Research Article - (2022) Volume 13, Issue 8
Heterocylcic Based Curcumin: Design, Synthesis and Anticancer Efficacy against HeLa Cells
Rana Al-Kerm1, Rola Al-Kerm1, Othman Hamed1*, Ashraf Swaft1,2, Mohammad Qneibi3, Avni Berisha4,5, Omar Dagdage6 and Ghaleb Adwan2Abstract
Developing a new effective anticancer agent becomes an urgent need to overcome of current drug-resistance. In this study, we demonstrated that curcumin with heterocyclic moiety can function as an anticancer agent in a human. A new series of curcumin-based benzodiazepines, diazepines and diazoles were prepared using a simple one pot process. The process involved a condensation reaction of curcumin with various 1,2 diamino compounds and hydrazine. The structures of the prepared heterocycles were identified by the spectroscopic methods Fourier-Transform Infrared Spectroscopy (FT-IR), 1H NMR (Proton Nuclear Magnetic Resonance), and 13C NMR. The in vitro anticancer activities of the synthesized curcumin-based heterocycles against HeLa cancer cells were evaluated by the 3-[4,5-dimethylthiazole-2-yl]-2,5-diphenyltetrazolium bromide (MTT) assay. The viability of HeLa cells was reduced in the range of 4.48%-14.57% within the studied concentrations. Curcumin-based diazepine 6 showed the highest cytotoxic effect on the HeLa cells at all concentrations. It reduced the viability of the tested HeLa cells in range of 4.48% for the 400 μg/ml concentration to 4.95% for the 12.5 μg/ml concentration. Moreover, heterocyclic 6 showed the highest cytotoxic and cytostatic effect among the tested heterocyclics against HeLa cells. It exhibited IC50 (Half-maximal inhibitory concentration) and a cytostatic effect of 0.4572 and 0.08515 μg/ ml, respectively at a nontoxic level, as the control L6 cells showed cytotoxic and cytostatic effect with IC50 values of 22.47 and 1.977 μg/ml, respectively. This study revealed that, the prepared curcumin-based compounds exhibit a promising anticancer activity against HeLa cancer cells at a nontoxic concentration.
Keywords
Curcumin, Benzodiazepine, Diazole, 3-[4,5-dimethylthiazole-2-yl]-2,5-diphenyltetrazolium bromide (MTT), Anticancer, HeLa cells
Introduction
Cancer is the second worldwide leading cause of death and one of the most common public health problems. World Health Organization (WHO) predicted a 70% increasing in cancer cases by the next few decades (Sawant S and Shegokar R, 2014). Despite the advances in the standard available therapies including chemotherapy, radiotherapy, surgery, immunotherapy and hormone therapy, Chemotherapy continues to be the most effective therapeutic option and widely used treatment for different types of malignancies. However, the side-effects associated with the cancer chemotherapy such as hair loss, fatigue, vomiting, nausea, and even death in severe cases which limits the scope of chemotherapeutic drugs. Therefore, continuous research for more efficient and less toxic cancer drug that can selectively target cancer cells with minimal or no side effects on normal cells is going on (Aslam MS, et al., 2014).
Natural plants products have traditionally been a magnificent source of pharmaceutical agents for centuries. Curcumin (1,7-bis(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione; diferuloylmethane), is a phenolic, natural yellow to orange compound isolated from the plant Curcuma longa L. (Zingiberaceae) (Figure 1). Curcumin has been used for centuries as a food colorant, and dietary spice in India, China, and Southeast Asia (Priyadarsini KI, 2013; Fang J, et al., 2005; Borik RM, et al., 2018; Ciochina R, et al., 2014; Ahmed M, et al., 2017; Sato T, et al., 2018).
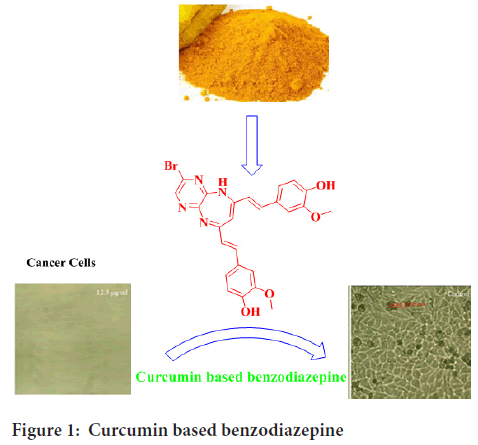
Figure 1: Curcumin based benzodiazepine
Curcumin and its derivatives possess numerous interesting pharmacological properties and wide variety of biological activities including anti-inflammatory, antioxidant, anti-microbial, antiviral, anti-fungal, antimalarial, anti-angiogenesis, anti-carcinogenic, antitumor, as well as medicinal applications, such as; their use against Alzheimer’s disease and in HIV therapies (Fang J, et al., 2005; Borik RM, et al., 2018, Li Q, et al., 2015; Ma'mani L, et al., 2014; Ding L, et al., 2015; Mishra S, et al., 2019; Ramya PS, et al., 2018). Further, curcumin is non-toxic and safe even at high dosages. The non-toxicity and the natural origin of curcumin and its wide range biological activities ensure that curcumin and its derivatives are promising lead compounds in medicinal chemistry. Curcumin contains three important functional groups that mainly contribute to its biological activities, α, β-unsaturated β-diketo group; a seven carbon olefinic linker, and an ortho-methoxy phenolic hydroxy group. Structure activity-based researches have investigated that α, β-unsaturated β-diketo group is an important functionality in anticancer activity, and the length of the olefinic linker is essential for the prevention of protein aggregation in Alzheimer’s disease models. Moreover, the ortho-methoxy phenolic hydroxy group and methylenic hydrogen are responsible for the antioxidant activity. Curcumin is insoluble in both water and ether but it is soluble in ethanol, acetone, chloroform, methanol, dichloromethane, and dimethylsulfoxide. It exists in enolic and β-diketonic forms. Therefore, it can undergo keto-enol tautomerisms that are stabilized by intra-molecular hydrogen bonding between the enolic hydrogen atom and keto carbonyl oxygen. The enol is the predominant form of curcumin in solution due to the conjugation and intramolecular hydrogen bonding that contribute to its stability (Ciochina R, et al., 2014; Ahmed M, et al., 2017; Li Q, et al., 2015; Mishra S, et al., 2019; Ramya PS, et al., 2018; Khor PY, et al., 2019).
To date, a series of novel curcumin derivatives that exhibit potential synergistic anticancer activity were synthesized. The results indicated that the prepared derivatives can inhibit breast cancer stem cells growth by hindering the mediated efflux mechanism of P-glycoprotein (P-gp). Glucoside of curcumin derivatives showed higher affinity to bind with P-glycoprotein than other derivatives of curcumin, which have reduced the breast cancer stem cells growth (Mbese Z, et al., 2019). The anticancer effect of the combination of 5-fluorouracil (5-FU) with curcumin against gastric cancer MKN45 and AGS cells (Human Gastric Adenocarcinoma Cells) was reported (Yang H, et al., 2017). The results indicated that the combination of 5-FU and curcumin (2:1, mol/mol) enhanced cytotoxic effect compared to curcumin or 5-FU alone and showed synergistic effect. Moreover, the combination of 5-FU and curcumin also potentiated cytotoxicity in AGS cells compared to curcumin or 5-FU alone, but the effect was moderate. Further, synthesis of new heterocyclic derivatives based on curcumin was reported (Borik RM, et al., 2018). The in vitro inhibition capacity of the synthesized compounds was screened in two human cancer cell lines (breast cancer (MCF-7) and Hepatocellular carcinoma (HEPG2) in addition to the normal cell line (human normal melanocyte, HFB4) in comparison to the known anticancer drugs: Doxorubicin and 5-flurouracil. The anticancer activity results investigated that the synthesized products showed growth inhibition activity against HEPG2 cell line and MCF-7 cell line, but with varying intensities compared to the known doxorubicin and 5-flurouracil anticancer drugs (Borik RM, et al., 2018).
In the present work, we report synthesis of new curcumin-based benzodiazepines, diazepines and diazoles via an acid catalyzed condensation cyclization reaction of curcumin with different 1,2 diamines, pyridine, pyrazine, or pyrimidine. Moreover, synthesis of hydrogenated curcumin (H-curcumin) based amine using a two-step process was also reported. The in vitro anticancer activities of the synthesized heterocycles against HeLa cancer cells were screened using the MTT colorimetric assay.
Materials and Methods
All chemicals used in this study were purchased from Aldrich Chemical Company and used as they were received. All prepared compounds were characterized by 1H NMR, 13C NMR, MS/MS (Tandem Mass Spectrometry) and Infra-Red (IR) spectroscopy. Nuclear Magnetic Resonance spectra were recorded on Varian Gemini 2000, 300 MHz instruments. Infrared spectra were recorded on a Shimadzu 820 PC (Polycarbonate) FT-IR spectrometer. The solvent used in the NMR was DMSO-d6 (Dimethyl sulfoxide-d6), 1H NMR experiments were reported in δ units, parts per million (ppm) downfield from Tetramethyl Silane (TMS). All 13C NMR spectra were reported in ppm relative to DMSO-d6 (39.52 ppm). The reactions progress was monitored using Thin Layer Chromatography (TLC) analysis performed on silica gel plates, pre-coated with Merck Kieselgel 60 F254, and visualization was done using an Ultraviolet (UV) lamp. The mobile phase used was hexane: Ethyl acetate (6:4) mixture. All melting points were uncorrected and were determined in an open capillary tube. Each melting point was carried out at least in duplicate. The sample purifications were carried out by either crystallization or flash chromatography with silica gel (100-200) mesh.
Preparation of curcumin-based heterocyclic compounds
In a round-bottomed flask equipped with a condenser and a magnetic stirring bar, curcumin (1.357 mmoles, 0.5 g) was dissolved in (30.0 mL) ethanol. The desired amine, pyridine, pyrazine, or pyrimidine (1.357 mmoles) was added to the curcumin solution followed by adding a few drops of the catalyst concentrated sulfuric acid. The reaction mixture was then refluxed in a paraffin oil bath until completion of the reaction (12 to 24 hr), which was monitored by TLC. The excess solvent was removed under reduced pressure using a rotary evaporator; the residue was washed with sodium bicarbonate solution (5%), filtered, washed again with water and dried. Residual starting materials were washed out from the product by suspending the product in either diethyl ether or ethyl acetate. The products were further purified by flash chromatography or crystallization. In the preparation of compound 2 glacial acetic acid was used as a solvent (Hamed O, et al., 2020).
5,7-bis [(E)-2-(4-hydroxy-3-methoxyphenyl) ethenyl]-1H-1,4-di- azepine-2,3-dicarbonitrile (2): A mixture of curcumin (1.357 mmole, 0.5 g) and diaminomaleonitrile (0.146 g, 1.357 mmol) were refluxed in glacial acetic acid (30.0 mL) for about 60 h. The product was recrystallized from hexane/EtOAc (2:1 by volume) to give 0.126 g (yield 21.1%), mp 250°C-254°C, IR: vmax cm-1 3417.74 (O-H), 3366.66 (N-H), 2362.39 (C≡N), 1650.55 (C=N), 1558.32 (C=C). 1H-NMR (400 MHz, DMSO-d6) δ: 3.82 (s, 6H, OCH3), 4.05 (bs, 1H, NH), 5.10 (1H, s), 5.41 (bs, 2H, OH), 5.71 (d, 1H, J=15.1 Hz), 6.82 (m, 4H), 7.03 m, 3H; 7.25 (d, 2H, J=7.5 Hz).13C-NMR (400 MHz, DMSO-d6) δ: 56.1, 103.6, 105.0, 11.3, 138, 1147.9, 115.2, 116.8, 120.2, 122.9, 124.3, 127.6, 135, 149.1, 149.4, 164.6. LC/MS [M+1] for C25H20N4O4: Calculated 441.15, found: 441.82.
4-[(E)-2-{2-[(E)-2-(4-hydroxy-3-methoxyphenyl) ethenyl]-5H-pyri- do [2,3-b][1,4] diazepin-4-yl} ethenyl]-2-methoxyphenol (3): A 2,3-diaminopyridine (0.148 g, 1.357 mmol) was added to a solution of curcumin (0.5 g, 1.357 mmol) in ethanol (30.0 mL), followed by adding a catalytic amount of H2SO4 (3 drops). The produced mixture was stirred under reflux until reaction completion. The product was recrystallized from hexane/EtOAc (2:1 by volume) to give 0.35 g (yield 58.5%), m.p is 118-120. IR: vmax cm-1 3344 (O-H, and N-H stretching), 3022 (=C-H), 2974 (C-H, aliphatic), 1605 (C=N), 1584 (C=C, conjugated), 1389 (C-N), 1085 (C-O ether and alcohol). 1H-NMR (400 MHz, DMSO-d6) δ: 3.83 (s, 6H, OCH3), 4.0 (bs, 1H, NH), 5.06 (1H, d), 5.35 (bs, 2H, OH), 5.67 (d, 1H, J=15.1 Hz), 6.80-6.98 (m, 7H); 7.13 (m, 1H), 7.26 (m, 2H); 7.36 (d, 1H, J=7.5 Hz); 8.12 (d, 1H).13C-NMR (400 MHz, DMSO-d6) δ: 56.1, 88.69, 111.9, 113.0, 116.8, 122.9, 124.0, 127.6, 132.6, 135.0, 138.1, 146.6, 147.9, 149.1, 149.5, 160.0, 164.6. LC/MS [M+1] for C26H23N3O4: Calculated 442.17, found: 442.43
6,8-bis [(E)-2-(4-hydroxy-3-methoxyphenyl) ethenyl]-5H-pyraz- ino [2,3-b] [1,4] diazepine-2,3-dicarbonitrile (4): A sample of 5,6-diamino-2,3-pyrazindicarbonitrile (0.217g, 1.357 mmol) was added to a solution of 1,7-curcumin (1.357 mmole, 0.5 g) in ethanol (30.0 mL) followed by adding catalytic amount of H2SO4 (3 drops). Then the solution was refluxed until reaction completion. The product was recrystallized from hexane/EtOAc (2:1 by volume) to give 0.67 g (Yield 99.55%), m.p. 206°C-208°C, IR: vmax cm-1 3338.69 (N-H), 3157.25 (=C-H), 2961.48 (C-H), 2231.89 (C≡N stretch), 1671 (C=N), 1628.62 (C=C). 1H-NMR (400 MHz, DMSO-d6) δ: 3.81 (s, 6H, OCH3), 4.1 (bs, 1H, NH), 5.09 (1H, s), 5.41 (bs, 2H, OH), 5.67 (d, 1H, J=15.1 Hz), 6.81 (m, 3H), 6.83 (d, 1H); 6.88 (d, 1H); 6.99 (d, 2H); 7.21 (d, 2H, J=7.5 Hz).13C-NMR (400 MHz, DMSO-d6) δ: 56.2, 112.1, 117.3, 122.9, 127.9, 131.3, 135.1, 124.4, 137.9, 148.1, 149.3, 149.7, 154.5, 147.5, 155.2, 160.2, 164.5. LC/MS [M+1] for C27H20N6O4: Calculated 493.15, found: 493.73.
4-[(E)-2-{3-bromo-8-[(E)-2-(4-hydroxy-3-methoxyphenyl)ethenyl]- 5H-pyrazino[2,3-b][1,4]diazepin-6-yl} ethenyl]-2-methoxyphenol (5): A sample of 2, 3-diamino-5-bromopyrizine (0.1276 g, 0.678 mmole) was added to a solution of curcumin (0.678 mmole, 0.25 g) in ethanol (30.0 mL), followed by adding catalytic amount of H2SO4 (1 drop). Then the solution was refluxed until reaction completion. The product was recrystallized from hexane/EtOAc (2:1 by volume) to give 0.31g (Yield 87.85%), mp 88°C-90°C, IR (KBr): vmax cm-1 1621.33 (-C=N), 542.15 (C-Br), and 3384.14 (-C-N-H). 1H-NMR (400 MHz, DMSO-d6) δ: 3.82 (s, 6H, OCH3), 3.98 (bs, 1H, NH), 5.06 (1H, s), 5.35 (bs, 2H, OH), 5.67 (d, 1H, J=15.1 Hz), 6.79 (m, 2H), 6.85 (d, 1H, J=15.1 Hz), 6.86 (d, 2H); 6.99 (d, 2H); 7.16 (d, 2H, J=7.5 Hz), 7.96 (s, 1H).13C-NMR (400 MHz, DMSO-d6) δ: 56.2, 103.1, 111.5, 116.4, 121.2, 122.5, 124.2, 135.6, 139.3, 147.2, 149.3, 150.6, 159.3, 164.8. LC/MS [M+1] for C25H21BrN4O4: Calculated 521.08, found: 521.38 and 533.43 (bromine isotope).
4-[(E)-2-{8-bromo-4-[(E)-2-(4-hydroxy-3-methoxyphenyl)ethenyl]- 1H-pyrido[2,3-b][1,4]diazepin-2-yl}ethenyl]-2-methoxyphenol (6): A 2, 3-diamino-5-bromopyridine (0.2538 g, 1.357 mmol) was added to a solution of curcumin (1.357 mmole, 0.5 g) in ethanol (30.0 mL), followed by the addition of concentrated H2SO4 (2 drops). The produced solution was then refluxed until reaction completion. The product was recrystallized from hexane/EtOAc (2:1 by volume) to give 0.34 g (Yield 48.14%), mp 108°C-110°C, IR (KBr): vmax cm-1 1623.19 (-C=N), 3374.27 (-C-NH), 568.88 (C-Br), and 1030.66 (C-O ether) of (-O-CH3). 1H-NMR (400 MHz, DMSO-d6) δ: 3.83 (s, 6H, OCH3), 4.02 (bs, 1H, NH), 5.05 (1H, s), 5.33 (bs, 2H, OH), 5.68 (d, 1H, J=15.1 Hz), 6.81 (m, 4H), 6.87 (d, 1H, J=15.1 Hz); 6.97 (d, 2H, J=7.5 Hz); 7.16 (s, 2H), 7.67 (s, 1H), 8.14 (s, 1H). 13C-NMR (400 MHz, DMSO-d6) δ: 56.2, 103.1, 111.5, 116.4, 121.2, 122.5, 123.2, 124.2, 135.6, 139.3, 147.2, 149.3, 150.6, 159.3, 164.8. LC/MS [M+1] for C26H22BrN3O4: Calculated 521.08, found: 521.38 and 533.43 (bromine isotope).
4-[(E)-2-{5-[(E)-2-(4-hydroxy-3-methoxyphenyl)ethenyl]-1-(pyrimi- din-2-yl)-1H-pyrazol-3-yl} ethenyl]-2-methoxyphenol (7): A 2-hydrazinopyrimidine hydrate (0.15 g, 1.357 mmol) was added to a solution of 1,7-Bis(4-hydroxy-3-methoxyphenyl)hepta-1,6-diene-3,5-dione (curcumin) (1.357 mmole, 0.5 g) in ethanol (30.0 mL), followed by the addition of concentrated H2SO4 (2 drops). The produced solution was refluxed for about 12 h. The product was recrystallized from hexane/EtOAc (2:1 by volume) to give 0.4 g (Yield 66.66%), mp 88°C-90°C, IR (KBr): vmax cm-1 1639.9 (-C=N), 1061 (C-O ether) of (-O-CH3), and 1214.80 (N-N). 1H-NMR (400 MHz, DMSO-d6) δ: 3.81 (s, 6H, OCH3), 5.38 (sb, 2H, OH), 6.76 (s, 1H), 6.97 (m, 6H); 7.12 (d, 2H, J=7.7 Hz), 7.18 (m, 2H, J=7.7 Hz); 7.68 (m, 1H), 8.83 (d, 2H, J=7.9 Hz). 13C-NMR (400 MHz, DMSO-d6) δ: 56.2, 107.5, 109.3, 116.4, 116.6, 118.5, 122.7, 123.8, 130.3, 131.4, 147.3, 147.5. 148.1, 149.3, 155.7, 156.6. LC/MS [M+1] for C25H22N4O4: Calculated 444.17, Found: 444.67.
Preparation of H-Curcumin Based Amine
1,7-bis (4-hydroxy-3-methoxyphenyl) heptane-3,5-dione (8): A low-pressure reaction bottle was charged with a solution of curcuminoids 10.0 g in absolute ethanol (100 ml) and in the presence of Pd/C (0.35 g) which was used as a heterogeneous catalyst. The bottle was attached to the low-pressure hydrogenation apparatus and evacuated, and then hydrogen was admitted to a pressure slightly above 5 atm. The contents of the flask were shaken until absorption of hydrogen stopped (about 4 hrs). The catalyst was removed by filtration and ethanol was removed under vacuum to afford 4.6 g (91.8%) of pale-yellow gummy material. The gummy material was purified by flash chromatography using ethyl acetate as eluent. The prepared compound 8, was analyzed by 1H NMR and 13C NMR. 1H NMR (300 MHz) (CDCl3): δ=6.8 (d, J=8.24, 2H), 6.62 (d, J=8.42=2H), 6.6 (s, 2H), 5.6 (br, 2H, OH), 5.4 (s, 0.75 H, vinylic), 3.90 (s, 0.5H, diketone), 3.85 (s, 6H, OCH3), 2.9 (t, J=7.97, 3H), 2.6 (t, J=7.14, 3H). 13C NMR (CDCl3 d6) δ: 193.2 (Cc and Ce), 144.5 (C4’), 143.9 (C3’), 132.5 (C1’), 120.7 (C6’), 114.3 (C2’), 110.9 (C5’), 99.8 (Cd), 55.8 (OMe), 40.4 (Cb, Cf), 31.1 (Ca, Cg).
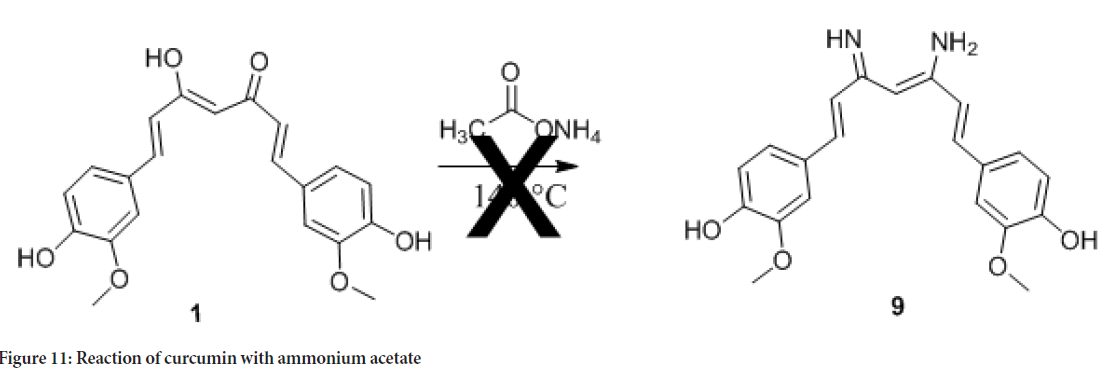
4-[(4Z)-5-amino-7-(4-hydroxy-3-methoxyphenyl)-3-iminohept-4-en-1-yl]-2-methoxyphenol (9): A sample of 1,7-Bis(4-hydroxy-3-methoxyphenyl) hepta-1,6-diene-3,5-dione (H-curcumin, 8) (0.5 g, 12.5 mL, 1.357 mmol) was placed in a single-neck round bottom flask (50 mL) and dissolved in 2.0 mL ethanol. To the solution was added ammonium acetate (0.313 g, 1.357 mmol). The produced mixture was stirred at 90°C until complete solvent evaporation. Then it was heated at 140°C until reaction completion (an hour), which was monitored by TLC. After cooling down to room temperature, it was purified by washing with water, isopropyl alcohol and diethyl ether. The purification was further completed by crystallization from ethanol/diethyl ether to give 0.3 g (yield 60%) of yellow solid. Melting point is 160°C-163°C, IR: vmax cm-1 3216.78 (C-NH), (C=N), 2939.29 (C-N), 1605.51 (C=C), 1515.17 (C=N). 1H NMR (400 MHz, DMSO-d6) δ: 1.86 (t, 2H, CH2), 2.29 (t, 2H, CH2), 2.56 (t, 4H, CH2), 3.83 (s, 6H, OCH3, methyl), 3.88 (s, 1H), 5.35 (bs, 2H, OH), 6.68 (d, 2H, CH benzene), 6.71 (s, 2H, CH benzene), 6.79 (d, 2H, CH benzene) 8.56 (bs, 2H, NH2).
13C NMR (400 MHz, DMSO-d6) δ: 29.7, 34.8, 35.7, 36.7, 51.7, 56.1, 113.2, 115.5, 122.5, 133.0, 145.9, 147.1, 147.4, 164.6.
Biological assays
Preparation of stock solutions: Solutions of curcumin-based heterocyclics were prepared at a concentration of 400 μg per 1 mL of Dimethyl Sulfoxide (DMSO) solvent and then incubated at 4°C.
Various concentrations of (200, 100, 50, 25, and 12.5 μg/ml) were then prepared using serial dilution method.
Cell lines: The human cervical cancer cell lines (HeLa cells) were obtained from the American Type Culture Collection [ATCC], Manassas, VA, USA. HeLa cells were grown in RPMI medium supplemented with 10% fetal calf serum, 1% non-essential amino acid, 1% l-glutamine, 1% penicillin streptomycin and 1% amphotericin B. All cells were grown in a humidified atmosphere of 95% air, 5% CO2 at 37°C, the culture medium was changed at least twice a week as needed. All chemicals used were purchased from Biological Industries except for the amphotericin B and MTT reagent from SIGMA Aldrich.
For screening experiment, the cells were grown into 12-well plates in 950 μl of RPMI medium (Roswell Park Memorial Institute Medium) (Biological Industries, USA) containing 5% FBS, 2 × 104 cells/well plating density. After that 50 μl of diverse concentrations (400, 200, 100, 50, 25, and 12.5 μg/ml) of curcumin-based heterocyclics was added in duplicates to the prepared 12-well plates and incubated for 24 hr at 37°C, 5% CO2, 95% air and 100% relative humidity. An inverted microscope (Labomed, USA) was used to observe the morphological changes of the cells.
Cytotoxicity assay
Cells at 70%-80% confluence were detached from culture flask by removing the culture medium then adding 0.05% trypsin-EDTA and a suspension of 100 µl (2.0 × 104 cells/well) of viable cells were seeded in a 96-well plate and incubated for 24 hr at 37°C. After the removal of media cells were treated with 50 µl stock solution (400 μg/ml) serially diluted to reach concentrations of (200, 100, 50, 25, and 12.5 μg/ml) of curcumin-based heterocyclics and then incubated for 24 hr at 37°C to perform the MTT assay.
MTT assay
The anticancer effect of curcumin-based heterocyclics against cell lines (both normal and cancer cell lines) was estimated by the 3-[4, 5-dimethylthiazole-2-yl]-2, 5-diphenyltetrazolium bromide (MTT) assay using (cell growth determination kit MTT based, Sigma). Cells (2 × 104 cells/well) for cytotoxic assay and (1.0 × 104 cells/well) for cytostatic Assay were incubated with various concentrations of the compounds (400, 200, 100, 50, 25, and 12.5 μg/ml) in 5% CO2, 95% air and 100% relative humidity at 37°C for 24 h in an Fetal bovine serum (FBS)-free medium. Aseptically MTT solution was added in an amount equal to 10% of the culture volume. Then cultures were returned to incubator and incubated for 4 hours. After the incubation period, the resulting MTT formazan crystals were dissolved by the addition of MTT solvent in an amount equal to the original culture volume. The addition of MTT solvent was performed after the removal and disposal of the culture fluid as HeLa cells and L6 were still attached to the culture surface. The absorbance at 570 nm was measured using microplate reader (Labtech, UK). The relative cell viability was determined by the amount of MTT converted to the insoluble formazan salt. The data are expressed as v the mean percentage of viable cells as compared to the respective control.
Results and Discussion
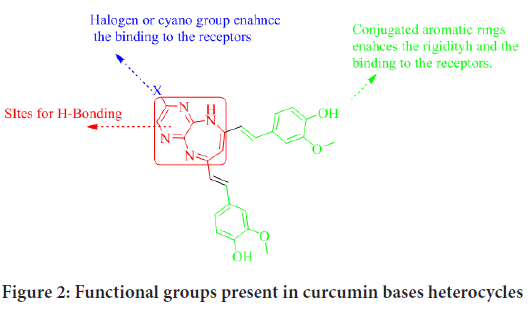
In preceding studies, a non-toxic and a naturally occurring curcumin was used as a skeleton for synthesizing various heterocyclic compounds including pyrazole, diazepine, benzodiazepine, diazoles, and isoxazole (Hamed O, et al., 2020; Hamed OA, et al., 2013; Qneibi M, et al., 2021). The antibacterial activity and cytotoxicity of the prepared curcuminbased heterocycles were tested against gram-positive and gram-negative bacteria. Some showed excellent antibacterial activities against the tested strains. Moreover, some of the prepared diazepines showed a synergistic effect with ampicillin antibiotic. The results indicated that the curcuminbased heterocycles are promising for designing a potentially active anticancer agent. The compound selected for this purpose are curcumin with heterocyclic moiety, since as shown in (Figure 2), they have the functionalities that make ideal for this purpose.
Figure 2: Functional groups present in curcumin bases heterocycles
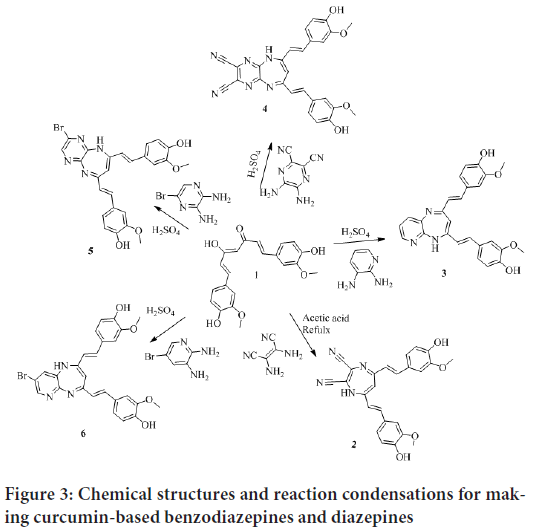
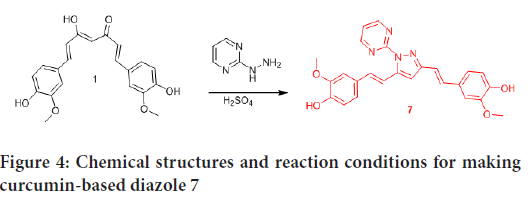
Based on these results, we intend to extend the work for development of curcumin-based reagents with better bioactivity. The anticancer activities of the prepared heterocycles against HeLa cancer cells were evaluated. Unsophisticated and convenient synthetic methods were employed in this work in preparation of benzodiazepines, diazepines, diazoles, and amines via condensation cyclization of curcumin with various 1, 2 diamino and hydrazine compounds. Schemes I, II and III show a summary of the prepared compounds structures and reaction conditions (Figures 3 and 4).
Figure 3: Chemical structures and reaction condensations for making curcumin-based benzodiazepines and diazepines
Figure 4: Chemical structures and reaction conditions for making curcumin-based diazole 7
In the preparation of curcumin-based benzodiazepines, diazepines and diazole, ethanol was used as a solvent and H2SO4 as a catalyst. However, in the condensation reaction for making diazepine 2, curcumin was refluxed with diaminomaleonitrile in a glacial acetic acid, which was performed as a catalyst and a solvent. The reaction progress was monitored by TLC. Curcumin-based benzodiazepines, diazepines 2, 3, 4, 5, and 6 required more reflux time than diazole 7. Two methods were employed in purification of the prepared heterocycles, either column chromatography or crystallization. The purified products were analyzed by FT-IR, 1H NMR, and 13C NMR; the detailed descriptions of all the procedures and of the final analytical characterization are summarized in the experimental part. The yield was quantitative for derivatives; it ranged between 21% and 99%.
Hydrogenated curcumin (H-Curcumin-based amine, 9) was prepared in a two-step process, as shown in Figure 5. The first step involved the hydrogenation of curcumin 1 using a hydrogenation apparatus charged, absolute ethanol was used as a solvent and Pd/C was the catalyst. The pale-yellow gummy product 8 was purified by flash chromatography using ethyl acetate as eluent and analyzed by FT-IR, 1H NMR, and 13C NMR as described in the experimental part.
Figure 5: Chemical structures and reaction conditions for making H-curcumin-based amine 9
The second step involves condensation reaction of amination of H-Curcumin 8 with Ammonium acetate as shown in Figure 5. The progress of reaction was monitored using TLC. The synthesized product was purified as previously described in the experimental part. The structure of compound 9 was identified by FT-IR, 1H NMR, and 13C NMR.
Anticancer activity
Curcumin and its derivatives have been known to have a wide variety of therapeutic effects, ranging from anti-inflammatory, chemo-preventive, anti-proliferative, and anti-metastatic. In this work a novel set curcuminbased heterocyclics were screened for their anti-tumor effect against HeLa cells. The in vitro cytotoxic and cytostatic effect of the prepared heterocyclics was evaluated using MTT test.
Cytotoxic effect of the curcumin-based heterocyclics
Cells (2 × 104 cells/well) with 40%-50% confluence were seeded on 96well plate, the cells were then treated with different concentrations of the prepared curcumin-based heterocyclics (400, 200, 100, 50, 25, and 12.5 μg/ ml) and incubated for 24 hours.
MTT assay was used to determine the cell viability by adding MTT solution to the plate and incubated for four hours, after that the isopropyl alcohol was added and incubated in dark for 15 minutes. The microplate reader (Labtech, UK) was used to measure the absorbance at 570 nm.
Cytotoxic effect of the curcumin-based heterocyclics on HeLa cells
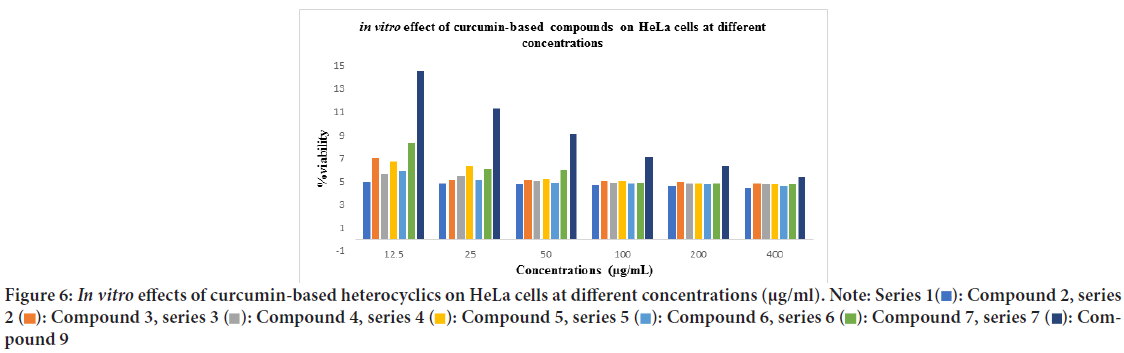
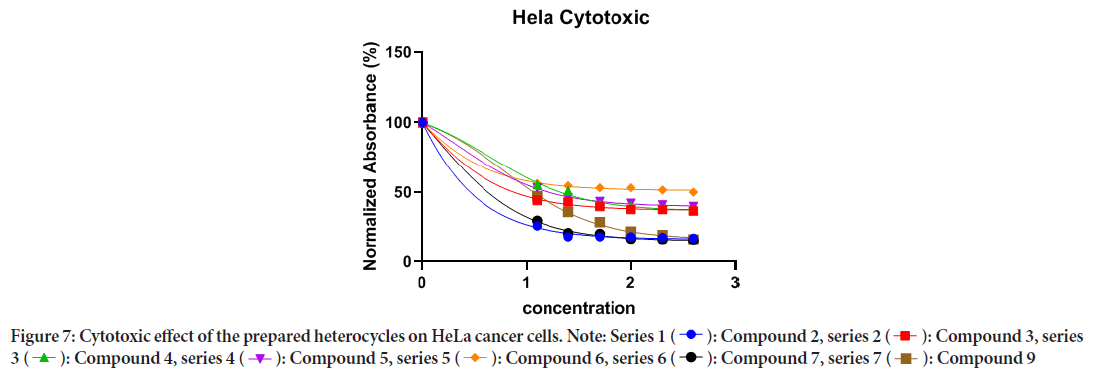
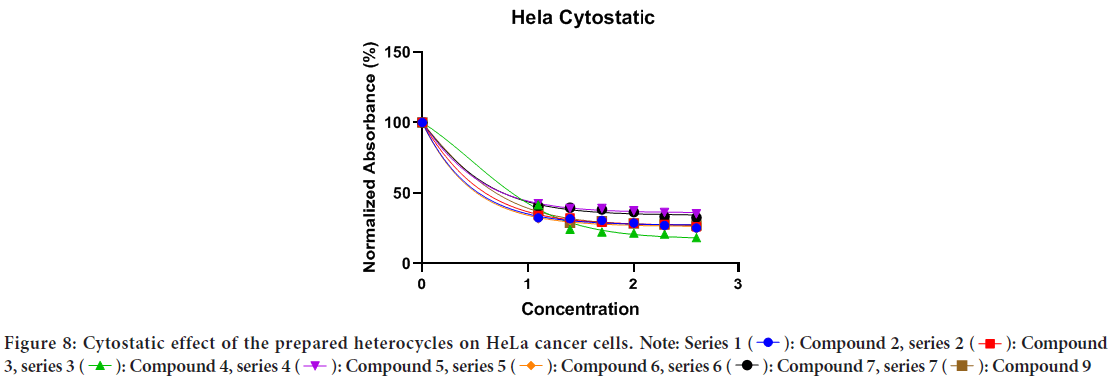
The in vitro anticancer activities of a novel set of curcumin-based benzodiazepine, diazepines, diazoles, and H-curcumin based amine against HeLa cancer cell were evaluated. The results indicate that the prepared curcumin-based heterocyclics have varying cytotoxic effect on the HeLa cells at different concentrations. The viability of HeLa cells has reduced in the range of 4.48%-14.57% within the studied concentration range as shown in (Table 1). In general, cells growth was decreased as the concentration of the prepared curcumin-based heterocyclics increased (Figure 6). Curcumin-based benzodiazepine and diazepines 2, 3, 4, 5, and 6 showed higher potency on HeLa cancer cells than the other tested compounds 7 and 9. The viability of HeLa cells that were treated with benzodiazepine and diazepines 2, 3, 4, 5, and 6 was reduced in the range of 4.48%-6.73%. Curcumin-based diazepines 6 showed the highest cytotoxic effect on the HeLa cells at all concentrations. It reduced the viability of the tested HeLa cells in range of 4.48% for the 400 μg/ml concentration to 4.95% for the 12.5 μg/ml concentration. Among the prepared curcumin-based benzodiazepine 3 and 5 are more effective against HeLa cancer cells than 4. As they reduced the viability of the tested HeLa cells in range of 4.78% and 4.63% for the 400 μg/ml concentration to 5.95% and 5.9% for the 12.5 μg/ml concentration for 3 and 5, respectively. IC50 of the prepared heterocycles tested on HeLa cancer cells were; compound 2:0.2490, compound 3:0.6811, compound 4:4.454, compound 5:2.120, compound 6:0.4572, and compound 7: 1.245. (Figures 7 and 8, Table 2). The lines represent the mean of three independent experiments carried out in triplicates.
| Curcumin derivatives | Concentration (μg/ml) | |||||
|---|---|---|---|---|---|---|
| 400 | 200 | 100 | 50 | 25 | 12.5 | |
| 2 | 4.84% | 4.95% | 5.07% | 5.08% | 5.10% | 6.96% |
| 3 | 4.78% | 4.84% | 4.88% | 5.07% | 5.44% | 5.59% |
| 4 | 4.72% | 4.84% | 5.07% | 5.19% | 6.32% | 6.73% |
| 5 | 4.63% | 4.75% | 4.82% | 4.88% | 5.12% | 5.90% |
| 6 | 4.48% | 4.60% | 4.70% | 4.72% | 4.84% | 4.95% |
| 7 | 4.71% | 4.84% | 4.90% | 5.95% | 6.02% | 8.31% |
| 9 | 5.43% | 6.37% | 7.08% | 9.07% | 11.28% | 14.57% |
Table 1: In vitro effects of curcumin-based heterocyclics on the viability of HeLa cells at different concentrations (μg/ml)
| Cancer cells | Curcumin | 2 | 3 | 4 | 5 | 6 | 7 | 9 |
|---|---|---|---|---|---|---|---|---|
| HeLa cytotoxic | 12.46 | 0.249 | 0.6811 | 4.454 | 2.12 | 0.4572 | 1.245 | 6.164 |
| HeLa cytostatic | 10.21 | ~0.1036 | 0.3536 | 2.947 | 0.3425 | ~0.08515 | 0.523 | 0.8659 |
| L6 cytotoxic | 12.7 | 12.48 | 13.76 | 9.307 | 10.75 | 22.47 | 25 | 15.61 |
| L6 cytostatic | 10.5 | 3.947 | 1.12 | 4.088 | 0.9463 | 1.977 | 1.202 | 1.09 |
Table 2: Cytotoxic and cytostatic effect of the prepared heterocycles on HeLa cancer cells and L6 Cells (normal cells)
Figure 6: In vitro effects of curcumin-based heterocyclics on HeLa cells at different concentrations (μg/ml).
 Compound 9
Compound 9
Figure 7: Cytotoxic effect of the prepared heterocycles on HeLa cancer cells. 
Figure 8: Cytostatic effect of the prepared heterocycles on HeLa cancer cells. 
Cytotoxic effect of the prepared heterocycles on L6 Cells (normal cells)
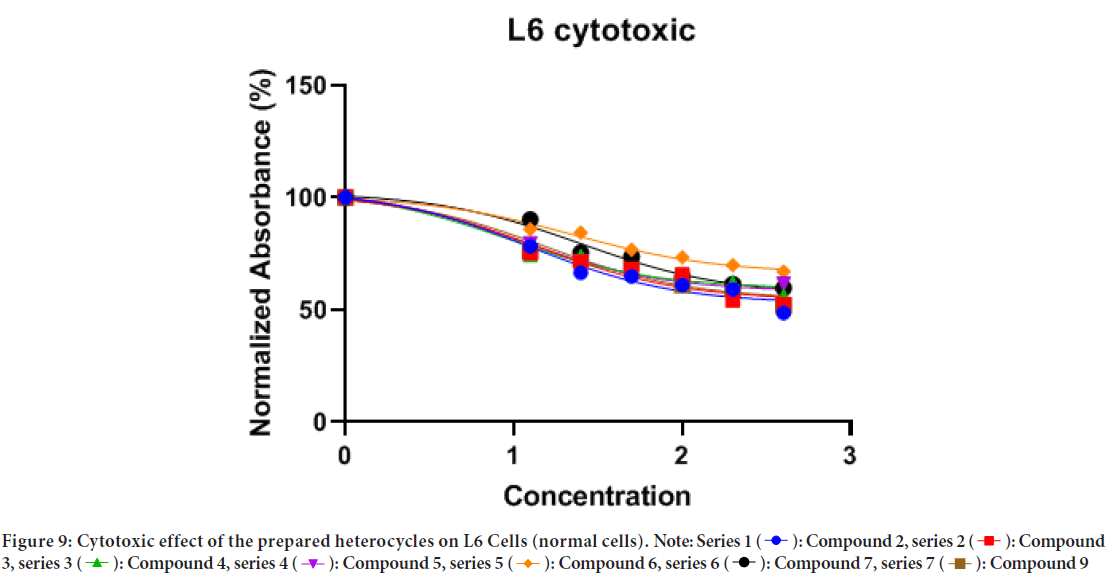
Results show that most of the prepared heterocycles have a cytotoxic effect on L6 cells at concentrations higher than 12.5 μg/ml. IC50 of the prepared heterocycles were; compound 2:12.43, compound 3:13.76, compound 4: 9.307, compound 5: 10.75, compound 6: 22.47, compound 7: 25.00 and compound 9: 15.61 (Figure 9, Table 2). The lines represent the mean of three independent experiments carried out in triplicates.
Figure 9: Cytotoxic effect of the prepared heterocycles on L6 Cells (normal cells).
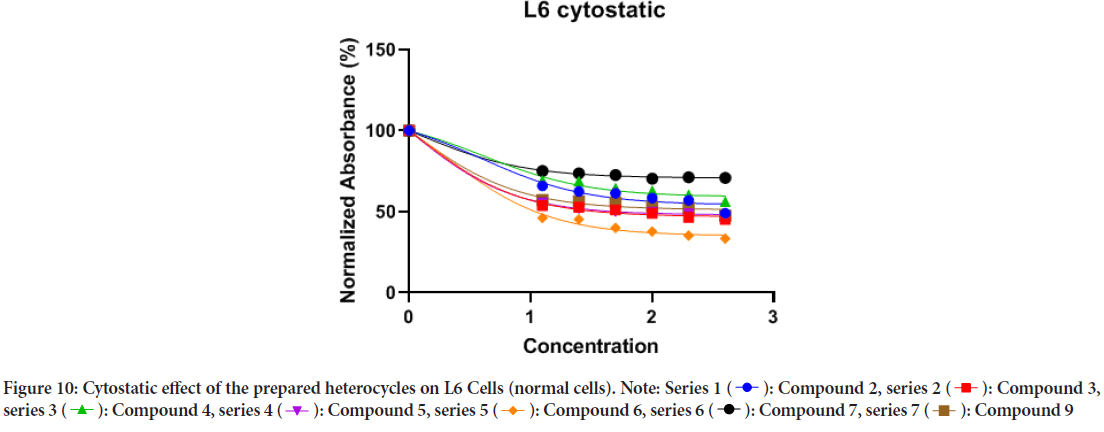
Results of L6 cells showed that all the tested curcumin-based heterocyclics exhibited cytotoxic and cytostatic effects in a dose-dependent manner as presented in Figures 9 and 10, and Table 2. Most of curcumin-based benzodiazepine and diazepines 2, 3, and 6 induced higher cytotoxic and cytostatic effects compared to curcumin-based diazole 7 and H-curcuminbased amine 9 at the same concentrations. Heterocyclic 2 exhibited cytotoxic and cytostatic effect against HeLa cells with IC50 values of 0.2490 and ~0.1036 µg/ml respectively at nontoxic levels as the control L6 cells showed cytotoxic and cytostatic effect with IC50 values of 12.48 and 3.947 µg/ml respectively. Heterocyclic 3 exhibited cytotoxic and cytostatic effect against HeLa cells with IC50 values of 0.6811 and 0.3536 µg/ml respectively at nontoxic levels as the control L6 cells showed cytotoxic and cytostatic effect with IC50 values of 13.76 and 1.120 µg/ml respectively. Heterocyclic 4 exhibited cytotoxic and cytostatic effect against HeLa cells with IC50 values of 4.454 and 2.947 µg/ml respectively at nontoxic levels as the control L6 cells showed cytotoxic and cytostatic effect with IC50 values of 2.947 and 4.088 µg/ml respectively. Heterocyclic 5 exhibited cytotoxic and cytostatic effect against HeLa cells with IC50 values of 2.120 and 0.3425 µg/ml respectively at nontoxic levels as the control L6 cells showed cytotoxic and cytostatic effect with IC50 values of 10.75 and 0.9463 µg/ml respectively. Heterocyclic 6 exhibited cytotoxic and cytostatic effect against HeLa cells with IC50 values of 0.4572 and ~0.08515 µg/ml respectively at nontoxic levels as the control L6 cells showed cytotoxic and cytostatic effect with IC50 values of 22.47 and 1.977 µg/ml respectively.
Figure 10: Cytostatic effect of the prepared heterocycles on L6 Cells (normal cells).
In general, heterocyclic 6 showed the highest cytotoxic and cytostatic effect among the tested heterocyclics against HeLa cells at same concentration. It exhibited IC50 values of 0.4572 and ~0.08515 µg/ml respectively at nontoxic levels as the control L6 cells showed cytotoxic and cytostatic effect with IC50 values of 22.47 and 1.977 µg/ml respectively.
Curcumin-based diazole 7 showed the lowest effect on the HeLa cells. It showed lower effect than curcumin-based benzodiazepine and diazepines 2, 3, 4, 5, and 6. Compound 7 reduced the viability of the tested HeLa cells in range of 4.71% for the 400 μg/ml concentration to 8.31% for the 12.5 μg/ml concentration. Curcumin-based diazole 7 exhibited cytotoxic and cytostatic effect against HeLa cells with IC50 values of 1.245 and 0.5230 µg/ ml respectively at nontoxic levels as the control L6 cells showed cytotoxic and cytostatic effect with IC50 values of 25.00 and 1.202 µg/ml respectively. The anticancer activities shown above could explain by structure activity relationship. Curcumin with benzodiazepine moiety 6 showed the highest activity. The presence of the hetero atom and nitrogen in addition to bromine gives this molecule the ability to accommodate well into the binding site and interact with the receptor site of the cancer cells and interact through H-bonding with functional groups present in the receptor site. As more substituents are introduced in the benzodiazepine ring the activity decreases as the case with compound 4, in this case the steric factor effect becomes the predominant factor that tend to reduce the interaction with the receptor sites and thus the potency drops.
On the other hand, H-curcumin amine 9 showed the lowest cytotoxic effect on the HeLa cells at all concentrations among the prepared heterocyclics. It reduced the viability of the tested HeLa cells in range of 5.43% for the 400 μg/ml concentration to 14.57% for the 12.5 μg/ml concentration. H-curcumin-based amine 9 exhibited cytotoxic and cytostatic effect against HeLa cells with IC50 values of 6.164 and 0.8659 µg/ml respectively at nontoxic levels as the control L6 cells showed cytotoxic and cytostatic effect with IC50 values of 15.61 and 1.090 µg/ml respectively.
The reason for the low bioactivity of compound 9 could be due to the loss of the olefinic part of curcumin due to hydrogenation. As mentioned in the introduction this functionality is important functionality in anticancer activity. For comparison purpose we tried converting curcumin to curcumin amine as shown in (Figure 11) but the effort was unsuccessful.
Figure 11: Reaction of curcumin with ammonium acetate
Conclusion
The in vitro anticancer activities of a novel synthesized set of heterocycles against HeLa cancer cells and normal muscle cells L6 as a control were evaluated. The results indicated that all the tested curcumin-based heterocyclics have varying cytotoxic effect on the HeLa cells at different concentrations. The viability of HeLa cells was reduced in the range of 4.48%-14.57% within the studied concentration. Cell growth was decreased as the concentration of the prepared curcumin-based heterocyclics increased. Curcumin-based benzodiazepine and diazepines showed higher potency on HeLa cancer cells than the other tested heterocyclics. The viability of HeLa cells that were treated with benzodiazepine and diazepines was reduced in the range of 4.48%-6.73%. On the other hand, curcumin-based diazepine 6 showed the highest cytotoxic effect on the HeLa cells at all concentrations. It reduced the viability of the tested HeLa cells in range of 4.48% for the 400 μg/ml concentration to 4.95% for the 12.5 μg/ml concentration. Heterocyclic 6 showed the highest cytotoxic and cytostatic effect among the tested heterocyclics against HeLa cells at same concentration. It exhibited IC50 values of 0.4572 and ~0.08515 µg/ml respectively at nontoxic levels as the control L6 cells showed cytotoxic and cytostatic effect with IC50 values of 22.47 and 1.977 µg/ml respectively. H-curcumin-based amine 9 showed the lowest cytotoxic effect on the HeLa cells at all concentrations among the prepared heterocyclics. It reduced the viability of the tested HeLa cells in range of 5.43% for the 400 μg/ml concentration to 14.57% for the 12.5 μg/ml concentration. Strictly speaking all the synthesized curcumin-based compounds exhibited promising anticancer activity against HeLa cancer cells, which indicates that these compounds have anticancer effect at nontoxic concentrations.
References
- Sawant S, Shegokar R. Cancer research and therapy: Where are we today. Int J Cancer Ther Oncol. 2014; 2(4): 2048.
- Aslam MS, Naveed S, Ahmed A, Abbas Z, Gull I, Athar MA. Side effects of chemotherapy in cancer patients and evaluation of patients opinion about starvation based differential chemotherapy. J Cancer Ther. 2014.
- Priyadarsini KI. Chemical and structural features influencing the biological activity of curcumin. Curr Pharm Des. 2013; 19(11): 2093-2100.
[Crossref] [Google Scholar] [Pubmed]
- Fang J, Lu J, Holmgren A. Thioredoxin reductase is irreversibly modified by curcumin: A novel molecular mechanism for its anticancer activity. J Biol Chem. 2005; 280(26): 25284-25290.
[Crossref] [Google Scholar] [Pubmed]
- Borik RM, Fawzy NM, Abu-Bakr SM, Aly MS. Design, synthesis, anticancer evaluation and docking studies of novel heterocyclic derivatives obtained via reactions involving curcumin. Molecules. 2018; 23(6): 1398.
[Crossref] [Google Scholar] [Pubmed]
- Ciochina R, Savella C, Cote B, Chang D, Rao D. Synthesis and characterization of new curcumin derivatives as potential chemotherapeutic and antioxidant agents. Drug Dev Res. 2014; 75(2): 88-96.
[Crossref] [Google Scholar] [Pubmed]
- Ahmed M, Qadir MA, Shafiq MI, Muddassar M, Hameed A, Arshad MN, et al. Curcumin: Synthesis optimization and in silico interaction with cyclin dependent kinase. Acta Pharm. 2017; 67(3): 385-395.
[Crossref] [Google Scholar] [Pubmed]
- Sato T, Hotsumi M, Makabe K, Konno H. Design, synthesis and evaluation of curcumin-based fluorescent probes to detect Aβ fibrils. Bioorganic Med Chem Lett. 2018; 28(22): 3520-3525.
[Crossref] [Google Scholar] [Pubmed]
- Li Q, Chen J, Luo S, Xu J, Huang Q, Liu T. Synthesis and assessment of the antioxidant and antitumor properties of asymmetric curcumin analogues. Eur J Med Chem. 2015; 93: 461-469.
[Crossref] [Google Scholar] [Pubmed]
- Ma'mani L, Nikzad S, Kheiri-Manjili H, Al-Musawi S, Saeedi M, Askarlou S, et al. Curcumin-loaded guanidine functionalized PEGylated I3ad mesoporous silica nanoparticles KIT-6: Practical strategy for the breast cancer therapy. Eur J Med Chem. 2014; 83: 646-654.
[Crossref] [Google Scholar] [Pubmed]
- Ding L, Ma S, Lou H, Sun L, Ji M. Synthesis and biological evaluation of curcumin derivatives with water-soluble groups as potential antitumor agents: An in vitro investigation using tumor cell lines. Molecules. 2015; 20(12): 21501-21514.
[Crossref] [Google Scholar] [Pubmed]
- Mishra S, Patel S, Halpani CG. Recent updates in curcumin pyrazole and isoxazole derivatives: Synthesis and biological application. Chem Biodivers. 2019; 16(2): 1800366.
[Crossref] [Google Scholar] [Pubmed]
- Ramya PS, Guntuku L, Angapelly S, Digwal CS, Lakshmi UJ, Sigalapalli DK, et al. Synthesis and biological evaluation of curcumin inspired imidazo [1, 2-a] pyridine analogues as tubulin polymerization inhibitors. Eur J Med Chem. 2018; 143: 216-231.
[Crossref] [Google Scholar] [Pubmed]
- Khor PY, Aluwi MF, Rullah K, Lam KW. Insights on the synthesis of asymmetric curcumin derivatives and their biological activities. Eur J Med Chem. 2019; 183: 111704.
[Crossref] [Google Scholar] [Pubmed]
- Mbese Z, Khwaza V, Aderibigbe BA. Curcumin and its derivatives as potential therapeutic agents in prostate, colon and breast cancers. Molecules. 2019; 24(23): 4386.
[Crossref] [Google Scholar] [Pubmed]
- Yang H, Huang S, Wei Y, Cao S, Pi C, Feng T, et al. Curcumin enhances the anticancer effect of 5-fluorouracil against gastric cancer through down-regulation of COX-2 and NF-κB signaling pathways. J Cancer. 2017; 8(18): 3697.
[Crossref] [Google Scholar] [Pubmed]
- Hamed O, Fares O, Taleeb S, Adwan G, Saadeh H, Jodeh S, et al. New insights towards 1, 4-Benzodiazepines from curcumin: Design, synthesis and antimicrobial activities. Med Chem. 2020; 16(8): 1112-1123.
[Crossref] [Google Scholar] [Pubmed]
- Hamed OA, Mehdawi N, Taha AA, Hamed EM, Al-Nuri MA, Hussein AS. Synthesis and antibacterial activity of novel curcumin derivatives containing heterocyclic moiety. Iran J Pharm Sci. 2013; 12(1): 47.
[Google Scholar] [Pubmed]
- Qneibi M, Hamed O, Jaradat N, Hawash M, Al-Kerm R, Al-Kerm R, et al. The AMPA receptor biophysical gating properties and binding site: Focus on novel curcumin-based diazepines as non-competitive antagonists. Bioorg Chem. 2021; 116: 105406.
[Crossref] [Google Scholar] [Pubmed]
Author Info
Rana Al-Kerm1, Rola Al-Kerm1, Othman Hamed1*, Ashraf Swaft1,2, Mohammad Qneibi3, Avni Berisha4,5, Omar Dagdage6 and Ghaleb Adwan22Department of Biology, An-Najah National University, Nablus, Palestinian Territory
3Department of Biomedical Sciences, An-Najah National University, Nablus, Palestinian Territory
4Department of Materials Science-Nanochemistry, NanoAlb-Unit of Albanian Nanoscience and Nanotechnology, Tirana, Albania
5Department of Chemistry, University of Prishtina, Prishtina, Kosovo
6Department of Process Engineering, Height School of Technology, Sidi Mohammed Ben Abdallah University, Fez, Morocco
Citation: Al-Kerm R: Heterocylcic Based Curcumin: Design, Synthesis and Anticancer Efficacy against HeLa Cells
Received: 01-Jul-2022 Accepted: 25-Jul-2022 Published: 01-Aug-2022, DOI: 10.31858/0975-8453.13.8.499-507
Copyright: This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
ARTICLE TOOLS
- Dental Development between Assisted Reproductive Therapy (Art) and Natural Conceived Children: A Comparative Pilot Study Norzaiti Mohd Kenali, Naimah Hasanah Mohd Fathil, Norbasyirah Bohari, Ahmad Faisal Ismail, Roszaman Ramli SRP. 2020; 11(1): 01-06 » doi: 10.5530/srp.2020.1.01
- Psychometric properties of the World Health Organization Quality of life instrument, short form: Validity in the Vietnamese healthcare context Trung Quang Vo*, Bao Tran Thuy Tran, Ngan Thuy Nguyen, Tram ThiHuyen Nguyen, Thuy Phan Chung Tran SRP. 2020; 11(1): 14-22 » doi: 10.5530/srp.2019.1.3
- A Review of Pharmacoeconomics: the key to “Healthcare for All” Hasamnis AA, Patil SS, Shaik Imam, Narendiran K SRP. 2019; 10(1): s40-s42 » doi: 10.5530/srp.2019.1s.21
- Deuterium Depleted Water as an Adjuvant in Treatment of Cancer Anton Syroeshkin, Olga Levitskaya, Elena Uspenskaya, Tatiana Pleteneva, Daria Romaykina, Daria Ermakova SRP. 2019; 10(1): 112-117 » doi: 10.5530/srp.2019.1.19
- Dental Development between Assisted Reproductive Therapy (Art) and Natural Conceived Children: A Comparative Pilot Study Norzaiti Mohd Kenali, Naimah Hasanah Mohd Fathil, Norbasyirah Bohari, Ahmad Faisal Ismail, Roszaman Ramli SRP. 2020; 11(1): 01-06 » doi: 10.5530/srp.2020.1.01
- Manilkara zapota (L.) Royen Fruit Peel: A Phytochemical and Pharmacological Review Karle Pravin P, Dhawale Shashikant C SRP. 2019; 10(1): 11-14 » doi: 0.5530/srp.2019.1.2
- Pharmacognostic and Phytopharmacological Overview on Bombax ceiba Pankaj Haribhau Chaudhary, Mukund Ganeshrao Tawar SRP. 2019; 10(1): 20-25 » doi: 10.5530/srp.2019.1.4
- A Review of Pharmacoeconomics: the key to “Healthcare for All” Hasamnis AA, Patil SS, Shaik Imam, Narendiran K SRP. 2019; 10(1): s40-s42 » doi: 10.5530/srp.2019.1s.21
- A Prospective Review on Phyto-Pharmacological Aspects of Andrographis paniculata Govindraj Akilandeswari, Arumugam Vijaya Anand, Palanisamy Sampathkumar, Puthamohan Vinayaga Moorthi, Basavaraju Preethi SRP. 2019; 10(1): 15-19 » doi: 10.5530/srp.2019.1.3