Research Article - (2022) Volume 13, Issue 4
Formulation and Evaluation of Micro Sponges Loaded with Acyclovir Using an Antiviral Drug
Shwetha TL*, Srinivasan S and Chandrakala VAbstract
The main objective of the present research work was to develop and evaluate the anti-viral drug (Acyclovir) loaded micro sponges using polymers. Micro sponges of acyclovir were prepared by quassi-emulsion solvent diffusion method by using EC and HPMC as polymers. The prepared acyclovir micro sponges were subjected to IR, SEM, Particle size and Size distribution, Entrapment efficiency, in vitro dissolution studies and release kinetics. The IR Spectras revealed that, there was no interaction between the polymer and acyclovir. Acyclovir micro sponges was spherical in nature, which was confirmed by SEM. Acyclovir micro sponges with normal frequency distribution were obtained. A maximum of 95.67% drug entrapment efficiency was obtained in the acyclovir micro sponges. F5 formulations ACV micro sponges show the maximum drug release. The coefficient of determination indicated that the release data was best fitted with higuchi model kinetics. The release kinetics data implies that the release mechanism of all formulation was Non Fickian diffusion mechanism. On the basis of particle size, entrapment efficiency and morphology, in vitro release studies and its kinetics data, F5 (formulation) was selected as optimized formulation.
Keywords
Hydroxypropyl methyl cellulose, Ethyl cellulose, Acyclovir, Scanning electron Microscopy (SEM)
Introduction
Viruses are the ultimate expression of parasitism. They are not only taking nutrition from host cell but also direct its metabolic machinery to synthesize new virus particles. Anti-viral drugs are a class of drugs which targets virus specific steps like cell penetration uncoating, virus assembly, maturation and reverse transcription etc. Anti-viral drugs classified as anti-herpes virus, anti-influenza virus, anti-hepatitis virus, and anti-retrovirus. Acyclovir is a first line antiviral drug which is used in the treatment of infection caused by Herpes Simplex Virus (HSV) (Healthline, 2021). Acyclovir is available as various dosage forms in the market such as capsules, creams, ointment, tablets and suspension (Yadav P and Nanda S, 2014).
Causative mechanism
HSV is a contagious virus that can be passed from person to person through direct contact. The microsponge-based polymeric microspheres uniquely overcome problems associate with above technologies. Microsponges are extremely small, inert, indestructible spheres that do not pass through the skin. Rather, they collect in the tiny nooks and crannies of the skin and slowly release the entrapped drug, as the skin needs it (Patel A, et al., 2012; Ravi R, et al., 2013). They are designed to deliver a pharmaceutical active ingredient efficiently at the minimum dose and also to enhance the stability, reduce side effects and modify drug release. The microsponge technology was developed by Won in the year 1987 and its original patents were assigned to Advanced Polymer Systems, Inc. Microsponges are polymeric delivery systems composed of porous microspheres (Hussain H, et al., 2014; Saxena S and Nacht S, 2005). They are tiny sponge-like spherical particles with a large porous surface. They may enhance stability, reduce side effects and modify drug release favorably. Microsponge technology has many favorable characteristics, which make it a versatile drug delivery vehicle (Osmani RA, et al., 2015). The Scanning Electron Microscopy of the microsponge particle reveals that its internal structure as the “bag of marbles”. The porosity is due to the interstitial spaces between the marbles. The interstitial pores can entrap many wide range of active ingredients such as emollients, fragrances, essential oils, sunscreens, anti-infective and anti-inflammatory agents (Patel EK and Oswal RJ, 2012). These entrapped microsponges may then integrated or formulated into product forms, such as creams, lotions, powders, soaps, capsules and tablets (Osmani AM, et al., 2015). Although the micro sponge size may vary, a typical 25 μm sphere can have up to 250000 pores and an internal pore structure equivalent to 10 ft in length, providing a total pore volume of about 1 ml/g. This results in a large reservoir within each micro sponge, which can be loaded with up to its own weight of the active agent. The micro sponge particles themselves are too large to be absorbed into the skin and this adds a measure of safety to these micro sponge materials (Ravi R and Senthikumar SK, 2013). Another safety concern is the potential bacterial contamination of the materials entrapped in the micro sponge. As the size of the pore diameter is smaller, the bacteria ranging from 0.007 to 0.2 μm cannot penetrate into the tunnel structure of the micro sponges (Pandey P, et al., 2013; Kale S and Shalini R; 2013).
Advantages of micro sponges drug delivery system
• Enhanced product performance
• Extended release
• Reduced irritation and hence improved patient compliance
• Improved product elegancy
• Improved formulation flexibility
• Improved thermal, physical and chemical stability
• Flexibility to develop novel product forms
• Non-irritating, non-mutagenic, non-allergenic and non-toxic
• Allows incorporation of immiscible substances
Materials and Methods
Acyclovir was received as a gift sample from Medopharma Pvt Ltd. HPMC and EC dichloromethane, PVA, Glycerine was provided by East Point College of Pharmacy.
Drug excipient compatibility studies by FTIR
Excipients are the integral components of almost all pharmaceutical dosage forms. The successful formulation of a stable and effective solid dosage form depends on the careful selection of the excipients, which are added to facilitate administration, promote the consistent release and bioavailability of the drug and protect it from degradation (El-Helw AM and Bayomi MA, 2000).
The compatibility of drug acyclovir and polymers was established by infrared absorption spectral analysis (IR). Any changes in the chemical composition of the drug after combining it with the excipients were investigated with IR spectral analysis. In the present study, the potassium bromide (KBr) disc pellets method was employed.
Method of preparation of acyclovir micro sponge
Microsponges were prepared by Quasi emulsion solvent diffusion method. Internal organic phase was prepared by dissolving ethyl cellulose and Hydroxy propyl methyl cellulose and drug in dichloromethane with addition of 15 ml of glycerine (Abdelmalak NS and El-Menshawe SF, 2012; Pavani S, et al., 2017). External phase was prepared by PVA and 150 ml water and heated at 40°C until it dissolved completely by using magnetic stirrer. The organic phase was added drop wise to the continuous stirring aqueous phase to form the discrete droplets at stirring speed 600 rpm to 750 rpm at temperature 40°C. The solution was filtered in Whattman filter paper and dried in hot air oven at 50°C for 2 hrs and sieved in a sieve No.30. (Hussein AA, 2014) (Table 1) (Figures 1 and 2).
| Sl. no | Ingredients | Formulation code | ||||||
|---|---|---|---|---|---|---|---|---|
| F1 | F2 | F3 | F4 | F5 | F6 | F7 | ||
| 1 | Acyclovir (mg) | 300 | 300 | 300 | 300 | 300 | 300 | 300 |
| 2 | Hpmc+Ec (g) | 3:3 | 3:4:5 | 3:6 | 3:12 | 3:9 | 3:10.5 | 3:7.5 |
| 3 | Dichloromethane (mml) | 70 | 70 | 70 | 70 | 70 | 70 | 70 |
| 4 | Pva (mg) | 225 | 225 | 225 | 225 | 225 | 225 | 225 |
| 5 | Glycerine (%) | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 |
| 6 | Distilled water (ml) | 450 | 450 | 450 | 450 | 450 | 450 | 450 |
Table 1: Formulation design

Figure 1: Formulation of microsponge loaded acyclovir
Figure 2: Microscopic view of microsponge
Preformulation studies
Melting point determination: The melting point of acyclovir was found to be 256.05 degree.
Solubility study: The solubility of Acyclovir was determined by solvents such as alcohols, 0.1 N HCl, water. Acyclovir as slightly soluble in water, soluble in 0.1 N HCl, partially insoluble in alcohols.
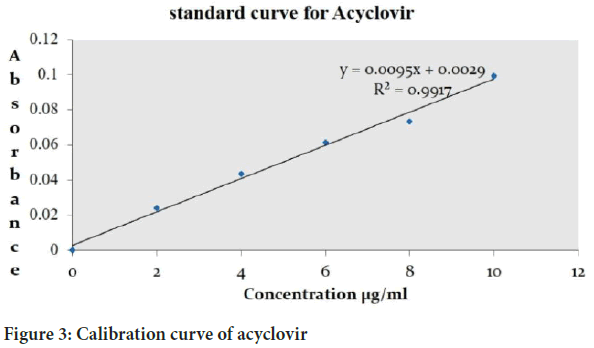
Calibration curve of acyclovir
For the preparation of the calibration curve sample were prepared from stock solution (0, 2, 4, 6, 8, 10 μg/ml) the absorbance of the sample was taken at 252 nm using 0.1 N HCl (Figure 3).
Figure 3: Calibration curve of acyclovir
Evaluation of micro sponges
Particle size analysis: Determination of the average particle size of acyclovir loaded micro sponges was determined with an optical microscope using a calibrated ocular and stage micrometer (Makwana R and Patel V, 2014). A minute quantity of microsponges was spread on a clean glass slide with a drop of liquid paraffin and a cover slip is placed on it. The average particle size was calculated by measuring 100 particles of each batch.
Where, dav is the average diameter of particles (μm), n is number of particles per group, and d is the middle value (μm).
Scanning electron microscopy: Scanning electron microscopy of optimized microsponge formulation was carried to determine the surface morphology. The sample was mounted directly onto the SEM sample holder using double sided sticking tape and images were recorded at different magnifications at acceleration voltage of 10 kV using scanning electron microscope.
Loading efficiency: The microsponges was determined spectrophotometrically (λmax=252 nm). A sample of Acyclovir microsponges (100 mg) was dissolved dissolved in 100 ml of phosphate buffer (pH 6.8) and kept for overnight. The drug content was determined and expressed as actual drug content in microsponge. The loading efficiency (%) of the microsponges was calculated according to the following equation,
Loading efficiency=Actual drug content in microsponges × 100/Theoretical drug content
In vitro dissolution studies: in vitro drug release study was carried out using USP type-II dissolution test apparatus. The dissolution medium 900 ml of 7.4 phosphate buffer was maintained at 37 ± 1⁰C and stirred at 50 rpm. Aliquots of samples (5 ml) at an interval of 1 hour were withdrawn and filtered through Whattman filter paper. The samples were analyzed for Acyclovir content by UV-Visible spectrophotometer at 252 nm (Swetha A, et al., 2011). Data obtained was also subjected to kinetic treatment to obtain the order of release and release mechanism (Mehta M, et al., 2012; Kaundal A, et al, 2014). To examine the drug release kinetics and mechanism, the cumulative release data were fitted to models representing zero order (Q v/s. t), first order [Log(Q0-Q) v/s t], Higuchi’s square root of time (Q v/s. t1/2) and KorsemeyerPeppas double log plot (log Q v/s log t) respectively, where Q is the cumulative percentage of drug released at time t and (Q0-Q) is the cumulative percentage of drug remaining after time t.
Results and Discussion
FTIR analysis
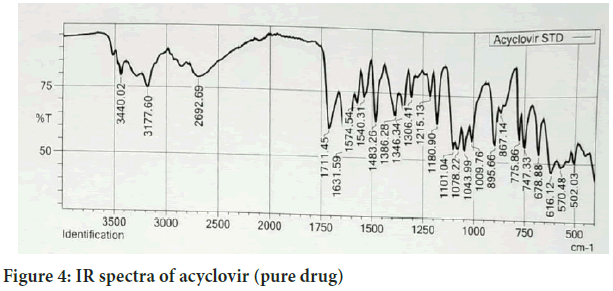
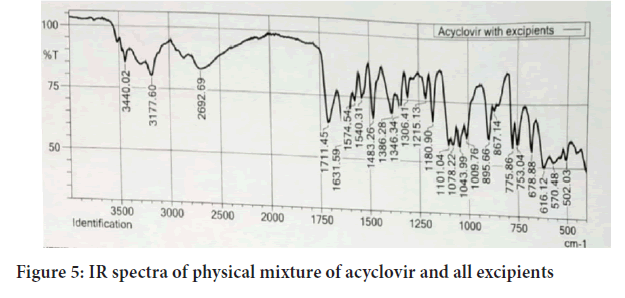
Fourier Transforms Infrared Radiation (FTIR) spectra of acyclovir and a mixture of acyclovir and the selected excipients were performed in conditions 40°C+2/75%+5 RH for one month storage. From the spectra of acyclovir, physical mixture of acyclovir and polymers. It was observed that all characteristic peaks of Acyclovir were present in the combination spectrum thus indicating compatibility of Acyclovir and polymer (Hussain H, et al., 2014) (Figures 4 and 5).
Figure 4: IR spectra of acyclovir (pure drug)
Figure 5: IR spectra of physical mixture of acyclovir and all excipients
Scanning Electron Microscopy (SEM)
Scanning electron microscopy was performed to characterize the surface of the formed micro sponge, the micro sponge particles are spherical for F1 and F5 formulation.
Loading efficiency
From the results obtained, it was observed that the F5 have higher entrapment as compared to other formulation like F1=89.34%, F2=85.65%, F3=91.48%, F4=88.67%, F5=95.88%, F6=92.43%, F7=94.34%.
Particle size distribution
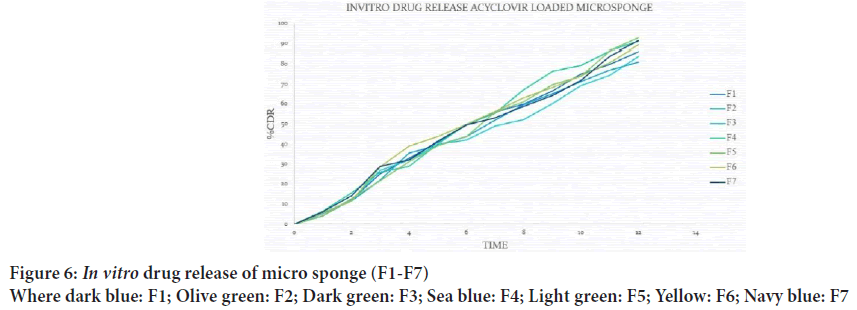
It was found that particle size distribution was in the range of 30.77 μm to 47.71 μm, F5 formulation shows less particle size as compared to other formulations, F4 formulation has maximum particle size range to 47.71 μm, and increase in amount of polymer increased the particle size of micro sponges (Figure 6).
Figure 6: In vitro drug release of micro sponge (F1-F7)
Where dark blue: F1; Olive green: F2; Dark green: F3; Sea blue: F4; Light green: F5; Yellow: F6; Navy blue: F7
Conclusion
A maximum of 95.67% drug entrapment efficiency was obtained in the acyclovir micro sponges. F5 formulations ACV micro sponges show the maximum drug release. On the basis of particle size, entrapment efficiency and morphology, in vitro release studies and its kinetics data, F5 (formulation) was selected as optimized formulation.
Acknowledgement
I am thankful to the HOD and entire staff of East Point College of Pharmacy Bengaluru for the support and Medopharma pvt ltd for gift sample of Acyclovir.
References
- Dock E. Herpes simplex. Healthline. 2021.
- Yadav P, Nanda S. Development and evaluation of some microsponge loaded medicated topical formulations of acyclovir. Int J Pharm Sci Res. 2014; 5(4): 1395-1410.
- Patel A, Upadhyay P, Trivedi J, Shah S, Patel J. Microsponges as the versatile tool for topical route: A review. Int J Pharm Sci Res. 2012; 3(9): 2926-2937.
[Crossref] [Google scholar] [Pubmed]
- Ravi R, Senthilkumar SK, Parthiban S. Microsponges drug delivery system: A review. Int J Pharm Sci Rev Res. 2013; 3(1): 6-11.
- Hussain H, Juyal D, Dhyani A. Microsponges: An overview. Int J Drug Deliv Technol. 2014; 4(4): 198-207.
- Saxena S, Nacht S. Delivery system handbook for personal care and cosmetic product: Technology, application and formulation. William Andrew publishing. 2005.
- Osmani RA, Aloorkarb NH, Ingale DJ, Kulkarni KP, Hani U, Bhosale RR, et al. Microsponge based novel drug delivery system for augmented arthritis therapy. Saudi Pharm J. 2015; 23(5): 562-572.
[Crossref] [Google scholar] [Pubmed]
- Patel EK, Oswal RJ. Nanosponges and micro sponges: A novel drug delivery system. Int J Res Pharm Chem. 2012; 2(2): 237-243.
- Pandey P, Jain V, Mahajan SC. A review: Microsponge drug delivery system. Int J Bio Pharm. 2013; 4(3): 225-230.
- Kale S, Shalini R, Kanchan M, Eknath P. Microsponge: Comprehensive review of application. Int J Pharm Bio Sci. 2013; 3(1): 214-226.
- El-Helw AM, Bayomi MA. Effect of core modification on the release of chlorpheniramine maleate from ethylcellulose and cellulose acetate propionate microcapsules. Saudi Pharm J. 2000; 8: 31-38.
- Makwana R, Patel V. Microsponge for topical drug delivery system. Int J pharm Tech. 2014: 5(4): 2839-2851.
- Swetha A, Rao GM, Ramana VK, Basha NB, Reddy KV. Formulation and in vitro evaluation of etodolac entrapped in microsponge based drug delivery system. Int J Pharm. 2011; 1(2): 73-90.
- Mehta M, Panchal A, Shah VH, Upadhayay U. Formulation and in vitro evaluation of controlled release microsponge gel for topical delivery of clotrimazole. Int J Adv pharm. 2012; 2(2): 93-101.
- Hussain H, Dhyani A, Juyal D, Bahuguna A. Formulation and evaluation of gel-loaded microsponges of diclofenac sodium for topical delivery. J Pharm Innov. 2014; 3(10): 58-63.
- Kaundal A, Bhatia R, Sharama A, Sukrial P. A review on microsponges drug delivery system. Int J Adv pharm. 2014; 4: 177-181.
- Abdelmalak NS, El-Menshawe SF. A new topical fluconazole microsponge loaded hydrogel: Preparation and characterization. Int J Pharm Pharm Sci. 2012; 4(1): 460-468.
- Pavani S, Mounika K, Naresh K. Formulation and evaluation of acyclovir microspheres. Iraqi J Pharm Sci. 2017; 27(1): 1-7.
- Hussein AA. Preparation and evaluation of oral microsponge drug delivery system of ketoconazole. Am j pharm sci. 2014; 14(1): 1-8.
Author Info
Shwetha TL*, Srinivasan S and Chandrakala VCitation: Shwetha TL: Formulation and Evaluation of Micro Sponges Loaded with Acyclovir Using an Antiviral Drug
Received: 08-Mar-2022 Accepted: 29-Mar-2022 Published: 05-Apr-2022, DOI: 10.31858/0975-8453.13.4.225-228
Copyright: This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
ARTICLE TOOLS
- Dental Development between Assisted Reproductive Therapy (Art) and Natural Conceived Children: A Comparative Pilot Study Norzaiti Mohd Kenali, Naimah Hasanah Mohd Fathil, Norbasyirah Bohari, Ahmad Faisal Ismail, Roszaman Ramli SRP. 2020; 11(1): 01-06 » doi: 10.5530/srp.2020.1.01
- Psychometric properties of the World Health Organization Quality of life instrument, short form: Validity in the Vietnamese healthcare context Trung Quang Vo*, Bao Tran Thuy Tran, Ngan Thuy Nguyen, Tram ThiHuyen Nguyen, Thuy Phan Chung Tran SRP. 2020; 11(1): 14-22 » doi: 10.5530/srp.2019.1.3
- A Review of Pharmacoeconomics: the key to “Healthcare for All” Hasamnis AA, Patil SS, Shaik Imam, Narendiran K SRP. 2019; 10(1): s40-s42 » doi: 10.5530/srp.2019.1s.21
- Deuterium Depleted Water as an Adjuvant in Treatment of Cancer Anton Syroeshkin, Olga Levitskaya, Elena Uspenskaya, Tatiana Pleteneva, Daria Romaykina, Daria Ermakova SRP. 2019; 10(1): 112-117 » doi: 10.5530/srp.2019.1.19
- Dental Development between Assisted Reproductive Therapy (Art) and Natural Conceived Children: A Comparative Pilot Study Norzaiti Mohd Kenali, Naimah Hasanah Mohd Fathil, Norbasyirah Bohari, Ahmad Faisal Ismail, Roszaman Ramli SRP. 2020; 11(1): 01-06 » doi: 10.5530/srp.2020.1.01
- Manilkara zapota (L.) Royen Fruit Peel: A Phytochemical and Pharmacological Review Karle Pravin P, Dhawale Shashikant C SRP. 2019; 10(1): 11-14 » doi: 0.5530/srp.2019.1.2
- Pharmacognostic and Phytopharmacological Overview on Bombax ceiba Pankaj Haribhau Chaudhary, Mukund Ganeshrao Tawar SRP. 2019; 10(1): 20-25 » doi: 10.5530/srp.2019.1.4
- A Review of Pharmacoeconomics: the key to “Healthcare for All” Hasamnis AA, Patil SS, Shaik Imam, Narendiran K SRP. 2019; 10(1): s40-s42 » doi: 10.5530/srp.2019.1s.21
- A Prospective Review on Phyto-Pharmacological Aspects of Andrographis paniculata Govindraj Akilandeswari, Arumugam Vijaya Anand, Palanisamy Sampathkumar, Puthamohan Vinayaga Moorthi, Basavaraju Preethi SRP. 2019; 10(1): 15-19 » doi: 10.5530/srp.2019.1.3