Research - (2023) Volume 14, Issue 8
Abstract
Introduction: Caspase a family of cysteine protease plays an important role in programmed cell death. Caspase-3 and Caspase-8 are key regulators of apoptosis. Caspase-8 plays principal role in conveying signals from death ligand receptors to intracellular pro-apoptotic machinery. It was found exclusively in the cytoplasm, however, its presence has also been observed in other intracellular compartments. The role of Caspase-8 in tumor progression is contentious.
Caspase-3 is a chief mediator of apoptosis and is cleaved to create an active form. The precursor form of Caspase-3 is localized in cytoplasm while active form is translocated to nucleus. Expression of active form of Cassspase-3 is studied in various forms of carcinomas but few reports regarding pro-caspase-3 are available. Some studies have also report the presence of inactive Caspase-3 in the nucleus.
Since caspases are located in different cellular compartments their deregulations might be responsible for the development of malignancies. We aimed to study the expression of pro-caspase-3 and caspase-8 in the various intercellular compartments of tumor area, adjacent normal and dysplastic area in multi-step carcinogenesis of oro-pharyngeal carcinoma.
Materials and methods: The research study was undertaken in Maulana Azad Medical College New-Delhi. Clinically diagnosed one hundred and fifty cases of squamous cell carcinoma of oro-pharyngeal region and one hundred and twenty eight control subjects were studied. Caspase-8 and pro-caspase-3 proteins were studied in tumor area and adjacent normal-dysplasia, by immunostaining based on avidin-biotin peroxides complex technique.
Results: The Caspase-8 cytoplasm expression was compared to adjacent normal and dysplasia group, significant increase in caspase-8 cytoplasm expression in tumor group was seen (P<0.001). Similar results were also observed in nuclear caspase-8 (P=0.0001). The cytoplasm pro-caspase-3 expression in tumor higher than adjacent normal-dysplasia and was significant (P<0.001). It was also higher as compared to nuclear pro-caspase-3 expression in tumor area (P<0.001). In overall assessment the cytoplasm expression of pro-caspase-3 was significantly higher than caspase-8 positivity in nucleus and cytoplasm.
Discussion: Caspase plays most important role in apoptosis, however its association with malignant transformation and prognosis is controversial. Cytoplasm expression of pro-caspase-3 in tumor region was significantly higher in comparison to adjacent normal epithelium and dysplasia. Since most of the pro-caspase-3 remains inactivated, its enhanced expression may be associated with poor prognosis in oral carcinoma.
A significant increase in nuclear caspase-8 in tumor area have been observed, the pro-caspase-3 may be directly activated in nucleus or are able to promote malignant progression by possibly assisting in mitotic cell division instead of assign the cells to p53-induced apoptosis. Thus possibly, altered caspases expression may be playing a decisive role in oral pharyngeal, multistep carcinoma progression.
Keywords
Carcinoma, Cysteine protease, Oral cancer, Cytoplasm
Introduction
India is leading in oral cancer and share one third of the total burden internationally (Gupta B, et al., 2017). The major etiology for oro-pharyngeal carcinoma is tobacco consumption (Laprise C, et al., 2016; Krishna Rao SV et al., 2013). Various forms includes smokeless tobacco, betel-quid chewing; excessive alcohol consumption, poor oral hygiene, nutrient-deficient diet, and sustained viral infections, i.e, Human Papillomavirus (HPV) are some of the risks associated with the occurrence of oral cancer. The International Agency for Research on Cancer (IARC) classified tobacco as a Group I carcinogenic substance in the oral cavity leading to mutations (International Agency for Research on Cancer, 2012). These genetic alterations may promote deregulation of apoptosis pathway, one of the most important mechanisms by which oral cancers are able to resist cell death (Loro L et al., 2005; Negrini S et al., 2010).
Apoptosis involve coordinated disassembly of intracellular machinery (Kumar S, 1999). Caspases, a group of cysteine proteases, undergoes a cascade of catalytic activation (McIlwain DR et al., 2013). The activation of extrinsic or death receptor pathway takes place in response to ligand binding of death receptors superfamily members (Reed JC, 2000). The initiator caspase-8 activates executioner pro-caspase-3 in cytoplasm which then organizes their activities to remove important structural proteins (Reed JC, 2000). Pro-caspase-3, as well as some other caspases, does not possess a nuclear localization signal however various mechanisms like passive diffusion and active transport have been suggested for nuclear accumulation of caspase-3 during apoptosis (Susin SA, et al., 1999; Enari M, et al., 1998; Liu X, et al., 1998). At the same time, some studies have reports also nuclear localization of Caspase-8 (Boege Y, et al., 2017).
Caspases are crucial for the removal of cells with mutations by apoptosis, thus their erroneous activation has strong consequence; it can assist in the perseverance of mutated cells, which support carcinogenesis (Philchenkov A et al., 2004). The zymogen precursor of executioner Caspase-3, is over expressed in multiple malignances, and this abnormal expression suggests the possibility for decisive roles of apoptotic machinery in oncogenic transformation (Soung YH, et al., 2004; Peterson QP, et al., 2009).
Reports of pro-caspase-3 and 8 expressions in multistep oral squamous cell carcinoma are contradictory. Some studies have reported elevated expression in squamous cell carcinoma as compared to adjacent normal and dysplasia some observed decrease (Coutinho-Camillo CM et al., 2011; Andressakis D, et al., 2008). The cytoplasmic positivity of pro-caspase-3 and 8 has been reported in many malignancies however their nuclear expression only few have reported.
In our study, we aimed to study expression and cellular localization of Pro-caspase-3 and Caspase-8 in different stages of oro-pharyngeal malignant transformation.
Materials and Methods
The research study was undertaken in Maulana Azad Medical College New-Delhi. One hundred and fifty clinically diagnosed cases of squamous cell carcinoma of oro-pharyngeal region and one hundred and twenty eight control subjects were included in the study. The oral biopsy specimens were taken from the representative area of each case of squamous cell carcinoma. The diagnosis was confirmed by the presence of characteristic histopathological features. The control group included those subjects having non-specific mild clinical changes on oral mucosa and histology showing minimum inflammation in sub epithelium, not specific for any pathology. The study was cleared by the ethical committee of the institute and an informed consent was obtained from each subject. The expression of Pro-caspase-3, Caspase-8 was studied in all the cases.
Immunohistochemical staining
Staining was done with monoclonal mouse anti-human Caspase-3, and Caspase-8 antibodies (Novacasta). According to the manufacturer specification Caspase-3 antibody recognizes unprocessed Caspase-3 and Caspase-8 antibody identifies active sub unit p-18 of Caspase-8.
Proteins were detected by immunostaining based on avidin-biotin peroxides complex technique. The tissue section were deparaffinised and rehydrated. The endogenous peroxidase was inactivated with 3% H2O2 in methanol and non-specific protein binding was blocked by incubation with 5 gm milk in 100 ml of phosphate buffer saline (pH-7). Slides were then incubated overnight with 40 µgm of diluted primary antibody (20:1) at 4°C. Biotinylated horse antimouse IgG secondary antibodies were then applied for 30 min (Novacastra, Newcastle, U.K). The sections were incubated for 30 min with avidin-horse peroxides complex (Novacastra, New-castle, U.K). All intermediate rinsing steps were performed by phosphate buffer saline (pH-7). The brown color was developed by diaminobenzidine. The positive expression of these antibodies was observed in the cytoplasm, nucleus and nucleus-cytoplasm. The expression was verified according to presence or absence of proteins.
Statistical analysis
The means and standard deviation for various parameters were calculated. The difference between two groups was tested by student t-test. The data which was not normally distributed was transformed logarithmically before applying student t-test. A P-value of <0.05 was considered as statistically significant.
Results
A total of two hundred and seventy eight (n=278) subjects were included in the study. The squamous cell carcinoma of oro-pharyngeal region was studied, in one hundred and fifty cases (n=150). The mean age of squamous cell carcinoma group was 51 yrs (SD=17.10).The control group comprised of one hundred and twenty eight cases (n=128) mean age was 28.91 yrs (SD=7.55).
Immune expressions of Pro-caspase-3, Caspase-8, were studied according to the cellular areas i.e. nucleus and cytoplasm in following area of squamous cell carcinoma:
a. Tumor area
b. Normal and Dysplasia adjacent to tumor area
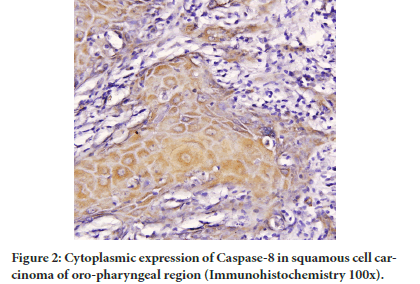
The Caspase-8 cytoplasm expression in tumor group was lower as compared to control group but the difference was not significant (P=0.2). However as compared to adjacent normal and dysplasia group significant increase in Caspase-8 cytoplasm expression in tumor group was observed (P<0.001) (Table 1).
| Caspase-8 | ||||
|---|---|---|---|---|
| Control-C Mean ± SD 49.3 ± 1.6 |
Normal-Adj-C Mean ± SD 13.8 ± 29.0 |
Dysplasia-Adj-C Mean ± SD 2.3 ± 13.3 |
Tumor-N Mean ± SD 8.3 ±20.8 |
|
| Tumor-C Mean ± SD 44.4 ± 40.1 P-value |
P=0.2 | P<0.001 | P<0.001 | P<0.001 |
Note: N-Nuclear, C-Cytoplasm, P ≤ 0.05 significant.
Table 1: Comparison of Caspase-8 expression in cytoplasm with controls and tumor group.
A significant decrease (P=0.002) in nuclear expression of Caspase-8 in tumor group was there in comparison to controls but significant increase in contrast to adjacent normal was observed (P=0.0001) (Table 2 and Figure 1). The nuclear expression of Caspase-8 in adjacent dysplasia was absent.
| Caspase-8 | |||
|---|---|---|---|
| Control-N Mean ± SD 2.2 ± 10.2 |
Normal-Adj-N Mean ± SD 13.8 ± 29.0 |
Tumor-C Mean ± SD 44.4 ± 40.1 |
|
| Tumor-N Mean ± SD 8.3 ± 20.8 P value |
P=0.002 | P<0.001 | P<0.001 |
Note: No nuclear expression observed in Adjacent Dysplasia
N-Nuclear, C-Cytoplasm, P ≤ 0.05 significant.
Table 2: Comparison of Caspase-8 expression in nucleus with controls and tumor group.
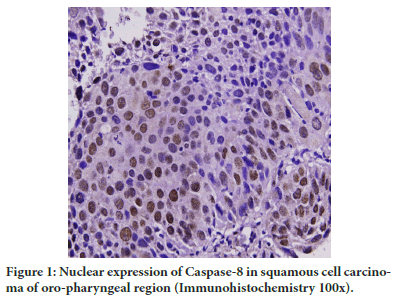
Figure 1: Nuclear expression of Caspase-8 in squamous cell carcinoma of oro-pharyngeal region (Immunohistochemistry 100x).
The cytoplasm Pro-caspase-3 positivity in tumor group was high as compared to controls not significant (P=0.6), it was also higher than adjacent normal and dysplasia and was significant (P<0.001) (Table 3 and Figure 2).
| Pro-Caspase-3 | ||||
|---|---|---|---|---|
| Control-C Mean ± SD 51.3 + 37.3 |
Normal-Adj-C Mean ± SD 10.6 + 4.8 |
Dysplasia-Adj-C Mean ± SD 4.6 + 17.9 |
Tumor-N Mean ± SD 4.2 + 14.4 |
|
| Tumor-C Mean ± SD 53.6 ± 37.9 |
P=0.6 | P<0.001 | P<0.001 | P<0.001 |
Note: P-value ≤ 0.05 is significant, N-Nuclear, C-Cytoplasm
No caspase-3 positivity was observed in controls, adjacent normal and dysplasia.
Table 3: Comparison of Caspase-3 expression in cytoplasm with controls and tumor group.
Figure 2: Cytoplasmic expression of Caspase-8 in squamous cell carcinoma of oro-pharyngeal region (Immunohistochemistry 100x).
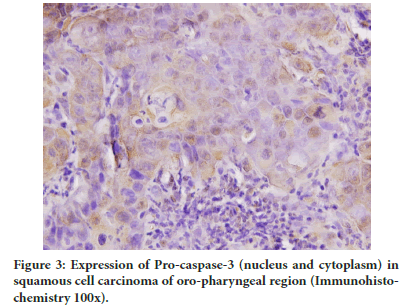
There was absence of Pro-caspase-3 expression in control and adjacent normal-dysplasia group. A significant increase in cytoplasm expression of Pro-caspase-3 as compared to nuclear expression was observed in tumor area (P<0.001) (Table 3 and Figure 3).
Figure 3: Expression of Pro-caspase-3 (nucleus and cytoplasm) in squamous cell carcinoma of oro-pharyngeal region (Immunohistochemistry 100x).
In the overall picture the cytoplasm expression of Pro-caspase-3 was significantly higher than Caspase-8 positivity in nucleus and cytoplasm in tumor area (Table 4).
| Caspase-8 | |||
|---|---|---|---|
| Tumor-C Mean ± SD 44.4 ± 40.1 |
Tumor-N Mean ± SD 8.3 ± 20.8 |
Dys-Adj-C Mean ± SD 2.2 ± 13.3 |
|
| Pro-Caspase-3 | P=0.03 | P<0 .01 | P<0.01 |
| Tumor-C Mean± SD 53.6 + 37.9 P value |
|||
| Tumour-N Mean ± SD 4.2 + 14.4 P value |
P<0.01 | P<0.01 | P=0.1 |
| Dys-Adj-C Mean ± SD 4.6 + 17.9 P value |
P<0.01 | P=0.002 | P=0.1 |
Note: P-value ≤ 0.05 is significant, N-Nuclear, C-Cytoplasm
Table 4: Comparison of Pro-caspase-3 and Caspase-8 expressions in tumor-group.
The comparison of cytoplasm and nuclear caspase-8 expression between tumor grades was statistically insignificant. A significant decrease in cytoplasm Pro-caspase-3 expression from well differentiated to moderately differentiated and poorly differentiated was observed.
Discussion
Apoptosis is a physiological phenomenon, in which activated caspases cleave vital proteins to execute cell death (Kumar S, 1999; McIlwain DR, 2013). They are synthesized as pro-caspases and they serve as the cytoplasmic regulator of apoptosis, conversely after being activated, they are translocated to other intracellular compartments, such as the Endoplasmic Reticulum (ER) and the nucleus (Shikama Y et al., 2001).
Caspase play main role in apoptosis however its association with malignant transformation and prognosis is controversial. Low expression levels or inactivation of caspases has been reported in cancer cells which quit possibly makes the cells resistant to environmental stresses and treatments (Xerri L et al., 1997; Winter RN et al., 2001; Nakopoulou L, et al., 2001). On the other hand, the over expression of caspases may release growth-stimulating signals that assist the non-apoptotic tumor cells to proliferate and survive under stress conditions (Estrov Z, et al., 1998). We studied change in the expression of Caspase-3 and Caspase-8 in cellular areas like cytoplasm and nucleus of tumor, adjacent regions like dysplasia and normal epithelium of oro-pharyngeal carcinoma.
Caspase-8 plays an important role in transmitting signals from, death receptors to intracellular pro-apoptotic proteins (Declercq W et al., 2009). On the other hand its role in tumor progression is contentious. Decline in Caspase-8 expression was observed in neuroblastoma (Barbero S, et al., 2009) and neuroendocrine lung tumors, (Harada K, et al., 2002) it is also associated with a poor prognosis in ovarian cancer (Kim M et al., 2016) and hepatocellular (Frisch SM, 2008) carcinoma. Conversely high Caspase-8 expression has been observed in many different carcinomas (Frisch SM, 2008; Helfer B, et al., 2006) including head and neck squamous cell carcinoma.
In our study we observed a insignificant decrease in cytoplasm expression of Caspase-8 in tumor areas as compared to controls, but significant increase, in contrast to adjacent dysplasia and normal epithelium. The significant increase in Caspase-8 expression indicated possibly promotion of tumor cells mortality resulting in improvement of prognosis.
It is established that apoptosis is triggered via activation of death receptors by Caspase-8 present in cytoplasm however its nuclear translocation has not been studied in detail. Apoptotic neurons studies have indicated that Caspase-8 may be involved in the cleavage of DNA repair enzyme PARP2 in nucleus (Benchoua A, et al., 2002). In addition to cytoplasmic, many studies have reported nuclear localization of Caspase-8 in cancer cells, signifying a possible non-apoptotic function of Caspase-8 in malignancy (Barbero S, et al., 2008).
Studies have reported high Caspase-8 nuclear expression. It correlated with disease progression in hepatocellular, (Koschny R, et al., 2013) melanoma (Müller I, et al., 2020), prostate (Müller I, et al., 2020) and breast carcinoma (de BA, et al., 2016). We studied gradual progression of nuclear Caspase-8 expression from adjacent normal to dysplasia then carcinoma. A significant increase in nuclear Caspase-8 in tumor cells was observed as compared to controls and adjacent dysplasia. However in comparison to cytoplasm expression a significant decrease was observed in nuclear expression of Caspase-8. Studies suggest that, cells expressing nuclear Caspase-8 levels are able to promote malignant progression by possibly assisting in mitotic cell division instead of assign the cells to p53-induced apoptosis. Our result suggests that, may be in squamous cell carcinoma oropharyngeal region, Caspase-8 plays non-apoptotic role however, it needs to be further investigated.
Caspase-3 plays central role in the execution of apoptosis process. In response to various death signals, the Caspase-3 proenzyme is cleaved to generate the active subunits (Julien O and Wells JA, 2017). The precursor form of Caspase-3 is localized in the cytoplasm and after initial cytoplasm activation it is translocates to the nucleus leading to morphological apoptotic changes (Enari M, et al., 1998). Research studies have established the cytoplasmic activation of procaspase-3 by immune-localization (Ramuz O, et al., 2003).
The Pro-caspase-3 expression has been studied in various types of cancers. However conflicting reports are available. Over expression of Pro-caspase-3 have been reported in neuroblastoma, (Nakagawara A, et al., 1997) solid tumors like breast, (Blazquez S, et al., 2006) esophageal, (Wang XS, et al., 2014) gastric, (Kania J, et al., 2003) pancreatic, (Jakubowska K, et al., 2016) and stomach carcinoma (Yoo NJ, et al., 2002). Under expression was observed in prostate (Winter RN et al., 2001) and hepatocellular carcinoma (Persad R, et al., 2004).
We studied the cytoplasmic expression of Pro-caspase-3 in tumor region, it was significantly higher in comparison to adjacent normal epithelium and dysplasia but no difference was observed as compared to normal epithelium. Our results are similar to other available studies in which levels of Pro-caspase-3, in oral squamous cell carcinoma of tongue (Liu PF, et al., 2017) and buccal mucosa (Huang JS, et al., 2017) were significantly higher compared to those in adjacent normal tissues. Since most of the Caspase-3 remains uncleaved and inactivated, its enhanced expression may be associated with poor prognosis.
Caspase-3 plays a significant role in the nuclear morphological changes in apoptotic cells despite the cytoplasmic localization of Pro-caspase-3. It has been suggested that the nuclear translocation of active Caspase-3 is dependent on active nuclear transport. Our immune histochemical staining was also observed Pro-caspase-3 in the tumor cells nucleus of oro-pharyngeal carcinoma.
Although some experimental studies have reported the nuclear presence of Pro-caspase-3, (Kamada S et al., 2005) however no immune histochemical studies have reported the nuclear presence of Pro-caspase-3 in cancer cells. In our study nuclear positivity was observed in tumor area. A significant decrease in nuclear expression in tumor as compared to cytoplasm and total positivity of pro-caspase-3 was observed. It is possible that since caspase-8 presence in nucleus have been reported, the pro-caspase-3 may be directly activated in nucleus.
The squamous cell carcinoma generally develops in stepwise manner from normal through the stages of premalignancy to invasive carcinoma. The available studies do not discuss about step wise transition of caspase expression and also the alterations in their cellular expression. We observed that cytoplasm and nuclear expressions of Pro-caspase-3 and Caspase-8 were significantly higher in tumor area as compared to adjacent dysplasia and normal epithelium. It is a protective mechanism by which caspases being important enzymes are stimulated to eliminate abnormal cells and avoid cancer growth.
Conclusion
Our results highlight the sub-cellular localization of Pro-caspase-3 and 8 in different stages of squamous cell carcinogenesis. More research needs to be done to study possible role of caspases in progression from dysplasia to carcinoma in oral cancer progression and prognosis.
References
- Gupta B, Bray F, Kumar N, Johnson NW. Associations between oral hygiene, habits, diet, tobacco and alcohol and risk of oral cancer: A case-control study from India. Cancer Epide miol. 2017; 51: 7-14.
[Crossref] [Google Scholar] [PubMed]
- Laprise C, Shahul HP, Madathil SA, Thekkepurakkal AS, Castonguay G, Varghese I, et al. Periodontal diseases and risk of oral cancer in Southern India: Results from the HeNCe life study. Int J Can. 2016; 139(7): 1512-1519.
[Crossref] [Google Scholar] [PubMed]
- Rao SV, Mejia G, Roberts-Thomson K, Logan R. Epidemiology of oral cancer in Asia in the past decade-an update (2000-2012). Asian Pac J Cancer Prev. 2013; 14(10): 5567-5577.
[Crossref] [Google Scholar] [PubMed]
- Associations between oral hygiene, habits, diet, tobacco and alcohol and risk of oral cancer: A case-control study from India
- Loro L, Vintermyr OK, Johannessen AC. Apoptosis in normal and diseased oral tissues. Oral Dis. 2005;11(5):274-287.
[Crossref] [Google Scholar] [PubMed]
- Negrini S, Gorgoulis VG, Halazonetis TD. Genomic instability-an evolving hallmark of cancer. Nat Rev Mol Cell Biol. 2010; 11(3): 220-228.
[Crossref] [Google Scholar] [PubMed]
- Kumar S. Regulation of caspase activation in apoptosis: Implications in pathogenesis and treatment of disease. Clin Exp Pharmacol Physiol. 1999; 26(4): 295-303.
[Crossref] [Google Scholar] [PubMed]
- McIlwain DR, Berger T, Mak TW. Caspase functions in cell death and disease. Cold Spring Harb Perspect Biol. 2013; 5(4): a008656.
[Crossref] [Google Scholar] [PubMed]
- Reed JC. Mechanisms of apoptosis. Am J Pathol. 2000; 157(5): 1415-1430.
[Crossref] [Google Scholar] [PubMed]
- Susin SA, Lorenzo HK, Zamzami N, Marzo I, Brenner C, Larochette N, et al. Mitochondrial release of caspase-2and-9during the apoptotic process. J Exp Med. 1999; 189: 381-393.
[Crossref] [Google Scholar] [PubMed]
- Enari M, Sakahira H, Yokoyama H, Okawa K, Iwamatsu A, Nagata S. A Caspase-activated DNase that degrades DNA during apoptosis, and its inhibitors ICAD. Nature. 1998; 391(6662): 43-50.
[Crossref] [Google Scholar] [PubMed]
- Liu X, Li P, Widlak P, Zou H, Luo X, Garrard W, et al. The 40-kDa subunit of DNA fragmentation factor induces DNA fragmentation and chromatin condensation during apoptosis. Proc Natl Acad Sci USA. 1998; 95(15): 8461-8466.
[Crossref] [Google Scholar] [PubMed]
- Boege Y, Malehmir M, Healy ME, Bettermann K, Lorentzen A, Vucur M, et al. A dual role of caspase-8 in triggering and sensing proliferation-associated DNA damage, a key determinant of liver cancer development. Cancer Cell. 2017; 32: 342-359.
[Crossref] [Google Scholar] [PubMed]
- Philchenkov A, Zavelevich M, Kroczak TJ, Los M. Caspases and cancer: Mechanisms of inactivation and new treatment modalities. Exp Oncol. 2004; 26: 82-97.
[Google Scholar] [PubMed]
- Soung YH, Lee JW, Kim SY, Park WS, Nam SW, Lee JY, et al. Somatic mutations of CASP3 gene in human cancers. Hum Genet. 2004; 115: 112-115.
[Crossref] [Google Scholar] [PubMed]
- Peterson QP, Hsu DC, Goode DR, Novotny CJ, Totten RK, Hergenrother PJ. Procaspase-3 activation as an anti-cancer strategy: Structure-activity relationship of Procaspase-Activating Compound-1 (PAC-1) and its cellular co-localization with caspase-3. J Med Chem. 2009; 52: 5721-5731.
[Crossref] [Google Scholar] [PubMed]
- Coutinho-Camillo CM, Lourenco SV, Nishimoto IN, Kowalski LP, Soares FA. Caspase expression in oral squamous cell carcinoma. Head Neck. 2011; 33(8): 1191-1198.
[Crossref] [Google Scholar] [PubMed ]
- Andressakis D, Lazaris AC, Tsiambas E, Kavantzas N, Rapidis A, Patsouris E. Evaluation of caspase-3 and caspase-8 deregulation in tongue squamous cell carcinoma, based on immunohistochemistry and computerised image analysis. J Laryngol Otol. 2008; 122: 1213-1218.
[Crossref] [Google Scholar] [PubMed]
- Shikama Y.U.M, Miyashita T, Yamada M. Comprehensive studies on subcellular localizations and cell death inducing activities of eight GFP-tagged apoptosis related caspases. Exp Cell Res. 2001; 264: 315-325.
[Crossref] [Google Scholar] [PubMed]
- Xerri L, Devilard E, Hassoun J, Haddad P, Birg F. Malignant and reactive cells from human lymphomas frequently express FAS ligand but display a different sensitivity to FAS-mediated apoptosis. Leukemia. 1997; 11: 1868-1877.
[Crossref] [Google Scholar] [PubMed]
- Winter RN, Kramer A, Borkowski A, Kyprianou N. Loss of caspase-1 and caspase-3 protein expression in human prostate cancer. Cancer Res. 2001; 61(3): 1227-1232.
[Google Scholar] [PubMed]
- Nakopoulou L, Alexandrou P, Stefanaki K, Panayotopoulou E, Lazaris AC, Davaris PS. Immunohistochemical expression of caspase-3 as an adverse indicator of the clinical outcome in human breast cancer. Pathobiology. 2001; 69(5): 266-273.
[Crossref] [Google Scholar] [PubMed]
- Estrov Z, Thall PF, Talpaz M, Estey EH, Kantarjian HM, Andreeff M, et al. Caspase-2 and caspase-3 protein levels as predictors of survival in acute myelogenous leukemia. Blood. 1998; 92(9):3090-3097.
[Google Scholar] [PubMed]
- Declercq W, Vanden Berghe T, Vandenabeele P. RIP kinases at the crossroads of cell death and survival. Cell. 2009; 138: 229-232.
[Crossref] [Google Scholar] [PubMed]
- Barbero S, Mielgo A, Torres V, Teitz T, Shields DJ, Mikolon D, et al. Caspase-8 association with the focal adhesion complex promotes tumor cell migration and metastasis. Cancer Res. 2009; 69: 3755-3763.
[Crossref] [Google Scholar] [PubMed]
- Harada K, Toyooka S, Shivapurkar N, Maitra A, Reddy JL, Matta H, et al. Deregulation of caspase 8 and 10 expression in pediatric tumors and cell lines. Cancer Res. 2002; 62: 5897-5901.
[Google Scholar] [PubMed]
- Kim M, Hernandez L, Annunziata CM. Caspase 8 expression may determine the survival of women with ovarian cancer. Cell Death Dis. 2016; 7: e2045.
[Crossref] [Google Scholar] [PubMed]
- Frisch SM. Caspase-8: Fly or die. Cancer Res. 2008; 68: 4491-4493.
[Crossref] [Google Scholar] [PubMed]
- Helfer B, Boswell BC, Finlay D, Cipres A, Vuori K, Bong KT, et al. Caspase-8 promotes cell motility and calpain activity under nonapoptotic conditions. Cancer Res. 2006; 66: 4273-4278.
[Crossref] [Google Scholar] [PubMed]
- Benchoua A, Couriaud C, Guegan C, Tartier L, Couvert P, Friocourt G, et al. Active caspase-8 translocates into thenucleus of apoptotic cells to inactivate poly(ADP-ribose) polymerase-2. J Biol Chem. 2002; 277(37): 34217-34222.
[Crossref] [Google Scholar] [PubMed]
- Barbero S, Barila D, Mielgo A, Stagni V, Clair K, Stupack D, et al. Identification of a critical tyrosine residue in caspase-8 that promotes cell migration. J Biol Chem. 2008; 283(19): 13031-13034.
[Crossref] [Google Scholar] [PubMed]
- Koschny R, Brost S, Hinz U, Sykora J, Singe S, Breuhahn K. Cytosolic and nuclear caspase-8 have opposite impact on survival after liver resection for hepatocellular carcinoma. BMC Cancer. 2013; 13: 532-543.
[Crossref] [Google Scholar] [PubMed]
- Müller I, Strozyk E, Schindle S, Beissert S, Zarni OH, Sauter T, et al. Cancer cells employ nuclear caspase-8 to overcome the p53- dependent G2/M checkpoint through cleavage of USP28. Mol Cell. 2020; 77(5): 970-984.
[Crossref] [Google Scholar] [PubMed]
- de BA, Di FR, Morreale M, Carlisi D, Drago-Ferrante R, Montalbano M, et al. Unusual roles of caspase-8 in triple-negative breast cancer cell line MDAmB-231. Int J Oncol. 2016; 48: 2339-2348.
[Crossref] [Google Scholar] [PubMed]
- Julien O, Wells JA. Caspases and their substrates. Cell Death Differ. 2017; 24: 1380-1389.
[Crossref] [Google Scholar] [PubMed]
- Enari M, Sakahira H, Yokoyama H, Okawa A, Iwamatsu S. A caspase-activated DNase that degrades DNA during apoptosis, and its inhibitor ICAD. Nature. 1998; 391: 43-50.
[Crossref] [Google Scholar] [PubMed]
- Ramuz O, Isnardon D, Devilard E, Jauffret M, Hassoun J, Birg O, et al. Constitutive nuclear localization and initial cytoplasmic apoptotic activation of endogenous Caspase-3 evidenced by confocal microscopy. Int J Exp Path. 2003; 84(2): 75-81.
[Crossref] [Google Scholar] [PubMed]
- Nakagawara A, Nakamura Y, Ikeda H, Hiwasa T, Kuida K, Su M, et al. High levels of expression and nuclear localization of Interleukin-1 beta Converting Enzyme (ICE) and CPP32in favorable human neuroblastomas. Cancer Res. 1997; 57: 4578-4584. [Crossref]
[Google Scholar] [PubMed]
- Blazquez S, Sirvent JJ, Olona M, Aguilar C, Pelegri A, Garcia JF, et al. Caspase-3 and caspase-6 in ductal breast carcinoma: A descriptive study. Histol Histopathol. 2006; 21(12): 1321-1329.
[Crossref] [Google Scholar] [PubMed]
- Wang XS, Luo KJ, Bella AE, Bu SS, Wen J, Zhang SS, et al. Caspase-3 expression in metastatic lymph nodes of esophageal squamous cell carcinoma is prognostic of survival. World J Gastroenterol. 2014; 20: 4414-4420.
[Crossref] [Google Scholar] [PubMed]
- Kania J, Konturek SJ, Mariiez K, Hahn EG, Konturek PC. Expression of survivin and caspase-3 in gastric cancer. Dig Dis Sci. 2003; 48: 266-271.
[Crossref] [Google Scholar] [PubMed]
- Jakubowska K, Guzinska-Ustymowicz K, Famulski W, Cepowicz D, Jagodzinska D, Pryczynicz A. Reduced expression of caspase-8 and cleaved caspase-3 in pancreatic ductal adenocarcinoma cells. Oncol Lett. 2016; 11: 1879-1884.
[Crossref] [Google Scholar] [PubMed]
- Yoo NJ, Kim Hs, Kim SY, Park WY, Kim SH, Lee JY, et al. Stomach cancer highly expresses both initiator and effector caspases: An immunohistochemical study. Apmis. 2002; 110: 825-832.
[Crossref] [Google Scholar] [PubMed]
- Winter RN, Kramer A, Borkowski A, Kyprianou N. Loss of caspase-1 and caspase-3 protein expression in human prostate cancer. Cancer Res. 2001; 61(3): 1227-1232.
[Google Scholar] [PubMed]
- Persad R, Liu C, Wu TT, Houlihan PS, Hamilton SR, Diehl AM, et al. Overexpression of caspase-3 in hepatocellular carcinomas. Mod Pathol. 2004; 17: 861-867.
[Crossref] [Google Scholar] [PubMed]
- Liu PF, Hu YC, Kang BH, Tseng YK, Wu PC, Liang CC, et al. Expression levels of cleaved caspase-3 and caspase-3 in tumorigenesis and prognosis of oral tongue squamous cell carcinoma. Plos one. 2017; 12(7): e0180620.
[Crossref] [Google Scholar] [PubMed]
- Huang JS, Yang CM, Wang JS, Liou HH, Hsieh IC, Li GC, et al. Caspase-3 expression in tumorigenesis and prognosis of buccal mucosa squamous cell carcinoma. Oncotarget. 2017; 8: 84237-84247.
[Crossref] [Google Scholar] [PubMed]
- Kamada S, Kikkawa U, Tsujimoto Y, Hunter T. Nuclear translocation of caspase-3 is dependent on its proteolytic activation and recognition of a substrate-like protein(s). J Biol Chem. 2005; 280 (2): 857-860.
[Crossref] [Google Scholar] [PubMed]
Author Info
Navneet Saini1*, Rashmi Babbar2 and Ashish Kumar Mandal32Department of Physiology, Maulana Azad Medical College, New Delhi, India
3Department of Pathology, Maulana Azad Medical College, New Delhi, India
Citation: Saini N: Importance of Sub-Cellular Localization of Caspase in Oro-Pharyngeal Carcinoma
Received: 24-Jul-2023 Accepted: 18-Aug-2023 Published: 25-Aug-2023, DOI: 10.31858/0975-8453.14.8.530-534
Copyright: This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
ARTICLE TOOLS
- Dental Development between Assisted Reproductive Therapy (Art) and Natural Conceived Children: A Comparative Pilot Study Norzaiti Mohd Kenali, Naimah Hasanah Mohd Fathil, Norbasyirah Bohari, Ahmad Faisal Ismail, Roszaman Ramli SRP. 2020; 11(1): 01-06 » doi: 10.5530/srp.2020.1.01
- Psychometric properties of the World Health Organization Quality of life instrument, short form: Validity in the Vietnamese healthcare context Trung Quang Vo*, Bao Tran Thuy Tran, Ngan Thuy Nguyen, Tram ThiHuyen Nguyen, Thuy Phan Chung Tran SRP. 2020; 11(1): 14-22 » doi: 10.5530/srp.2019.1.3
- A Review of Pharmacoeconomics: the key to “Healthcare for All” Hasamnis AA, Patil SS, Shaik Imam, Narendiran K SRP. 2019; 10(1): s40-s42 » doi: 10.5530/srp.2019.1s.21
- Deuterium Depleted Water as an Adjuvant in Treatment of Cancer Anton Syroeshkin, Olga Levitskaya, Elena Uspenskaya, Tatiana Pleteneva, Daria Romaykina, Daria Ermakova SRP. 2019; 10(1): 112-117 » doi: 10.5530/srp.2019.1.19
- Dental Development between Assisted Reproductive Therapy (Art) and Natural Conceived Children: A Comparative Pilot Study Norzaiti Mohd Kenali, Naimah Hasanah Mohd Fathil, Norbasyirah Bohari, Ahmad Faisal Ismail, Roszaman Ramli SRP. 2020; 11(1): 01-06 » doi: 10.5530/srp.2020.1.01
- Manilkara zapota (L.) Royen Fruit Peel: A Phytochemical and Pharmacological Review Karle Pravin P, Dhawale Shashikant C SRP. 2019; 10(1): 11-14 » doi: 0.5530/srp.2019.1.2
- Pharmacognostic and Phytopharmacological Overview on Bombax ceiba Pankaj Haribhau Chaudhary, Mukund Ganeshrao Tawar SRP. 2019; 10(1): 20-25 » doi: 10.5530/srp.2019.1.4
- A Review of Pharmacoeconomics: the key to “Healthcare for All” Hasamnis AA, Patil SS, Shaik Imam, Narendiran K SRP. 2019; 10(1): s40-s42 » doi: 10.5530/srp.2019.1s.21
- A Prospective Review on Phyto-Pharmacological Aspects of Andrographis paniculata Govindraj Akilandeswari, Arumugam Vijaya Anand, Palanisamy Sampathkumar, Puthamohan Vinayaga Moorthi, Basavaraju Preethi SRP. 2019; 10(1): 15-19 » doi: 10.5530/srp.2019.1.3