Research Article - (2022) Volume 13, Issue 11
Enhancing Cationic Surfactants’ Inactivation of Non-Enveloped Viruses by Sulfate Ions
Rei Saito1*, Seiichi Tobe1, Miyuki Miyake1, Yukari Sekine1, Takeshi Takizawa1, Junichi Sugiyama1, Yasushi Kakizawa1 and Shigeru Morikawa2Abstract
Quaternary cationic surfactants with bactericidal activity such as Didecyldimethylammonium chloride (DDAC) show an inactivation effect on enveloped viruses, but they have little effect on non-enveloped viruses such as noroviruses. Therefore, we examined additives that enhance the inactivation effect of cationic surfactants by using Feline calicivirus (FCV) as a representative of a non-enveloped virus. The result was that SO42- ions, which are a general-purpose salt, had a strong salting-out effect that reduced the solubility of proteins, greatly enhancing the inactivation ability of DDAC. The SO42- ions also enhanced FCV inactivation by other cationic surfactants such as Cetylpyridinium Chloride (CPC) and Benzalkonium chloride (ADBAC). To clarify the mechanism, we evaluated the denaturation and binding process of DDAC to Bovine Serum Albumin (BSA) as a model protein by means of Circular Dichroism (CD) spectrum and Isothermal Titration Calorimeter (ITC), respectively. The SO42- ions disturbed the protein structure by their salting- out effect and promoted cooperative binding from lower DDAC concentrations by reducing the Critical Micelle Concentration (CMC), indicating that these synergistic effects caused a large structural change in the protein. These results suggested that increasing the protein denaturation of the cationic surfactants by adding SO42- ions enhanced the inactivation effect on the non-enveloped virus.
Keywords
Non-enveloped virus, Feline calicivirus (FCV), Inactivation effect, Cationic surfactant, SO42- ions, Protein denaturation, Interaction
Introduction
Based on the experience of the COVID-19 (SARS-CoV-2) pandemic, we expect that safer and easier-to-handle agents for virus inactivation will appear as a technology against viruses that may threaten our daily lives in the future (Ban M, 2021). SARS-CoV-2 is an enveloped virus with a phospholipid membrane on its surface. This virus can be easily inactivated by using solvents such as 30% ethanol (Kratzel A, et al., 2020). Anionic, cationic, nonionic and amphoteric surfactants used in detergents and cosmetics also exhibit the inactivation effects (Yokohata R, et al., 2020). In particular, cationic surfactants used as bactericides, such as Benzalkonium Chloride (ADBAC) and Didecyldimethylammonium Chloride (DDAC), have inactivation effects at a very low concentration on enveloped viruses such as SARS-CoV-2 and influenza viruses, so they are used for effective disinfectants (NITE, 2022).
On the other hand, non-enveloped viruses such as noroviruses are more difficult to inactivate than enveloped viruses because they do not have a lipid membrane and are composed of capsid, which is an outer shell of protein tightly bound to viral DNA or RNA (Block SS, 2002). Thus, it is considered to be difficult to disintegrate the outer shell of non-enveloped viruses and inactivate them by surfactants alone.
Recently, it is generally considered that a reduction in viral infection titer of 99% or 99.9% more (Δ log ≥ 2 or 3) is required to demonstrate sufficient viral inactivation (e.g., ISO, 2014; EPA, 2022). By this standard, no cationic surfactants, even those with bactericidal effects, are sufficiently effective (Doultree JC, et al., 1999; Bélec L, et al., 2000).
Belec L, et al. reported the inactivation effect of ADBAC on non-enveloped viruses such as Adenovirus (ADV) and Enterovirus (ENV) (Bélec L, et al., 2000). ADBAC inactivated more than 99% of ADV at 1.4 mM for 20 minutes of contact, and only 90% (Δ log=1) inactivation of ENV was observed at 1.4 mM for 60 minutes of contact. Therefore, cationic surfactants have been combined with strong oxidants and other agents with safety and handling issues in order to enhance inactivation (Zonta W, et al., 2016; Whitehead K and McCue KA, 2010).
Zonta W, et al. found that the addition of isopropanol and glutaraldehyde to DDAC in the formulation enhanced the reduction of infectivity of Feline calicivirus (FCV) (Zonta W, et al., 2016). Whitehead K and McCue KA reported that an increase in pH of DDAC solution from 8.0 to 12.2 resulted in enhancement of virus inactivation (Whitehead K and McCue KA, 2010). However, these simulative and highly alkaline conditions have safety issues for human use. Accordingly, there is a need to develop combination agents that safely and effectively enhance the inactivation ability of cationic surfactants against non-enveloped viruses.
Zonta W, et al. found that the addition of isopropanol and glutaraldehyde to DDAC in the formulation enhanced the reduction of infectivity of Feline calicivirus (FCV) (Zonta W, et al., 2016). Whitehead K and McCue KA reported that an increase in pH of DDAC solution from 8.0 to 12.2 resulted in enhancement of virus inactivation (Whitehead K and McCue KA, 2010). However, these simulative and highly alkaline conditions have safety issues for human use. Accordingly, there is a need to develop combination agents that safely and effectively enhance the inactivation ability of cationic surfactants against non-enveloped viruses.
Materials and Methods
Materials
Feline calicivirusF-9 (VR-782) (FCV) for inactivation evaluation, which is an internationally recommended non-enveloped virus as a surrogate virus of norovirus (EPA, 2022), and Crandell Rees Feline Kidney (CCL-94) (CRFK) cells for FCV inactivation assay were purchased from American Type Culture Collection (ATTC). Didecyldimethylammonium Chloride (DDAC>80%, supplied by Lion Specialty Chemicals, Inc.), Cetylpyridinium chloride monohydrate (CPC, special grade, code number 190177, Fuji Film Wako Pure Chemical Industries Co., Ltd.), Cetyltrimethylammonium chloride (CTAC>95%, code number H0082, Tokyo Kasei Inc.) and dodecyl Benzalkonium chloride (ADBAC>99%, code number 13373-10G-F, Sigma-Aldrich) were obtained. Sodium sulfate (special grade, code number 37280-00, Kanto Chemical Co., Ltd.) and sodium chloride (special grade, code number 19015-0301, Junsei Chemical Co., Ltd.), and calcium chloride (special grade, code number 135-00165, Fuji Film Wako Pure Chemical Industries Co., Ltd.) and Bovine Serum Albumin (BSA>98%, Lot. SLCH8449, code number A7906-50G, Sigma-Aldrich) were purchased. The virus inactivation evaluation samples were diluted with distilled water (Milli-Q).
Inactivation effects of virus inactivation assay
The methods for culturing CRFK cells and for evaluating the viral infection titer were conducted in accordance with JIS 1922 (ISO, 2014). The CRFK cells were cultured in Eagle’s Minimal Essential Medium (EMEM, code number M4655-500ML, Sigma-Aldrich) supplemented with 1% Penicillin and Streptomycin (PS, code number P4333-100ML, Sigma-Aldrich) as an antibiotic and 10% Fetal bovine serum (code number 173012, Sigma-Aldrich) under a CO2 concentration of 5% at 37℃. FCV was mixed with various surfactants with or without additives and the inactivation effect of surfactants on FCV was determined as an infectivity titer by the following steps: First, the 0.05 ml of FCV solution that showed the average initial virus infectivity titer of 3.5 × 108 50% Tissue Culture Infectious Dose (TCID50/ml) was exposed to 0.45 ml of the sample solution of the cationic surfactants for an indicated time. Then the mixture of the FCV and sample solution was serially diluted in the culture medium, and inoculated on the CRFK cells prepared in 96 well microplates. The infectivity of FCV after treatment of the sample solution was obtained as TCID50 and the virus inactivation effect of the cationic surfactants was expressed by the reduction rate in log10, Δ log (Equation 1).
Δ log(Reduction rate of infectivity titer)=log(Infectivity titer upon treatment with control solution)-log(Infectivity titer upon treatment with sample solution) (1)
The control solution means EMEM supplemented with 1% PS which was used for FCV cultivation.
Δ log=1 means that 90% of the tested viruses have been inactivated.
Analysis of protein denaturation upon Didecyldimethylammonium Chloride (DDAC) treatment
DDAC solutions with and without additives, and BSA (100 ppm) solution as a model protein, were prepared using the medium-mixed solution of Water:EMEM medium containing 1% PS=9:1. After 10 minutes of adjusting the solution, the Circular Dichroism polarization spectrum of BSA was measured by means of a Circular Dichroism spectrometer (Jasco J-720, cell path length=1 mm) at room temperature. Since the negative molecular ellipticity, [θ], with the α-helix structure of BSA has double minimums near 210 nm and 220 nm, the protein denaturation rate was calculated from the change in molecular ellipticity (θ) at 220 nm (Obe K, et al., 1980) (Equation 2).
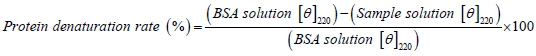
Interaction of Didecyldimethylammonium Chloride (DDAC) with protein
The interaction of DDAC that caused the denaturation of the model protein BSA was evaluated by means of an Isothermal Titration Calorimeter (VP-ITC, MicroCal). Solutions with BSA and DDAC were prepared with the medium-mixed solution. The BSA (300 ppm) solution was put into the cell of the calorimeter, and 16.6 mM DDAC and 16.6 mM DDAC+10 mM Na2SO4 solutions in the syringe were titrated by 50 drops of 2 μL at 30℃.
The interaction heat of DDAC with BSA was calculated by subtracting the heat of dilution generated by titrated DDAC to the medium-mixed solution from the heat of the reaction of titrated DDAC to BSA solution (Equation 3).
Heat of interaction of DDAC with BSA=(Heat of reaction of DDAC and BSA)-(Heat of dilution of DDAC in medium-mixed solution) (3)
Results and Discussion
Effects of salts on inactivation of Feline calicivirus (FCV) by Didecyldimethylammonium Chloride (DDAC)
First, we confirmed the inactivation level of the bactericidal cationic surfactants with activity against FCV used in this study. At a contact time of 1 minute, DDAC solutions at the concentration from 0.5 to 3 mM showed the inactivation of FCV at Δ Log=1 or less, indicating a little inactivation effect of DDAC on the non-enveloped FCV (data not shown). The inactivation of FCV requires denaturation and/or disruption of the capsid structure (McDonnell G and Russell AD, 1999). Originally, the protein denaturation ability of surfactants was reported to be increased with their concentration and saturated at CMC (Obe K, et al., 1980). In the mediummixed solution in which the virus inactivation was evaluated, the concentration of DDAC range tested in this study was above the CMC, 0.44 mM, thus its inactivation effect on FCV was considered to be saturated. As a result, we confirmed that DDAC alone did not sufficiently inactivate FCV.
Therefore, we investigated the effects of salt on FCV inactivation. Salts dissolve and dissociate in water, and the dissolved state of proteins is affected by the salting-out effect of ions, which is determined by their concentration, charge, and size. The effect of DDAC on FCV inactivation was examined in the presence of Na2SO4, which are known to have a high salting-out effect. As shown in Figure 1, the inactivation effect of DDAC at 0.5 mM was significantly increased with 10 mM of Na2SO4 by Δ log=2 at 10 mM for 1 minute. This enhancement tended to decrease inversely with higher concentration of Na2SO4 such as 30 mM and 50 mM.
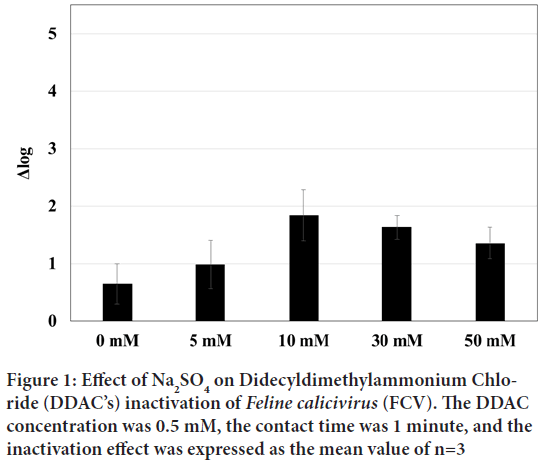
Figure 1: Effect of Na2SO4 on Didecyldimethylammonium Chloride (DDAC’s) inactivation of Feline calicivirus (FCV). The DDAC concentration was 0.5 mM, the contact time was 1 minute, and the inactivation effect was expressed as the mean value of n=3
Since the addition of Na2SO4 enhanced significantly the effect of DDAC on inactivation of FCV, we also investigated the effects of ion species with other salts. The concentrations of various salts were set under the same ionic strength conditions as the 10 mM Na2SO4 that showed the greatest enhancement effect of DDAC.
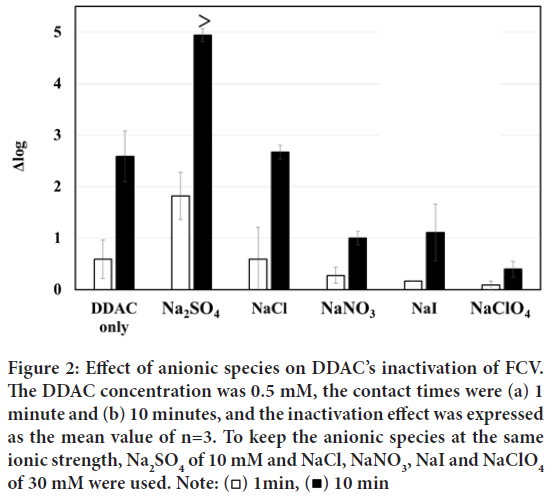
First, the cation species of the various salts were fixed to Na+ ion, and the effects of anionic species (SO42-, Cl-, NO3-, I-, ClO4-) were evaluated as shown in Figure 2.
Figure 2: Effect of anionic species on DDAC’s inactivation of FCV.
The DDAC concentration was 0.5 mM, the contact times were (a) 1
minute and (b) 10 minutes, and the inactivation effect was expressed
as the mean value of n=3. To keep the anionic species at the same
ionic strength, Na2SO4 of 10 mM and NaCl, NaNO3, NaI and NaClO4 of 30 mM were used. Note:  10 min
10 min
At a contact time of 1 minute between the sample solutions (DDAC+salts) and FCV solution, the additions of Cl-, NO3-, I-, and ClO4- ions hardly increased any Δ log value, except for SO42- ions. When the contact time was increased to 10 minutes, the inactivation ability of DDAC alone increased to Δ log=2 or more. In contrast, the added SO42- ions at a contact time of 10 minutes extremely enhanced the inactivation effect of DDAC resulting in Δ log ≥ 5, which was more than 103 times enhancement compared to that of DDAC without SO42- ions.
On the other hand, the addition of Cl- ions to DDAC resulted in the same level of inactivation as DDAC alone at contact times of both 1 minute and 10 minutes, respectively, indicating no enhancing effect. The additions of NO3-, I-, and ClO4- ions to DDAC resulted in less inactivation of FCV (Δ log=2 or less) than that of DDAC alone at both contact times of 1 and 10 minutes, respectively.
Then, the anionic species were fixed to Cl- ions, and the effects of the cationic species (Ca2+, Mg2+, Na+, (CH3)4N+) were evaluated in the same way. The inactivation effect at 10 minutes of contact time was approximately Δ log=2 for Ca2+, Na+, (CH3)4N+ ions, and less than Δ log=2 for Mg2+ ion, respectively. The results showed that the cationic ions had no effect, if any, on the enhancement of FCV inactivation by DDAC.
In conclusion, the enhancement of FCV inactivation among anionic species added to DDAC was demonstrated in the order of SO42->Cl->NO3->I- >ClO4-. This trend correspond
The effect of SO42- ions on inactivation of Feline calicivirus (FCV) by various cationic surfactants
We found the enhancement of FCV inactivation by adding SO2- ions to DDAC, as mentioned above, thus that to other cationic surfactants: CPC, CTAC, and ADBAC were investigated. The concentrations of DDAC and CPC in this analysis were 0.5 mM, while those of CTAB and ADBAC were 3 mM, respectively, since the inactivation effect of CTAB or ADBAC alone was very small at 0.5 mM (data not shown). The cationic surfactants alone at the concentrations described above showed only a low level of FCV in- activation at a contact time of 1 minute as shown in Figure 3a. The addition of SO2- ions to CPC and ADBAC slightly enhanced the inactivation effect, but that to CTAC was no effect on enhancement (Figure 3a).
In contrast, the inactivation effects of CPC and ADBAC alone were approximately Δ log=2 at a 10-minute contact time, and the addition of SO42- ions greatly enhanced their inactivation ability as shown in Figure 3b. This suggested that the combination of several cationic surfactants (DDAC, CPC, and ADBAC) and SO4 Figure 3b. This suggested that the combination of several cationic surfactants (DDAC, CPC, and ADBAC) and SO42- ions is able to promote the inactivation of non-enveloped viruses.
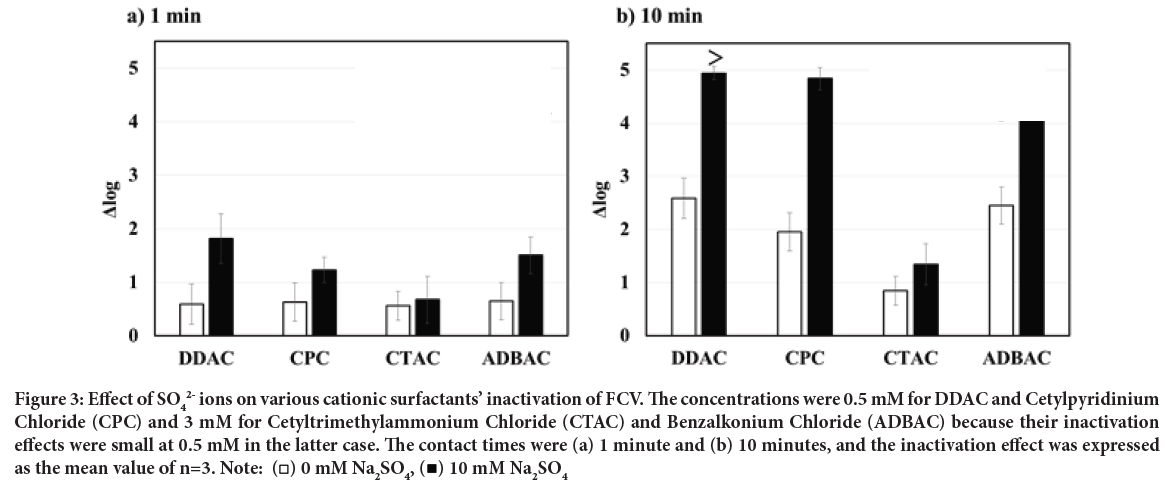
Figure 3: Effect of SO42- ions on various cationic surfactants’ inactivation of FCV. The concentrations were 0.5 mM for DDAC and Cetylpyridinium
Chloride (CPC) and 3 mM for Cetyltrimethylammonium Chloride (CTAC) and Benzalkonium Chloride (ADBAC) because their inactivation
effects were small at 0.5 mM in the latter case. The contact times were (a) 1 minute and (b) 10 minutes, and the inactivation effect was expressed
as the mean value of n=3. Note:  10 mM Na2SO4
10 mM Na2SO4
Furthermore, the inactivation enhancement effects of SO42- ions differed among the cationic surfactants in descending order of DDAC>CPC>ADBAC>CTAC. It is of particular interest that DDAC is a double-chain type with a large total carbon number, while CPC and ADBAC have an aromatic ring in the molecule even though they are single-chain types, thus it is possible that such a hydrophobic molecular structure affects the interaction of cationic surfactants in the protein denaturation process.
Effect of SO42- ions on the protein denaturation of Didecyldimethylammonium chloride (DDAC)
The enhancement of FCV inactivation by cationic surfactants and SO42- ions may be induced by the denaturation of the capsid structure of FCV. However, the capsids were not commercially available and were difficult to obtain. Therefore, we used BSA as a model protein, which is often used in studies of protein denaturation by surfactants, and compared the denaturation effect of DDAC alone to a mixture of DDAC and SO42- ions on the α-helical structure of BSA.
The CD spectra of BSA after adding DDAC with and without SO42- ions are shown in Figure 4. The molar molecular ellipticity of BSA before denaturation was reduced by the addition of 0.5 mM DDAC, and the denaturation ratio calculated from the molecular ellipticity at 222 nm was 33.5%. Strikingly, when the DDAC with 10 mM SO42- ions was added to BSA, the negative peak disappeared, the calculated denaturation ratio exceeded 100% in the measured solution, and precipitation of the BSA and DDAC complexes was observed. Furthermore, SO42- ions alone showed a denaturation ratio of only about 7.4%, suggesting that SO42- ions and DDAC synergistically affected the protein structure and induced a significant denaturation effect. We presume that this large denaturation effect affected the capsid of FCV, enhancing its inactivation ability.
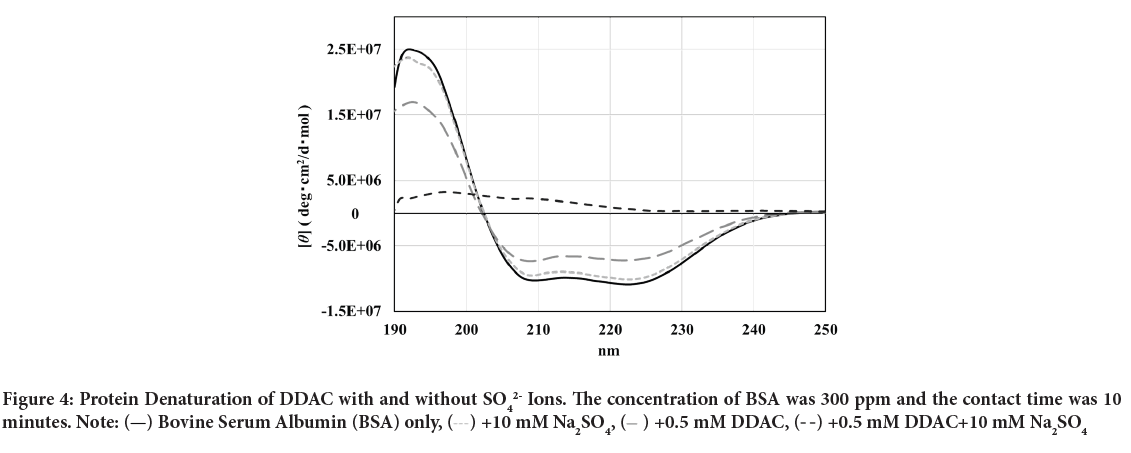
Figure 4: Protein Denaturation of DDAC with and without SO42- Ions. The concentration of BSA was 300 ppm and the contact time was 10
minutes. Note: 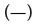 Bovine Serum Albumin (BSA) only,
Bovine Serum Albumin (BSA) only, 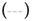 +10 mM Na2SO4,
+10 mM Na2SO4, 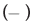 +0.5 mM DDAC,
+0.5 mM DDAC, 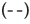 +0.5 mM DDAC+10 mM Na2SO4
+0.5 mM DDAC+10 mM Na2SO4
Effect of SO4 2- ions on the interaction of Didecyldimethylammonium Chloride (DDAC) with Bovine Serum Albumin (BSA)
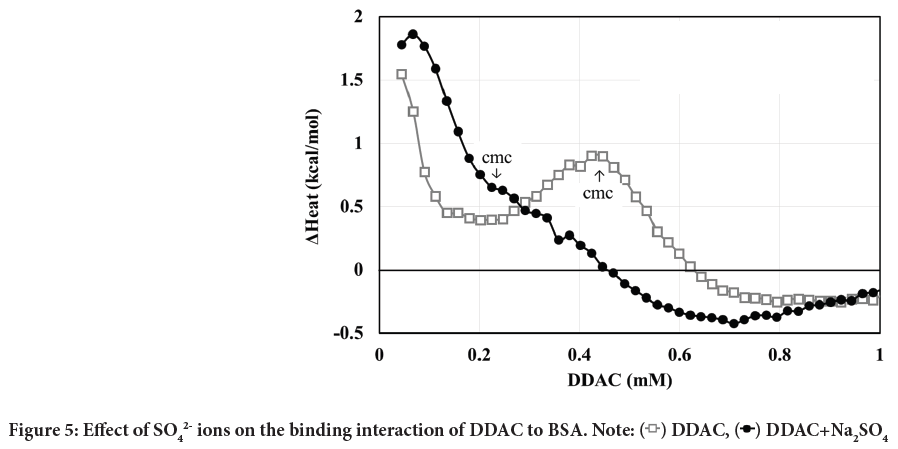
As described above, DDAC and SO42- ions seemed to synergistically enhance the protein denaturation effect. Therefore, we investigated how the SO42- ions changed the interaction of DDAC with BSA during the denaturation process of BSA. Figure 5 shows the heat of interaction curves of DDAC and BSA with and without SO42- ions by means of ITC.
Figure 5: Effect of SO42- ions on the binding interaction of DDAC to BSA. Note:  DDAC,
DDAC,  DDAC+Na2SO4
DDAC+Na2SO4
In general, when ionic surfactants interact with BSA, the monomers first bind to the high-affinity binding sites by electrostatic interaction at the lower concentration range of the surfactants. Thereafter, the amounts of bound surfactants increase through cooperative binding in which the hydrophobic interaction among surfactants is increased, and saturates at the concentration near CMC (Zhang S, et al., 2016; Aoki K, 1985). During the binding process, heat related to the interaction of surfactant molecules and the structural changes of protein induced by the interaction is observed, and converges to zero after all the changes are completed (Takeda K and Moriyama Y, 2011).
In the case of DDAC alone, an endothermic heat was observed during the binding process at the lower concentration side, and the endothermic heat decreased once and then increased again. Since the CMC of DDAC was 0.44 mM in the medium mixed solution in which ITC measurements were performed, the peak at lower concentrations is attributed to high-affinity binding and the peak near the CMC corresponds to cooperative binding. The endothermic heat at concentrations above CMC suggests that the structure of BSA tends to aggregate.
In contrast, in the case of DDAC with SO42- ions, a large endothermic peak was observed, which was larger than that of DDAC alone. The presence of SO42- ions decreased CMC by 0.23 mM, suggesting that this large peak was due to simultaneous progression of high-affinity binding and cooperative binding. The exothermic level at concentrations above CMC is also greater than for DDAC alone. The denaturation rates of BSA at concentrations near the saturation of these binding processes, 0.4 mM for DDAC alone and 0.2 mM for DDAC with SO42- ions, were 45% and 96%, respectively. In fact, a slight precipitation of complexes composed of BSA and DDAC was observed at 0.2 mM DDAC with SO42- ions.
On the other hand, the protein denaturation rate in Figure 4 showed a denaturation rate of 7.4% for the SO42- ion alone, but no heat of interaction of the SO42- ions themselves with BSA was observed by means of ITC. This means that the SO42- ions disturbed the protein structure by the salting-out effect.
From these results, we found that the SO42- ions acted synergistically in protein denaturation to disrupt the structure by salting-out and to promote the cooperative binding of DDAC by lowering CMC. In other words, this synergistic effect of protein denaturation can be considered to affect the outer shell protein of non-enveloped viruses and enhance their inactivation ability.
Conclusion
We investigated the inactivation effects of cationic surfactants known as disinfectants on non-enveloped viruses and the influence of salts as an additive to enhance them. The enhancement effect of ionic species on inactivation by DDAC was greater for anionic species than cationic species. We found that the addition of anionic species with higher salting-out effects, such as SO42- ions, greatly enhanced the inactivation effect of non-enveloped viruses. These results suggested that the tendency of ionic species to increase the interaction ability of cationic surfactants correlated well with the Hofmeister series.
In addition, inactivation effects of FCV by the cationic surfactants were enhanced by the addition of SO42- ions in descending order of DDAC>CPC> ADBAC>CTAC. Their inactivation ability grew with larger hydrophobic moieties, such as two hydrophobic chains with a larger carbon number or a single chain with an aromatic ring. This suggested that the hydrophobic moieties of cationic surfactants were largely responsible for the FCV inactivation.
To consider the contribution of SO42- ions in the inactivation of non-enveloped viruses, we used BSA as a model protein and compared the protein denaturation and the binding process of DDAC with and without SO42- ions. First, in the case of DDAC alone, the binding behavior evaluated by ITC suggested that the binding process that included monomer and cooperative bindings was already saturated at 0.5 mM of DDAC concentration, at which point inactivation was tested. Many surfactants show around 30% protein denaturation rates above CMC, where the binding was saturated. The denaturation rate of DDAC alone was at the same level.
On the other hand, we found that the addition of SO42- ions to DDAC caused a large denaturation, in which the monomer and cooperative binding progresses simultaneously from lower concentrations, due to a decrease in the CMC, and the DDAC-BSA complexes aggregated. Furthermore, the SO42- ions themselves did not directly bind to BSA, but slightly denatured the BSA. These results indicated that the salting-out effect of SO42- ions disrupted the protein structure and promoted the binding of DDAC, thereby amplifying the disruption of the protein structure, which induced a major protein denaturation effect.
As described above, the combination of a salt with strong salting-out effect, such as SO42- ions, and an antibacterial cationic surfactant with double-chain or hydrophobic moieties were effective for the inactivation of non-enveloped viruses such as FCV. In the future, we plan to further investigate the denaturation of β-sheet type proteins close to capsids by applying the combination of cationic surfactants and salts with stronger salting-out affects, and to further elucidate the inactivation mechanism of non-enveloped viruses.
Data Availability Statement
All data generated or analysed during this study are included in this published article, its supplementary information files and also available from the corresponding author on reasonable request.
References
- Ban M. Trends of antibacterial, antivirus and antibiofilm surface treatments. J Surf Finish Soc Jpn. 2021; 72(5): 252-258.
- Kratzel A, Todt D, V’kovski P, Steiner S, Gultom M, Thao TT, et al. Inactivation of severe acute respiratory syndrome coronavirus 2 by WHO-recommended hand rub formulations and alcohols. Emerg Infect Dis. 2020; 26(7): 1592.
[Crossref] [Google Scholar] [Pubmed]
- Yokohata R, Ishida Y, Nishio M, Yamamoto T, Mori T, Suzuki F, et al. A systematic review of the potential disinfectants against coronavirus toward risk mitigation strategies for COVID-19 contact infection route. Jpn J Risk Anal. 2020; 30(1): 5-28.
- NITE. Final report on efficacy assessment of disinfecting substances alternative to alcohol for use against Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2). National Institute of Technology and Evaluation (NITE). 2022.
- Block SS. Disinfection, sterilization, and preservation. Infect Control Hosp Epidemiol. 2002; 23(2): 109.
- ISO. Textiles-Determination of antiviral activity of textile products. International Organization for Standardization (ISO 18184:2014). 2014: 42.
- EPA. Product Performance Test Guideline, OCSPP 810.2200, Disinfectants for use on Environmental Surfaces, Guidance for Efficacy Testing, (EPA 712-C-17-004). Environmental Protection Agency (EPA). 2018.
- Doultree JC, Druce JD, Birch CJ, Bowden DS, Marshall JA. Inactivation of Feline calicivirus, a Norwalk virus surrogate. J Hosp Infect. 1999; 41(1): 51-57.
[Crossref] [Google Scholar] [Pubmed]
- Bélec L, Tevi-Benissan C, Bianchi A, Cotigny S, Beumont-Mauviel M, Si-Mohamed A, et al. In vitro inactivation of Chlamydia trachomatis and of a panel of DNA (HSV-2, CMV, adenovirus, BK virus) and RNA (RSV, enterovirus) viruses by the spermicide benzalkonium chloride. J Antimicrob Chemother. 2000; 46(5): 685-693.
[Crossref] [Google Scholar] [Pubmed]
- Zonta W, Mauroy A, Farnir F, Thiry E. Comparative virucidal efficacy of seven disinfectants against murineNorovirus and Feline calicivirus, surrogates of human norovirus. Food Environ Virol. 2016; 8(1): 1-2.
[Crossref] [Google Scholar] [Pubmed]
- Whitehead K, McCue KA. Virucidal efficacy of disinfectant actives against Feline calicivirus, a surrogate for Norovirus, in a short contact time. Am J Infect Control. 2010; 38(1): 26-30.
[Crossref] [Google Scholar] [Pubmed]
- Obe K, Tsunena N, Miyajima N, Mizushima N, Kashiwa I. Evaluation of protein denaturation effect of various surfactants by circular dichroism. J Jpn Oil Chem Soc. 1980; 29(11): 866-871.
- McDonnell G, Russell AD. Antiseptics and disinfectants: Activity, action, and resistance. Clin Microbiol Rev. 1999; 12(1): 147-179.
[Crossref] [Google Scholar] [Pubmed]
- Wang J, Satoh M. Novel PVA-based polymers showing an anti-Hofmeister series property. Polymer. 2009; 50(15): 3680-3685.
- Zhang S, Chen X, Ding S, Lei Q, Fang W. Unfolding of human serum albumin by gemini and single-chain surfactants: A comparative study. Colloids Surf A. 2016; 495: 30-38.
- Aoki K. The interactions of surfactants with proteins (I). J Jpn Oil Chem Soc. 1985; 34(9): 718-729.
- Takeda K, Moriyama Y. Surfactant-protein interaction. Oreoscience. 2011; 11(1): 3-10.
Author Info
Rei Saito1*, Seiichi Tobe1, Miyuki Miyake1, Yukari Sekine1, Takeshi Takizawa1, Junichi Sugiyama1, Yasushi Kakizawa1 and Shigeru Morikawa22Department of Microbiology, Faculty of Veterinary Medicine, Okayama University of Science, Ehime, Japan
Citation: Saito R: Enhancing Cationic Surfactantsâ?? Inactivation of Non-Enveloped Viruses by Sulfate Ions
Received: 20-Oct-2022 Accepted: 03-Nov-2022 Published: 10-Nov-2022, DOI: 10.31858/0975-8453.13.11.751-755
Copyright: This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
ARTICLE TOOLS
- Dental Development between Assisted Reproductive Therapy (Art) and Natural Conceived Children: A Comparative Pilot Study Norzaiti Mohd Kenali, Naimah Hasanah Mohd Fathil, Norbasyirah Bohari, Ahmad Faisal Ismail, Roszaman Ramli SRP. 2020; 11(1): 01-06 » doi: 10.5530/srp.2020.1.01
- Psychometric properties of the World Health Organization Quality of life instrument, short form: Validity in the Vietnamese healthcare context Trung Quang Vo*, Bao Tran Thuy Tran, Ngan Thuy Nguyen, Tram ThiHuyen Nguyen, Thuy Phan Chung Tran SRP. 2020; 11(1): 14-22 » doi: 10.5530/srp.2019.1.3
- A Review of Pharmacoeconomics: the key to “Healthcare for All” Hasamnis AA, Patil SS, Shaik Imam, Narendiran K SRP. 2019; 10(1): s40-s42 » doi: 10.5530/srp.2019.1s.21
- Deuterium Depleted Water as an Adjuvant in Treatment of Cancer Anton Syroeshkin, Olga Levitskaya, Elena Uspenskaya, Tatiana Pleteneva, Daria Romaykina, Daria Ermakova SRP. 2019; 10(1): 112-117 » doi: 10.5530/srp.2019.1.19
- Dental Development between Assisted Reproductive Therapy (Art) and Natural Conceived Children: A Comparative Pilot Study Norzaiti Mohd Kenali, Naimah Hasanah Mohd Fathil, Norbasyirah Bohari, Ahmad Faisal Ismail, Roszaman Ramli SRP. 2020; 11(1): 01-06 » doi: 10.5530/srp.2020.1.01
- Manilkara zapota (L.) Royen Fruit Peel: A Phytochemical and Pharmacological Review Karle Pravin P, Dhawale Shashikant C SRP. 2019; 10(1): 11-14 » doi: 0.5530/srp.2019.1.2
- Pharmacognostic and Phytopharmacological Overview on Bombax ceiba Pankaj Haribhau Chaudhary, Mukund Ganeshrao Tawar SRP. 2019; 10(1): 20-25 » doi: 10.5530/srp.2019.1.4
- A Review of Pharmacoeconomics: the key to “Healthcare for All” Hasamnis AA, Patil SS, Shaik Imam, Narendiran K SRP. 2019; 10(1): s40-s42 » doi: 10.5530/srp.2019.1s.21
- A Prospective Review on Phyto-Pharmacological Aspects of Andrographis paniculata Govindraj Akilandeswari, Arumugam Vijaya Anand, Palanisamy Sampathkumar, Puthamohan Vinayaga Moorthi, Basavaraju Preethi SRP. 2019; 10(1): 15-19 » doi: 10.5530/srp.2019.1.3