Research Article - (2023) Volume 14, Issue 2
Abstract
Background: PIPAC (Pressurized Intraperitoneal Aerosol Chemotherapy) acts by applying aerosolized chemotherapy in the peritoneal cavity, enhancing tissue penetration of chemotherapeutic agents. This method of chemotherapy delivery still raises several concerns related to the operating room’s environmental exposure, arousing discussions related to the occupational risks of this technique. This work aims to demonstrate the pattern of aerosolization distribution in the absence of safety mechanisms in an operating room.
Materials and Methods: A cross-sectional experimental work of 31 aerosol applications was carried out. Aerosolization was performed with a 1% aqueous solution of caffeine Cellulose. Nitrate membranes were used to capture the concentration of caffeine in different sites within the operating room for 5 periods of fixed exposure times.
Results: 930 samples are obtained in 31 rounds of aerosolization. Comparing the changes in concentration per minute between the different time intervals. There were statistically significant differences between the 0-2 minutes interval and the 15-30 interval (P<0.001). Surgeon site shows a significant difference between the times (P=0.010). There were no difference between changes in concentrations in the time intervals for the anesthetist site (P=0.094). At the injector site, there is a statistically significant difference (P<0.001). The time assessment between 30-35 exposure showed a median of 0.
Conclusion: The study pointed out that the moment of greatest risk of contamination of the surgical environment occurs during aerosolization, especially during the first 15 minutes after the start of aerosolization. The sites that were most exposed to contamination were the patient, the surgeon and the injector, respectively.
Keywords
Carcinomatosis, Occupational safety, Pressurized Intraperitoneal Aerosol Chemotherapy (PIPAC)
Introduction
The use of intraperitoneal chemotherapy has been used as a therapeutic option in the treatment of neoplastic diseases since the 1980’s (Markman M, 2003). Unlike the traditional intravenous use, the use of liquid form directly in the peritoneal space has been marginally performed for the treatment of carcinomatosis. This is considered a new approach to the delivery of traditionally used drugs, such as oxaliplatin, mitomycin C, cisplatin and doxorubicin (Yonemura Y, et al., 2020). However, this results in controlling the peritoneal carcinomatosis in challenging scenarios, such as gastrointestinal (Huang CQ, et al., 2017), gynecological (Zhang G, et al., 2019) and primary peritoneal carcinoma (Govaerts K, et al., 2021) highlighted the importance of pharmacokinetics, dynamic action and behavior of chemotherapeutic agents in the peritoneal space through different forms of application. Reymond MA, et al., 2000 presented a new procedure of delivery of chemotherapeutic agents in the peritoneal space called “Pressurized Intraperitoneal Aerosol Chemotherapy” (PIPAC). The difference is the provision of aerosolized chemotherapy in the peritoneal cavity through video laparoscopy to enhance the penetration and distribution of therapeutic agents within that space. This new way of delivery still raises concerns related to the exposure of the operating room environment and its teams to chemotherapeutic agents. This scenario raises discussions related to the occupational risks that did not exist before and that could affect the teams and the surgical environment.
Several articles have been written on the assessment of safety in the application of aerosolized chemotherapy for both the assistant team and the surgical environment. However, all studies focus on assessing aerosolization and its possible safety flaws during the procedure and do not demonstrate the contamination pattern during eventual inadvertent aerosolization. This study aims to demonstrate the distribution pattern of contamination during the inadvertent aerosolization of the therapeutic substance, using PIPAC in an operating room.
Materials and Methods
Experimental design
A cross-sectional experimental study was carried out in the operation room of Hospital Santa Rita do complexo Santa Casa de Porto Alegre from August 15, 2019, to April 15, 2020 with a total of 31 aerosolizations. PIPAC applications were simulated in a negative pressure operating room with unidirectional (laminar) airflow ventilation and open sealing doors to analyze contamination in the operating room corridor. The first stage of validation of the methodology was under analysis, 10 aerosolization tests were carried out simulating different levels of leakage to assess the contamination scenarios. The first scenario was the simulation of leakage keeping the trocar’s luer lock open throughout the procedure. In this scenario, the contamination was hard to measure. The second scenario was the aerosolization 20 cm above the center of the operating table in a 10-liter open container. The third model was free aerosolization 20 cm above the center of the operating table (Figure 1). After assessing the first 10 applications, the scenario that proved to be most suitable for the purpose was scenario 3 during all procedures, no contamination control method was used. This study was submitted to the Ethics Committee, however, it was exempt from analysis because it is an experimental study that does not involve living beings or toxic substances.
Aerosolization procedure
The following equipment was used: BhioQAP registered at the Agência Nacional de Vigilância Sanitária (ANVISA) under No. 80381210072 and IV contrast injection system with remote actuation (Empower CTA-Bracco). The aerosolized equipment was kept in the center and 20 cm above the operating table with the aid of a mechanical arm that supported the system (Figure 1).
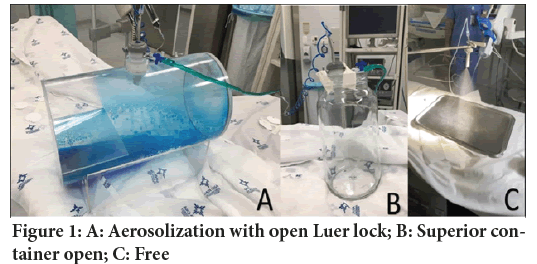
Figure 1: A: Aerosolization with open Luer lock; B: Superior container open; C: Free
The assessment of environmental contamination during the PIPAC procedure was performed using a 1% aqueous solution of caffeine (Sigma-Aldrich®). This substance was specifically chosen because caffeine has low toxicity and is easy to detect. Some physical and chemical characteristics of caffeine are described in Table 1.
| Parameter described | Substance-caffeine |
|---|---|
| Formula | 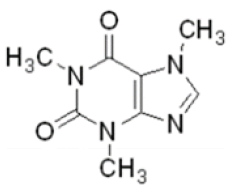 |
| Molecular weight | 194.19 g/mol |
| Water solubility | 20 g/L (20°C) |
| Application concentration | 10 mg/mL=10 g/L=1.0% |
Table 1: Physical and chemical characteristics of caffeine
The injector (Empower CTA-Bracco) was configured to apply the fixed volume for each 200 ml application. The injection equipment was always programmed with fixed infusion parameters of 3.0 ml/s with a maximum pressure of 200 to 300 PSI. The injection equipment adjusts the infusion flow to keep the pressure below 300 PSI. The average injection variation was 0.6 ml/s ± 0.2 ml/s. All injections were carried out without locking the injection equipment or changing the parameters described.
Cellulose nitrate membranes (8.00 µm pores and 47 mm diameter/UNIFIL®) were used to capture the caffeine concentration in different parts of the operating room for 5 fixed time periods of exposure. The membrane exposure times after the start of aerosolization were 2, 5, 15 and 30 minutes. The 5th exposure period of the cellulose membrane was started 30 minutes after the beginning of the aerosolization up to 35 minutes. The time was chosen based on critical periods in the aerosolization process.
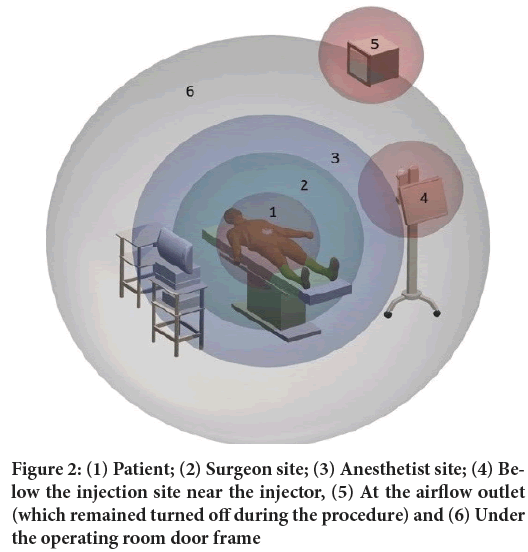
Time 2 min is related to average of half the aerosolization time. Time 5 min regards the end of aerosolization in the vast majority of applications. Time 15 min relates to half the PIPAC procedure time in clinical practice. Time 30 min is related to the end of the proposed procedure in clinical practice. The time between 30-35 mins relates to the end of PIPAC procedure and entry of the assistant team in the surgical environment. The areas of interest for determining the contamination were established at 6 points. The determination of these points was chosen according to the occupational risk of healthcare professionals or by the places which has high risk of environmental contamination (Figure 2). The sites chosen for data collection, representing the following positions during PIPAC procedure: (1) patient, (2) surgeon site, (3) anesthetist site, (4) below the injection site near the injector, (5) at the airflow outlet (which remained turned off during the procedure) and (6) under the operating room door frame.
Figure 2: (1) Patient; (2) Surgeon site; (3) Anesthetist site; (4) Below the injection site near the injector, (5) At the airflow outlet (which remained turned off during the procedure) and (6) Under the operating room door frame
Environment contamination analysis
Cellulose nitrate membranes collected in each situation were analyzed to determine the environmental concentration of caffeine. After the collection period in each different scenario, the membranes were separately packed in plastic bags and sent to Central Analítica da Universidade Federal de Ciências da Saúde de Porto Alegre (UFCSPA). Immediately after arriving at the assessment site, the samples were placed in an amber flask containing 3 ml of water supplemented with 0.1% formic acid, protected from light and incubated at 4°C-8°C for 24 hours. Aliquots of the solutions were directly injected into a Liquid Chromatography system together with High-Resolution Mass Spectrometry (LC-HRMS). This system was integrated into a mass spectrometer composed of a hybrid system with quadrupole analyzer and TOF (Time of Flight, Bruker Daltonics, micrO-TOF-QIII model) series and in an orthogonal position for high resolution and mass accuracy. Data were processed using Data Analysis HyStar™ software. The analyses were carried out through Electrospray Ionization (ESI) and the parameters such as ionization method, temperature, gas flow, collision energy and capillary energy were tested and optimized. Elution was made on a Shim-pack XR-ODS II chromatography column (75 × 2 mm, particle size 2.2 µm, Shimadzu®, Tokyo, Japan) with a water and methanol gradient supplemented with 0.1% formic acid, 0.4 ml/min flow, and temperature of 50°C. The total assessment time was on average 4 minutes.
Statistical analysis
The sample size was calculated using the WINPEPI program (Abramson JH, WINPEPI updated: Computer programs for epidemiologists, and their teaching potential: Epidemiologic Perspectives and Innovations 2011) considering a 5% significance level and 90% power. The first 10 aerosolizations were used to validate the experimental design and optimize the analytical methodology, define better packaging, train the research team, and choose the best-proposed scenario. After the tenth aerosolization, 21 consecutive aerosolizations were considered for analysis. The variable distribution was assessed by the Kolmogorov-Smirnov test. Concentration differences in the intervals of 0 to 2 minutes, 2 to 5 minutes, 5 to 15 minutes, and 15 to 30 minutes were calculated, and this difference was divided by 2, 3, 10 and 15 minutes to determine the variation per minute. The variations per minute were described by the median and the 25%-75% interquartile range and compared using the Friedman test between the intervals. A 5% significance level was considered.
Results
The 930 samples obtained in 31 rounds of aerosolization with 5 different exposure times and 6 different sites in the operating room were analyzed. The median of the concentration of each site in different time analysis was presented by the median of the contamination values. The differences in the intervals were calculated and corrected for the exposure time interval and its interquartile range from 25% to 75% is due to the large variability of the assessment. Comparing the changes in concentration per minute between the different time intervals, there were statistically significant differences between the 0-2 minutes interval and the 15-30 interval (P<0.001); and between the 2-5 minutes interval and the 5-15 minutes interval (P=0.014) and 15-30 (P<0.001) (Table 2).
| Interval | 0-2 | 0-5 | 0-15 | 0-30 | P |
|---|---|---|---|---|---|
| Patient | 5.57 (2.01-12.57) | 7.34 (0.53-14.46) | 1.56 (-0.86-4.78) | -1.08 (-2.09-0.54) | <0.001 |
| Surgeon | 0.50 (0-2.65) | 0.56 (0–2.91) | 0 (-0.74-0.27) | 0 (-0.25-0.15) | 0.01 |
| Anesthetist | 0 (0-4.19) | 0 (-0.02-0.81) | 0 (-0.11-0.17) | 0 (-0.14-0.07) | 0.094 |
| Injector | 0.87 (0-5.07) | -0.01 (-1,24-0.11) | 0 (-0.12-0.10 ) | 0 (0-0.18) | <0.001 |
| Air conditioning | 0 (0-0) | 0 (0–0.32) | 0 (-0.02-0) | 0 (0-0.03) | 0.097 |
| Outside operating room | 0 (0-0.25) | 0 (-0.01-0) | 0 (0-0) | 0 (-0.02-0) | 0.124 |
Table 2: Comparative table of variations in concentrations over time intervals for different sites (n=31). Data given by the median (interquartile range: 25th and 75th percentiles) and compared using Friedman test
Although the changes in concentrations at the surgeon-2-site shows a significant difference between the times (P=0.010), these differences did not remain after the adjustment for multiple comparisons in peer-to-peer comparisons. There were no differences between changes in concentrations in the time intervals for the anesthetist-3-site (P=0.094). At the injector-4-site, a statistically significant difference (P<0.001) was detected for intervals 0-2 in relation to 2-5 minutes interval (P=0.002) and 5-15 minutes interval (P=0.008). With regard to the changes in concentrations at the air outlet and outside the operating room, there were no statistically significant differences (P=0.097 and P=0.124 respectively). The time assessment between 30-35 mins exposure showed a median of 0.
Discussion
The use of intraperitoneal chemotherapy has gained increasing importance in tackling metastases, mainly with the increased use of hyper thermic chemotherapy associated with surgical cytoreduction. That way, different chemotherapeutic agents are being used more in surgical centers around the world. The advent of PIPAC, which turns liquid chemotherapy into an aerosol, has brought new challenges regarding safety in the handling of these substances in clinical practice. By creating a therapeutic mist, this procedure raised extra concerns regarding the safety of healthcare professionals and the workplace. Methods of continuous assessment of contamination of the operating room for 60 minutes during the application of PIPAC procedure with 3-level safety mechanisms (closed abdomen, laminar flow room/protective cover, isolated operating room) (Hübner M, et al., 2017) proved to be safe for the members of the assistant team taking part in the procedure (Oyais A, et al., 2016; Graversen M, et al., 2018). Unlike previous studies, the purpose of this study is to assess whether or not there is a pattern of aerosol contamination inside the operating room during a failure in the abdominal wall sealing mechanisms and in the total absence of any safety barrier. During the validation of the best model, the observations made as project’s pilots, even with the video laparoscopy insufflator turned on and the trocar’s luer lock opened, were not sufficient to detect residues in the analyzed samples (Figure 1). A second pilot model with the acrylic box lid open showed the same difficulty in detecting the substance used (caffeine) at the different collection points (Figure 1). The first study to assess contamination of the surgical environment was presented by Sollas W, et al., 2013 during the first PIPAC applications to determine the safety standards of the procedure (Solaß W, et al., 2013). A system leak simulation was carried out with the opening of the trocar’s luer lock, keeping the “pneumoperitoneum” active during aerosolization in a model with an acrylic box. However, only the aerosol’s behavior was described. The degree of contamination at different sites in the operating room or the behavior of the therapeutic mist was not measured. The first group that assessed mathematical models that indicate the maximum dose inhaled in the event of a failure in the PIPAC procedure. The possible contamination identified is between 1:1, 00,000 and 1:10, 00,000 of the total dose used during the 30 minutes of the procedure (Tempfer C and Reymond MA, 2015). Subsequent analyses were carried out to identify the sites in the surgical environment that were most exposed to the therapeutic mist. During the procedures, traces of cisplatin were not detected in the surgeon and anesthetist sites (Solaß W, et al., 2013), but there were no flaws or leakages in these procedures. This meets the levels of contamination measured during the PIPAC procedure without signs of leakages in the operating room. Doxorubicin levels below 0.00002 ng/m3 were observed during the entire procedure, which represents only 1% of the maximum allowed dose of exposure to healthcare professionals (Delhorme JB, et al., 2019). Graversen M analyzed the presence of particles in both the anesthetist and surgeon sites, and he found no trace of contamination by a chemotherapeutic agent in the volume of air analyzed during two consecutive procedures (Graversen M, et al., 2016). Willaert W, et al., 2017 assessed contamination on PIPAC applications and identified, which preventive measures are effective in reducing contamination in the surgical environment during PIPAC. This data demonstrates the safety of the procedure but does not determine the behavior of the aerosol in the event of a failure in the process of applying aerosolized chemotherapy.
The contamination pattern does not seem to have a normal curve. However, the data collected pointed out some critical sites which are at high risk when using PIPAC. Our samples showed that the patient site is the most exposed during inadvertent aerosolization. This leads us to discuss the use of impermeable and disposable fields as the only measure to reduce the risk of aerosol contact with the patient’s skin. The unprotected surgical site becomes an important exposure point and should be a concern that justifies the use of skin protection films. The surgeon and the anesthetist sites and the area under the injector are critical during inadvertent aerosolization, which supports Sollas W initial concern. Ndaw S, et al., 2018 assessed the presence of platinum after PIPAC, detecting contamination two meters from the operating table and on the injector. However, there was substantially lower than 3 meters from the operating table. Our observations support this idea. The first 5 minutes seem to be the most critical period for contamination of the surgical environment. The anesthetist site, despite showing a variation in the 75% percentile up to 5 minutes (0 to 2.91), still shows a lower value than the surgeon site. However, it seems to be within the critical exposure range of less than 3 meters suggested by Ndaw S, et al., 2018. Also the first 2 minutes of the injector shows a significant difference. The first 2 minutes on the injector showed a significant difference, pointing to corroborating the idea that this location, even though it is farther from the centre of the aerosolization, there is a possible contamination at that site mainly during the injection. Aerosolization occurs mostly up to 5 minutes in our study, no aerosolization exceeded 6 minutes. At this point, the injection pressure and the therapeutic mist turbulence are at their highest, as pointed out, reaching the highest levels of contamination. The simulation of two leakage scenarios during the procedure evaluated by Tempfer C and Reymond MA showed an insignificant substance concentration in the environment after 12-15 minutes (Tempfer C and Reymond MA, 2015; Delhorme JB, et al., 2019). Our samples present a median of 0 in the difference in contamination corrected by the exposure time in all 6 sites evaluated in this study after 15 minutes. This tendency continues in the 30-35 exposure period. In any sample, all sites showed an occasional presence of caffeine. Even though there may be some bias and due to the limitation of being an analysis of the surfaces contaminated with the substance under analysis, we believe this tendency indicates that after 15 minutes, the therapeutic mist is no longer dispersed and it has already condensed. This idea is supported by the tendency of negativity in the assessment of the 75th percentiles corrected by the exposure time in the different sites analyzed after 15 minutes. This analysis must be carefully extrapolated to the closed therapeutic pneumoperitoneum systems, since the therapeutic mist faces other challenges in the closed abdomen environment and must have a different distribution behavior. However, we must point out the need to assess the impact of these findings in the ultimate objective of the treatment of peritoneal metastases through PIPAC. In this laparoscopic environment with reduced space and under 12 mmHG, this build-up tendency should be more dramatic and possibly below 15 minutes.
Conclusion
The moment of major contamination risk identified in the samples analyzed in the surgical environment during aerosolization occurs in the first 15 minutes after the start of aerosolization in different sites: Patient, surgeon, anesthetist, and injector. The patient and surgeon sites and the area near the injector showed the highest levels of contamination. The anesthetist site tends to get contaminated.
Declarations
Conflict of interest
I would like inform that Rafael Seitenfus, Eduardo Dipp de Barros and Paulo Roberto Walter Ferreira have a part of the patent device. (Br-1020180757415, Br-3020180557379)
Funding source
The company Bhiosupply financed 32 aerosolizers and all the necessary inputs for data collection (cellulose membranes). None of the authors received any personal funding or even sponsored by Bhiosupply.
References
- Markman M. Intraperitoneal antineoplastic drug delivery: Rationale and results. Lancet Oncol. 2003; 4(5): 277-283.
[Crossref] [Google Scholar] [Pubmed]
- Yonemura Y, Iahibashi H, Sako S, Mizumoto A, Takao N, Ichinose M, et al. Advances with pharmacotherapy for peritoneal metastasis. Expert Opin Pharmacother. 2020; 21(16): 2057-2066.
[Crossref] [Google Scholar] [Pubmed]
- Huang CQ, Min Y, Wang SY, Yang XJ, Liu Y, Xiong B, et al. Cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy improves survival for peritoneal carcinomatosis from colorectal cancer: A systematic review and meta-analysis of current evidence. Oncotarget. 2017; 8(33): 55657.
[Crossref] [Google Scholar] [Pubmed]
- Zhang G, Zhu Y, Liu C, Chao G, Cui R, Zhang Z. The prognosis impact of Hyperthermic Intraperitoneal Chemotherapy (HIPEC) plus Cytoreductive Surgery (CRS) in advanced ovarian cancer: The meta-analysis. J Ovarian Res. 2019; 12(1): 1-9.
[Crossref] [Google Scholar] [Pubmed]
- Govaerts K, Lurvink RJ, de Hingh IH, van der Speeten K, Villeneuve L, Kusamura S, et al. Appendiceal tumours and pseudomyxoma peritonei: Literature review with PSOGI/EURACAN clinical practice guidelines for diagnosis and treatment. Eur J Surg Oncol. 2021; 47(1): 11-35.
[Crossref] [Google Scholar] [Pubmed]
- Reymond MA, Hu B, Garcia A, Reck T, Köckerling F, Hess J, et al. Feasibility of therapeutic pneumoperitoneum in a large animal model using a microvaporisator. Surg Endosc. 2000; 14(1): 51-55.
[Crossref] [Google Scholar] [Pubmed]
- Hübner M, Grass F, Teixeira-Farinha H, Pache B, Mathevet P, Demartines N. Pressurized intraperitoneal aerosol chemotherapy-practical aspects. Eur J Surg Oncol. 2017; 43(6): 1102-1109.
[Crossref] [Google Scholar] [Pubmed]
- Oyais A, Solass W, Zieren J, Reymond MA, Giger-Pabst U. Occupational health aspects of Pressurised Intraperitoneal Aerosol Chemotherapy (PIPAC): Confirmation of harmlessness. Zentralbl Chir. 2016; 141(4): 421-424.
[Crossref] [Google Scholar] [Pubmed]
- Graversen M, Detlefsen S, Bjerregaard JK, Fristrup CW, Pfeiffer P, Mortensen MB. Prospective, single-center implementation and response evaluation of Pressurized Intraperitoneal Aerosol Chemotherapy (PIPAC) for peritoneal metastasis. Ther Adv Med Oncol. 2018; 10: 1758835918777036.
[Crossref] [Google Scholar] [Pubmed]
- Solaß W, Giger-Pabst U, Zieren J, Reymond MA. Pressurized Intraperitoneal Aerosol Chemotherapy (PIPAC): Occupational health and safety aspects. Ann Surg Oncol. 2013; 20(11): 3504-3511.
[Crossref] [Google Scholar] [Pubmed]
- Tempfer C, Reymond MA. Pressurized Intraperitoneal Aerosol Chemotherapy (PIPAC): Occupational health and safety management. Adv Med Biol. 2015;157(10).
- Delhorme JB, Klipfel A, D’Antonio F, Greget MC, Diemunsch P, Rohr S, et al. Occupational safety of Pressurized Intraperitoneal Aerosol Chemotherapy (PIPAC) in an operating room without laminar airflow. J Visc Surg. 2019; 156(6): 485-488.
[Crossref] [Google Scholar] [Pubmed]
- Graversen M, Pedersen PB, Mortensen MB. Environmental safety during the administration of Pressurized Intraperitoneal Aerosol Chemotherapy (PIPAC). Pleura Peritoneum. 2016; 1(4): 203-208.
[Crossref] [Google Scholar] [Pubmed]
- Willaert W, Sessink P, Ceelen W. Occupational safety of Pressurized Intraperitoneal Aerosol Chemotherapy (PIPAC). Pleura Peritoneum. 2017; 2(3): 121-128.
[Crossref] [Google Scholar] [Pubmed]
- Ndaw S, Hanser O, Kenepekian V, Vidal M, Melczer M, Remy A, et al. Occupational exposure to platinum drugs during intraperitoneal chemotherapy. Biomonitoring and surface contamination. Toxicol Lett. 2018; 298: 171-176.
[Crossref] [Google Scholar] [Pubmed]
Author Info
Rafael Seitenfus1*, Eduardo Dipp de Barros1, Gustavo Andreazza Laporte1, Thais Spohr Christ2, Thiago Franco de Oliveira3, Viviane de Moura Linck2, Rodrigo de Pieri Coan1, Cassio Bona Alves1, Paulo Walter Ferreira4 and Marcelo Dutra Arbo2,32Department of Pharmaceuctical Sciences, Universidade Federal do Rio Grande do Sul (UFRGS), Porto Alegre, Brazil
3Department of Analysis, Laboratory of Toxicology (LATOX), Universidade Federal do Rio Grande do Sul (UFRGS), Porto Alegre, Brazil
4Department of Engineering, Innovation and Entrepreneurship, Faculdade de Desenvolvimento do RioGrande do Sul (FADERGS), Porto Alegre, Brazil
Citation: Seitenfus R: Environmental Contamination Assessment in the Process of Application of Aerosolized Therapeutic Substances
Received: 02-Jan-2023 Accepted: 27-Jan-2023 Published: 03-Feb-2023, DOI: 10.31858/0975-8453.14.2.94-97
Copyright: This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
ARTICLE TOOLS
- Dental Development between Assisted Reproductive Therapy (Art) and Natural Conceived Children: A Comparative Pilot Study Norzaiti Mohd Kenali, Naimah Hasanah Mohd Fathil, Norbasyirah Bohari, Ahmad Faisal Ismail, Roszaman Ramli SRP. 2020; 11(1): 01-06 » doi: 10.5530/srp.2020.1.01
- Psychometric properties of the World Health Organization Quality of life instrument, short form: Validity in the Vietnamese healthcare context Trung Quang Vo*, Bao Tran Thuy Tran, Ngan Thuy Nguyen, Tram ThiHuyen Nguyen, Thuy Phan Chung Tran SRP. 2020; 11(1): 14-22 » doi: 10.5530/srp.2019.1.3
- A Review of Pharmacoeconomics: the key to “Healthcare for All” Hasamnis AA, Patil SS, Shaik Imam, Narendiran K SRP. 2019; 10(1): s40-s42 » doi: 10.5530/srp.2019.1s.21
- Deuterium Depleted Water as an Adjuvant in Treatment of Cancer Anton Syroeshkin, Olga Levitskaya, Elena Uspenskaya, Tatiana Pleteneva, Daria Romaykina, Daria Ermakova SRP. 2019; 10(1): 112-117 » doi: 10.5530/srp.2019.1.19
- Dental Development between Assisted Reproductive Therapy (Art) and Natural Conceived Children: A Comparative Pilot Study Norzaiti Mohd Kenali, Naimah Hasanah Mohd Fathil, Norbasyirah Bohari, Ahmad Faisal Ismail, Roszaman Ramli SRP. 2020; 11(1): 01-06 » doi: 10.5530/srp.2020.1.01
- Manilkara zapota (L.) Royen Fruit Peel: A Phytochemical and Pharmacological Review Karle Pravin P, Dhawale Shashikant C SRP. 2019; 10(1): 11-14 » doi: 0.5530/srp.2019.1.2
- Pharmacognostic and Phytopharmacological Overview on Bombax ceiba Pankaj Haribhau Chaudhary, Mukund Ganeshrao Tawar SRP. 2019; 10(1): 20-25 » doi: 10.5530/srp.2019.1.4
- A Review of Pharmacoeconomics: the key to “Healthcare for All” Hasamnis AA, Patil SS, Shaik Imam, Narendiran K SRP. 2019; 10(1): s40-s42 » doi: 10.5530/srp.2019.1s.21
- A Prospective Review on Phyto-Pharmacological Aspects of Andrographis paniculata Govindraj Akilandeswari, Arumugam Vijaya Anand, Palanisamy Sampathkumar, Puthamohan Vinayaga Moorthi, Basavaraju Preethi SRP. 2019; 10(1): 15-19 » doi: 10.5530/srp.2019.1.3