Research Article - (2022) Volume 13, Issue 3
Abstract
Introduction: Health Organization declared COVID-19 to be a global pandemic. This had led to the development of many novel vaccines for which effective post-vaccination surveillance is essential. This study aims to synthesize post-vaccination surveillance of physical side-effects to learn the best method that can be applied to the surveillance of novel COVID-19 vaccines. Methods: A full systematic search was performed on four databases following the PRISMA guidelines. Following the inclusion and exclusion criteria, and hand-searching the eligible papers, a total of seven studies were included in this review. The risk of bias within studies and their quality was assessed using the critical appraisal for public health checklist. Results: The seven studies included were from six countries representing both active and passive surveillance systems. The results showed reliability and reproducibility between data across countries. They also provided a good framework for how post-vaccination surveillance can be performed in low-income countries. Conclusions: The reporting of post-vaccine side-effects is essential. Active and passive systems were essential to encourage reporting of AEFIs (Adverse Event Following Immunization) and should be encouraged globally. A combination of active and passive surveillance should be used to monitor adverse events relating to the novel COVID-19 vaccines.
Keywords
AEFIs (Adverse Event Following Immunization), COVID-19, Post-vaccination
Introduction
On the 30th of January 2020, the coronavirus outbreak was declared a public health emergency of international concern by The World Health Organization (WHO) and within two months it was declared to be a global pandemic (WHO, 2021).
This pandemic has had devastating effects globally. A report showed that in the year 2020, there were approximately one million excess deaths in 29 high-income countries (HICs), showing the direct and indirect effects of COVID-19 on mortality (Islam N, et al., 2021) and emphasizing the drastic need for global vaccination against the deadly virus.
The necessity of having a vaccine to fight the war against COVID-19 has led to the rapid development of many novel vaccines, and in the UK alone there are already three vaccines licensed for use (Pfizer/BioNTech, AstraZeneca, Moderna) (GOV. UK, 2021). At the time of writing this report, according to the WHO draft landscape and tracker of COVID-19 candidate vaccines, there are 101 vaccines in clinical development and 183 vaccines in pre-clinical development (WHO, 2021). With the sudden emergence of so many novel vaccines, countries must have a robust and reliable post-vaccination surveillance and reporting system. Effective AEFI surveillance enables the correct management of AEFIs, as inappropriate or incorrect responses can be catastrophic to public health (WHO, 2014). In the post-market setting, it is also vital as it can detect any unexpected or rare reactions, as well as any late-onset reactions that are challenging to detect in a trial (Dey A, et al., 2020).
An AEFI is any post-immunization adverse medical occurrence, although it can be coincidental to the vaccine. This can be a reaction to the vaccine itself, an administrative error regarding the vaccine, or an error in the handling of the vaccine, for example breaking the cold chain (WHO, 2014).
Reporting of AEFIs can either be done passively or actively. Passive surveillance is how most countries across the world monitor AEFIs and typically involves a platform or a database where people can report any post-vaccination adverse events. Active surveillance involves actively following up on vaccines and asking them specifically about adverse events, which can be done in many ways.
This systematic review aims to analyze data of reporting systems in place for post-vaccination surveillance of physical side-effects to propose the best method for COVID-19 vaccine surveillance. This review aims to look specifically at the true adverse events following immunization, the number of vaccines administered, and how the adverse event data is collected.
Methodology
Search strategy
A systematic literature review was performed of studies that assessed AEFIs in line with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. The search aimed to identify reports that focused on the reporting of AEFIs in a mass vaccination setting. The search was carried out across four databases: Ovid Embase, Ovid Medline, Web of Science, and Scopus; and was limited to the past three years to aim to get the most up-to-date evidence and limited to English. The search strategy can be found in appendix 1.
Inclusion and exclusion criteria
Studies were deemed eligible if they met the inclusion criteria of human vaccination studies looking at the physical post-vaccination side effects with a sample size greater than one hundred. Reasons for exclusion included studies that were confined to one sex, control groups that had comorbidities, and studies where surveillance was not complete. Figure 1 shows a flow diagram of the selection process of the studies.
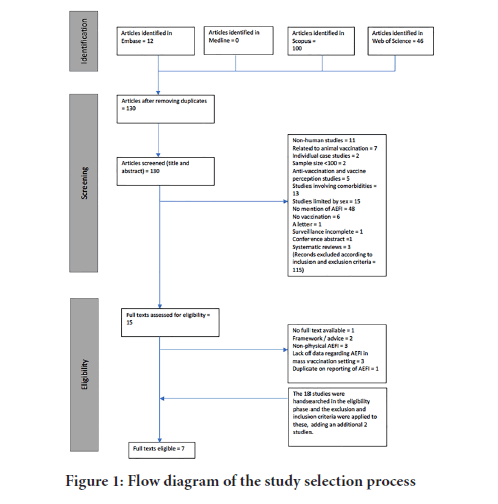
Figure 1: Flow diagram of the study selection process
Quality assessment and risk of bias
The quality of the included studies was assessed using the critical appraisal for public health checklist (Heller RF, et al., 2008), this also helped check for bias and aided the certainty assessment. This was performed by one reviewer.
Data extraction and synthesis
Data were extracted from the eligible studies that related to the number and types of AEFIs reported, population demographics, reporting rates, vaccine characteristics, geographical distribution, response rates (if related to active surveillance), and the period covered. One reviewer was responsible for collecting the data. Data were grouped according to relevance, and this allowed for the determination of the correct statistical analysis. Odds ratios were used to compare female to male AEFI reporting whereas disorder groupings of AEFIs were converted to the percentage of total adverse events reported. This allowed for a comparison of reporting trends that should be less sensitive to the period they were reported. Where studies had figures regarding rates per population or rates per dose these were also grouped and converted to the same denominator to allow direct comparison.
From each study, tables were made that extracted data considered as relevant. These tables will be visible in appendix 2. Meta-analysis was not possible due to the heterogeneity of the included studies. Due to this, data were put into subgroups to allow for comparison. The reason for the heterogeneity is discussed throughout the review.
Results
Study characteristics
There were variations geographically of the studies included in this systematic review: Two studies in China (Wu W, et al., 2019; Zhang MX, et al., 2021), one study in South Korea (Yoon D, et al.,2020), one in Canada (deSerres G, et al., 2018), one in Brazil (Sato APS, et al., 2018), one in Australia (Dey A, et al., 2020), and one in the Democratic Republic of Congo (DRC) (Nzolo D, et al., 2018). Within these studies, three looked at national reporting of adverse effects (AEs), and four studies were limited by a region of the country.
Of the reporting systems involved, three looked at passive national reporting systems, one looked at passive reporting to a regional system, two looked at active surveillance via online surveys, and one involved a mixture of reporting through face-to-face methods, phone calls, SMS and WhatsApp.
There was also a variation in the vaccines involved. The four studies looking at national surveillance looked at all vaccines licensed in the respective countries. One study looked specifically at the SARS-CoV-2 vaccine (CoronaVac), one study looked at the four-component meningococcal B vaccine (4CMenB) and one study looked at the 17DD Yellow Fever vaccine (specifically at fractionated dosing).
Variation also existed in the ages of the participants of the study. Three studies included participants of all ages, one study included participants aged 18 to 59 years, one study included participants older than 2 years old, one study included participants less than 2 years old, and one study included participants between the ages of 2 months to 2 years.
The study entitled ‘Nephrotic syndrome following four-component meningococcal B vaccination: Epidemiologic investigation of a surveillance signal’ (de Serres G,et al., 2019) fit the inclusion criteria however was excluded. This was because it was a duplication of the surveillance system of the 4CMenB (Canadian) study, written by the same authors using the same data but it specifically focused on one AEFI (nephrotic syndrome). It was removed to avoid duplicating results.
Comparison of vaccine AEFIs
Due to discrepancies in the periods studied and the length of time over which the data was collected, where possible, the rate per 100,000 cases was calculated.
There were similarities between seven of the top ten vaccines causing adverse events in South Korea and eight of the top ten in Australia. In Australia, the other vaccines causing the top ten adverse reactions were DTPa-IPV-HepB-Hib and DTPa-IPV. In South Korea, the other top three vaccines were Hib, BCG, and Japanese encephalitis. This differs from Australia as these three collectively only account for 0.4% of reported AEFIs.
The report on deaths related to AEFIs in China only focused on one serious and rare adverse event (death). The data correlates with South Korea as the report showed DTaP and Japanese Encephalitis as being in the top ten vaccines that caused death. All three (China, Australia, and South Korea) had the DTaP and its various variations in the top ten causes of vaccine reactions.
Type of AEFIs reported
Specific types of AEs were reported from four studies: SARS-CoV-2 (China), Yellow Fever (DRC), Araraquara (Brazil), and the national database in Australia. Data from these studies were compared to see if they followed similar reporting trends. AEs were grouped in alignment with the grouping of the Yellow Fever adverse reactions. Any additional AEFIs mentioned were classified accordingly and when it was unclear which group to assign them to, they were classified as ‘other’. The percentage of the grouping of the AEFIs in comparison to the AEs reported in total was calculated to show the overall trend in a way that was not affected by the timespan the data covered. In Brazil and Australia, the largest proportion of disorders were general disorders (28.6% and 40.1% respectively). This result replication is a positive finding towards the efficacy of the surveillance systems. Figure 2 shows pie charts that allow for a visual comparison of the proportion of disorders reported through the passive surveillance systems in Brazil and Australia.
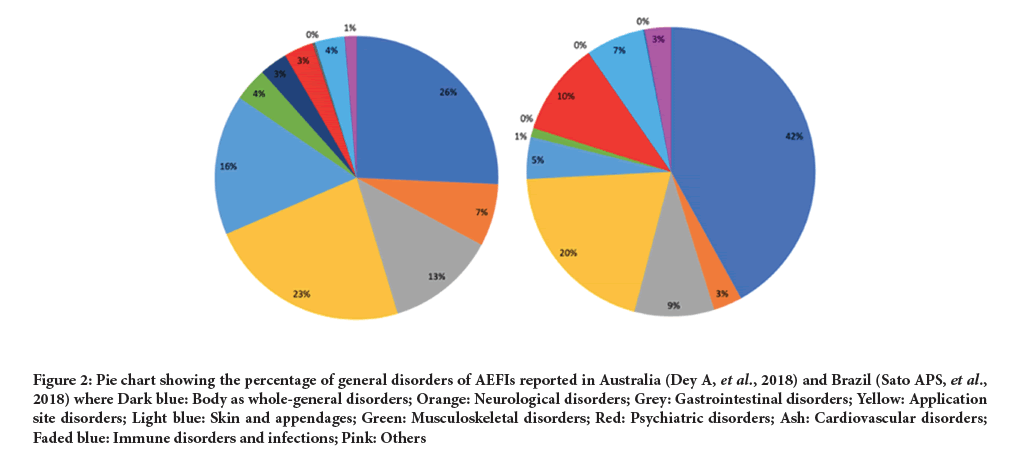
Figure 2: Pie chart showing the percentage of general disorders of AEFIs reported in Australia (Dey A, et al., 2018) and Brazil (Sato APS, et al., 2018) where Dark blue: Body as whole-general disorders; Orange: Neurological disorders; Grey: Gastrointestinal disorders; Yellow: Application site disorders; Light blue: Skin and appendages; Green: Musculoskeletal disorders; Red: Psychiatric disorders; Ash: Cardiovascular disorders; Faded blue: Immune disorders and infections; Pink: Others
Serious AEFIs
The report in China only looked at death. 753 deaths were reported as AEFI and 120 were confirmed. There was a death rate of 0.26 per million doses administered or 0.09 per million population. In South Korea 107 of the 1,949 serious AEs resulted in death (5.5%). Australia had reporting rate of serious AEFIs of 1.8 per 100,000 population (Figure 3).
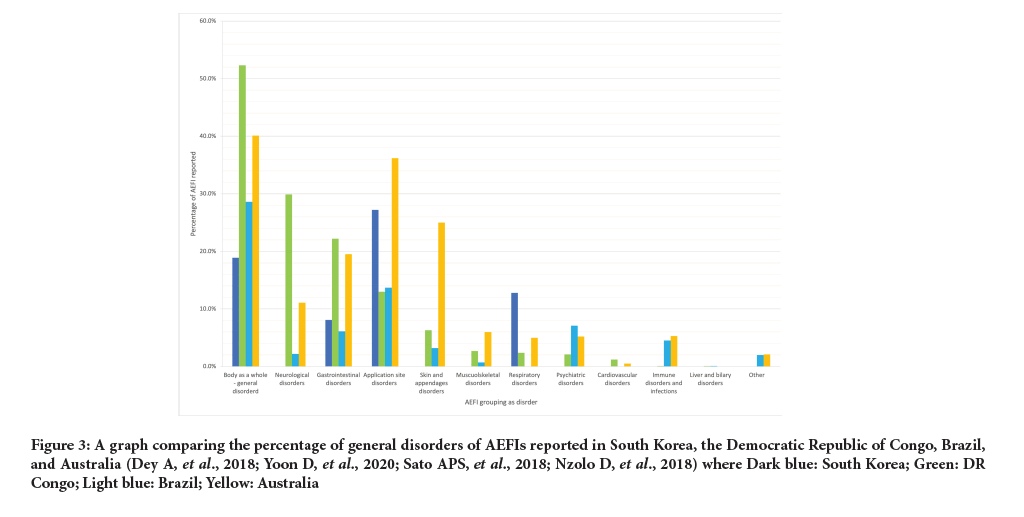
Figure 3: A graph comparing the percentage of general disorders of AEFIs reported in South Korea, the Democratic Republic of Congo, Brazil, and Australia (Dey A, et al., 2018; Yoon D, et al., 2020; Sato APS, et al., 2018; Nzolo D, et al., 2018) where Dark blue: South Korea; Green: DR Congo; Light blue: Brazil; Yellow: Australia
Sex: The SARS-CoV-2 (China) study found that women, on average, were 2.26 times more likely to report adverse events than men, with a 95% CI it’s between 1.12 to 4.55 times more likely. In Australia more AEFIs related to women (56.6%) were reported than those related to men (41.8%). In South Korea, males accounted for fewer AEFIs than females (Yoon D, et al., 2020).
In China (national surveillance) (Table 1) 38.91% of deaths were female. In Brazil, the rate of occurrence per 10,000 doses of vaccine for HHE in males and females was 3.9 and 4 respectively. The rate of occurrence per 10,000 doses of vaccine for seizures in males and females was 2 and 1 respectively. These figures contrast to the figures from China (SARS) and Australia.
| Author | Country | Regional? If so what region | Vaccine studied | Age of study sample | Active or passive surveillance |
|---|---|---|---|---|---|
| Wu W, Liu D, Nourti J, et al. | China | No | All licensed vaccines | All ages | Passive |
| Zhang M, Zhang T, Shi G, et al. | China | Limited to one hospital Taizhou, China | SARS-CoV-2 | 18-59 years | Active |
| Yoon D, Kim J, Lee H, et al. | South Korea | No | All licensed vaccines | All ages | Passive |
| Nzolo D, Biongo EA, Kuemmerle A, et al. | Democratic Republic of Congo | Yes, Kinshasa | 17DD Yellow Fever | >2 years | Active |
| Dey A, Wang H, Quinn H, et al. | Australia | No | All licensed vaccines | All ages | Passive |
| Sato A, Ferreira V, Tauil M, et al. | Brazil | Yes, Araraquara, São Paulo | All licensed vaccines | <2 years | Passive |
| de Serres G, Billard M, Gariépy M, et al. | Canada | Yes, Saguenay-Lac-Saint-Jean region, Quebec | 4CMenB | 2 months -20 years | Active |
Table 1: The geographical location of the studies involved whether it was national or regional, and the type of surveillance (Dey A, et al.,2020; Wu W, et al., 2019; Zhang MX, et al., 2021; Yoon D, et al., 2020; de Serres G, et al., 2018; Sato APS,et al., 2018; Nzolo D, et al., 2018)
Survey uptake rate
The two studies looking at active surveillance of adverse vaccine events were the SARS-CoV-2 study (China) and the 4CMenB study (Quebec). The SARS-CoV-2 study participants were contacted via email or sent an e-poster which asked them to fill out an online survey on WeChat. All vaccines were contacted but only 36.4% of vaccines filled in the survey. In Quebec, Canadian vaccines were asked for an email and similarly were contacted to fill out an online questionnaire. The total response rate among all vaccines for doses 1 and 2 were 27% and 22% respectively and amongst all vaccines with an email address for doses 1 and 2 was 39% and 31% respectively.
Discrepancies in active and passive surveillance
As previously stated, both the 4CMenB (Quebec) and the Yellow Fever (DRC) studies were active surveillance studies. However, both countries do have a passive national surveillance system in place and both studies reported discrepancies between these systems.
In Canada, an additional 4 febrile seizures were picked up from the Quebec VAERS that were missed through active surveillance. 1,642 more adverse events were reported through the questionnaire than to the Quebec VAERS.
In the DRC, only 5 serious AEFIs were captured through community-based active surveillance. Whereas 7 serious AEFIs were reported through a passive surveillance system, 41 suspected serious AEFIs were picked up through a hospital-based alert system, and 4 cases were reported of Yellow Fever symptoms post-vaccination via a country-wide surveillance system (cited from (WHO, 2016) in the report).
Discussion
Main findings of this study
This report achieved its aim of synthesizing post-vaccination surveillance of physical side effects. This report was able to synthesize both active and passive surveillance systems as well as their application in both HICs (High-income countries) and LICs (Low-income countries), allowing the data discussed to be more representative of AEFIs globally.
What is already known on this topic?
There is typically a large underreporting of adverse events and a French study found that across 37 studies spanning 12 countries there was a median underreporting rate of 94% (Hazell L, and Shakir SAW, 2006).
What this study adds
Currently, most COVID-19 vaccination programmes are targeted at the adult population. In China (national surveillance) neonatal deaths account for 10.4% of the serious AEFI reported, thus, it may be less useful when predicting reporting rates of AEFIs following COVID-19 vaccinations.
Most mass vaccination campaigns are targeted at people younger than eighteen (for example MMR vaccine) and so similarly this is difficult to compare to COVID-19. However, since the reporting of most AEFIs in under eighteen-year-olds typically would be done by a parent or guardian or doctor, this creates space for the encouragement of adults receiving COVID-19 vaccinations to reports any adverse events.
The Yellow Fever (DRC) study showed a framework for adverse event reporting that can be done in LICs. Providing education about AEFI before data collection will help encourage the reporting of events. It also highlighted various platforms through which it could be done: Face-to-face discussions, phone calls, SMS, and WhatsApp messages (Nzolo D, et al., 2018). The targeting of community areas such as schools or universities, churches, and community centers allows AEFIs to be reported to known and trusted members of the community and may increase reporting rates. This also helped with the financial constraints. The use of innovative methods to report AEFIs and reach the community will be vital in adverse event reporting of COVID-19 vaccines, particularly in rural areas in LICs.
Limitations of the Study
There were several limitations to this report. As with the inherent nature of passive surveillance, there is vast underreporting of AEFIs. Due to there being no control groups, there were also limitations of the data analysis that could be performed. However, this report was successful in comparing data and analyses where possible.
The disadvantage of passive surveillance was partially compensated in the Brazil study by using an electronic immunization registry as their source of data (Sato APS, et al., 2018).
The studies did not specifically span the same time and the same age groups. The emergence of pandemics and the fluctuation of a person’s immune system during different stages of life could have led to discrepancies in the reporting of adverse events and the characteristics of the adverse events reported.
There is also variation from country to country on national vaccination programmes and vaccinations required which makes it very difficult to directly compare all AEFIs and trends.
In both the Yellow Fever (DRC) and the SARS (China) study, the sample demographics were affected by how they collected the data. As stated in the Yellow Fever report there was selection bias that most likely led to a healthier, younger, and more educated vaccine reporting group. This can also be inferred by the SARS study as all the vaccine recipients were employees of the hospital and thus age was also limited by participants 18 to 59 years old.
The studies were limited geographically to six countries, and four of the studies were limited to either one region or one hospital. Thus, the data could be less representative of the population as a whole. A limitation of this study is not having access to the raw data in the national reporting systems.
Conclusion
In conclusion, there is no gold standard model to work from when recommending post-vaccination surveillance. From this systematic review, it can be deduced that it is something that is struggled to be done effectively and reliably in HICs thus one can expect that it will be an uphill battle for LICs. LICs will need help and guidance from organizations such as the WHO to achieve successful post-vaccination surveillance.
Data can also be improved by using an electronic immunization reporting system like that used in Araraquara, Brazil. In rural areas, we should learn from the models used in the Yellow Fever (DRC) study. Educating citizens on adverse events following immunization encourages monitoring of adverse events and promotes communities to talk about them and acknowledge if the vaccine is safe. This in turn would hopefully increase uptake rates of vaccination. An increase in reporting of adverse events would also allow for trends and patterns of vaccines and reactions to be identified more rapidly.
Having people in the community such as people in churches, schools and community centers where AEFIs can be reported should also encourage reporting. Taking emails from vaccines and sending them an online survey also encourages the reporting of AEFIs however this may be a hurdle in LICs when faced with a lack of electricity and smartphones.
There needs to be a global push to encourage the reporting of AEFIs to passive national surveillance systems from both medical professionals and the public. This could be done through social media campaigns and educational platforms.
Recommendations
Going forward, I recommend a combination of strategies used in the reports mentioned and a combination of active and passive surveillance. In LICs, citizens should be educated about AEFIs before vaccination and community leaders and respected figures should be appointed as figures that people can report adverse events to. Vaccines should be asked for email addresses or mobile numbers (where possible) which would allow online surveys to be sent out to encourage the reporting of AEFIs.
References
- Timeline of WHO’s response to COVID-19. WHO. 2021.
- Islam N, Shkolnikov VM, Acosta RJ, Klimkin I, Kawachi I, Irizarry RA, et al. Excess deaths associated with COVID-19 pandemic in 2020: Age and sex disaggregated time series analysis in 29 high income countries. BMJ. 2021; 373: n1137.
- Coronavirus vaccine-weekly summary of yellow card reporting. GOV.UK. 2021.
- Draft landscape and tracker of COVID-19 candidate vaccines. WHO. 2021.
- WHO library cataloguing-in-publication data global manual on surveillance of adverse events following immunization. WHO. 2014.
- Dey A, Wang H, Quinn H, Hiam R, Wood N, Beard F, et al. Surveillance of adverse events following immunisation in Australia: Annual report, 2018. Commun Dis Intell. 2020; 44.
- Heller RF, Verma A, Gemmell I, Harrison R, Hart J, Edwards R, et al. Critical appraisal for public health: A new checklist. Public Health. 2008; 122: 92-98.
[Crossref] [Google scholar] [Pubmed]
- Wu W, Liu D, Nuorti JP, Keli L, Disha Xu, Jiakai Y, et al. Deaths reported to national surveillance for adverse events following immunization in China, 2010-2015. Vaccine. 2019; 37: 1182-1187.
[Crossref] [Google scholar] [Pubmed]
- Zhang MX, Zhang TT, Shi GF, Cheng FM, Zheng YM, Tung TH, et al. Safety of an inactivated SARS-CoV-2 vaccine among healthcare workers in China. Expert Rev Vaccines. 2021; 20(7).
[Crossref] [Google scholar] [Pubmed]
- Yoon D, Kim JH, Lee H, Shin YJ. Updates on vaccine safety and post-licensure surveillance for adverse events following immunization in south korea, 2005-2017. Yonsei Med J. 2020; 61: 623.
[Crossref] [Google scholar] [Pubmed]
- de Serres G, Billard MN, Gariépy MC, Rouleau I, Toth E, Landry M, et al. Short-term safety of 4CMenB vaccine during a mass meningococcal B vaccination campaign in Quebec, Canada. Vaccine. 2018; 36: 8039-8046.
[Crossref] [Google scholar] [Pubmed]
- Sato APS, Ferreira VL de R, Tauil M de C, Rodrigues LC, Barros MB, Martineli E, et al. Use of electronic immunization registry in the surveillance of adverse events following immunization. Rev Saude Publica. 2018; 52: 1-10.
[Crossref] [Google scholar] [Pubmed]
- Nzolo D, Biongo AE, Kuemmerle A, Lusakibanza M, Lula Y, Nsengi N, et al. Safety profile of fractional dosing of the 17DD yellow fever vaccine among males and females: Experience of a community-based pharmacovigilance in Kinshasa, DR Congo. Vaccine. 2018; 36: 6170-6782.
[Crossref] [Google scholar] [Pubmed]
- de Serres G, Billard MN, Gariépy MC, Roy MC, Boucher FD, Gagne H, et al. Nephrotic syndrome following four-component meningococcal B vaccination: Epidemiologic investigation of a surveillance signal. Vaccine. 2019; 37: 4996-5002.
[Crossref] [Google scholar] [Pubmed]
- Yellow fever mass vaccination campaign using fractional dose in kinshasa, DRC. WHO. 2016.
- Hazell L, Shakir SAW. Under-reporting of adverse drug reactions: A systematic review. Drug Saf. 2006; 29: 385-396.
Author Info
Alice Reid*, Finn Highfield and Arpana VermaCitation: Reid A: Identifying the Best Methods to Report on the Post-Vaccination Surveillance of Physical Side-Effects of the COVID-19 Vaccines: A Systematic Review
Received: 07-Feb-2022 Accepted: 22-Feb-2022 Published: 01-Mar-2022, DOI: 10.31858/0975-8453.13.3.158-162
Copyright: This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
ARTICLE TOOLS
- Dental Development between Assisted Reproductive Therapy (Art) and Natural Conceived Children: A Comparative Pilot Study Norzaiti Mohd Kenali, Naimah Hasanah Mohd Fathil, Norbasyirah Bohari, Ahmad Faisal Ismail, Roszaman Ramli SRP. 2020; 11(1): 01-06 » doi: 10.5530/srp.2020.1.01
- Psychometric properties of the World Health Organization Quality of life instrument, short form: Validity in the Vietnamese healthcare context Trung Quang Vo*, Bao Tran Thuy Tran, Ngan Thuy Nguyen, Tram ThiHuyen Nguyen, Thuy Phan Chung Tran SRP. 2020; 11(1): 14-22 » doi: 10.5530/srp.2019.1.3
- A Review of Pharmacoeconomics: the key to “Healthcare for All” Hasamnis AA, Patil SS, Shaik Imam, Narendiran K SRP. 2019; 10(1): s40-s42 » doi: 10.5530/srp.2019.1s.21
- Deuterium Depleted Water as an Adjuvant in Treatment of Cancer Anton Syroeshkin, Olga Levitskaya, Elena Uspenskaya, Tatiana Pleteneva, Daria Romaykina, Daria Ermakova SRP. 2019; 10(1): 112-117 » doi: 10.5530/srp.2019.1.19
- Dental Development between Assisted Reproductive Therapy (Art) and Natural Conceived Children: A Comparative Pilot Study Norzaiti Mohd Kenali, Naimah Hasanah Mohd Fathil, Norbasyirah Bohari, Ahmad Faisal Ismail, Roszaman Ramli SRP. 2020; 11(1): 01-06 » doi: 10.5530/srp.2020.1.01
- Manilkara zapota (L.) Royen Fruit Peel: A Phytochemical and Pharmacological Review Karle Pravin P, Dhawale Shashikant C SRP. 2019; 10(1): 11-14 » doi: 0.5530/srp.2019.1.2
- Pharmacognostic and Phytopharmacological Overview on Bombax ceiba Pankaj Haribhau Chaudhary, Mukund Ganeshrao Tawar SRP. 2019; 10(1): 20-25 » doi: 10.5530/srp.2019.1.4
- A Review of Pharmacoeconomics: the key to “Healthcare for All” Hasamnis AA, Patil SS, Shaik Imam, Narendiran K SRP. 2019; 10(1): s40-s42 » doi: 10.5530/srp.2019.1s.21
- A Prospective Review on Phyto-Pharmacological Aspects of Andrographis paniculata Govindraj Akilandeswari, Arumugam Vijaya Anand, Palanisamy Sampathkumar, Puthamohan Vinayaga Moorthi, Basavaraju Preethi SRP. 2019; 10(1): 15-19 » doi: 10.5530/srp.2019.1.3






