos UR. Dolutegravir: Virologic response and tolerability of initial antiretroviral regimens for adults living with HIV. PLoS One. 2020; 15(8): e0238052.
[Crossref] [Google Scholar] [Pubmed]
- Taiwo BO, Zheng L, Stefanescu A, Nyaku A, Bezins B, Wallis CL, et al. ACTG A5353: A pilot study of dolutegravir plus lamivudine for initial treatment of human immunodeficiency virus-1 (HIV-1)-infected participants with HIV-1 RNA<500000 copies/mL. Clin Infect Dis. 2018; 66(11): 1689-1697.
[Crossref] [Google Scholar] [Pubmed]
- Teshome W, Assefa A. Predictors of immunological failure of antiretroviral therapy among HIV infected patients in Ethiopia: A matched case-control study. PloS One. 2014; 9(12): e115125.
[Crossref] [Google Scholar] [Pubmed]
- Ilovi CS, Lule GN, Obel O, Irimu M. Correlation of WHO clinical staging with CD4 counts in adult HIV/AIDS patients at Kenyatta National Hospital, Nairobi. East Afr Med J. 2011; 88(2): 65-70.
[Google Scholar] [Pubmed]
- Maldonado-Martínez G, Hunter-Mellado RF, Fernández-Santos D, Ríos-Olivares E. Persistent HIV viremia: Description of a cohort of HIV infected individuals with ART failure in Puerto Rico. Int J Environ Res Public Health. 2016; 13(1): 50.
[Crossref] [Google Scholar] [Pubmed]
- Safren SA. Infected men who have sex with men. 2014;12(5):319-24.
Author Info
Teshager Gebremedhin1, Melak Ayenalem2, Mohammed Adem3, Demeke Geremew4, Yetemwork Aleka3* and Amare Kiflie32Department of Hematology and Immunohematology, University of Gondar, Gondar, Ethiopia
3Department of Immunology and Molecular Biology, University of Gondar, Gondar, Ethiopia
4Department of Medical Laboratory Sciences, Immunology and Molecular Biology, Bahir Dar University, Bahir Dar, Ethiopia
Citation: Gebremedhin T: Dolutegravir Based Therapy Showed CD4+ T Cell Count Recovery and Viral Load Suppression among Art Naive HIV Positive Individuals: A Longitudinal Evaluation
Received: 20-Mar-2023 Accepted: 04-Apr-2023 Published: 11-Apr-2023, DOI: 10.31858/0975-8453.14.4.264-271
Copyright: This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
ARTICLE TOOLS
- Dental Development between Assisted Reproductive Therapy (Art) and Natural Conceived Children: A Comparative Pilot Study Norzaiti Mohd Kenali, Naimah Hasanah Mohd Fathil, Norbasyirah Bohari, Ahmad Faisal Ismail, Roszaman Ramli SRP. 2020; 11(1): 01-06 » doi: 10.5530/srp.2020.1.01
- Psychometric properties of the World Health Organization Quality of life instrument, short form: Validity in the Vietnamese healthcare context Trung Quang Vo*, Bao Tran Thuy Tran, Ngan Thuy Nguyen, Tram ThiHuyen Nguyen, Thuy Phan Chung Tran SRP. 2020; 11(1): 14-22 » doi: 10.5530/srp.2019.1.3
- A Review of Pharmacoeconomics: the key to “Healthcare for All” Hasamnis AA, Patil SS, Shaik Imam, Narendiran K SRP. 2019; 10(1): s40-s42 » doi: 10.5530/srp.2019.1s.21
- Deuterium Depleted Water as an Adjuvant in Treatment of Cancer Anton Syroeshkin, Olga Levitskaya, Elena Uspenskaya, Tatiana Pleteneva, Daria Romaykina, Daria Ermakova SRP. 2019; 10(1): 112-117 » doi: 10.5530/srp.2019.1.19
- Dental Development between Assisted Reproductive Therapy (Art) and Natural Conceived Children: A Comparative Pilot Study Norzaiti Mohd Kenali, Naimah Hasanah Mohd Fathil, Norbasyirah Bohari, Ahmad Faisal Ismail, Roszaman Ramli SRP. 2020; 11(1): 01-06 » doi: 10.5530/srp.2020.1.01
- Manilkara zapota (L.) Royen Fruit Peel: A Phytochemical and Pharmacological Review Karle Pravin P, Dhawale Shashikant C SRP. 2019; 10(1): 11-14 » doi: 0.5530/srp.2019.1.2
- Pharmacognostic and Phytopharmacological Overview on Bombax ceiba Pankaj Haribhau Chaudhary, Mukund Ganeshrao Tawar SRP. 2019; 10(1): 20-25 » doi: 10.5530/srp.2019.1.4
- A Review of Pharmacoeconomics: the key to “Healthcare for All” Hasamnis AA, Patil SS, Shaik Imam, Narendiran K SRP. 2019; 10(1): s40-s42 » doi: 10.5530/srp.2019.1s.21
- A Prospective Review on Phyto-Pharmacological Aspects of Andrographis paniculata Govindraj Akilandeswari, Arumugam Vijaya Anand, Palanisamy Sampathkumar, Puthamohan Vinayaga Moorthi, Basavaraju Preethi SRP. 2019; 10(1): 15-19 » doi: 10.5530/srp.2019.1.3