Case Report - (2022) Volume 13, Issue 12
Herpes Zoster involving the maxillary branch of the trigeminal nerve after viral vector COVID-19 vaccine: is it a possible adverse effect?
Rebeh Bougossa*, Wafa Marrakchi, Ikbel Kooli, Hajer ben Brahim, Adnene Toumi, Abir Aouam and Mohammed ChakrounAbstract
Introduction: Several vaccines against COVID-19 have been developed and approved. Many cases of Varicella-zoster virus reactivation following mRNA and inactivated COVID-19 vaccines have been described in the literature, suggesting a link between these vaccinations and the virus reactivation. However, reported cases of herpes zoster after viral vector COVID-19 vaccines were few.
Case presentation: We report a case of a maxillary zoster in a 66 year-old immunocompetent female, occurring 5 days after the first dose of a viral vector COVID-19 vaccination. The patient was treated with intravenous acyclovir for 10 days with a favourable outcome.
Conclusion: To the best of our knowledge, this is the first case of a maxillary zoster after viral vector COVID-19 vaccine. It may be a potential side effect of this vaccination. Further investigations are needed to elucidate these results.
Keywords
Herpes zoster, Varicella zoster virus, COVID-19, Vaccine, Maxillary nerve
Introduction
The COVID-19 pandemic has negatively impacted the global health. Several vaccines against this disease have been developed and approved. Safety and adverse effects of these vaccines are being evaluated.
Herpes zoster is a reactivation of a latent infection with Varicel-la-zoster Virus (VZV) in a sensory ganglion. Maxillary branch of the trigeminal nerve is rarely affected in this infection and approximately seen in 1.7% of the cases (Patil S, et al., 2013). This reactivation is often triggered by age-related immunosenescence and immunocompromised state. Vaccines are not common risk factors for this reactivation. However, many cases of VZV reactivation following mRNA and inactivated COVID-19 vaccines have been recently described, suggesting a link between these vaccinations and the virus reactivation. Reported cases of Herpes zoster after viral vector COVID-19 vaccines were few (Rodríguez-Jiménez P, et al., 2021; Chiu HH, et al., 2021). Here, we report a case of a 66 year-old immunocompetent female who developed a maxillary zoster, 5 days after the first dose of viral vector COVID-19 vaccination.
Case Presentation
A 66-year-old female, with a history of arterial hypertension, was admitted to the infectious diseases department for vesicular eruption associated with a severe pain on her right side of the face. She reported that she received her first dose of AstraZeneca COVID-19 vaccine 5 days ago. No other symptoms such as fever, dyspnea or cough were accompanied. The physical examination revealed: A temperature of 37°C, heart rate 92 beats per minute, respiratory rate 16 breaths per minute, blood pressure 120/70 mmHg and Glasgow coma score of 15. The vesicular lesions contained purulent fluid upon an erythematous base and were localized in the right cheek, side of nose and upper lip, associated with palpebral edema (Figure 1). The oral mucosa was intact.
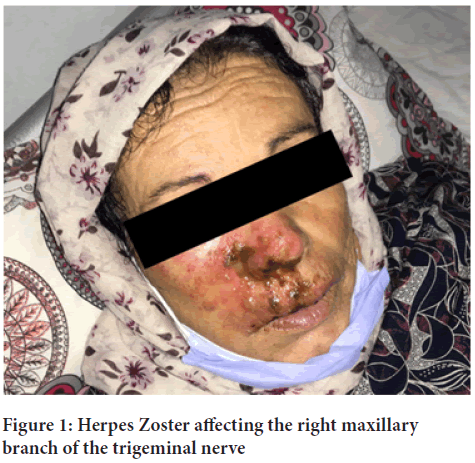
Figure 1: Herpes Zoster affecting the right maxillary branch of the trigeminal nerve
The biological findings of the serum showed-blood cell count, renal and hepatic functions within normal limits and an elevated c-reactive protein (35 mg/L). The serological tests of Human Immunodeficiency Virus (HIV) 1 and 2 were negative. The patient didn’t report the use of immunosuppressive drugs or coticosteroid therapy, a history of malignancy, physical trauma or psychological stress. The diagnosis of herpes zoster in the rignt maxillar branch of trigeminal nerve with bacterial superinfection was made based on the clinical dermatomal presentaton of the lesions. Antiviral treatment was started with acyclovir 750 mg thrice daily for 10 days associated with antibiotic treatment: Amoxicillin-clavulanic acid 1 g three times a day for a week to treat the bacterial superinfection. The outcome was favourable unless a moderate postherpetic neuralgia.
Discussion
Varicella-zona Virus reactivation in immunocompetent cases infected with Severe Acute Respiratoy Syndrome Coronavirus 2 (SARS-Cov2) has been published (Ferreira AD, et al., 2020, Tartari F, et al., 2020). This association can be explained by cytokine release and lymphopenia during the COVID-19 (Tavakolpour S, et al., 2020). A fair number of Herpes zoster cases developing after the administration of mRNA and inactivated COVID-19 vaccines have been reported in the literature (Rodríguez Jiménez P, et al., 2021; Chiu HH, et al., 2021; Aksu SB and Öztürk GZ, 2021; Bostan E and Yalici-Armagan B, 2021). The majority of the cases developed after the first dose versus the second dose of the vaccine. However, reported cases of reactivation of VZV after viral vector COVID-19 vaccines were few (Massip E, et al., 2021).
Most commonly reported adverse effects of COVID-19 vaccine were short term, mild to moderate pain in injection site, fatigue, fever and headache, but other side effects remain unknown (Polack FP, et al., 2020). The short delay of onset after vaccination and the occurrence in immunocompetent patients suggests a strong link between COVID-19 vaccine and herpes zoster emergence. The exact mechanism remains unsolved, but it is possible that the VZV reactivation is one of the side effects of the novel vaccination. A case control study of Alhasawi A, et al., 2021 enrolling 186 patients, showed that the COVID-19 vaccination had significant statistical association with herpes zoster (adjusted matched odds ratio=4.87, 95% confidence interval: 2.40-9.89, p<0.001).
These vaccines don’t cause lymphompenia or cytokine release, but they can induce an immunomodulaion that may be responsible for herpes zoster (Walter R, et al., 1999). Another theory is suggesting similarities with the immune reconstitution syndrome wich is a paradoxical worsening of a pre-existing infection seen in immunocompromised HIV patients, following the initiation of antiretroviral therapy (Psichogiou M, et al., 2021).
The ophtalmic division of the trigeminal nerve is the most involved branch in herpes zoster, while the maxillary nerve, the second division wich is affected in our case, is the least frequently affected and rarely causes ocular injury (14). In the study of Psichogiou et al, there were two cases of herpes zoster of the second division of the trigeminal nerve following mRNA COVID-19 vaccine (Psichogiou M, et al., 2021).
Conclusion
There are increasing case reports about herpes zoster following mRNA COVID-19 vaccines. But few case reports of reactivation of VZV after viral vector COVID-19 vaccines have been reported. To the best of our knowledge, this is the first case of herpes zoster involving the maxillary branch of trigeminal nerve after viral vector COVID-19 vaccine. Although, we are aware that the association could be coincidental and further studies are needed to verify these results.
Consent for Publication
It was obtained from the patient for publication of this case report and accompanying images.
References
- Patil S, Srinivas K, Reddy BS, Gupta M. Prodromal herpes zoster mimicking odontalgia-A diagnostic challenge. Ethiop J Health Sci. 2013; 23(1): 73-77.
[Google scholar] [Pubmed]
- Rodríguez-Jiménez P, Chicharro P, Cabrera LM, Seguí M, Morales-Caballero Á, Llamas-Velasco M, et al. Varicella-zoster virus reactivation after SARS-CoV-2 BNT162b2 mRNA vaccination: Report of 5 cases. JAAD case rep. 2021; 12: 58-59.
[Crossref] [Google scholar] [Pubmed]
- Chiu HH, Wei KC, Chen A, Wang WH. Herpes zoster following COVID-19 vaccine: A report of three cases. QJM. 2021; 114(7): 531-532.
[Crossref] [Google scholar] [Pubmed]
- Ferreira AD, Romão TT, Macedo YS, Pupe C, Nascimento OJ, Fellow of the American Academy of Neurology (FAAN). COVID‐19 and herpes zoster co‐infection presenting with trigeminal neuropathy. Eur J Neurol. 2020; 27(9): 1748-1750.
[Crossref] [Google scholar] [Pubmed]
- Tartari F, Spadotto A, Zengarini C, Zanoni R, Guglielmo A, Adorno A, et al. Herpes zoster in COVID‐19‐positive patients. Int J Dermatol. 2020.
[Crossref] [Google scholar] [Pubmed]
- Tavakolpour S, Rakhshandehroo T, Wei EX, Rashidian M. Lymphopenia during the COVID-19 infection: What it shows and what can be learned. Immunol Lett. 2020; 225: 31.
[Crossref] [Google scholar] [Pubmed]
- Aksu SB, Öztürk GZ. A rare case of shingles after COVID-19 vaccine: Is it a possible adverse effect? Clin Exp Vaccine Res. 2021; 10(2): 198.
[Crossref] [Google scholar] [Pubmed]
- Bostan E, Yalici-Armagan B. Herpes zoster following inactivated COVID-19 vaccine: A coexistence or coincidence? J Cosmet Dermatol. 2021; 20(6): 1566-1567.
[Crossref] [Google scholar] [Pubmed]
- Massip E, Marcant P, Font G, Faiz S, Véron M, Macaire C, et al. Zona après vaccination anti-COVID-19: série descriptive de 10 cas. Ann Dermatol Venereol. 2021; 1(8): A250-A251.
- Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N Engl J Med. 2020; 383(27): 2603‑2615.
[Crossref] [Google scholar] [Pubmed]
- Alhasawi A, Kamel MI, Elmasry S, Kamel WA, Hassan A. Impact of COVID-19 vaccination on Varicella zoster virus reactivation: A case control study. Microbiol Infect Dis. 2021; 5(4): 1-6.
- Walter R, Hartmann K, Fleisch F, Reinhart WH, Kuhn M. Reactivation of herpes virus infections after vaccinations? Lancet. 1999; 353(9155): 810.
[Crossref] [Google scholar] [Pubmed]
- Psichogiou M, Samarkos M, Mikos N, Hatzakis A. Reactivation of Varicella zoster virus after vaccination for SARS-CoV-2. Vaccines. 2021; 9(6): 572.
[Crossref] [Google scholar] [Pubmed]
- Patro S, Jena PK, Misra GC, Rath KC, Khatua P. Herpes zoster infection involving the maxillary branch of the right trigeminal nerve-a rare case report. The Antiseptic. 2013; 110(1): 36-38.
Author Info
Rebeh Bougossa*, Wafa Marrakchi, Ikbel Kooli, Hajer ben Brahim, Adnene Toumi, Abir Aouam and Mohammed ChakrounCitation: Bougossa R: Herpes Zoster involving the Maxillary Branch of the Trigeminal Nerve after Viral Vector COVID-19 Vaccine: Is it a Possible Adverse Effect?
Received: 01-Nov-2022 Accepted: 25-Nov-2022 Published: 02-Dec-2022, DOI: 10.31858/0975-8453.13.12.831-832
Copyright: This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
ARTICLE TOOLS
- Dental Development between Assisted Reproductive Therapy (Art) and Natural Conceived Children: A Comparative Pilot Study Norzaiti Mohd Kenali, Naimah Hasanah Mohd Fathil, Norbasyirah Bohari, Ahmad Faisal Ismail, Roszaman Ramli SRP. 2020; 11(1): 01-06 » doi: 10.5530/srp.2020.1.01
- Psychometric properties of the World Health Organization Quality of life instrument, short form: Validity in the Vietnamese healthcare context Trung Quang Vo*, Bao Tran Thuy Tran, Ngan Thuy Nguyen, Tram ThiHuyen Nguyen, Thuy Phan Chung Tran SRP. 2020; 11(1): 14-22 » doi: 10.5530/srp.2019.1.3
- A Review of Pharmacoeconomics: the key to “Healthcare for All” Hasamnis AA, Patil SS, Shaik Imam, Narendiran K SRP. 2019; 10(1): s40-s42 » doi: 10.5530/srp.2019.1s.21
- Deuterium Depleted Water as an Adjuvant in Treatment of Cancer Anton Syroeshkin, Olga Levitskaya, Elena Uspenskaya, Tatiana Pleteneva, Daria Romaykina, Daria Ermakova SRP. 2019; 10(1): 112-117 » doi: 10.5530/srp.2019.1.19
- Dental Development between Assisted Reproductive Therapy (Art) and Natural Conceived Children: A Comparative Pilot Study Norzaiti Mohd Kenali, Naimah Hasanah Mohd Fathil, Norbasyirah Bohari, Ahmad Faisal Ismail, Roszaman Ramli SRP. 2020; 11(1): 01-06 » doi: 10.5530/srp.2020.1.01
- Manilkara zapota (L.) Royen Fruit Peel: A Phytochemical and Pharmacological Review Karle Pravin P, Dhawale Shashikant C SRP. 2019; 10(1): 11-14 » doi: 0.5530/srp.2019.1.2
- Pharmacognostic and Phytopharmacological Overview on Bombax ceiba Pankaj Haribhau Chaudhary, Mukund Ganeshrao Tawar SRP. 2019; 10(1): 20-25 » doi: 10.5530/srp.2019.1.4
- A Review of Pharmacoeconomics: the key to “Healthcare for All” Hasamnis AA, Patil SS, Shaik Imam, Narendiran K SRP. 2019; 10(1): s40-s42 » doi: 10.5530/srp.2019.1s.21
- A Prospective Review on Phyto-Pharmacological Aspects of Andrographis paniculata Govindraj Akilandeswari, Arumugam Vijaya Anand, Palanisamy Sampathkumar, Puthamohan Vinayaga Moorthi, Basavaraju Preethi SRP. 2019; 10(1): 15-19 » doi: 10.5530/srp.2019.1.3






