Research Article - (2022) Volume 13, Issue 10
Abstract
Introduction: Plants, due to presence of secondary metabolites like flavonoids, phenols, have been used for various skin care purposes. The ethanolic extracts of different parts of various plants were screened for phytochemicals. Total phenols, total flavonoids, in vitro Sun Protection Factor (SPF) were determined and sunscreen cream was formulated and evaluated from the most potent plant extract.
Method: Extraction was done by double maceration. The total phenol content was determined colorimetrically using Folin-Ciocalteu reagent and flavonoid content by aluminium chloride colorimetric method. In vitro SPF was determined by spectrophotometric analysis using Mansuer equation. Formulation was done by incorporating oil phase into aqueous phase.
Results: Phytochemical analysis suggested the presence of various phytoconstituents. Among nine plants, the maximum total phenolic and flavonoid content was of Magnifera indica extract. Four formulations were prepared containing 0%, 1%, 2.5% and 5% of extract in a cream base. The evaluation of all the formulations has been done by analysis of different parameters such as pH, acid value, saponification value, in vitro SPF, stability for 21 days. The pH of the formulation was within the range of 5.01-6.22. Acid value was within the range of 5.98-14.21. The saponification value of cream was between 23.27-33.25. The formulations containing 5% plant extract showed the highest SPF value. The stability study of the formulation with 5% plant extract showed homogeneity under 4°C and room temperature but non-homogeneity under higher temperature i.e. 40°C.
Conclusion: This will be a better, cheaper and safer alternative to harmful chemical sunscreens which are abundantly available in the market.
Keywords
Flavonoids, In vitro sun protection factor, Medicinal plants, Phenols, Sunscreen cream
Introduction
Before the introduction of orthodox drugs, different plant extracts have been used for the treatment of several diseases. Due to the presence of secondary metabolites, plants poses antifungal, antimicrobial, anticancer, antidiabetic and other pharmacological activities (Larbie CO, et al., 2019). Similarly, natural ingredients have been traditionally used for skin care purposes. Source of natural ingredients can be herbs, flowers, fruits, roots and leaves (Rebeiro AS, et al., 2015). Natural ingredients are gaining more popularity as consumers seek more natural ingredients in their personal care product because they are less hypo allergic compared to synthetic cosmeceutical ingredients and consumer need not to worry about skin irritations. Cosmeceutical preparations with natural ingredients supply the skin with nutrients like vitamin A, vitamin C, vitamin E, phenolic compounds, flavonoids and terpenoids which act as antioxidant, anti-inflammatory, antiaging and anti-melanogenic effect on skin and enhance the skin health (Lohani A et al., 2018).
Exposure to Ultraviolet (UV) radiation triggers the rapid generation and accumulation of Reactive Oxygen Species (ROS) in the skin. When there is imbalance between ROS and antioxidants, they can also take part in the pathological process known as oxidative stress and leads to cell damage and aging (Petruk G et al., 2018). UV radiation consists of three components, UVA, UVB and UVC. Among these components UVA and UVB reach the earth surface in sufficient amount to damage the skin whereas UVC is almost completely absorbed by the ozone layer (Yang Y and Li S, 2015). UVB is responsible for erythema and sunburn. In contrast to UVB, UVA is more efficient in inducing immediate and delayed pigment darkening and delayed tanning. UVA induces several adverse effects including immunosuppression, photoaging, ocular damage and skin cancer. UV radiations also have beneficial effect as it increases synthesis of vitamin D in skin. There are a lot of different types of sunscreen products (oils, sticks, gels, creams, lotions) which absorb UV rays and prevents them from penetrating the skin (Korac RR and Kambholja KM, 2011).
Different class of antioxidants such as flavonoids, polyphenols, carotenoids and vitamins show protectant activity against UV radiation. Different bioactive compounds responsible for skin protection against UV radiation are quercetin, resveratrol, lycopene, beta-carotene, vitamin C and vitamin E (Petruk G et al., 2018). Phenolic compounds act as antioxidant and protect leaf from photodamage. Different physical sunblock such as zinc oxide, titanium dioxide are also used. Besides that, the skin’s natural sun blockers are proteins, lipids and nucleotides (Korac RR and Kambholja KM, 2011).
Sun protection factor is defined as the UV energy required to produce a Minimal Erythema Dose (MED) on protected skin divided by the UV energy required producing a minimal erythema dose on unprotected skin. MED is defined as the dose of UV irradiation sufficient to produce a minimal erythema on unprotected skin. The higher the SPF, more effective is the product in preventing sunburn. Photo protection afforded by sunscreen against solar radiation exposure can be determined by photo testing in human volunteer (Dutra EA, et al., 2004).
Topical cream formulations are considered to be more acceptable to patients as they can be effortlessly applied on various part of the skin without imparting the greasy feel. Creams are more potent and occlusive for long period of time as compared to lotions. Topical creams are prepared by the fusion process wherein the oil phase and the water phase components are separately heated and mixed with continuous agitation (Mendonsa NS, et al., 2019). Creams and ointments containing plant extract are being formulated due to rapid expansion of demand for herbal cosmetics (El-Gied AA, et al., 2015). Plants or their parts used in herbal cosmetic preparation should have various properties like antioxidant, anti-inflammatory, antiseptic, emollient and antibacterial (Aswal A, et al., 2013) (Table 1).
| S.No | Scientific name | Common name | Part used |
|---|---|---|---|
| 1 | Allium cepa L. | Onion | Bulb |
| 2 | Allium sativum L. | Garlic | Leaves |
| 3 | Cinnamomum tamala | Bay leaf | Leaves |
| 4 | Ficus religiosa L. | Banyan | Bark |
| 5 | Magnifera indica L. | Mango | Bark |
| 6 | Mimosa pudica L. | Touch me not plant | Roots |
| 7 | Ocimum tenuiflorum L. | Holy basil | Leaves |
| 8 | Rubus ellipticus Sm. | Himalayan raspberry | Leaves |
| 9 | Solanum tuberosum L. | Potato | Peels |
Table 1: List of selected plants and parts used for the study
Materials and Methods
Plant collection and identification
All the plants were collected from Rupatal, Deurali, and Khudi of Kaski district at an altitude ranging from 800-1200 m. The herbaria of collected plants sample were prepared. Then it was identified by comparing the morphological characteristics and was confirmed by taxonomist at National Herbarium and Vegetation laboratories, Godawari, Lalitpur, Nepal. All the plant samples were made clean from mud, dirt, or lichens present and then cut into pieces with the help of plant cutter and knives and the left for shade drying until dried completely before extraction started.
Extraction of plants
Bark, leaves, roots, bulb and peels of the selected plants were separated, dried in shade at room temperature and grinded to coarse powder using blender. The 100 gram dried powder of each plant was extracted with 800 ml ethanol for 24 hours. Then the residue obtained from the initial extraction was again extracted with 800 ml ethanol for 24 hours. All the extract was concentrated to dryness, under reduced pressure and controlled temperature, using rotary evaporator.
Phytochemical screening
The phytochemical analysis was performed for testing the different chemical groups present in the ethanolic extract of various plants. The presence of phytochemical like alkaloids, flavonoids and phenols was evaluated according to methods described by Zhang J, et al., 2011 with some modifications.
Reagents used for phytochemical screening
Mayer’s reagent: About 1.358 gram of HgCl2 was dissolved in 60 ml of water and it was mixed with a solution of 5 g KI in 10 ml water and volume was adjusted upto 100 ml by water.
Wagner’s reagent: Potassium iodide of about 16.6 gram of was dissolved in 100 ml of distilled water and few crystals of iodine were added to the solution and stirred properly.
Ferric chloride solution: Fifteen grams of ferric chloride hexahydrate was dissolved in 100 ml of distilled water.
Lead acetate solution: Ten grams of lead acetate was dissolved in 100 ml of carbondioxide free water.
Sodium hydroxide solution: Twenty grams of NaOH was dissolved in 100 ml of distilled water.
Phytochemical tests
Alkaloids test: Each of the sample extracts were dissolved individually in dilute hydrochloric acid and filtered. Then the filtrates were treated with
Mayer’s reagent and Wagner’s reagent to test for the presence of alkaloids.
Phenolic test: All extracts were treated with 5 ml FeCl3 solution.
Flavonoids test: In alkaline test, all extracts were treated with 5 ml of sodium hydroxide and observed. In lead acetate test, all extracts were treated with 5 ml of lead acetate solution and observed.
Determination of total phenolic content
The total phenolic content of plant extract was determined using a spectrophotometric method according to Zhang J, et al., 2011 with slight modifications.
Preparation of gallic acid solutions and test samples
Gallic acid was taken as standard phenolic compound. Different concentrations of gallic acid (7.82125 µg/ml, 15.625 µg/ml, 31.25 µg/ml, 62.5 µg/ ml, 125 µg/ml, 250 µg/ml, 500 µg/ml and 1000 µg/ml) were prepared. From dried extract of plants 1 mg/ml concentration was prepared as a test sample.
Preparation of 10% sodium carbonate solution: Ten grams of sodium carbonate was dissolved in distilled water and diluted up to 50 ml to make 10% of solution (Indian Pharmacopoeia).
Determination of total phenolic content
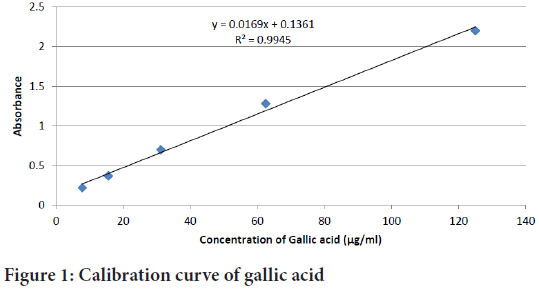
About 1 ml of test sample was added to 1 ml of 2 N Folin reagent followed by the addition of 5 ml of distilled water and left for five minutes. Then, 1 ml of 10% NaCO3 was added and incubated at room temperature for 1 hour. Finally, the absorbance of the reaction mixture was measured at 760 nm against blank ethanol. The total phenol content was expressed as µg gallic acid equivalents (GAE)/mg of extract, using the calibration curve of gallic acid (Figure 1) (7.8125-1000 µg/ml) standards. All the determinations were carried in triplicate.
Figure 1: Calibration curve of gallic acid
Determination of total flavonoid content
The content of flavonoids in plant extracts was determined using a spectrophotometric method according to Zhishen J, et al., 1999 with slight modifications.
Preparation of quercetin and test samples
Quercetin was taken as standard flavonoid compound. Different concentrations of quercetin (7.8125 µg/ml, 15.625 µg/ ml, 31.25 µg/ ml, 62.5 µg/ ml, 125 µg/ml, 250 µg/ml, 500 µg/ml and 1000 µg/ml) were prepared. From the stock solution 1 mg/ml concentration of extract were prepared as test samples.
Preparation of aluminium chloride (10%): Ten grams of aluminium chloride was dissolved in 100 ml of water to prepare 10% of aluminium chloride solution.
Determination of total flavonoid content
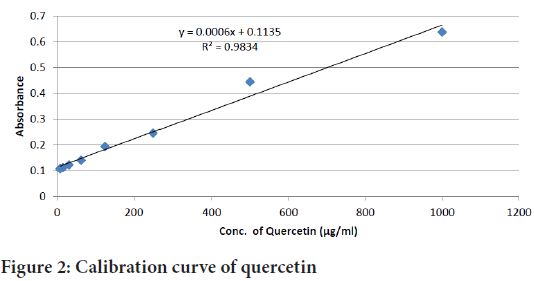
Total flavonoid content was determined by Aluminium chloride colorimetric method. 1 ml of each extract solution (1 µg/ml) was mixed with 4 ml of distilled water. Then, 0.3 ml of 5% sodium nitrate was added. After 5 minutes, 0.3 ml of 10% aluminium chloride was added and allowed to stand for 6 minutes. Then, 2 ml of 1 M sodium hydroxide was added and absorbance was measured at 510 nm using UV spectrophotometer. The calibration curve was prepared using quercetin as the standard (Figure 2). Total flavonoid content was calculated from the calibration curve and results were expressed as µg quercetin equivalent per gram dry extract weight.
Figure 2: Calibration curve of quercetin
Determination of sun protection factor
In vitro SPF was determined using spectrophotometric analysis. For the determination of SPF, 1% w/v solution was prepared in ethanol and from this 0.01% w/v concentration was prepared. The absorbance of the sample solutions were taken by UV-visible spectrophotometer in the range of 290- 320 nm, in every 5 nm interval using ethanol as a blank. Three readings were taken at every interval. The SPF value was calculated by following equation-
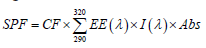
Where,
CF=Correlation factor
EE=Erythemogenic effect of radiation with wavelength
Abs=Spectrophotometric absorbance values of wavelength
The values of EE(λ) × I(λ) is constant at a fixed wavelength (3).
Formulation of cream
For the formulation of cream in laboratory method described by Farboud ES, et al., 2011 with slight modifications was used. Required amount of both aqueous phase and lipid phase were heated separately at 70 ± 2°C. The aqueous phase was added to the lipid phase, with continuous stirring, using magnetic stirrer until congealed. Four formulations, having concentrations of 0%, 1%, 2.5% and 5% plant extract, were prepared. All the formulations, containing certain level of plant extracts, were evaluated for different parameters. The formulation components, used during formulation of cream, are listed below (Table 2).
| Ingredients | Amount | Uses | |||
|---|---|---|---|---|---|
| Cream base |
1% extract |
2.50% extract |
5% extract |
||
| Stearic acid | 5 g | 4.95 g | 4.87 g | 4.75 g | Emollient and co-emulsifier |
| Cetostearyl alcohol | 3.75 g | 3.713 g | 3.66 g | 3.56 g | Emulsifier |
| Lanolin | 5 g | 4.95 g | 4.88 g | 4.85 g | Emollient |
| Triethanolamine | 0.62 ml | 0.61 ml | 0.604 ml | 0.58 ml | PH adjuster |
| Glycerol | 5 ml | 4.95 ml | 4.88 ml | 4.75 ml | Humectant |
| Propyl parabean | 0.04 g | 0.039 g | 0.039 g | 0.038 g | Preservatives |
| Distilled water | 80.64 ml | 79.83 ml | 78.624 ml | 76.58 ml | Solvent |
| Magnifera indica extract | 0 g | 1 g | 2.5 g | 5 g | Active ingredient |
Table 2: List of components used during the formulation of the cream
Evaluation of pH of cream
The pH meter was calibrated using standard buffer solution. pH of 0.5 g was measured by digital pH meter (Aswal A, et al., 2013).
Appearance
The appearances of the creams were evaluated visually for their color and homogeneity (Joshi P, et al., 2019).
Evaluation of SPF of cream
Sunscreen samples were diluted using ethanol, final concentration of cream having 1 µg/ml, and analyzed by UV-Visible spectrophotometry ranging from 290 nm to 320 nm, at an interval of 5 nm.
Evaluation of acid value
Acid values of creams were determined according to method described by Aswal A, et al., 2013. Four grams of cream was dissolved in 20 ml mixture of equal volume of ether and ethanol and heated until sample dissolved. Five ml of this mixture was taken in a conical flask and titrated with 0.1 N NaOH until fairly pink color persisted for 30 seconds. 1 ml of phenol- phthalein was used as indicator.
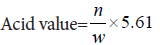
where,
n=No.of ml of NaOH required
w=The weight of substance (Aswal A, et al., 2013).
Evaluation of saponification value
Saponification values of creams were determined according to method by Aswal A, et al., 2013. 2 g of cream was refluxed with 25 ml of 0.5 N of alcoholic KOH, for 30 minutes, and the sample was titrated with 0.5 N HCl, phenolphthalein as an indicator. Then, saponification value was calculated from following formula.
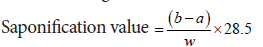
Where,
a=Volume of titrant (in ml)
b=Volume of titrate (in ml)
w=Weight of substance (in grams) (Aswal A, et al., 2013).
Stability testing
Formulated creams were stored at three different temperatures, 4°C, room temperature (21 ± 5°C) and 40 ± 2°C, and the stability study were carried out at 0th, 7th, 14th, and 21st day (Smaoui S, et al., 2019).
Results
Extraction yield value
Obtained yield values of each ethanolic extracts were expressed as extract yield percentage (Table 3).
| S.No | Plants | Amount of crude drug(g) | Dry extract (g) | Yield value (%) |
|---|---|---|---|---|
| 1 | O. tenuiflorum | 100 | 16.88 | 16.88 |
| 2 | F. religiosa | 100 | 8.9 | 8.9 |
| 3 | C. tamala | 100 | 8.54 | 8.54 |
| 4 | M. pudica | 100 | 7.52 | 7.52 |
| 5 | M. indica | 100 | 14.57 | 14.57 |
| 6 | R.ellipticus | 100 | 10.11 | 10.11 |
| 7 | S. tuberosum | 100 | 6.54 | 6.54 |
| 8 | A. cepa | 100 | 15.53 | 15.53 |
| 9 | A. sativum | 100 | 3.2 | 3.2 |
Table 3: Yield value of the extract of selected plants
Phytochemical screening
Phytochemical screening of various plant extract confirmed absence or the presence of alkaloids, flavonoids and phenols. The results are as follows (Table 4).
| Phytochemicals | Tests | A1X1 | A1G1 | A1C1 | A1M1 | A1R1 | A1T1 | A1P1 | A1B1 | A1Z1 | Results |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Alkaloids | Mayer’s test | + | + | - | - | + | - | - | - | - | Yellowish white precipitate |
| Wagner’s test | - | - | - | - | - | - | - | + | + | Yellow cream precipitate | |
| Flavonoids | Alkaline reagent test | + | + | + | + | + | + | + | + | + | Intense yellow color with alkali and colorless with acids |
| Lead acetate test | + | + | + | + | + | + | + | + | + | Formation of yellow precipitate | |
| Phenols | Ferric chloride test | + | + | + | + | + | + | + | + | + | Bluish black precipitate |
| Lead acetate test | + | + | + | + | + | + | + | + | + | Yellow color isformed |
Table 4: Phytochemical screening of selected plants
Total phenol content
The quantitative determination of total phenolic content was carried out using Folin-Ciocalteu reagent in terms of gallic acid equivalent (GAE). The calibration curve of gallic acid. Table 5shows total phenolic content expressed as µg gallic acid equivalent per milligram dry extract weight.
| S.No | Plants | Total phenolic content (µg GAE/mg dry wt. of extract |
|---|---|---|
| 1 | Allium cepa | 22.22 ± 0.46 |
| 2 | Allium sativum | 16.48 ± 0.65 |
| 3 | Cinnamomum tamala | 11.35 ± 0.44 |
| 4 | Ficus religiosa | 124.24 ± 0.70 |
| 5 | Magnifera indica | 182.09 ± 0.3 |
| 6 | Mimosa pudica | 70 ± 0.73 |
| 7 | Ocimum tenuiflorum | 167.87 ± 0.30 |
| 8 | Rubus ellipticus | 51.59 ± 0.53 |
| 9 | Solanum tuberosum | 10.68 ± 0.70 |
Note: Data were expressed in mean ± SD (n=3)
Table 5: Total phenolic content of selected plant samples
Total flavonoids content
Quantitative determination of total flavonoid was performed by precipitating with aluminium chloride in an alkalinized medium. The calibration curve with quercetin as the standard is shown in figure while Table 6shows the total flavonoid content of plant extracts
| S.No | Plants | Total flavonoid content (µg QE/mg dry wt. of extract |
|---|---|---|
| 1 | Allium cepa | 143.44 ± 10 |
| 2 | Allium sativum | 766 ± 24.20 |
| 3 | Cinnamomum tamala | 712.85 ± 9.92 |
| 4 | Ficus religiosa | 788.34 ± 6.26 |
| 5 | Magnifera indica | 2949.49 ± 8.97 |
| 6 | Mimosa pudica | 402 ± 4.48 |
| 7 | Ocimum tenuiflorum | 514.33 ± 13.38 |
| 8 | Rubus ellipticus | 1569.36 ± 9.15 |
Table 6: Total flavonoid content of selected plant samples
Discussion
In our study, nine different plants were collected from Deurali, Rupatal, Rajakochautara and Bijaypur of Kaski district. Ethnobotanical information revealed that the selected plants in this study are traditionally used for various medicinal purposes and possess different pharmacological properties. The thesis work included phytochemical screening, determination of total phenol and flavonoid content, in vitro SPF of selected nine plants, formulation and evaluation of sunscreen cream from most potent plant extract. Phytochemical screening was performed for different phytochemical constituents such as alkaloids, phenols and flavonoids. Ethanolic extract of all the plants showed the presence of phenols and flavonoids. According to Mustapha AA, et al., 2014, M. indica showed the presence of phenols, flavonoids and alkaloids which supports this research work. According to Makhija IK, et al., 2010, Ficus religiosa showed the presence of flavonoids which supports this research work.
M. indica showed highest amount of phenolic content. Total phenolic content of M. indica was found to be in the range of 63.89 to 116.80 mg GAE/g dry weight of extract in 80% methanol (Sultana B, et al., 2012), but in this research work total phenolic content of this plant was found to be 182 mg GAE/g dry weight of extract in ethanol. This variation may be due to variation in solvent. The plant having high phenolic content showed the highest SPF value (Ebrahimzadeh MA, et al., 2014), which supports our study. Flavonoids do not seem to be simply detectable by any method therefore AlCl3 was used as a complexing reagent. The method is based on the formation of a stable complex between AlCl3 keto and hydroxyl group of flavones and flavonoids (Hassan SM, et al., 2013). M. indica showed the highest flavonoid content. Total phenolic content of the M. indica was found to be 925.55 CE/g dry weight of extract in 80% methanol (Sultana et al., 2012). In this research, total flavonoids content of ethanolic extract of M. indica bark was found to be 2949 mg QE/g dry weight of extract. This may be due to variation in solvent and may be due to variation in comparison with standard where catechin was used in previous work and quercetin in this work. The plant having high flavonoid content showed the highest SPF value (Costa SC, et al., 2015). From above discussion we can conclude that there may be correlation between phenolic flavonoid compounds and SPF value. Flavonoids and phenols have been reported as an important functional compound in plants which play a vital role in management of inflammation due to solar radiation (Imam S, et al., 2015).
There is growing demand for herbal cosmetics in the world (Aswal A, et al., 2013). Therefore, an herbal sunscreen cream containing ethanolic extract of M. indica was prepared. Four formulations were prepared and evaluated for various parameters such as pH, acid value, saponification value, in vitro SPF and stored at three different temperatures for their stability testing (Table 7). The measurement of SPF is an ultimate way to determine the effectiveness of the sunscreen formulation. The higher the SPF the more protection the sunscreen offers against UV light. Sunscreen is used to aid the body’s defense mechanism to protect against harmful UV-radiation from the Sun (Lohani A, et al., 2018). Here, in this research work, the formulation containing 5% plant extract showed the highest SPF value. Therefore, this formulation may protect skin against UV-radiation. Acid value is the mass of KOH in mg that is required to neutralize one gram of chemical substance. Higher the acid value, it will cause irritation to skin after application (Fatima S, et al., 2017; Swarnlata S, et al., 2011). Saponification value number represents the number of mg of KOH required to saponify 1g of fat. Saponification value influences pH and stability of cream. Higher saponification value means higher amount of free fatty acid which are prone to hydrolysis and can cause rancidification (Saraf S, et al., 2011). In our research work it is found that the saponification value is decreasing on increasing the concentration of plant extract.
| EE*I | Absorbance | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| A1X1 | A1G1 | A1C1 | A1M1 | A1R1 | A1T1 | A1P1 | A1B1 | A1Z1 | ||
| 290 | 0.015 | 0.172 | 0.206 | 0.396 | 0.207 | 0.917 | 0.547 | 0.481 | 0.182 | 0.439 |
| 295 | 0.081 | 0.161 | 0.187 | 0.334 | 0.176 | 0.89 | 0.505 | 0.385 | 0.173 | 0.431 |
| 300 | 0.287 | 0.129 | 0.171 | 0.307 | 0.149 | 0.87 | 0.465 | 0.28 | 0.168 | 0.431 |
| 305 | 0.327 | 0.143 | 0.158 | 0.295 | 0.135 | 0.847 | 0.428 | 0.237 | 0.161 | 0.437 |
| 310 | 0.186 | 0.142 | 0.146 | 0.292 | 0.124 | 0.819 | 0.394 | 0.208 | 0.16 | 0.446 |
| 315 | 0.037 | 0.135 | 0.135 | 0.294 | 0.112 | 0.776 | 0.362 | 0.191 | 0.159 | 0.448 |
| 320 | 0.018 | 0.127 | 0.123 | 0.297 | 0.1 | 0.707 | 0.331 | 0.181 | 0.158 | 0.443 |
| SPF | - | 1.39 ± 0.3 | 1.6 ± 0.13 | 3.03 ± 0.16 | 1.39 ± 0.08 | 8.44 ± 0.35 | 4.33 ± 0.43 | 2.55 ± 0.01 | 1.64 ± 0.06 | 4.38 ± 0.13 |
Note: EE-Erythemal Effect spectrum; I-Solar intensity Spectrum and the data was expressed in mean ± SD (n=3).
Table 7: SPF value of selected plant samples
This study revealed that formulations I,II and III were found to be stable at all storage conditions except the formulation IV, which was unstable and resulted in breakdown of emulsion, at 40 ± 2°C from the 7th day. The formulation IV, which is kept at 40 ± 2°C, was found to be unstable i.e. liquefaction started at increased temperature along with increase in concentration of extract. Monitoring the pH of cream is necessary for determining the stability of pharmaceutical and cosmeceuticals (Table 8). Any changes in the pH of the product indicate the possible interaction which may provide an idea on the quality of the product (Smaoui S, et al., 2012). The pH of the human skin normally ranges from 4.5 to 6. Due to frequent washing and use of soap, the acidity of the skin is lost.
| Temperature | Cream | pH | Appearance (Color, homogeneity) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 0th day | 7th day | 14th day | 21st day | 0th day | 7th day | 14th day | 21th day | ||
| 4°C | Base | - | 6.12 | 6.13 | 6.14 | White, homogenous | White, homogenous | White, homogenous | white, homogenous |
| 1% Extract | - | 5.57 | 6.09 | 6.11 | Light brown, homogenous | Light brown, homogenous | Light brown, homogenous | Light brown, homogenous | |
| 2.5% Extract | - | 5.46 | 5.79 | 5.86 | Light brown, homogenous | Light brown, homogenous | Light brown, homogenous | Light brown, homogenous | |
| 5% Extract | 5.04 | 5.38 | 5.38 | Dark brown, homogenous | Dark brown, homogenous | Dark brown, homogenous | Dark brown, homogenous | ||
| Room temperature | Base | 6.16 | 6.22 | 6.3 | 6.55 | White, homogenous | White, homogenous | White, homogenous | white, homogenous |
| 1% Extract | 5.15 | 5.48 | 5.68 | 5.8 | Light brown, homogenous | Light brown, homogenous | Light brown, homogenous | Light brown, homogenous | |
| 2.5% Extract | 5.3 | 5.43 | 5.63 | 5.69 | Light brown, homogenous | Light brown, homogenous | Light brown, homogenous | Light brown, homogenous | |
| 5% Extract | 5.03 | 5.13 | 5.16 | 5.3 | Dark brown, homogenous | Dark brown, homogenous | Dark brown, homogenous | Dark brown, homogenous | |
| 40°C | Base | - | 5.37 | 5.42 | 5.88 | White, homogenous | White, homogenous | White, homogenous | white, homogenous |
| 1% Extract | - | 5.01 | 5.1 | 5.26 | Light brown, homogenous | Light brown, homogenous | Light brown, homogenous | Light brown, homogenous | |
| 2.5% Extract | - | 5.01 | 5.09 | 5.2 | Light brown, homogenous | Light brown, homogenous | Light brown, homogenous | Light brown, homogenous | |
| 5% Extract | - | 5.04 | 5.23 | 5.29 | Dark brown, non- homogenous | Dark brown, non- homogenous | Dark brown, non- homogenous | Dark brown, non- homogenous | |
Note: All the results were expressed in mean ± SD
Table 8: Stability results of different creams at 3 different temperatures
Therefore, moisturizer has an acidic range which is used to normalize the skin. Acceptable pH range of the moisturizer should be between 5-8. The pH of the cream was found to be in the range 5.01-6.76. The result revealed that the pH of the cream was within the range (Saraf S, et al., 2011). The formulations have almost constant pH throughout the study. The appearance of the cream was not changed. In this study sunscreen cream containing 1% extract showed acid value and saponification value 8.6 ± 0.41 and 30.4 ± 0.83 respectively. In a previous research, antiaging facial cream containing 1% curcumin extract was formulated, which showed acid value and saponification value of 5.7 and 25.7 respectively (Panda S, et al., 2018). This difference in acid value and saponification value may be due to ingredients used in cream and chemical constituents present in extract (Table 9). According to Akter S, et al. 2013, on varying the concentration of stearic acid and cetyl alcohol, saponification value also varies. Saponification value goes on decreasing on increasing the concentration of those constituents. In this study, the saponification value goes on decreasing on increasing the concentration of extract.
| Cream | Acid value | Saponification value | SPF |
|---|---|---|---|
| Base | 10.86 ± 0.32 | 33.25 ± 0.822 | 0.64 ± 0.011 |
| 1% extract | 8.60 ± 0.41 | 30.4 ± 0.83 | 1.26 ± 0.05 |
| 2.5% extract | 14.21 ± 0.33 | 24.7 ± 0.91 | 2.35 ± 0.03 |
| 5% extract | 5.98 ± 0.647 | 23.27 ± 1.645 | 7.19 ± 0.12 |
Table 9: Acid value, saponification value and SPF of the cream stored at room temperature
According to Pratama G, et al., 2019, the cream having high amount of extract was very good for counteracting UV rays because it has high SPF value when compared to other formulations. Also, in this study, the formulation containing 5% extract showed the highest SPF i.e. 7.19 ± 0.12.
Conclusion
The study provided reasonable data to conclude that Magnifera indica has highest phenolic and flavonoid value which may have resulted in the highest SPF value among the nine medicinal plants taken during the study. The sunscreen cream, which is o/w type emulsion, containing different concentration yields good physical characteristics formulation. Method used for the evaluation of sunscreen cream, used in this work, is simple, fast, economical and easy to use.
Acknowledgement
We would like to express our humble gratitude to our supervisor, Kalpana Parajuli, Assistant Professor, School of Health and Allied Sciences, Pokhara University, for her keen and continuous interest, valuable suggestion and guidance throughout the course of research.
We would like to express our sincere thanks to Dr. Khem Raj Joshi, former Dean, School of Health and Allied Sciences, Pokhara University and Dr. Damaru Prasad Paneru, Director of School of Health and Allied Sciences, Mr. Nim Bahadur Dangi, Assistant Professor and program coordinator of B. Pharm. and Mr. Atismmodavardhana Kaundinnyayana, Assistant Professor and program coordinator of M. Pharm, Pokhara University for their continuous correspondence which helped us to work efficiently during our research.
We are thankful to Associate Professor Dr. Nirmala Jamarkattel, Assistant Professor Dr. Sushil Panta, Assistant Professor Dr. Shila Gurung, Assistant Professor Dr. Namraj Dhami, and all the faculty members of School of Health and Allied Sciences, Pokhara University, for their kind support and suggestions to perform our research work.
We are also thankful to Santosh Gurung and Durga Bahadur BK, Lab Assistants, and all the staffs of School of Health and Allied Sciences, Pokhara University, for their support and help.
Our best regard goes to investigation officers Krishna Dahal, Ganga Dutta Bhatta and their colleagues for helping us for the identification of plant species.
We are also thankful to our seniors and all our classmates for their kind support and guidance throughout our research work.
References
- Larbie CO, Nyarkoh CO, Adjei CO. Phytochemical and safety evaluation of hydroethanolic leaf extract of Tecoma stans (L.) Juss. ex Kunth. Evid Based Complement Alternat Med. 2019.
[Crossref] [Google scholar] [Pubmed]
- Rebeiro AS, Estanqueiro M, Oliveira MB, Lobo JMS. Main benefits and applicability of plant extracts in skin care products. Cosmetics. 2015; 2(2): 48-65.
- Lohani A, Miasra AK, Verma A. Cosmeceutical potential of geranium and calendula essential oil: Determination of antioxidant activity and in vitro sun protection factor. J Cosmet Dermatol. 2018; 18: 163-172.
[Crossref] [Google scholar] [Pubmed]
- Petruk G, Giudice RD, Rigano MM, Monti DM. antioxidants from plants protect against skin photoaging. Oxidative Med Cell Longev. 2018.
[Crossref] [Google scholar] [Pubmed]
- Yang Y, Li S. Dandelion extracts protect human skin fibroblasts from uvb damage and cellular senescence. Oxid Med Cell Longev. 2015; 15: 12-22.
[Crossref] [Google scholar] [Pubmed]
- Korać RR, Khambholja KM. Potential of herbs in skin protection from ultraviolet radiation. Pharmacog rev. 2011; 5(10): 164.
[Crossref] [Google scholar] [Pubmed]
- Dutra EA, Oliveira DA, Hackmann EKR, Santoro MI. Determination of Sun Protection Factor (SPF) of sunscreens by ultraviolet spectrophotometry. Rev Ciênc Farm Básica Apl. 2004; 40: 381-385.
- Mendonsa NS, Pradhan A, Sharma P, Prado RM, Murthy SN, Kundu S, et al. A quality by design approach to develop topical creams via hot-melt extrusion technology. Eur J Pharm Sci. 2019; 136: 104948.
[Crossref] [Google scholar] [Pubmed]
- El-Gied AA, Abdelkareem AM, Hamedelniel EI. Investigation of cream and ointment on antimicrobial activity of Mangifera indica extract. J Adv Pharm Technol Res. 2015; 6(2): 53.
[Crossref] [Google scholar] [Pubmed]
- Aswal A, Kalra M, Rout A. Preparation and evaluation of polyherbal cosmetic cream. Der Pharm Lett. 2013; 5(1): 83-88.
- Zhang J, Yuan K, Zhou WL, Zhou J, Yang P. Studies on the active components and antioxidant activities of the extracts of Mimosa pudica Linn. from Southern China. Pharmacogn Mag. 2011; 7(25): 35.
[Crossref] [Google scholar] [Pubmed]
- Zhishen J, Mengcheng T, Jianming W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food chem. 1999; 64(4): 555-559.
- Farboud ES, Nasrollahi SA, Tabbakhi Z. Novel formulation and evaluation of a Q10-loaded solid lipid nanoparticle cream: In vitro and in vivo studies. Int J Nanomedicine. 2011; 6: 611.
[Crossref] [Google scholar] [Pubmed]
- Joshi P, Joshi S, Rajani U, Semwal RB, Semwal DK. Formulation and evaluation of polyherbal cream and lotion for the treatment of psoriasis-induced secondary infections. Curr Rev Clin Exp Pharmacol Curr Clin Pharmacol. 2021; 16(1): 79-96.
[Crossref] [Google scholar] [Pubmed]
- Smaoui S, Hlima HB, Jarraya R, Kamoun NG, Ellouze R, Damak M. Cosmetic emulsion from virgin olive oil: Formulation and bio-physical evaluation. Afr J Biotechnol. 2012; 11(40): 9664-9671.
- Mustapha AA, Enemali MO, Olose M, Owuna G, Ogaji JO, Idris MM et al. Phytoconstituents and antibacterial efficacy of mango (Magnifer indica) leaves extracts. J Med Plants Stud. 2014; 2(5): 19-23.
- Makhija IK, Sharma IP, Khamar D. Phytochemistry and pharmacological properties of Ficus religiosa: An overview. Ann Biol Res. 2010; 1(4): 171-180.
- Sultana B, Hussain Z, Asif M, Munir A. Investigation on the antioxidant activity of leaves, peels, stems bark, and kernel of mango (Mangifera indica L.). J Food Sci. 2012; 77(8): 849-852.
[Crossref] [Google scholar] [Pubmed]
- Ebrahimzadeh MA, Enayatifard R, Khalili M, Ghaffarloo M, Saeedi M, Charati JY. Correlation between sun protection factor and antioxidant activity, phenol and flavonoid contents of some medicinal plants. Iran J Pharm Res. 2014; 13(3): 1041-1047.
[Google scholar] [Pubmed]
- Hassan SM, Al Aqil AA, Attimarad M. Determination of crude saponin and total flavonoids content in guar meal. Adv Med Plant Res. 2013; 1(2): 24-28.
- Costa SC, Detoni CB, Branco CR, Botura MB, Branco A. In vitro photoprotective effects of Marcetia taxifolia ethanolic extract and its potential for sunscreen formulations. Rev Bras Farmacogn. 2015; 25: 413-418.
- Imam S, Azhar I, Mahmood Za. In-vitro evaluation of sun protection factor of a cream formulation prepared from extracts of Musa accuminata (L.), Psidium gujava (L.) and Pyrus communis (L.). In-vitro. 2015; 8(3): 234-237.
- Fatima S, Zaman R, Haider N, Shamsi S, Alam A. Design and development of Unani anti-inflammatory cream. J Ayurveda Integr Med. 2017; 8(3): 140-144.
[Crossref] [Google scholar] [Pubmed]
- Swarnlata S, Gunjan J, Chanchal DK, Shailendra S. Development of novel herbal cosmetic cream with Curcuma longa extract loaded transfersomes for antiwrinkle effect. Afr J Pharmacy Pharmacol. 2011; 5(8): 1054-1062.
- Saraf S, Sahu S, Kaur CD, Saraf S. Comparative measurement of hydration effects of herbal moisturizers. Pharmacog res. 2010; 2(3): 146.
[Crossref] [Google scholar] [Pubmed]
- Panda S. Extraction, formulation and evaluation of antiaging curcumin facial cream. J emerg technol innov res. 2018; 5(3): 1369-1371.
- Akter S, Ali MA, Barman RK, Rahman BM, Imam M, Wahed I. In vitro antioxidant and cytotoxic activity of ethanolic extract of Cinnamomum tamala (Tejpat) leaves. Int J Pharm Sci Res. 2015; 6(3): 532-536.
- Pratama G, Yanuarti R, Ilhamdy AF, Suhana MP. Formulation of sunscreen cream from Eucheuma cottonii and Kaempferia galanga (zingiberaceae). IOP Conf Ser.: Earth Environ Sci. 2019; 278(1): 012062.
Author Info
Bindu Poude*, Akash Gurung, Hari Prasad Subedi, Sagar Babu KC and Anita Tiwari Kalpana ParajuliCitation: Poudel B: In Vitro Sun Protection Factor Determination of Selected Medicinal Plants and Formulation of Sunscreen Cream
Received: 15-Sep-2022 Accepted: 10-Oct-2022 Published: 17-Oct-2022, DOI: 10.31858/0975-8453.13.10.664-671
Copyright: This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
ARTICLE TOOLS
- Dental Development between Assisted Reproductive Therapy (Art) and Natural Conceived Children: A Comparative Pilot Study Norzaiti Mohd Kenali, Naimah Hasanah Mohd Fathil, Norbasyirah Bohari, Ahmad Faisal Ismail, Roszaman Ramli SRP. 2020; 11(1): 01-06 » doi: 10.5530/srp.2020.1.01
- Psychometric properties of the World Health Organization Quality of life instrument, short form: Validity in the Vietnamese healthcare context Trung Quang Vo*, Bao Tran Thuy Tran, Ngan Thuy Nguyen, Tram ThiHuyen Nguyen, Thuy Phan Chung Tran SRP. 2020; 11(1): 14-22 » doi: 10.5530/srp.2019.1.3
- A Review of Pharmacoeconomics: the key to “Healthcare for All” Hasamnis AA, Patil SS, Shaik Imam, Narendiran K SRP. 2019; 10(1): s40-s42 » doi: 10.5530/srp.2019.1s.21
- Deuterium Depleted Water as an Adjuvant in Treatment of Cancer Anton Syroeshkin, Olga Levitskaya, Elena Uspenskaya, Tatiana Pleteneva, Daria Romaykina, Daria Ermakova SRP. 2019; 10(1): 112-117 » doi: 10.5530/srp.2019.1.19
- Dental Development between Assisted Reproductive Therapy (Art) and Natural Conceived Children: A Comparative Pilot Study Norzaiti Mohd Kenali, Naimah Hasanah Mohd Fathil, Norbasyirah Bohari, Ahmad Faisal Ismail, Roszaman Ramli SRP. 2020; 11(1): 01-06 » doi: 10.5530/srp.2020.1.01
- Manilkara zapota (L.) Royen Fruit Peel: A Phytochemical and Pharmacological Review Karle Pravin P, Dhawale Shashikant C SRP. 2019; 10(1): 11-14 » doi: 0.5530/srp.2019.1.2
- Pharmacognostic and Phytopharmacological Overview on Bombax ceiba Pankaj Haribhau Chaudhary, Mukund Ganeshrao Tawar SRP. 2019; 10(1): 20-25 » doi: 10.5530/srp.2019.1.4
- A Review of Pharmacoeconomics: the key to “Healthcare for All” Hasamnis AA, Patil SS, Shaik Imam, Narendiran K SRP. 2019; 10(1): s40-s42 » doi: 10.5530/srp.2019.1s.21
- A Prospective Review on Phyto-Pharmacological Aspects of Andrographis paniculata Govindraj Akilandeswari, Arumugam Vijaya Anand, Palanisamy Sampathkumar, Puthamohan Vinayaga Moorthi, Basavaraju Preethi SRP. 2019; 10(1): 15-19 » doi: 10.5530/srp.2019.1.3