Research Article - (2022) Volume 13, Issue 12
Abstract
Echinacea purpurea extract, one of the main compounds of which is caffeic acid, has anti-cancer and anti-angiogenic properties. In the present study, the anticancer effect of E. purpurea’s extract was investigated on the growth of gastric cancer cell line AGS, induction of apoptosis and inhibition of cell migration, tube formation and colony formation as well as the expression level of the VEGF-A gene. E. purpurea’s extract was extracted using a Soxhlet device and HPLC chromatography was used to confirm the presence of caffeic acid in the extract. The result showed that the cell viability percentage as well as the formation of colonies in AGS cancer cells after treatment of the plant extract were reduced in a dosedependent manner. The orange-red fluorescence after treatment was increased indicating the emergence of apoptosis. The assessment of the wound healing area showed no significant differences between 24 and 48 h compared to 0 h in the treatment groups (P>0.01). The expression level of the VEGF-A gene at a concentration of 2.0, 2.75, and 3.25 mg/mL of the plant extract was reduced by 0.91, 0.87, and 0.85 fold, respectively. Total tube length and number of branches in treatment groups were significantly reduced in a dose-dependent manner. E. purpurea’s extract could effectively inhibit AGS gastric cancer cell proliferation, migration, colony formation, the formation of tubules, reduce VEGF-A expression and induce apoptosis.
Keywords
Angiogenesis, Caffeic acid, Cytotoxic, Echinacea purpurea, Gastric cancer
Introduction
Echinaceais a perennial plant from the Asteraceae family, and this genus contains a small number of species. Three of the species, Echinacea angustifolia, Echinacea pallida, and Echinacea purpureaare popularly used as an herbal medicine (Barnes J, et al., 2005).
The plant extract of Echinacea has shown immunostimulating, immune-regulator, antioxidant, anti-inflammatory, antivirus, antitumor, antiviral and antifungal activities. Echinaceaextract has been prescribed as a cancer chemotherapy supplement because of its protective effects on the immune system (Cichello SA, et al., 2016). The secondary metabolites of Echinaceainclude Cichoric acid, Echinacin, Cynarin Echinacoside, Chlorogenic acid, and Caffeic acid derivatives (Cichello SA, et al., 2016; Binns S, et al., 2002; Dalby-Brown L, et al., 2005; Driggins S, et al., 2017; Merali S, et al., 2003). Cichoric acid effectively induced apoptosis in colon cancer cells, characterized by DNA fragmentation, caspase-9 activation, PARP cleavage, and downregulation of β-catenin (Tsai YL, et al., 2012). Caffeic acid is an organic compound consists of both phenolic and acrylic functional groups. It is found in all plants with different values because it is a key intermediate in the biosynthesis of lignin (Boerjan W, et al., 2003). Caffeic acid has demonstrated to possess interesting biological activities and pharmacological properties including antioxidants, antithrombosis, antihypertensive, antifibrosis, antiviral, and anti-tumor (Bruni R, et al., 2018; Hudson EA, et al., 2000; Johnson AA, et al., 2004; Maurya DK and Devasagayam TP, 2010; Morton LW, et al., 2000; Park EH and Kahng JH, 1999; Sroka Z and Cisowski W, 2003).
Caffeic acid showed various potential pharmacological effects in vitroand in vivoand it’s an inhibitory effect on cancer cell proliferation has recently been established (Prasad RN, et al., 2011). Caffeic acid and it’s derivative, caffeic acid phenethyl ester showed inhibition of invasion, angiogenesis, and metastasis in different cancer (Jung JE, et al., 2007).
Caffeic acid with a relatively large amount can be found in E. purpurea which extensively as a medicinal plant used in many treatments in Europe and North America (Kumar K and Ramaiah S, 2011). In the previous study has been reported caffeic acid have antioxidant properties in normal cells and pro-oxidant in cancer cells. Due to its pro-oxidant properties, it is able to cause oxidative damage in DNA and finally induction of apoptosis in cancer cells (Kanimozhi G and Prasad NR, 2015). Tumor development needs angiogenesis which stimulates using angiogenic inducers such as Vascular Endothelial Growth Factor (VEGF).VEGF had a crucial function in angiogenesis and tumor progression (Ferrara N, 2005). VEGF could suppress using the type of polyphenolic compounds including caffeic acid (Balan B, et al., 1999; Maksimenko A, et al., 2014).
Stomach cancer is the second cause of death from cancer and the third most common cancer in the world with the high mortality rate in central, south and east of Asia; east and central of Europe and South America (Han H, et al., 2010; Takahashi T, et al., 2013). This disease is diagnosed at advanced stage and treatment options are limited therefore still is the medical challenges (Takahashi T, et al., 2013).
In this study, the effects of toxicity of E. purpurea extract from Iranian species on growth prevention in a gastric cancer cell line (AGS), inhibition of cell invasion and colony formation, inducing of apoptosis as well as the expression of VEGF-Agene, for the first time were evaluated.
Materials and Methods
Extraction of Echinacea purpurea’s extract
Echinacea purpureaplant was cultivated in National Institute of Genetic Engineering and Biotechnology. The seeds of E. purpurea were purchased from BazrCo (Tehran, Iran). Flowers leaves and stems of the plant were dried in the shade and then turned into powder. After that, the powder was placed in a soxhlet, with 70% ethanol at 60°C for 4 h. The crude extract was placed in a freeze dryer to remove the ethanol and powdered. Thus in this type of extraction, the sufficient amount of caffeic acid in the extract was more. The extract powder was stored at -20°C in dark. Finally, the extract powder was dissolved in DMSO and then filtered with a 0.2 µm filter. The plant extract was used due to the synergistic effects of the extract.
High Performance Liquid Chromatography (HPLC)
Since caffeic acid is one of the indicators of E. purpurea extract, it was chromatographed with standard caffeic acid. HPLC chromatography was used to confirm the presence of caffeic acid in the extract (KNAUER, Germany). In this experiment, the C18 column was used and the solvent of the mobile phase was acetonitrile/deionized water and trifluoroacetic acid 0.01% (70:30). The sample was eluted at a flow rate of 0.8 mL/min with 20 minutes of the retention time. The presence of caffeic acid in samples was confirmed by injection of each sample and drawing of chromatograms.
Determination of the amount of caffeic acid in the extract
The amount of caffeic acid in the extract was calculated based on the HPLC chromatogram for standard caffeic acid.
Cell lines and culture
Gastric cancer cell line AGS, as well as fibroblast normal cell (HFF), provided from National Institute of Genetic Engineering and Biotechnology (Iran). The cells were cultured in DMEM media with 10% FBS. Incubation was done in 5% CO2 incubator (BINDER, UK) at 37°C until they reached to 80% growth confluence then the cells counted and subculture in 96 well plates with 1 × 104 cells for each well.
MTT assay
After appropriate AGS cells growth, the different concentration of the extract (0, 2.0, 2.75, 3.25 mg/mL) was added to each well in triplicates. Incubation continued for 24 hours. For fibroblast normal cells, different concentration of the extract (0, 2.0, 2.75, 3.25 mg/mL) was added to each well in triplicates for 24 hours. After that MTT assay was done and absorption of the purple color of formazan crystal formation read at 580 nm using ELISA reader (Multiskan RC microplate reader of LabSystem, Vantaa, Finland).
Apoptosis assay
Apoptosis assay was performed using AO/EB double staining. Untreated cells (control) showed normal structure with AO green staining. 1 × 104 AGS cells were cultured in 96 well plates. After 24 hours of appropriate cell growth, the cells were treated with E. purpurea’s extract at a concentration of 0, 2, 2.75, and 3.25 mg/mL, respectively for 24 h. After incubation during 24 hrs, the medium was removed and washed with fresh PBS buffer. Then, 50 µl of PBS buffer containing 3 µg/mL of the AO/EB dye mix was added to each well and the apoptotic cells were observed under the fluorescent microscope at 32X magnification (ZEISS Axiovert 25, Germany).
Scratch wound healing assay in AGS cells
2 × 105 AGS cells were cultured for 24 hours on a 12-well plate containing DMEM with 10% FBS medium. After incubation and the cell growth reached until 80% confluency, DMEM was removed and the adherent cells layer was scratched with a sterile 100 μl pipette tip. The wells were washed with phosphate buffer saline (PBS) to remove the cellular debris. The cells were treated with 2.75 mg/mL concentration of E. purpurea’s extract (2.75 mg/mL). The positive control was considered only DMEM. The scratch area images were taken using a microscope (ZEISS Axiovert25, Germany) at 0, 24, and 48 hrs, and wound area analyzed with Image J version1.52v software. Migration rates were calculated as a part of the area at 24 and 48 compared to 0 hrs in both positive control and treatment. All experiments were repeated three times.
Colony formation assay
3 × 105 cells/well were seed on 6-well plates and incubated for 24 h. After incubation, the cells were treated with 2, 2.75, and 3.25 mg/mL concentration of E. purpurea’s extract at 24 hrs. After that, the cells were trypsinized and 5 × 102 cells cultured on 35 × 10 mm plates for 10 days, and once every 3 days, the media of the culture was changed. Then, the media was removed and washed with PBS and fixed with ethanol. The colonies were stained using 1% methylene blue. Colonies more than 50 cells were counted under a microscope. The experiment was repeated three times.
RNA extraction and quantitative real time PCR analysis
To determination of the gene expression level of VEGF-A, 5 × 105 cells/well of AGS were cultured into six-well plates and incubated for 24 hours. The cells were treated with the plant extract with a concentration of 2.0, 2.75 and 3.25 mg/mL for 24 hours. Total RNA was extracted from cells using the RNX-plus kit (SinaClon) according to the manufacturer’s instructions. cDNA synthesized using Maxime RT PreMix Kit (iNtRON, Korea). The primers used for real-time PCR were as follows-
Forward 5’-AACTTTCTGCTGTCTTGGGTG-3’ and reverse 5’-ATGTCCACCAGGGTCTCGATT-3’ for VEGF-A, and forward 5’-CCTGGGCATGGAGTCCTGT-3’ and reverse 5’-ATCTCCTTCTGCATCCTGTCG-3’ for the housekeeping gene (β-actin) as control.
The amplification was done using the SYBR green PCR master mix (Amplicon, Denmark). PCR conditions as follows for initial denaturation, 95°C for 15 min; followed 40 cycles of second denaturation 95°C for 15 s, annealing 62°C for 30 s and extension 62°C for 30 s and a final extension at 72°C for 5 min (Corbett, QIAGEN, Germany). The data was analyzed using the Rotor-Gene 6000 and Rest software and the graphs were performed by Excel.
Angiogenesis assay
Angiogenesis assay was performed to evaluate the effect of Echinacea purpurea extract on tube formation in Huvec cells. At first, type 1 collagen solution (Roche Germany), DMEM and 1 N sterile NaOH were mixed in a ratio of 8:1:1, respectively and 50 μl of the mixture poured into 96- well plates and incubated at 37°C for 2 hours. Then HUVEC cells (31,000 cells/well) in 50 μl DMEM were seeded on the collagen gel followed by the addition of 2, 2.75, and 3.25 mg/mL concentrations of Echinacea purpur ea extract, with or without conditioned medium (CM) derived from AGS cells (50 μl). The wells without treatment and conditioned medium were considered as negative control. The cells were incubated at 37°C for 18-20 hours. After incubation tube formation was observed, and photographed using light microscopy (ZEISS Axiovert 405M). Complete networks were counted and the number of branches and total tube lengths were analyzed using Image J software.
Statistical analysis
All data from the experiments in the treated cells with the plant extract as well as the control cells were compared using the Tukey test in One-way ANOVA analysis of SPSS 26 software and significant data were analyzed. P values <0.01 were considered to be significant.
Results
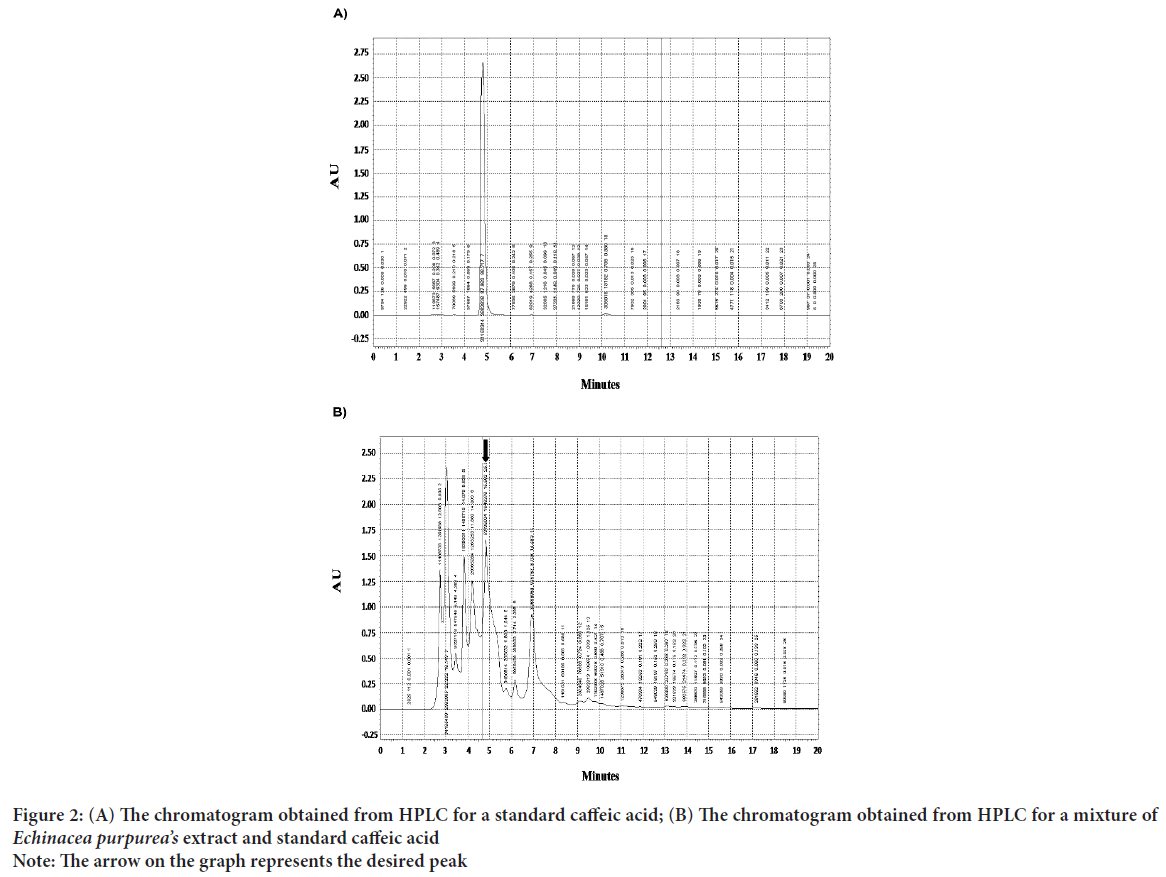
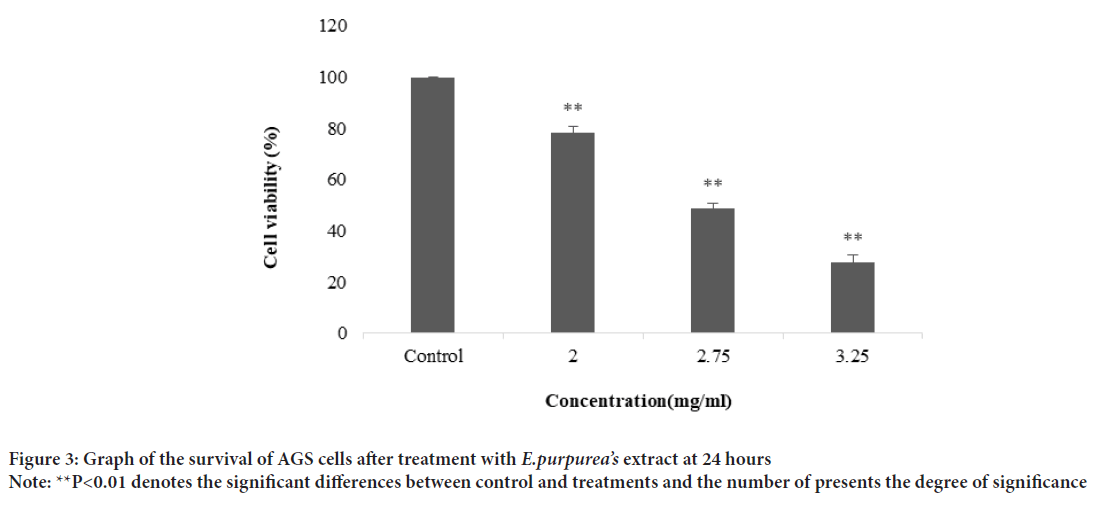
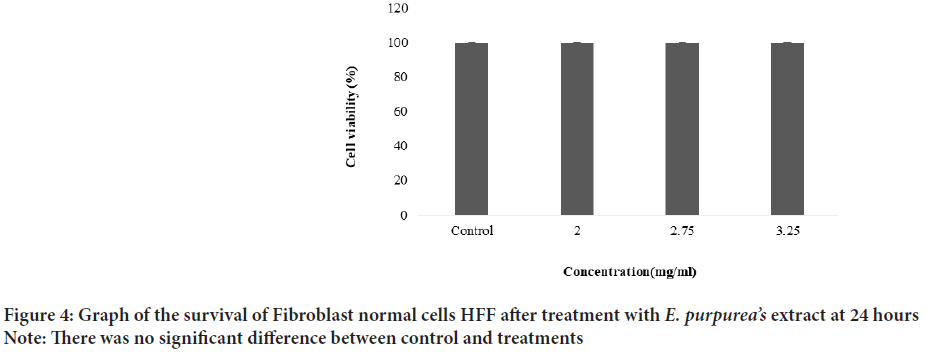
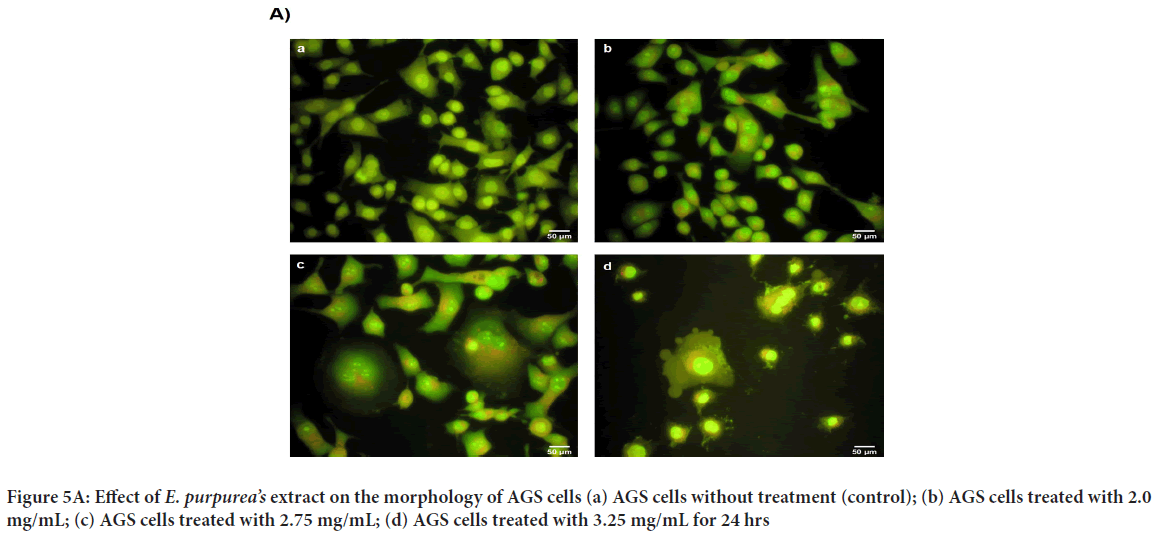
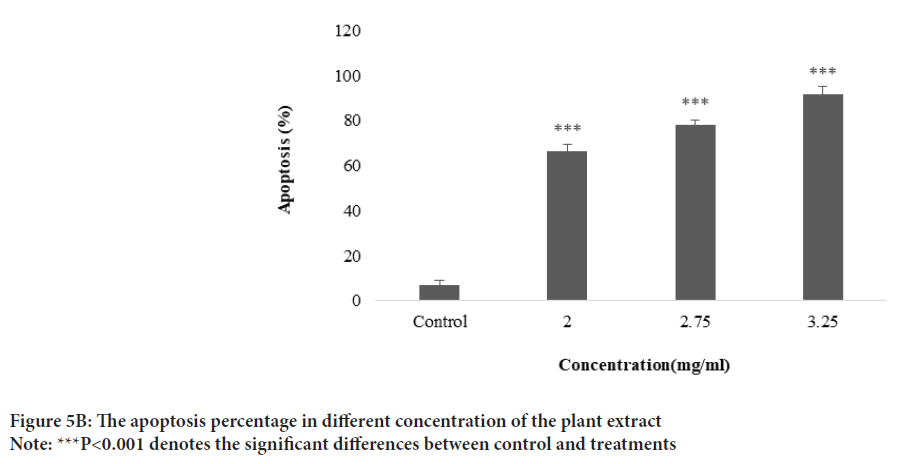
The plant extract and standard caffeic acid and a mixture of both them were injected into the HPLC and the graphs were drawn up. A comparison of the peaks and positions revealed that the extract was rich in caffeic acid (Figures 1 and 2). The caffeic acid concentration in 1 mg of the plant extract was 0.023 mg. The viability of AGS cells after treatment with different concentration of the E. purpurea’s extract showed that in a concentration of 2.0, 2.75 and 3.25 mg/mL was 78.3, 49.0 and 27.6%, respectively (Figure 3). The result of the effect of E. purpurea’s extract on fibroblast normal cells showed that the extracts didn’t inhibit the cell growth and no significant difference in cell growth between control and treatments (Figure 4). The effect of E. purpurea’s extract on the morphology of AGS cells and apoptosis showed that the control AGS cells were green under the view of a fluorescence microscope after staining with AO/EB. The color and morphology of the nucleus cells did not differ significantly. In AGS cells treated with the concentration of 2.0, 2.75, and 3.25 mg/mL, in a dose-dependent manner, the orange-red fluorescence was increased and indicating the emergence of apoptosis. Nuclear condensation and lysis occurred in the number of treated cells. Apoptotic bodies are also formed in these cells (Figure 5A). The percentage of apoptosis in different concentration of the plant extract (0, 2.0, 2.75, and 3.25 mg/mL) was 6.8%, 66.6%, 78.3%, and 91.6%, re spectively, which showed a significant difference compared to the control in a dose dependent manner (P<0. 01) (Figure 5B).
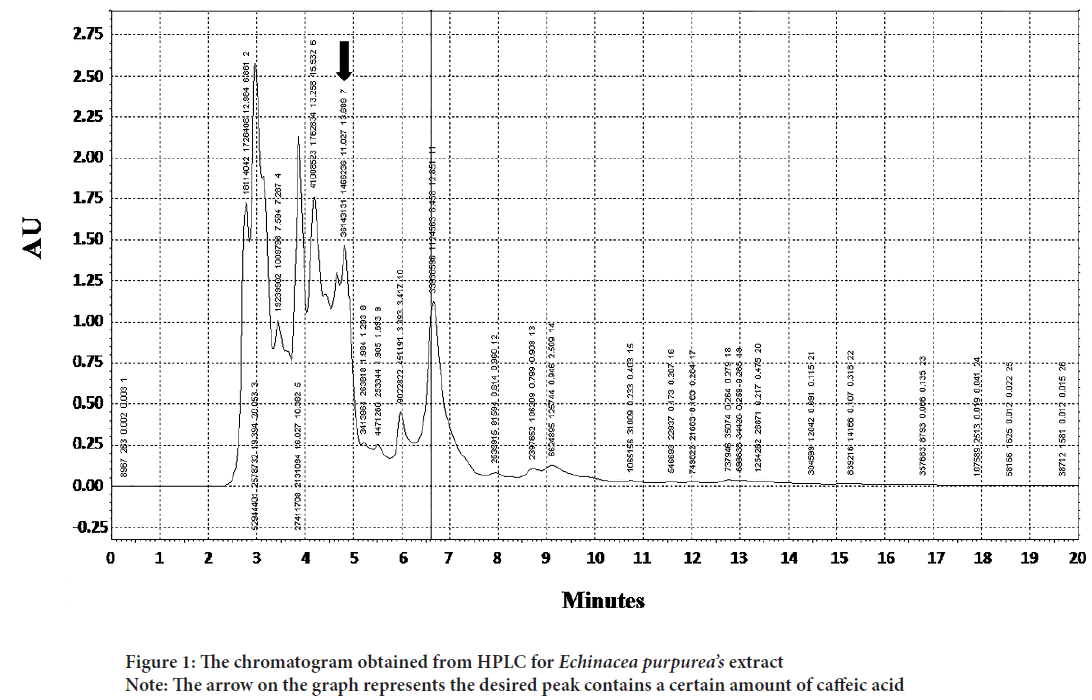
Figure 1: The chromatogram obtained from HPLC for Echinacea purpurea’s extract
Note: The arrow on the graph represents the desired peak contains a certain amount of caffeic acid
Figure 2: (A) The chromatogram obtained from HPLC for a standard caffeic acid; (B) The chromatogram obtained from HPLC for a mixture of Echinacea purpurea’s extract and standard caffeic acid
Note: The arrow on the graph represents the desired peak
Figure 3: Graph of the survival of AGS cells after treatment with E.purpurea’s extract at 24 hours
Note: **P<0.01 denotes the significant differences between control and treatments and the number of presents the degree of significance
Figure 4:Graph of the survival of Fibroblast normal cells HFF after treatment with E. purpurea’s extract at 24 hours
Note: There was no significant difference between control and treatments
Figure 5: Effect of E. purpurea’s extract on the morphology of AGS cells (a) AGS cells without treatment (control); (b) AGS cells treated with 2.0 mg/mL; (c) AGS cells treated with 2.75 mg/mL; (d) AGS cells treated with 3.25 mg/mL for 24 hrs
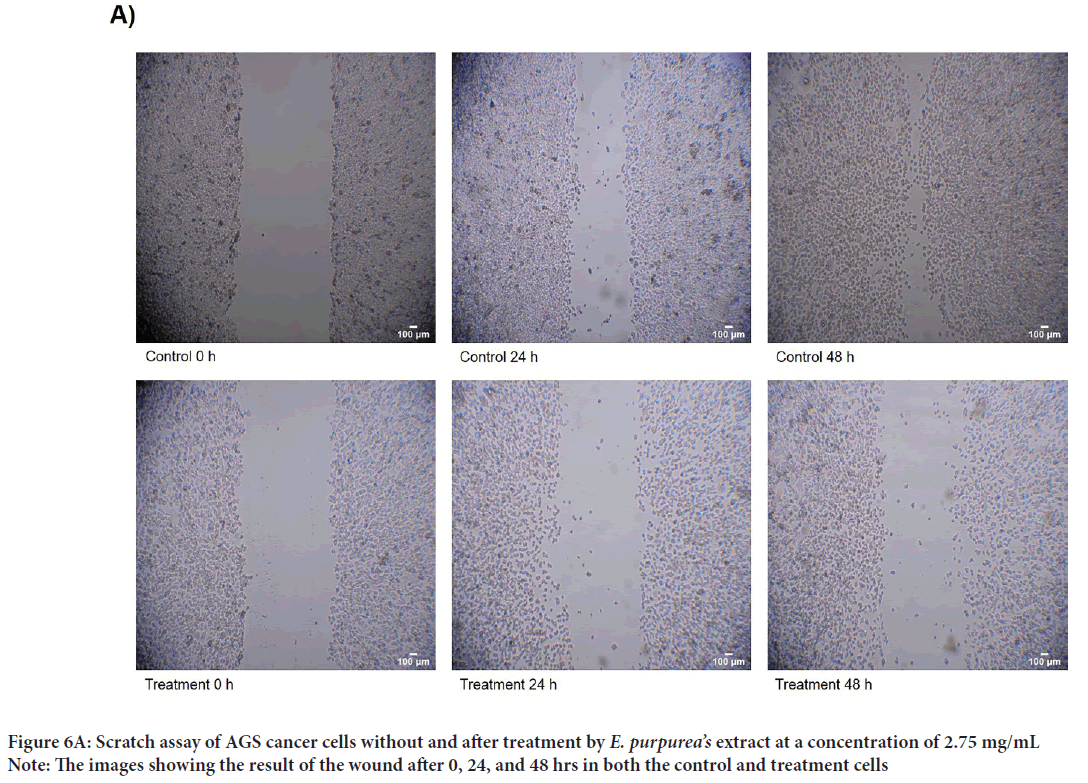
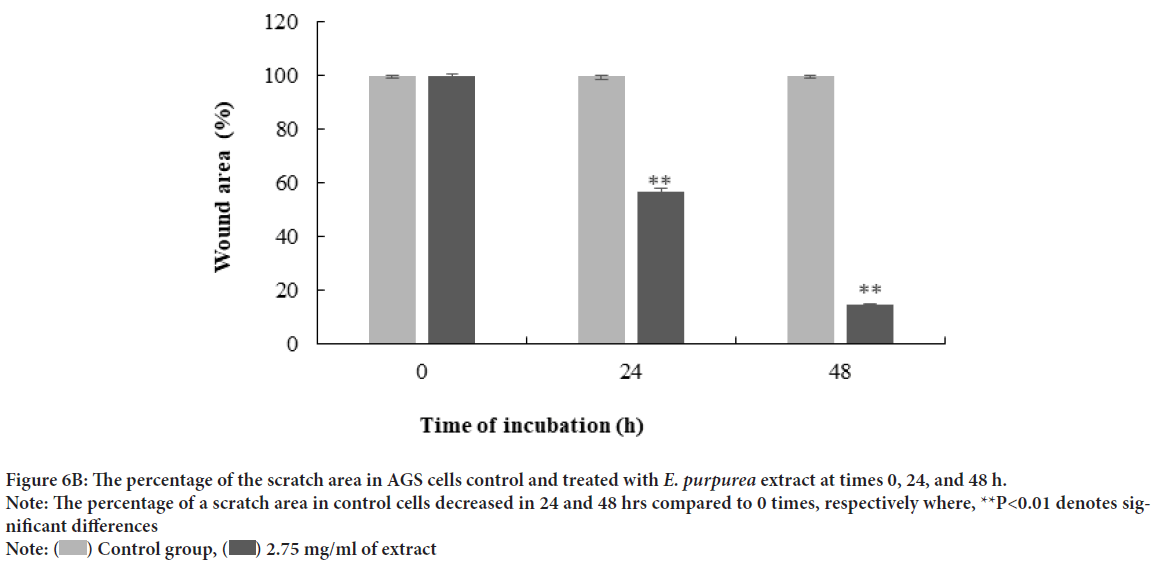
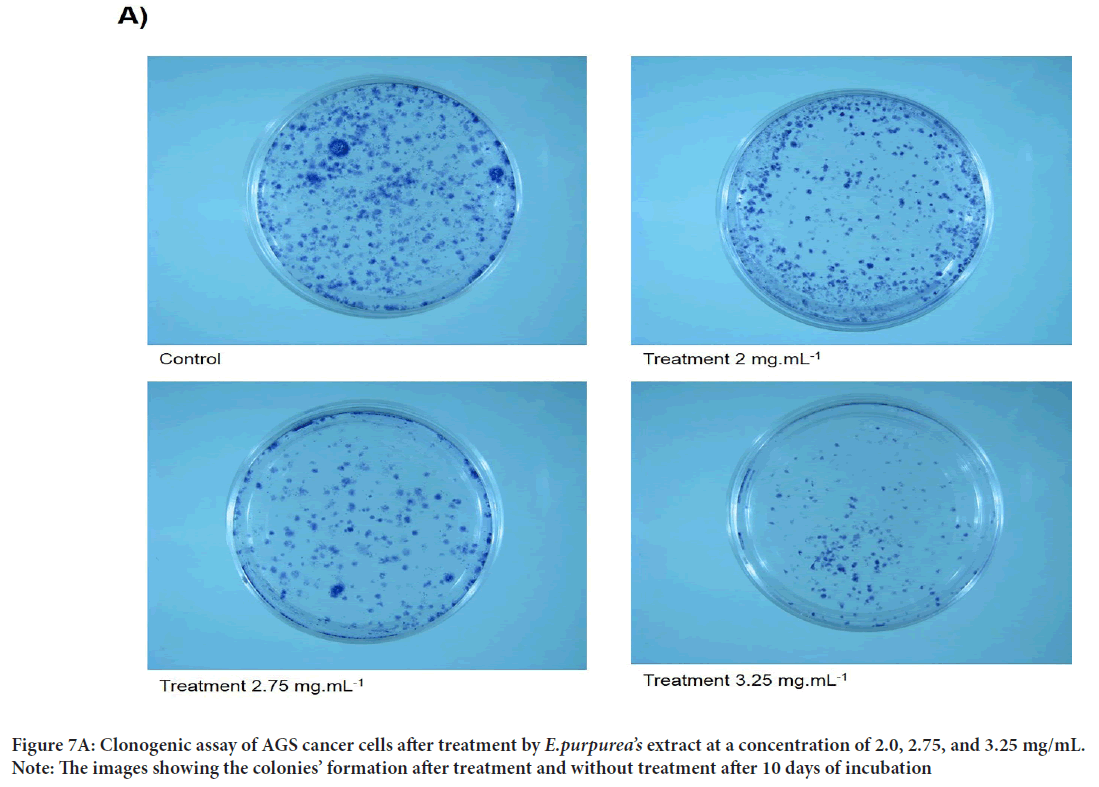
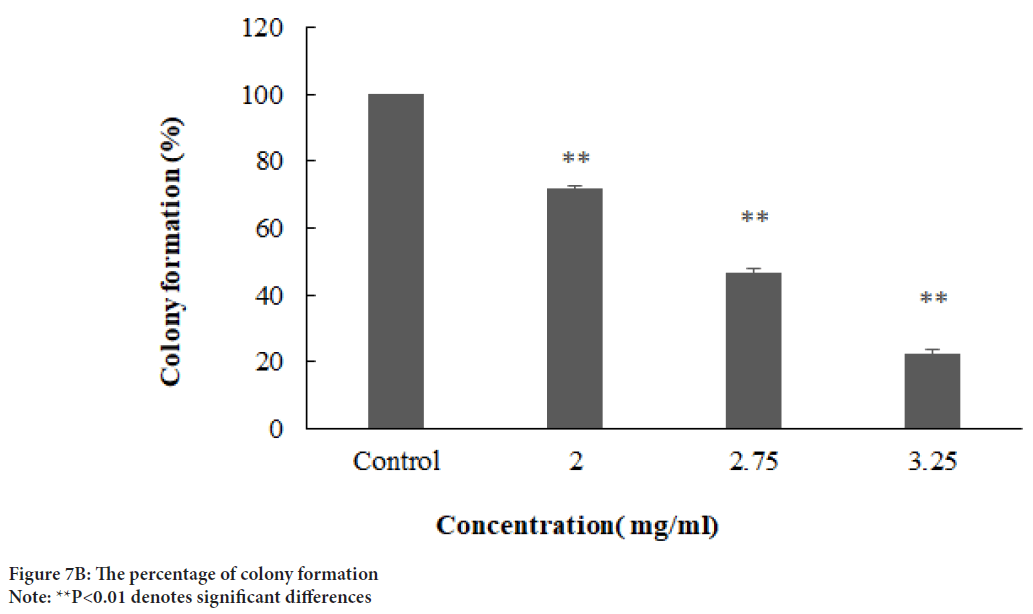
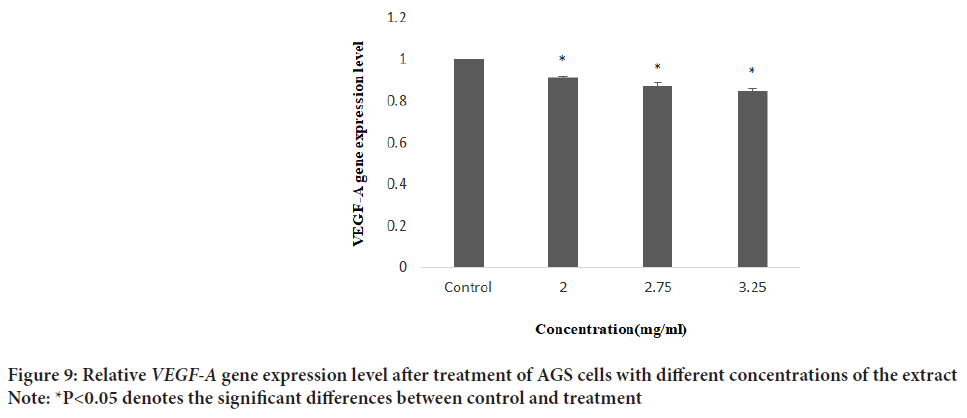
A scratch wound healing assay was used to evaluate cellular proliferation and invasion of AGS cells with E. purpurea’sextract. The assessment of the wound healing area after 0, 24, and 48 h in two positive control and treatment groups performed. The results showed no significant differences between 24 and 48 hrs compared to 0 h in the treatment groups (P>0.01). Whereas, in positive controls, the percentage of the scratch area was reduced to 57% and 15% after 24 and 48 hrs, respectively, compared to 0 h (P<0.01) (Figures 6A and 6B). One of the cytotoxic effect of E. purpurea’s extract is reducing the servival of cancer cells. Anti-clonogenic effect of E. purpurea’s extract show that after 10 days of treatment, E. purpurea’sextract supressed the formation of colonies in AGS cancer cells in a dose dependent manner. The treatments at 2, 2.75, and 3.25 mg/mL inhibited colony formation by 28%, 53.4%, and 77.7%, respectively. The results of statistical analysis at the level of P<0.01 indicated a significant difference between the treatment and control (Figures 7A and 7B). After the synthesis of cDNA, the amplification of the VEGF-Agene performed by PCR and the 179 bp bands amplified were observed in the agarose gel (Figure 8). According to Delta Delta C(T) (ΔΔCt) obtained after Real-time PCR, the level of VEGFgene expression at a concentration of 2.0, 2.75 and 3.25 mg/mL of E. purpurea’sextract were 0.91, 0.87 and 0.85 fold, respectively compared to control (Figure 9). The result showed that reducing the expression level was significantly different between 3.25 mg/mL concentration of extract and control (P<0.01).
Figure 5B: The apoptosis percentage in different concentration of the plant extract
Note: ***P<0.001 denotes the significant differences between control and treatments
Figure 6A: Scratch assay of AGS cancer cells without and after treatment by E. purpurea’s extract at a concentration of 2.75 mg/mL
Note: The images showing the result of the wound after 0, 24, and 48 hrs in both the control and treatment cells
Figure 6B: The percentage of the scratch area in AGS cells control and treated with E. purpurea extract at times 0, 24, and 48 h.
Note: The percentage of a scratch area in control cells decreased in 24 and 48 hrs compared to 0 times, respectively where, **P<0.01 denotes significant differences
Note:  Control group,
Control group,  2.75 mg/ml of extract
2.75 mg/ml of extract
Figure 7A: Clonogenic assay of AGS cancer cells after treatment by E.purpurea’s extract at a concentration of 2.0, 2.75, and 3.25 mg/mL.
Note: The images showing the colonies’ formation after treatment and without treatment after 10 days of incubation
Figure 7B: The percentage of colony formation
Note: **P<0.01 denotes significant differences
Figure 8: Gel agarose electrophoresis of the amplification of the VEGF-A gene by PCR after cDNA synthesis (M): Standard DNA molecular weight (100bp, SINACLON); Line 1: The cells without treatment as control; Lines 2,3 and 4: The 179 bp bands of amplification of VEGF-A gene after treatment with 2.0, 2.75, and 3.25 mg/mL of the extract, respectively
Figure 9: Relative VEGF-A gene expression level after treatment of AGS cells with different concentrations of the extract
Note: *P<0.05 denotes the significant differences between control and treatment
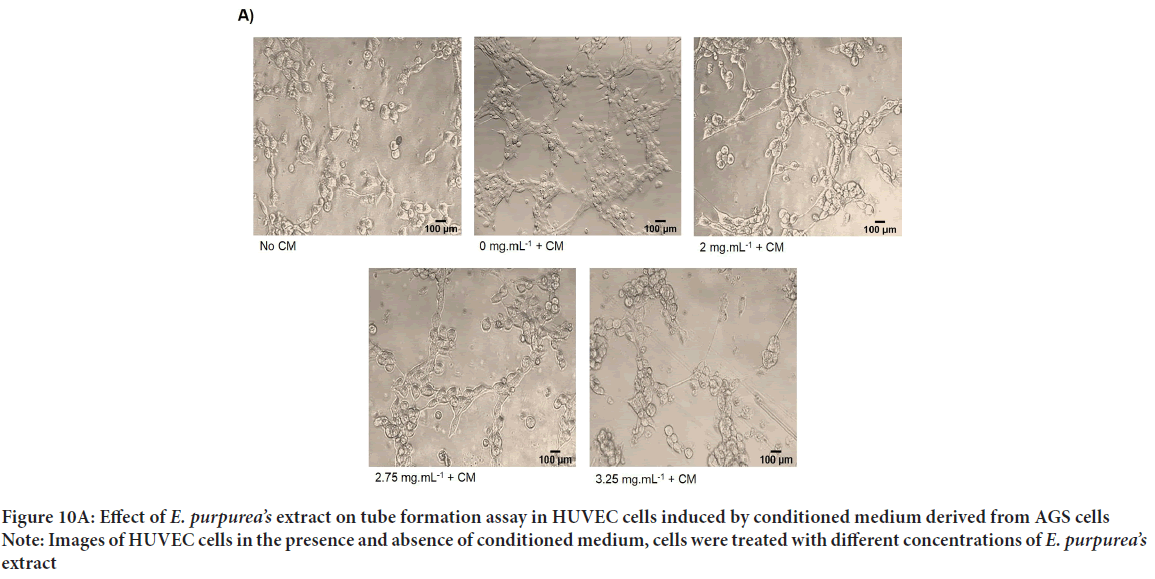
Percentage of tube formation in samples induced with conditioned medium and treated with concentrations of 2, 2.75, and 3.25 mg/mL of Echinacea purpurea’sextract compared to control samples decreased by 70.9%, 80.2%, and 91.7%, respectively. It has been found that this decrease was concentration dependent and significant (Figures 10A and 10B). In addition, the results obtained from the analysis with Image J software show a significant reduction of total tube length and number of branches in treatment group compared to control cells with a dose-dependent manner (Figure 10A). Our results revealed that conditioned medium derived from AGS cells induce tube formation of HUVEC cells and E. purpurea’sextract was able to inhibit the formation of tubules in induced HUVEC cells in a dose dependent manner.
Figure 10A: Effect of E. purpurea’s extract on tube formation assay in HUVEC cells induced by conditioned medium derived from AGS cells
Note: Images of HUVEC cells in the presence and absence of conditioned medium, cells were treated with different concentrations of E. purpurea’s extract
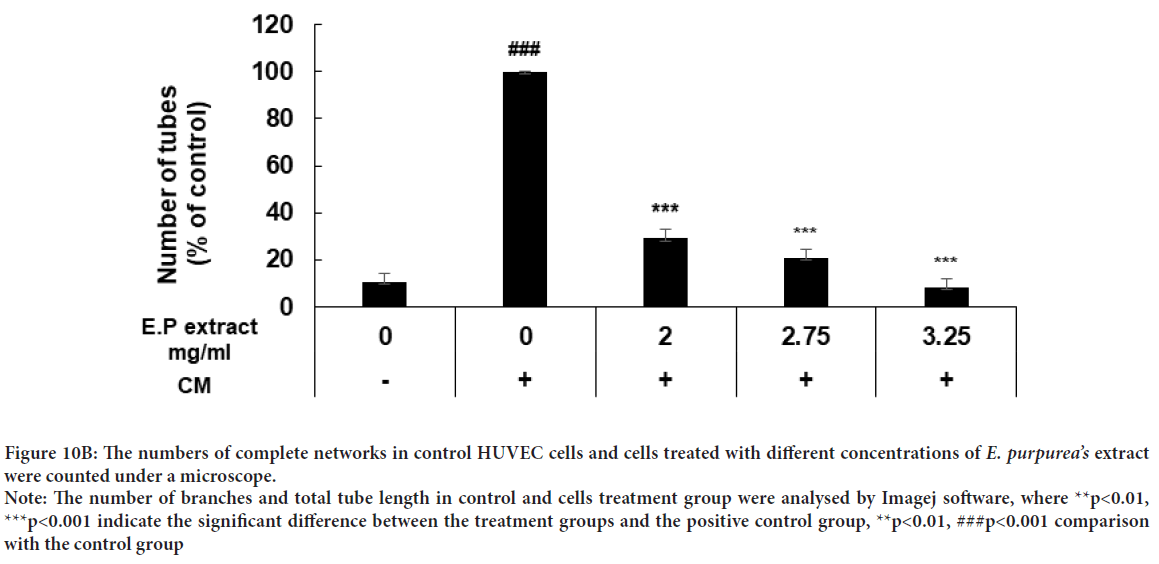
Figure 10B: The numbers of complete networks in control HUVEC cells and cells treated with different concentrations of E. purpurea’s extract were counted under a microscope.
Note: The number of branches and total tube length in control and cells treatment group were analysed by Imagej software, where **p<0.01, ***p<0.001 indicate the significant difference between the treatment groups and the positive control group, **p<0.01, ###p<0.001 comparison with the control group
Discussion
In this study, we investigated for the first time, the effect of E. purpurea’sextract on gastric cancer cell line AGS with regards to cell viability, cell migration, and invasion, colony formation, apoptosis as well as expression of VEGF-Agene, which is important in angiogenesis. Many studies have been performed on the AGS cell line and the effect of various reagents on inhibiting the growth of this cell, but the anticancer effect of E. purpurea’sextract specific to the Iranian species is reported for the first time on this cell line. The study of the effect of toxicity and anticancer of Echinacea purpureaextract on AGS cell line showed that IC50 of the extract of the plant was obtained 2.75 mg/mL with an inhibition percentage of 49. Today, the use of plants and their extracts for the treatment of diseases has been con sidered. Despite the various treatments for gastric cancer, the treatment of this cancer is still a challenge. Previous studies have reported that Echinacea purpurea plant and one of its derivatives, caffeic acid, regulated the immune system and inhibited tumor invasion and angiogenesis in cancer cells (Jung JE, et al., 2007; Jayanthi R and Subash P, 2010). Echinacea is a popular herbal supplement that acts as an immune system stimulant. It is used to treat common colds, coughs, bronchitis and upper respiratory infections. It is also a popular product used in the treatment of cancer, but the effects of Echinaceatoxicity on cancer cells are still unclear (Tsai YL, et al., 2012).
Echinaceahas a long history of use as a medication and is widely used in the treatment and prevention of upper respiratory tract infections. It is claimed to boost the immune system during cancer, reduce the side effects of chemotherapy and radiotherapy, and has anti-cancer effects. In vitro studies have shown immunostimulating and anti-cancer activity of various Echinaceacompounds (Pilkington K, 2007). It is reported that Echinaceaextracts have been able to induce apoptosis of human cancer cell lines by performing nuclear DNA fragmentation and increasing caspase activity (Chicca A, et al., 2007; Kim MH, 2003). The effect of the organic extract as well as the water-soluble extract of E. purpurea leaf and root, respectively indicated that the extracts decreased the proliferation of a breast cancer cell line (BT-549). Also, the extract of E. purpurea had anti-tumor activity on breast cancer cell line, MCF7 (Driggins S, et al., 2017). The concentration of 25 µg/mL Echinacea extract inhibited HeLa cells up to 100%; further inhibitory effects were observed in QBC-939 cancer cells, from 9% inhibition at 25 µg/mL to 38% inhibition at 100 µg/mL concentration (Cichello SA, et al., 2016). E. purpurea, previously used by native Americans to increase the immune system (Kumar K and Ramaiah S, 2011). Many studies have shown that herbal drugs may have anti-cancer effects by promoting the immune system, inhibiting the activity of telomerase, and inducing apoptosis in cancer cells (Baum M, et al., 2006; Lian Z, et al., 2003). Caffeic acid showed antioxidant and anti-inflammatory properties in diabetic rats. Also, caffeine and caffeic acid phenyl ester inhibited the activity of the metalloprotease 9 enzyme that plays an important role in metastasis (Chao PC, et al., 2009; Chung TW, et al., 2004). The results of the study of the effect of E. purpurea extract on the survival of PBMCs and RAW264.7 cells showed that in the concentration of 3.2 mg/mL, the viability of the cells was 89% and 81%, respectively (Chung TW, et al., 2004). Prasad RN, et al., 2011 showed that the concentration of 50 µg/mL caffeic acid could inhibit the growth of fibrosarcoma cells HT-1080. Caffeic acid inhibited T47D breast cancer cells in time and concentration-dependent manner (Rininger JA, et al., 2000; Kampa M, et al., 2004). To observe the antiproliferative effect of E. purpureaextract, the apoptosis morphological changes were performed by the acridine orange-ethidium bromide dual staining method. Acridine Orange (AO) and Ethidium bromide (EtBr) staining with DNA allowed observation of the condensed chromatin in apoptotic cells. During apoptosis DNA becomes condensed and the nucleus of the cells fragmented (Lakshmi S, et al., 2008). Acridine-orange is absorbed by the membrane and enters DNA of the living cells, turning the cell nucleus green and Ethidium bromide is only able to stain the nuclei of cells whose membranes have been destroyed. In our study, AGS cells without treatment were green color and the treatment cells were orange-red color represented apoptosis. The percentage of apoptotic cells in control and treatment samples with E. purpurea’sextract (0, 2.0, 2.75 and 3.25 µg/ mL) were 6.8, 66.6, 78.3, and 91.6%, respectively. It has been shown that a concentration of 50 µg/mL caffeic acid can greatly inhibit the growth of human HT-1080 fibrosarcoma cells and significantly increased apoptotic morphological changes in these cells (Prasad RN, et al., 2011).
Metastasis is a multi-step process that involves the migration and invasion of cancer cells and is a sign of malignancy (Tahtamouni L, et al., 2019). Inhibition of migration of cells treated with E. purpurea’s extract can indicate the anti-cancer properties of Echinacea. Echinacoside is a small molecule derived from the natural plant like Echinacea. Echinacoside has been reported to have anti-cancer effects by inducing apoptosis in colorectaltumor cells (Tang C, et al., 2020).
In the previous study on Echinacoside and caffeic acid showed that effectively inhibits the proliferation, migration, and invasion of breast cancer MDA-MB-231 and MCF7 cells, respectively. Both of these compounds are found in E. purpurea’sextract. A dose of 50 μM of caffeic acid inhibited migration in MCF-7 cells migration became more pronounced when the dose caffeic acid was increased to 100 μM. Thus, caffeic acid can be used as a chemopreventive agent (Tang C, et al., 2020; Kabała-Dzik A, et al., 2018). 50 μmol/L of caffeic acid reduced the motility rate and viability of Oral Carcinoma Cells SCC-25 cells (Dziedzic A, et al., 2014).
In our study, E. purpurea’s extract inhibited the formation of colonies in AGS cancer cells in a dose dependent manner. In the previous study showed that 60 and 80 μM Echinacoside decreased the colony formation of SW480 cells (Dong L, et al., 2015). Colony formation of MDA-MB-231 breast cancer cells significantly was reduced by Echinacoside (Tang C, et al., 2020). Caffeic acid inhibited HCT15 colon cancer cell proliferation and colony formation ability like to some anticancer agents (Jaganathan SK, 2012). Caffeic acid phenethyl ester (CAPE) extracted from honeybee suppressed cell proliferation and colony formation of TW2.6 human Oral Squamous Cell Carcinoma (OSCC) cells dose dependently. In that study, suggested that the administration of caffeic acid phenethyl ester was a potential adjuvant therapy for patients with oral cancer (Kuo YY, et al., 2013). Caffeic acid phenethyl ester and CAPE-pNO2 inhibited the colony formation ability in MDA-MB-231 breast cancer cell line (Huang Q, et al., 2019). CAPE inhibited colony formation in laryngeal carcinoma cells HEp2 in a dose-dependent manner (Ren X, et al., 2019). Caffeic acid 3,4-dihydroxy-phenethyl ester (CADPE) prevented colony formation in AGS and HGC27 cell lines (Dong A, et al., 2011).
In this study, the effect of the plant extract on VEGF-A gene expression showed that the level of expression somewhat reduced. One of the most important factors in the development of tumor growth is angiogenesis and one of the genes involved in vein formation is VEGF-Agene, which is an important regulator of the formation and functioning of blood vessels (Gerhardt H, et al., 2003). In the previous study, a number of pills containing 0.48, 0.96 and 1.92 mg of Echinaceaspp were given to mice subcutaneously injected with the kidney and lung cancer cells and the results showed that the performance of VEGF gene has decreased. It has been shown that flavonoids in Echinacea extract, are able to inhibit the activity of metalloproteinases and some serine proteases that are important for angiogenesis. Also, other phenolic compounds like caffeic acid have the ability to block VEGF binding to its receptor (Radomska-Leśniewska DM, et al., 2017).
It has been reported that treatment of mice with 0.6 mg/day of drugs derived from E. purpureafor 5 days led to decreasing the angiogenesis in L-1 sarcoma tumor (Wasiutyński A, et al., 2009). The extract of E. purpureahas given to pregnant mice and tissue VEGF production in their fetuses were lower than control. The inhibitory effect of E. purpureahas seen on tumor angiogenic activity (Bany J, et al., 2004). E. purpurea could reduce angiogenesis in murine L-1 sarcoma cells after three days of sarcoma cells injection. Also, it reduced VEGF concentration in L-1 sarcoma tumor tissue so that there were fewer small blood vessels in the margin of the tumor (Wasiutyński A, et al., 2009). It was reported, that E. purpureaextract reduced neovascular reaction in mice skin after inducing with human cancer cells (Radomska-Leśniewska DM, et al., 2017). Polyphenols present in Echinacea could inhibit the activities of metalloproteases and some serine proteases that were important for angiogenesis and also, inhibited VEGF receptor phosphorylation (Chicca A, et al., 2007; Kim MH, 2003).
In the present study, our data demonstrated that E. purpurea’sextract could reduce the formation of tubules in induced HUVEC cells. It has been reported that Echinacoside reduced the angiogenesis of serous ovarian carcinoma cells in a dose-dependent manner (Liu J, et al., 2022). VEGF-induced tube formation in human retina microvascular endothelial cells (HRMECs) was inhibited by treatment with 100 μM CA (Kim JH, et al., 2009). The previous study on CA and CADPE showed that 100 μM CA or 30 μM CADPE reduce tubule formation by HUVECs (Jung JE, et al., 2007). The results of a previous study indicated that 1.5 μM CAPE can prevent the formation of blood vessels in BCE (bovine capillary endothelial) cells under normoxic and hypoxic conditions (Wu J, et al., 2011).
Conclusion
Several possible uses of Echinaceain cancer management have been investigated so far, but there is currently insufficient evidence to support or refute these claims. Therefore, further study on this useful plant is necessary. In vitrostudy, we confirmed that E. purpurea’sextract could effectively inhibit AGS gastric cancer cell proliferation, migration, colony formation, the formation of tubules, and reduce VEGF-A expression and induce apoptosis. One can hope that E. purpurea’sextract will be used as pharmaceutical agent for treatment of cancer. These results could be used for further in vivostudies with Echinaceaextract in different models of normal cells and cancerous tumors.
Author Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Azar Sadeghi. The first draft of the manuscript was written by Azar Sadeghi and Fatemeh Moradian and then Farzaneh Sabouni and Forough Sanjaraian commented on the last version of the manuscript. All authors read and approved the final manuscript.
Funding
The source of funding for the project was the corresponding author’s grant, and the student was responsible for collecting and analyzing the information, and the student and the corresponding author were responsible for writing the article.
Acknowledgment
This original research was performed in the National Institute of Genetic Engineering and Biotechnology of Iran. The authors are thankful for providing facilities and assistance. Also, the authors are thankful of Department of Basic Sciences, Sari Agricultural Sciences and Natural Resources University for financial support.
Data Availability
The datasets analyzed during the current study are available from the corresponding author on reasonable request.
Consent for Publication
Informed consent was obtained from all individual participants included in the study.
References
- Barnes J, Anderson LA, Gibbons S, Phillipson JD. Echinacea species (Echinacea angustifolia (DC.) Hell., Echinacea pallida (Nutt.) Nutt., Echinacea purpurea (L.) Moench): A review of their chemistry, pharmacology and clinical properties. J Pharm Pharmacol. 2005; 57(8): 929-954.
[Crossref] [Google scholar] [Pubmed]
- Cichello SA, Yao Q, He XQ. Proliferative activity of a blend of Echinacea angustifolia and Echinacea purpurea root extracts in human vein epithelial, HeLa, and QBC-939 cell lines, but not in Beas-2b cell lines. J tradit complement med. 2016; 6(2): 193-197.
[Crossref] [Google scholar] [Pubmed]
- Binns S, Hudson J, Merali S, Arnason J. Antiviral activity of characterized extracts from Echinacea spp.(Heliantheae: Asteraceae) against Herpes simplex virus (HSV-I). Planta Med. 2002; 68(9): 780-783.
[Crossref] [Google scholar] [Pubmed]
- Dalby-Brown L, Barsett H, Landbo A-KR, Meyer AS, Mølgaard P. Synergistic antioxidative effects of alkamides, caffeic acid derivatives, and polysaccharide fractions from Echinacea purpurea on in vitro oxidation of human low-density lipoproteins. J Agric Food Chem. 2005; 53(24): 9413-9423.
[Crossref] [Google scholar] [Pubmed]
- Driggins S, Whalen M, Myles E. The inhibitory effect of Echinacea purpurea and Echinacea pallida on BT-549 and natural killer cells. MOJ Cell Sci Rep. 2017; 4(3): 00091.
- Merali S, Binns S, Paulin-Levasseur M, Ficker C, Smith M, Baum B, et al. Antifungal and anti-inflammatory activity of the genus Echinacea. Pharm Biol. 2003; 41(6): 412-420.
- Tsai YL, Chiu CC, Chen JYF, Chan KC, Lin SD. Cytotoxic effects of Echinacea purpurea flower extracts and cichoric acid on human colon cancer cells through induction of apoptosis. J Ethnopharmacol. 2012; 143(3): 914-919.
[Crossref] [Google scholar] [Pubmed]
- Boerjan W, Ralph J, Baucher M. Lignin biosynthesis. Annu Rev Plant Biol. 2003; 54(1): 519-546.
[Crossref] [Google scholar] [Pubmed]
- Bruni R, Brighenti V, Caesar LK, Bertelli D, Cech NB, Pellati F, et al. Analytical methods for the study of bioactive compounds from medicinally used Echinacea species. J Pharm Biomed Anal. 2018; 160: 443-477.
[Crossref] [Google scholar] [Pubmed]
- Hudson EA, Dinh PA, Kokubun T, Simmonds MS, Gescher A. Characterization of potentially chemopreventive phenols in extracts of brown rice that inhibit the growth of human breast and colon cancer cells. Cancer Epidemiol Biomark Prev. 2000; 9(11): 1163-1170.
[Google scholar] [Pubmed]
- Johnson AA, Marchand C, Pommier Y. HIV-1 integrase inhibitors: A decade of research and two drugs in clinical trial. Curr Top Med Chem. 2004; 4(10): 1059-1077.
[Crossref] [Google scholar] [Pubmed]
- Maurya DK, Devasagayam TP. Antioxidant and prooxidant nature of hydroxycinnamic acid derivatives ferulic and caffeic acids. Food Chem Toxicol. 2010; 48(12): 3369-3373.
[Crossref] [Google scholar] [Pubmed]
- Morton LW, Croft KD, Puddey IB, Byrne L. Phenolic acids protect low density lipoproteins from peroxynitrite-mediated modification in vitro. Redox Rep. 2000; 5(2-3): 124-125.
[Crossref] [Google scholar] [Pubmed]
- Park EH, Kahng JH. Suppressive effects of propolis in rat adjuvant arthritis. Arch Pharm Res. 1999; 22(6): 554-558.
[Crossref] [Google scholar] [Pubmed]
- Sroka Z, Cisowski W. Hydrogen peroxide scavenging, antioxidant and anti-radical activity of some phenolic acids. Food Chem Toxicol. 2003; 41(6): 753-758.
[Crossref] [Google scholar] [Pubmed]
- Prasad RN, Karthikeyan A, Karthikeyan S, Reddy BV. Inhibitory effect of caffeic acid on cancer cell proliferation by oxidative mechanism in human HT-1080 fibrosarcoma cell line. Mol Cell Biochem. 2011; 349(1): 11-19.
[Crossref] [Google scholar] [Pubmed]
- Jung JE, Kim HS, Lee CS, Park DH, Kim YN, Lee MJ, et al. Caffeic acid and its synthetic derivative CADPE suppress tumor angiogenesis by blocking STAT3-mediated VEGF expression in human renal carcinoma cells. Carcinogenesis. 2007; 28(8): 1780-1787.
[Crossref] [Google scholar] [Pubmed]
- Kumar K, Ramaiah S. Pharmacological importance of Echinacea purpurea. Int J Pharma Bio Sci. 2011; 2(4): 304-314.
- Kanimozhi G, Prasad NR. Anticancer effect of caffeic acid on human cervical cancer cells. Academicc Press. 2015.
- Ferrara N. VEGF as a therapeutic target in cancer. Oncology. 2005; 69(Suppl 3): 11-6.
[Crossref] [Google scholar] [Pubmed]
- Balan B, Skopinska-Rózewska E, Barcz E, Sokolnicka I, Gawrychowski K, Strzelecka H. The effect of selected phenolic acids on angiogenic activity of ovarian cancer cells-preliminary report. Onkol Pol. 1999; 2(4): 203-208.
- Maksimenko A, Alami M, Zouhiri F, Brion JD, Pruvost A, Mougin J, et al. Therapeutic modalities of squalenoyl nanocomposites in colon cancer: An ongoing search for improved efficacy. ACS nano. 2014; 8(3): 2018-2332.
[Crossref] [Google scholar] [Pubmed]
- Han H, Du B, Pan X, Liu J, Zhao Q, Lian X, et al. CADPE Inhibits PMA-stimulated gastric carcinoma cell invasion and matrix metalloproteinase-9 expression by FAK/MEK/ERK-mediated AP-1 activation. Mol Cancer Res. 2010; 8(11): 1477-1488.
[Crossref] [Google scholar] [Pubmed]
- Takahashi T, Saikawa Y, Kitagawa Y. Gastric cancer: Current status of diagnosis and treatment. Cancers. 2013; 5(1): 48-63.
[Crossref] [Google scholar] [Pubmed]
- Jayanthi R, Subash P. Antioxidant effect of caffeic acid on oxytetracycline induced lipid peroxidation in albino rats. Indian J Clin Biochem. 2010; 25(4): 371-375.
[Crossref] [Google scholar] [Pubmed]
- Pilkington K. Echinacea spp. CAM-Cancer Consortium. 2017.
- Chicca A, Adinolfi B, Martinotti E, Fogli S, Breschi MC, Pellati F, et al. Cytotoxic effects of Echinacea root hexanic extracts on human cancer cell lines. J Ethnopharmacol. 2007; 110(1): 148-153.
[Crossref] [Google scholar] [Pubmed]
- Kim MH. Flavonoids inhibit VEGF/bFGF-induced angiogenesis in vitro by inhibiting the matrix-degrading proteases. J Cell Biochem. 2003; 89(3): 529-538.
[Crossref] [Google scholar] [Pubmed]
- Baum M, Ernst E, Lejeune S, Horneber M. Role of complementary and alternative medicine in the care of patients with breast cancer: Report of the European Society of Mastology (EUSOMA) Workshop, Florence, Italy, December 2004. Eur J Cancer. 2006; 42(12): 1702-1710.
[Crossref] [Google scholar] [Pubmed]
- Lian Z, Niwa K, Gao J, Tagami K, Mori H, Tamaya T. Association of cellular apoptosis with anti-tumor effects of the Chinese herbal complex in endocrine-resistant cancer cell line. Cancer Detect Prev. 2003; 27(2): 147-154.
[Crossref] [Google scholar] [Pubmed]
- Chao PC, Hsu CC, Yin MC. Anti-inflammatory and anti-coagulatory activities of caffeic acid and ellagic acid in cardiac tissue of diabetic mice. Nutr Metab. 2009; 6(1): 1-8.
[Crossref] [Google scholar] [Pubmed]
- Chung TW, Moon SK, Chang YC, Ko JH, Lee YC, Cho G, et al. Novel and therapeutic effect of caffeic acid and caffeic acid phenyl ester on hepatocarcinoma cells: Complete regression of hepatoma growth and metastasis by dual mechanism. FASEB J. 2004; 18(14): 1670-1681.
[Crossref] [Google scholar] [Pubmed]
- Rininger JA, Kickner S, Chigurupati P, McLean A, Franck Z. Immunopharmacological activity of Echinacea preparations following simulated digestion on murine macrophages and human peripheral blood mononuclear cells. J Leukoc Biol. 2000; 68(4): 503-510.
[Crossref] [Google scholar] [Pubmed]
- Kampa M, Alexaki VI, Notas G, Nifli AP, Nistikaki A, Hatzoglou A, et al. Antiproliferative and apoptotic effects of selective phenolic acids on T47D human breast cancer cells: Potential mechanisms of action. Breast Cancer Res. 2004; 6(2): 1-2.
[Crossref] [Google scholar] [Pubmed]
- Lakshmi S, Dhanya GS, Joy B, Padmaja G, Remani P. Inhibitory effect of an extract of Curcuma zedoariae on human cervical carcinoma cells. Med Chem Res. 2008; 17(2): 335-344.
- Tahtamouni L, Ahram M, Koblinski J, Rolfo C. Molecular regulation of cancer cell migration, invasion, and metastasis. Anal Cell Pathol. 2019.
[Crossref] [Google scholar] [Pubmed]
- Tang C, Gong L, Qiu K, Zhang Z, Wan L. Echinacoside inhibits breast cancer cells by suppressing the Wnt/ß-catenin signaling pathway. Biochem Biophys Res Commun. 2020; 526(1): 170-175.
[Crossref] [Google scholar] [Pubmed]
- Kabala-Dzik A, Rzepecka-Stojko A, Kubina R, Wojtyczka RD, Buszman E, Stojko J. Caffeic acid versus caffeic acid phenethyl ester in the treatment of breast cancer MCF-7 cells: Migration rate inhibition. Integr Cancer Ther. 2018; 17(4): 1247-1259.
[Crossref] [Google scholar] [Pubmed]
- Dziedzic A, Kubina R, Kabala-Dzik A, Wojtyczka RD, Morawiec T, Buldak RJ. Caffeic acid reduces the viability and migration rate of oral carcinoma cells (SCC-25) exposed to low concentrations of ethanol. Int J Mol Sci. 2014; 15(10): 18725-18741.
[Crossref] [Google scholar] [Pubmed]
- Dong L, Yu D, Wu N, Wang H, Niu J, Wang Y, et al. Echinacoside induces apoptosis in human SW480 colorectal cancer cells by induction of oxidative DNA damages. Int J Mol Sci. 2015; 16(7): 14655-14658.
[Crossref] [Google scholar] [Pubmed]
- Jaganathan SK. Growth inhibition by caffeic acid, one of the phenolic constituents of honey, in HCT 15 colon cancer cells. Sci World J. 2012.
[Crossref] [Google scholar] [Pubmed]
- Kuo YY, Lin HP, Huo C, Su LC, Yang J, Hsiao PH, et al. Caffeic acid phenethyl ester suppresses proliferation and survival of TW2. 6 human oral cancer cells via inhibition of Akt signaling. Int J Mol Sci. 2013; 14(5): 8801-8817.
[Crossref] [Google scholar] [Pubmed]
- Huang Q, Li S, Zhang L, Qiao X, Zhang Y, Zhao X, et al. CAPE-p NO2 inhibited the growth and metastasis of triple-negative breast cancer via the EGFR/STAT3/Akt/E-cadherin signaling pathway. Front Oncol. 2019; 9: 461.
[Crossref] [Google scholar] [Pubmed]
- Ren X, Liu J, Hu L, Liu Q, Wang D, Ning X. Caffeic acid phenethyl ester inhibits the proliferation of HEp2 cells by regulating Stat3/Plk1 pathway and inducing S phase arrest. Biol Pharm Bull. 2019: b19-00315.
[Crossref] [Google scholar] [Pubmed]
- Dong A, Fang Y, Zhang L, Xie J, Wu X, Zhang L, et al. Caffeic acid 3, 4-dihydroxy-phenethyl ester induces cancer cell senescence by suppressing twist expression. J Pharmacol Exp Ther. 2011; 339(1): 238-247.
[Crossref] [Google scholar] [Pubmed]
- Gerhardt H, Golding M, Fruttiger M, Ruhrberg C, Lundkvist A, Abramsson A, et al. VEGF guides angiogenic sprouting utilizing endothelial 1005 tip cell filopodia. J Cell Biol. 2003; 161(6): 1163-1177.
[Crossref] [Google scholar] [Pubmed]
- Radomska-Lesniewska DM, Balan BJ, Skopinski P. Angiogenesis modulation by exogenous antioxidants. Cent Eur J Immunol. 2017; 42(4): 370-376.
- Wasiutynski A, Balan B, Skopinska-Rózewska E. The effect of Echinacea purpurea on the morphology, angiogenic activity and Vascular Endothelial Growth Factor (VEGF) concentration of murine L-1 sarcoma tumors. Cent Eur J Immunol. 2009; 34: 38-41.
- Bany J, Skopinska-Rózewska E, Chorostowska-Wynimko J, Rogala E, Sommer E, Zdanowska D, et al. The effect of complex herbal remedy on the angiogenic activity of L-1 sarcoma cells, L-1 sarcoma tumour growth, and on the bacterial infection in mice. Cent Eur J Immunol. 2004; 29(1): 29-34.
- Liu J, Tang N, Liu N, Lei P, Wang F. Echinacoside inhibits the proliferation, migration, invasion and angiogenesis of ovarian cancer cells through PI3K/AKT pathway. J Mol Histol. 2022; 53(2): 1-10.
[Crossref] [Google scholar] [Pubmed]
- Kim JH, Lee BJ, Kim JH, Yu YS, Kim KW. Anti-angiogenic effect of caffeic acid on retinal neovascularization. Vasc Pharmacol. 2009; 51(4): 262-267.
[Crossref] [Google scholar] [Pubmed]
- Wu J, Omene C, Karkoszka J, Bosland M, Eckard J, Klein CB, et al. Caffeic acid phenethyl ester (CAPE), derived from a honeybee product propolis, exhibits a diversity of anti-tumor effects in pre-clinical models of human breast cancer. Cancer Lett. 2011; 308(1): 43-53.
[Crossref] [Google scholar] [Pubmed]
Author Info
Azar Sadeghi1, Fatemeh Moradian1*, Farzaneh Sabouni2 and Forough Sanjarian32Department of Molecular Medicine, National Institute of Genetic Engineering and Biotechnology, Tehran, Iran
3Department of Agriculture Biotechnology, National Institute of Genetic Engineering and Biotechnology, Tehran, Iran
Citation: Sadeghi A: Effect of Echinacea purpureaâ??s Extract on Gastric Cancer Cell (AGS) Growth, Migration, Colony Formation, Tube Formation, Apoptosis, and VEGF-A Gene Expression
Received: 01-Nov-2022 Accepted: 25-Nov-2022 Published: 02-Dec-2022, DOI: 10.31858/0975-8453.13.12.870-881
Copyright: This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
ARTICLE TOOLS
- Dental Development between Assisted Reproductive Therapy (Art) and Natural Conceived Children: A Comparative Pilot Study Norzaiti Mohd Kenali, Naimah Hasanah Mohd Fathil, Norbasyirah Bohari, Ahmad Faisal Ismail, Roszaman Ramli SRP. 2020; 11(1): 01-06 » doi: 10.5530/srp.2020.1.01
- Psychometric properties of the World Health Organization Quality of life instrument, short form: Validity in the Vietnamese healthcare context Trung Quang Vo*, Bao Tran Thuy Tran, Ngan Thuy Nguyen, Tram ThiHuyen Nguyen, Thuy Phan Chung Tran SRP. 2020; 11(1): 14-22 » doi: 10.5530/srp.2019.1.3
- A Review of Pharmacoeconomics: the key to “Healthcare for All” Hasamnis AA, Patil SS, Shaik Imam, Narendiran K SRP. 2019; 10(1): s40-s42 » doi: 10.5530/srp.2019.1s.21
- Deuterium Depleted Water as an Adjuvant in Treatment of Cancer Anton Syroeshkin, Olga Levitskaya, Elena Uspenskaya, Tatiana Pleteneva, Daria Romaykina, Daria Ermakova SRP. 2019; 10(1): 112-117 » doi: 10.5530/srp.2019.1.19
- Dental Development between Assisted Reproductive Therapy (Art) and Natural Conceived Children: A Comparative Pilot Study Norzaiti Mohd Kenali, Naimah Hasanah Mohd Fathil, Norbasyirah Bohari, Ahmad Faisal Ismail, Roszaman Ramli SRP. 2020; 11(1): 01-06 » doi: 10.5530/srp.2020.1.01
- Manilkara zapota (L.) Royen Fruit Peel: A Phytochemical and Pharmacological Review Karle Pravin P, Dhawale Shashikant C SRP. 2019; 10(1): 11-14 » doi: 0.5530/srp.2019.1.2
- Pharmacognostic and Phytopharmacological Overview on Bombax ceiba Pankaj Haribhau Chaudhary, Mukund Ganeshrao Tawar SRP. 2019; 10(1): 20-25 » doi: 10.5530/srp.2019.1.4
- A Review of Pharmacoeconomics: the key to “Healthcare for All” Hasamnis AA, Patil SS, Shaik Imam, Narendiran K SRP. 2019; 10(1): s40-s42 » doi: 10.5530/srp.2019.1s.21
- A Prospective Review on Phyto-Pharmacological Aspects of Andrographis paniculata Govindraj Akilandeswari, Arumugam Vijaya Anand, Palanisamy Sampathkumar, Puthamohan Vinayaga Moorthi, Basavaraju Preethi SRP. 2019; 10(1): 15-19 » doi: 10.5530/srp.2019.1.3