Brief Report - (2024) Volume 15, Issue 7
Abstract
Fungal infections are pervasive and challenging health issue, impacting millions of individuals globally. With the rise of antifungal resistance and the adverse effects associated with synthetic antifungal agents, there is a growing interest in natural alternatives. This study focuses to study the formulation and development of an antifungal herbal gel, leveraging the therapeutic potential of various medicinal plants. The primary objective is to create topical gel with potent antifungal properties with minimal side effects and enhanced patient compliance. Fruits and leaves of Schinus terebinthifolius (Brazilian pepper tree) were selected for this formulation. The extraction was carried by the maceration and Soxhlet apparatus. The extracts were incorporated into the base gel-prepared formulation prepared in the laboratory. The prepared products were further evaluated for their color, spread ability, pH, washability, consistency and zone of inhibition.
Keywords
Maceration, Antifungal agent, Soxhlet apparatus, Base gel, Herbal extraction, Schinus terebinthifolius
Introduction
Schinus terebinthifolius popularly known as the Brazilian pepper tree has been useful in the field of herbal medicine. The phytochemical study of the plant has resulted in the isolation of terpenes, monoterpenes, sesquiterpenes and flavonoids (Table 1). In this study we have focused on the antifungal properties that are exerted by Schinus terebinthifolius (da Silva Nascimento M, et al., 2023; Marangoni JA, et al., 2023). In a previous study where the antifungal properties of essential oil made by Schinus terebinthifolius were observed and it has been reported that the presence of o-cymene and limonene have been shown to have strong anti-fungal properties. It has been suggested that the phytoconstituents present in the plant species are likely to disrupt the permeability barrier of cell membrane of the fungi which eventually disrupts the respiration of the cell leading to its death. The plant species has also been shown to have astringent, anti-bacterial, diuretic, anti-viral and wound healing properties (Almeida-Silva F, et al., 2022; de Oliveira DM, et al., 2021; Rosas EC, et al., 2019).
| Phytoconstituents | Brazilian pepper tree |
|---|---|
| Glycosides | ✔ |
| Phenolic compounds | ✔ |
| Carotenoids | ✔ |
| Flavonoids | ✔ |
| Alkaloids | ✔ |
| Steroids | ✔ |
| Terpenoids | ✔ |
| Saponins | ✔ |
| Tannins | ✔ |
Table 1: Phytochemical screening of herbal plant
Materials and Methods
Plant profile
Schinus terebinthifolius, also called the Brazilian pepper tree, belongs to the Anacardiaceae family. It can be used to tighten pores, kill bacteria, viruses, aid digestion and can be used as a stimulant, diuretic, wound healer and antifungal (Corrêa FR, et al., 2017; Silva MM, et al., 2017; Uliana MP, et al., 2016; Rosas EC, et al., 2015; Fedel-Miyasato LE, et al., 2014).
Preparation of extracts
Both the fruit and leaf extracts were prepared by maceration and Soxhlet apparatus technique.
Maceration: Brazilian pepper tree extract was collected by the maceration process. The freshly collected fruits and leaves were dried at room temperature and these were ground separately. 3 g of Brazilian pepper tree powder was dissolved in 50 ml of ethyl acetate. The preparation was left undisturbed for 72 hours and the mixture was filtered by Whatman filter paper, grade 40. 30 ml of leaf extract and 40 ml of fruit extract was reported (Figures 1A and 1B) (Nickerson K and Flory SL, 2015; Estevão LR, et al., 2013).
Figure 1: Extract by maceration, (A): Leaf and (B): Fruit
Soxhlet extraction: Leaves and fruit of Brazilian pepper tree were dried and powered. 10 g of the powered drug form was placed in a thimble. Soxhlet extractor was placed onto a flask containing the extraction solvent (250 ml of ethyl acetate); Soxhlet apparatus was equipped with a condenser. The solvent in round bottom flask was heated to reflux (Figures 2A-2C). The evaporated solvent vapors pass through the side tube of the extractor and condensed in the condenser, which was fitted at the top of the extractor and drips into the body of the extractor as pure menstruum. The condensed hot solvent runs into the thimble and soaks the material and extracts the constituents. When the Soxhlet chamber holding the thimble becomes full, the chamber is emptied by siphon and the solvent runs back down the distillation flask. This cycle is allowed to repeat many times over hours or days. During each cycle, a portion of the compound dissolves in the solvent. After many cycles (6 hours) the desired extract was obtained.
Figure 2: (A): Soxhlet apparatus; (B): Leaf extract by Soxhlet extraction and (C): Fruit extract by Soxhlet extraction
Results and Discussion
Evaluation and phytochemical screening of extracts
All the extracts were analyzed for their phytoconstituents, such as saponins, alkaloids, tannins, phenolic compounds, flavonoids, polyphenols and carotenoids (Alves LA, et al., 2013; Gomes FS, et al., 2013; Overholt WA, 2012).
Preparation of base gel
Carbopol gel preparation: Carbopol 934, triethanloamine, propylene glycol, disodium edetate, methyl paraben and propyl paraben were used to make the gel preparation. 1.5 g of carbapol 934 was dissolved slowly with stirring in 60 ml of distilled water for 1 hour to avoid agglomeration (Figures 3A, 3B and Table 2). Then 0.5 g of di-sodium edetate and 2.5 ml of triethanolamine were dissolved in 10 ml of distilled water separately and stirred for 10 minutes. 5 ml of propylene glycol was mixed in 12 ml of distilled water, by stirring for 10 minutes. Di-sodium edetate and triethanolamine were added to the carbopol solution and the pH was adjusted to 7.5 by stirring the solution for 10 minutes (Table 3). Then propylene glycol solution was added by stirring for 10 minutes, until a clear, consistent base gel was obtained.
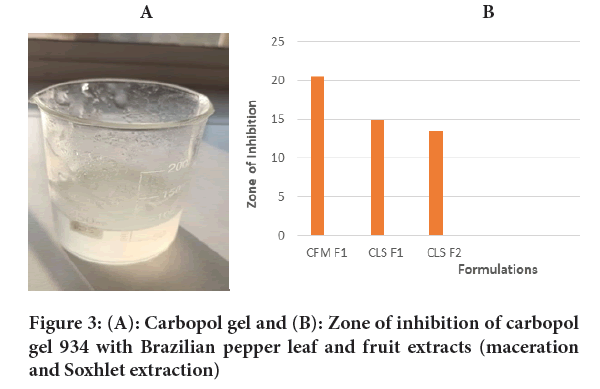
Figure 3: (A): Carbopol gel and (B): Zone of inhibition of carbopol gel 934 with Brazilian pepper leaf and fruit extracts (maceration and Soxhlet extraction)
| Ingredients | Quantity required |
|---|---|
| Carbopol 934 | 1.5 g |
| Disodium edetate | 0.5 g |
| Triethanolamine | 2.5 ml |
| Propylene glycol | 5 ml |
| Methyl paraben | 0.05 g |
| Propyl paraben | 0.05 g |
| Distilled water | Quantity sufficient |
Table 2: Preparation of base gel
| Extract type | Gel type | Formulation (g) | Extract quantity (ml) | pH |
|---|---|---|---|---|
| Brazilian pepper tree leaf extract maceration | Carbopol gel | F1 (1.5 g) | 5 | 7.4 |
| F2 (2 g) | 10 | 7.3 | ||
| Xanthum gum gel | F1 (1.5 g) | 5 | 6.7 | |
| F2 (2 g) | 10 | 6 | ||
| Brazilian pepper tree fruit extract maceration | Carbopol | F1 (1.5 g) | 5 | 7.4 |
| F2 (2 g) | 10 | 7.1 | ||
| Xanthum gum gel | F1 (1.5 g) | 5 | 6.8 | |
| F2 (2 g) | 10 | 7 | ||
| Brazilian pepper tree leaf Soxhlet extraction | Carbopol gel | F1 (1.5 g) | 5 | 7.9 |
| F2 (2 g) | 10 | 7.6 | ||
| Xanthum gum gel | F1 (1.5 g) | 5 | 6.7 | |
| F2 (2 g) | 10 | 7.2 | ||
| Brazilian pepper tree fruit Soxhlet extraction | Carbopol gel | F1 (1.5 g) | 5 | 7.2 |
| F2 (2 g) | 10 | 6.6 | ||
| Xanthum gum gel | F1 (1.5 g) | 5 | 6.9 | |
| F2 (2 g) | 10 | 7 |
Table 3: pH of different formulations of Brazilian pepper tree
Xanthum gel: Xanthum gum is dispersed in 50 ml of distilled water in a beaker. The beaker was kept aside for 30 minutes until the xanthum gum swells up and then stirred by mechanical stirrer at 1200 revolutions per minute (rpm) for 30 minutes. Propylene glycol, methyl and propyl paraben were taken in another beaker and were stirred properly. With constant stirring, the herbal extract was added to the above prepared gel (Figures 4A and 4B).
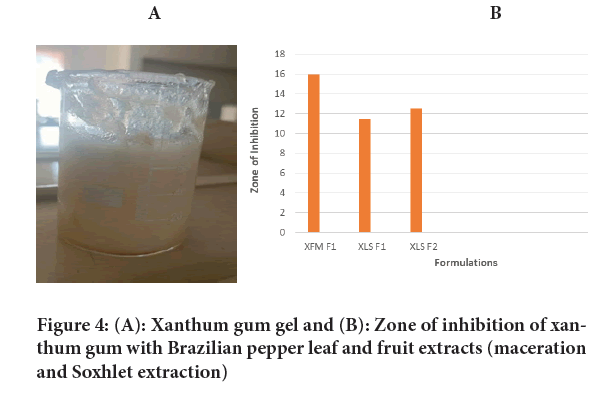
Figure 4: (A): Xanthum gum gel and (B): Zone of inhibition of xanthum gum with Brazilian pepper leaf and fruit extracts (maceration and Soxhlet extraction)
Evaluation parameters of antifungal herbal gel
Various tests have been performed on herbal gel, which included color, pH, washability, zone of inhibition, consistency and viscosity.
Color: The color of Brazilian pepper leaf gel was light moss green color while the fruit gel was dark moss green color (Geiger JH, et al., 2011; Johann S, et al., 2010; El-Massry KF, et al., 2009; Lima LB, et al., 2009; Mc Kay F, et al., 2009; Cavalher-Machado SC, et al., 2008).
pH: 1 gm of gel was dissolved in 100 ml of distilled water and was measured by pH meter (Figure 5).
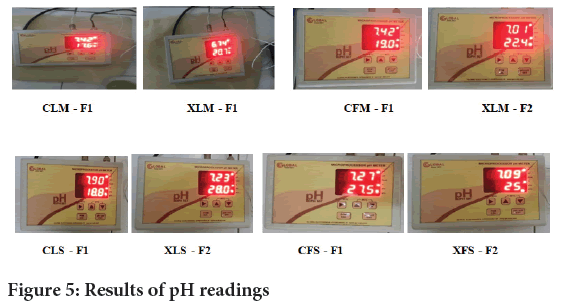
Figure 5: Results of pH readings
Washability: The prepared herbal gels are much easier to wash away from the skin surface.
Zone of inhibition: The effect of herbal gel was studied on Candida albicans by disc plate method (Table 4). Disc plate method utilized different concentrations of antibiotics which are put into cups, wells or paper discs and are placed on top of or punched into agar plates that already have the test bacterial strain on them.
| Gels | Candida albicans |
|---|---|
| Carbopol gel | |
| CFM-F2 | 20.5 mm |
| CLS-F1 | 15 mm |
| CLS-F2 | 13.5 mm |
| Xanthum gum gel | |
| XFM-F1 | 16 mm |
| XLS-F1 | 11.5 mm |
| XLS-F2 | 12.5 mm |
Table 4: Antifungal property of herbal formulation
Procedure: All the ingredients were weighted using weighing balance. They were gently heated with continuous stirring, till the peptone and glucose is completely dissolved. The mixture should not be heated above 115°C of temperature and was sterilized at 121°C by autoclave for 15 minutes. Then the mixture was transferred to a suitable container and was labelled properly with tight cap. Then, antifungal activity was studied and inoculum was prepared by suspending 24 hours fresh culture of bacteria (Candida albicans) in 25 ml of sterile water (Table 5).
| Composition | Working formula |
|---|---|
| Glucose | 6 g |
| Peptone | 3 g |
| Agar | 4.5 g |
| Distilled water | 300 ml |
Table 5: Preparation of culture media
Determination of the zone of inhibition:
Procedure: The zone of inhibition must be carried out in laminar air flow to prevent contamination and for better bacterial growth (Figure 6). The agar media was cooled and the Candida albicans was added to the conical flask. The agar media was allowed to solidify and after solidification, holes were made with a borer. Once the wells were formed, wells were marked properly and the extracts were pipetted into the wells. Petri dishes were kept in an incubator for 48 hours to avoid microbial growth and prevent contamination (Figures 7 and 8).

Figure 6: Laminar air flow
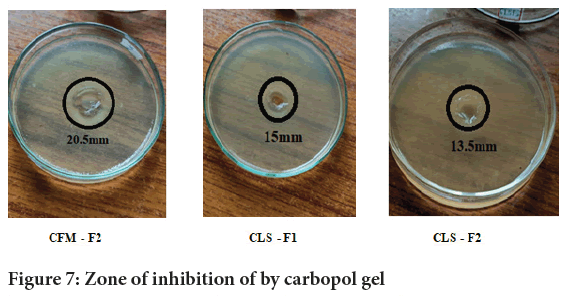
Figure 7: Zone of inhibition of by carbopol gel

Figure 8: Zone of inhibition by xanthum gum gel
Consistency and viscosity: The polyherbal gel had very smooth consistency and texture, while the viscosity was performed using the Brookfield viscometer, spindle No: 62, at 12 rpm, and 2500 cP (centipoise) (Figure 9).

Figure 9: Brookfield viscometer
Conclusion
In present paper, we summarized various formulated antifungal gel with Schinus teribinthifolius carbopol 934 as gelling agent and xanthum gum gel for topical administratiom. As per the reports of maceration and Soxhlet apparatus, carbopol 934 gel with maceration technique reported better antifungal activity. So, various antifungal gels can be formulated for better safety, efficacy and patient compliance in the future. Various research works on herbal formulations with less side effects and contraindications would be formulated, for topical administration gel is the good choice to deliver the drug through skin providing excellent results.
References
- da Silva Nascimento M, Dos Santos PH, de Abreu FF, Shan AY, Amaral RG, Andrade LN, et al. Schinus terebinthifolius Raddi (Brazilian pepper) leaves extract: In vitro and in vivo evidence of anti-inflammatory and antioxidant properties. Inflammopharmacology. 2023; 31(5): 2505-2519.
[Crossref] [Google Scholar] [Pubmed]
- Marangoni JA, da Costa Pinto JV, Kassuya CA, de Oliveira Junior PC, Dos Santos SM, Cardoso CA, et al. Geographical variation in the chemical composition, anti-inflammatory activity of the essential oil, micromorphology and histochemistry of Schinus terebinthifolia Raddi. J Ethnopharmacol. 2023; 301: 115786.
[Crossref] [Google Scholar] [Pubmed]
- Almeida-Silva F, Bernardes-Engemann AR, Bérenger AL, da Silva VP, Figueiredo MR, Freitas DF. In vitro activity of Schinus terebinthifolius extract and fractions against Sporothrix brasiliensis. Mem Inst Oswaldo Cruz. 2022; 117: e220063.
[Crossref] [Google Scholar] [Pubmed]
- de Oliveira DM, Menezes DB, Andrade LR, Lima FD, Hollanda L, Zielinska A, et al. Silver nanoparticles obtained from Brazilian pepper extracts with synergistic anti-microbial effect: Production, characterization, hydrogel formulation, cell viability, and in vitro efficacy. Pharm Dev Technol. 2021; 26(5): 539-48.
[Crossref] [Google Scholar] [Pubmed]
- Rosas EC, Correa LB, das Henriques GM. Antiinflammatory properties of Schinus terebinthifolius and its use in arthritic conditions. InBioactive food as dietary interventions for arthritis and related inflammatory diseases. 2019.
- Corrêa FR, Schanuel FS, Moura-Nunes N, Monte-Alto-Costa A, Daleprane JB. Brazilian red propolis improves cutaneous wound healing suppressing inflammation-associated transcription factor NFκB. Biomed Pharmacother. 2017; 86: 162-171.
[Crossref] [Google Scholar] [Pubmed]
- Silva MM, Iriguchi EK, Kassuya CA, Vieira MD, Foglio MA, Carvalho JE, et al. Schinus terebinthifolius: Phenolic constituents and in vitro antioxidant, antiproliferative and in vivo anti-inflammatory activities. Revista Brasileira de Farmacognosia. 2017; 27(4): 445-452.
- Uliana MP, Fronza M, da Silva AG, Vargas TS, de Andrade TU, Scherer R. Composition and biological activity of Brazilian rose pepper (Schinus terebinthifolius Raddi) leaves. Ind Crop Prod. 2016; 83: 235-240.
[Crossref] [Google Scholar] [Pubmed]
- Rosas EC, Correa LB, de Pádua AT, Costa TE, Mazzei JL, Heringer AP, et al. Anti-inflammatory effect of Schinus terebinthifolius Raddi hydroalcoholic extract on neutrophil migration in zymosan-induced arthritis. J Ethnopharmacol. 2015; 175: 490-498.
[Crossref] [Google Scholar] [Pubmed]
- Fedel-Miyasato LE, Kassuya CA, Auharek SA, Formagio AS, Cardoso CA, Mauro MO, et al. Evaluation of anti-inflammatory, immunomodulatory, chemopreventive and wound healing potentials from Schinus terebinthifolius methanolic extract. Rev Bras Farmacogn. 2014; 24(5): 565-575.
- Nickerson K, Flory SL. Competitive and allelopathic effects of the invasive shrub Schinus terebinthifolius (Brazilian pepper tree). Biol Invasions. 2015; 17: 555-564.
- Estevão LR, Mendonça FD, Baratella-Evêncio L, Simões RS, Barros ME, Arantes RM, et al. Effects of aroeira (Schinus terebinthifoliu Raddi) oil on cutaneous wound healing in rats. Acta Cir Bras. 2013; 28: 202-209.
[Crossref] [Google Scholar] [Pubmed]
- Alves LA, Freires ID, Pereira TM, Souza AD, Lima ED, Castro RD. Effect of Schinus terebinthifolius on Candida albicans growth kinetics, cell wall formation and micromorphology. Acta Odontol Scand. 2013; 71(3-4): 965-971.
[Crossref] [Google Scholar] [Pubmed]
- Gomes FS, Procópio TF, Napoleão TH, Coelho LC, Paiva PM. Antimicrobial lectin from Schinus terebinthifolius leaf. J Appl Microbiol. 2013; 114(3): 672-679.
[Crossref] [Google Scholar] [Pubmed]
- Overholt WA. Can novel weapons favor native plants? Allelopathic interactions between Morella cerifera (L.) and Schinus terebinthifolia Raddi1. J Torrey Bot Soc. 2012; 139(4): 356-366.
- Geiger JH, Pratt PD, Wheeler GS, A. Williams D. Hybrid vigor for the invasive exotic Brazilian pepper tree (Schinus terebinthifolius Raddi., Anacardiaceae) in Florida. Int J Plant Sci. 2011; 172(5): 655-663.
- Johann S, Sá NP, Lima LA, Cisalpino PS, Cota BB, Alves TM, et al. Antifungal activity of schinol and a new biphenyl compound isolated from Schinus terebinthifolius against the pathogenic fungus Paracoccidioides brasiliensis. Ann Clin Microbiol Antimicrob. 2010; 9: 1-6.
[Crossref] [Google Scholar] [Pubmed]
- El-Massry KF, El-Ghorab AH, Shaaban HA, Shibamoto T. Chemical compositions and antioxidant/antimicrobial activities of various samples prepared from Schinus terebinthifolius leaves cultivated in Egypt. J Agric Food Chem. 2009; 57(12): 5265-5270.
[Crossref] [Google Scholar] [Pubmed]
- Lima LB, Vasconcelos CF, Maranhão HM, Leite VR, Ferreira PA, Andrade BA, et al. Acute and subacute toxicity of Schinus terebinthifolius bark extract. J Ethnopharmacol. 2009; 126(3): 468-473.
[Crossref] [Google Scholar] [Pubmed]
- Mc Kay F, Oleiro M, Walsh GC, Gandolfo D, Cuda JP, Wheeler GS. Natural enemies of Brazilian pepper tree (Sapindales: Anacardiaceae) from Argentina: Their possible use for biological control in the USA. Florida Entomologist. 2009; 92(2): 292-303.
- Cavalher-Machado SC, Rosas EC, de Brito AF, Heringe AP, de Oliveira RR, Kaplan MA, et al. The anti-allergic activity of the acetate fraction of Schinus terebinthifolius leaves in IgE induced mice paw edema and pleurisy. Int Immunopharmacol. 2008; 8(11): 1552-1560.
[Crossref] [Google Scholar] [Pubmed]
Author Info
Nabamita Basu*, Sainuthana G, Rasmeen Fathima, Lohithalekha Sree and HephzibhaCitation: Basu N: Formulation, Development and Evaluation of Antifungal Herbal Gel
Received: 30-Jul-2024 Accepted: 13-Aug-2024 Published: 20-Aug-2024, DOI: 10.31858/0975-8453.15.7.225-229
Copyright: This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
ARTICLE TOOLS
- Dental Development between Assisted Reproductive Therapy (Art) and Natural Conceived Children: A Comparative Pilot Study Norzaiti Mohd Kenali, Naimah Hasanah Mohd Fathil, Norbasyirah Bohari, Ahmad Faisal Ismail, Roszaman Ramli SRP. 2020; 11(1): 01-06 » doi: 10.5530/srp.2020.1.01
- Psychometric properties of the World Health Organization Quality of life instrument, short form: Validity in the Vietnamese healthcare context Trung Quang Vo*, Bao Tran Thuy Tran, Ngan Thuy Nguyen, Tram ThiHuyen Nguyen, Thuy Phan Chung Tran SRP. 2020; 11(1): 14-22 » doi: 10.5530/srp.2019.1.3
- A Review of Pharmacoeconomics: the key to “Healthcare for All” Hasamnis AA, Patil SS, Shaik Imam, Narendiran K SRP. 2019; 10(1): s40-s42 » doi: 10.5530/srp.2019.1s.21
- Deuterium Depleted Water as an Adjuvant in Treatment of Cancer Anton Syroeshkin, Olga Levitskaya, Elena Uspenskaya, Tatiana Pleteneva, Daria Romaykina, Daria Ermakova SRP. 2019; 10(1): 112-117 » doi: 10.5530/srp.2019.1.19
- Dental Development between Assisted Reproductive Therapy (Art) and Natural Conceived Children: A Comparative Pilot Study Norzaiti Mohd Kenali, Naimah Hasanah Mohd Fathil, Norbasyirah Bohari, Ahmad Faisal Ismail, Roszaman Ramli SRP. 2020; 11(1): 01-06 » doi: 10.5530/srp.2020.1.01
- Manilkara zapota (L.) Royen Fruit Peel: A Phytochemical and Pharmacological Review Karle Pravin P, Dhawale Shashikant C SRP. 2019; 10(1): 11-14 » doi: 0.5530/srp.2019.1.2
- Pharmacognostic and Phytopharmacological Overview on Bombax ceiba Pankaj Haribhau Chaudhary, Mukund Ganeshrao Tawar SRP. 2019; 10(1): 20-25 » doi: 10.5530/srp.2019.1.4
- A Review of Pharmacoeconomics: the key to “Healthcare for All” Hasamnis AA, Patil SS, Shaik Imam, Narendiran K SRP. 2019; 10(1): s40-s42 » doi: 10.5530/srp.2019.1s.21
- A Prospective Review on Phyto-Pharmacological Aspects of Andrographis paniculata Govindraj Akilandeswari, Arumugam Vijaya Anand, Palanisamy Sampathkumar, Puthamohan Vinayaga Moorthi, Basavaraju Preethi SRP. 2019; 10(1): 15-19 » doi: 10.5530/srp.2019.1.3






