Short Communication - (2024) Volume 15, Issue 8
Abstract
In this study, the theoretical molecular structure of 3’-hydroxy-5,7-dimethoxy-4-O-2’-cyclflavan (C17H16O5) compound was computed using Density Functional Theory/Becke,3-parameter Lee-Yang-Parr (DFT/B3LYP) methods with the 6-311++G(d,p) basis set. Additionally, Highest Occupied Molecular Orbital- Least Unoccupied Molecular Orbital (HOMO-LUMO) energies were calculated at the DFT/6-311G(d,p) level and three Dimensional (3D) molecular orbital levels were depicted. The calculated molecular geometry of the title compound was compared with experimental data, showing good agreement, particularly in optimized bond lengths along with bond angles, which closely matched X-ray data. Furthermore, the stable state molecular properties and molecular electrostatic potential surface maps were comprehensively investigated.
Keywords
Flavan, Highest occupied molecular orbital, Least unoccupied molecular orbital, Molecular electrostatic potential
Introduction
Flavonoids (Bostan SZ and Karadeniz T, 2006) are naturally occurring and biologically active compounds found in plants. These compounds are typically present in various parts of plants such as flowers, leaves, and fruits, where they serve as pigments responsible for determining the color and taste of plants (Tranfić M, et al., 2011). Additionally, flavonoids are known for their potent antioxidant properties. Antioxidants can help protect cells and tissues from oxidative stress by neutralizing the harmful effects of free radicals. Therefore, regular consumption of foods containing flavonoids may support heart health, reduce inflammation, and even exhibit protective effects against certain types of cancer.
Recent research provides significant insights into the effects of flavonoids on human metabolism and potential benefits for overall health (Dragovich PS, et al., 2002). For instance, due to their antioxidant activities, flavonoids have been shown to potentially play a role in reducing oxidative stress, thereby lowering the risk of chronic diseases such as cardiovascular diseases. Moreover, flavonoids are noted for their anti-inflammatory (Li Q, et al., 2000) properties and their potential regulatory effects on the immune system. This article will comprehensively examine the biological (Dragovich PS, et al., 2002) significance of flavonoids and their effects on health, while discussing studies on the electronic structures and chemical interactions of flavonoid molecules using theoretical calculation methods like DFT/B3LYP.
Description
Details of computational analysis
The molecular simulation of the molecule in its ground state was conducted using the Gaussian 09W program package (Frish MJ, et al., 2009). DFT methods were employed to intricately resolve the complex interactions of molecular forces and electronic structure. The rich data obtained from these calculations were subsequently analyzed and visualized in detail using the powerful visualization tools provided by Gaussian view 5 (Dennington R, et al., 2009), facilitating a comprehensive understanding of molecular dynamics.
All computations were meticulously performed using the B3LYP method, a hybrid functional developed by Becke AD, 1992 incorporating the LYP correlation functional. This methodological choice was further supported by the use of the 6-311++G(d,p) basis set (Lee C, et al., 1988), known for its wide applicability in calculating electronic structures and reaction energies of molecular systems. The 6-311++G(d,p) basis set enables detailed examination of molecular interactions, enhancing the accuracy of our study.
Analysis of molecular geometry structure
The crystal structure of the flavonoid-containing compound 3’-hydroxy-5,7-dimethoxy-4-O-2’-cyclflavan (C17H16O5) was synthesized by Cui YM, et al., 2011. The synthesized molecule’s X-ray single crystal structure is available in the Cambridge Structural Database (CSD) under the code CCDC 796429. The experimental structure of the flavonoid compound is depicted in Figure 1. The theoretical geometric structure of the compound containing flavan, C17H16O5 was computed DFT in Gaussian 09, based on crystal structure data obtained from the CSD. The calculations were performed using the B3LYP/6-311++G(d,p) method and the optimized geometry structure is shown in Figure 2. The bond lengths (Å) and bond angles (º) of the compound containing flavan, C17H16O5 were theoretically calculated in the stable state and vacuum phase using the Gaussian 09 package. The optimized theoretical bond lengths and bond angles were compared with experimental results obtained from single-crystal X-ray diffraction data sourced from the literature, and are listed in Table 1.
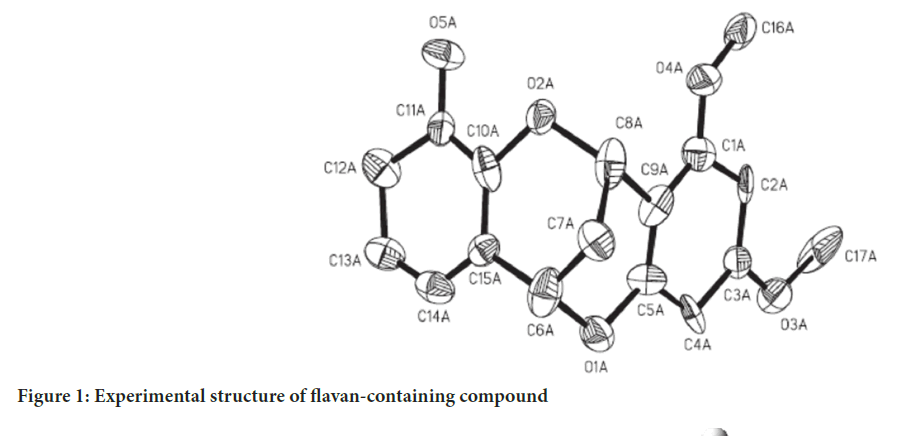
Figure 1: Experimental structure of flavan-containing compound
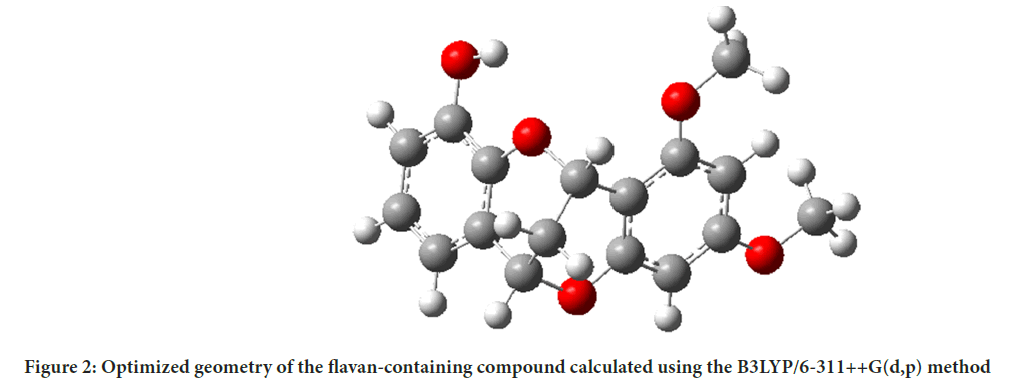
Figure 2: Optimized geometry of the flavan-containing compound calculated using the B3LYP/6-311++G(d,p) method
| Bond lengths | Bond angles | ||||
|---|---|---|---|---|---|
| Flavan-containing compound | X-ray | B3LYP | Flavan-containing compound | X-ray | B3LYP |
| C17-O3 | 1.3972 | 1.42069 | C17-O3-C3 | 119.4928 | 119.1397 |
| O3-C3 | 1.36046 | 1.36373 | O3-C3-C2 | 122.3485 | 115.568 |
| C3-C2 | 1.39865 | 1.40303 | O3-C3-C4 | 116.4094 | 115.568 |
| C2-C1 | 1.38514 | 1.39593 | C3-C4-C5 | 119.4179 | 119.0825 |
| C1-O4 | 1.36494 | 1.36521 | C4-C5-C9 | 121.7382 | 121.7072 |
| O4-C16 | 1.42058 | 1.42143 | C9-C1-C2 | 121.7135 | 121.6354 |
| C1-C9 | 1.40003 | 1.40487 | C3-C2-C1 | 118.3194 | 118.6757 |
| C9-C5 | 1.39381 | 1.40362 | C9-C1-O4 | 115.1605 | 115.2232 |
| C5-C4 | 1.37872 | 1.39258 | C1-O4-C16 | 117.558 | 119.1714 |
| C4-C3 | 1.37036 | 1.39048 | C9-C8-C7 | 109.6136 | 109.7156 |
| C5-O1 | 1.37078 | 1.36405 | C8-C7-C6 | 106.1242 | 105.8798 |
| O1-C6 | 1.44797 | 1.45397 | O1-C6-C7 | 106.1242 | 109.237 |
| C6-C7 | 1.50218 | 1.52454 | O1-C6-C15 | 111.0062 | 111.4194 |
| C7-C8 | 1.50382 | 1.52494 | C8-O2-C10 | 113.8194 | 115.3884 |
| C9-C8 | 1.49909 | 1.50484 | C6-C15-C10 | 119.1686 | 118.8789 |
| C6-C15 | 1.51352 | 1.51274 | C7-C8-O2 | 108.8068 | 108.5367 |
| C15-C10 | 1.38144 | 1.395 | C7-C6-C15 | 109.9552 | 110.0424 |
| C10-O2 | 1.3643 | 1.371 | C15-C14-C13 | 121.3186 | 120.3851 |
| O2-C8 | 1.46113 | 1.46267 | O2-C10-C11 | 114.9268 | 115.2496 |
| C15-C14 | 1.38919 | 1.40039 | C10-C11-O5 | 119.7083 | 119.9124 |
| C14-C13 | 1.36612 | 1.38954 | C12-C11-O5 | 120.1047 | 120.4562 |
| C13-C12 | 1.38975 | 1.39755 | C10-C11-C12 | 120.1821 | 119.6313 |
| C12-C11 | 1.37375 | 1.38763 | C12-C13-C14 | 120.5066 | 120.3945 |
| C11-O5 | 1.36453 | 1.3637 | C5-O1-C6 | 114.7414 | 116.6118 |
| C11-C10 | 1.39415 | 1.40438 | C1-C9-C8 | 121.7732 | 121.6654 |
Table 1: Geometric parameters of the flavan-containing compound, their bond lengths (Å) and bond angles (º)
Electronic properties
Molecular orbitals can be quantitatively calculated using quantum chemical computational methods such as DFT. In this study, Energy of the Highest Occupied Molecular Orbital (EHOMO) and Energy of the Lowest Unoccupied Molecular Orbital (ELUMO) energy values of the compound containing flavan were computed using the DFT method. The calculated EHOMO and ELUMO energies were used to determine energy difference (ΔE), electron Affinity (A), Ionization potential (I), Electronegativity (χ), chemical Softness (S) and hardness (η) parameters based on formulas derived from electron density distributions. The results are listed in Table 2. LUMO and HOMO are critical concepts in determining the electronic structure of a molecule. These terms refer to the energies of the LUMO and HOMO in the molecule, respectively. ELUMO and EHOMO specifically denote the energy levels of these orbitals. In the detailed analysis aimed at exploring the electronic structural properties (Francesca M, et al., 2024) of the compound containing flavan, LUMO and HOMO orbitals have been visualized in detail in Figure 3. These analyses not only provide insights into the nature of molecular interactions but also serve as a fundamental source of information for understanding the physical and chemical properties of the molecule. Such insights form an important starting point for advanced pharmacological and therapeutic research. The molecular structure of the compound containing flavan has been thoroughly examined through detailed 3D graphics depicted in Figure 3. These graphics visually represent electron distributions and charge densities in different regions of the molecule. Particularly, regions carrying negative charges are highlighted in green, while those with positive charges are emphasized in red. This color scheme provides a significant visual tool for understanding the electronic structural characteristics of the molecule and offers an in- depth insight into the analysis of molecular interactions.
| Parameters | B3LYP/6-311++G(d,p) |
|---|---|
| EHOMO (eV) | -5.84532 |
| ELUMO (eV) | -0.57498 |
| ΔE=E(LUMO-HOMO) (eV) | 5.27034 |
| I (eV) | 5.84532 |
| A (eV) | 0.57498 |
χ (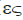 ) ) |
3.21015 |
η (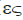 ) ) |
2.63517 |
| S (eV-1) | 0.085539 |
| ETotal (a.u) | -0.25457 |
Note: ΔE: Energy difference; electron; A: Affinity; I: Ionization potential; χ: Electronegativity; S: Chemical softness; η: Hardness and a.u: astronomical unit
Table 2: Molecular orbital energy calculations of the flavan-containing compound
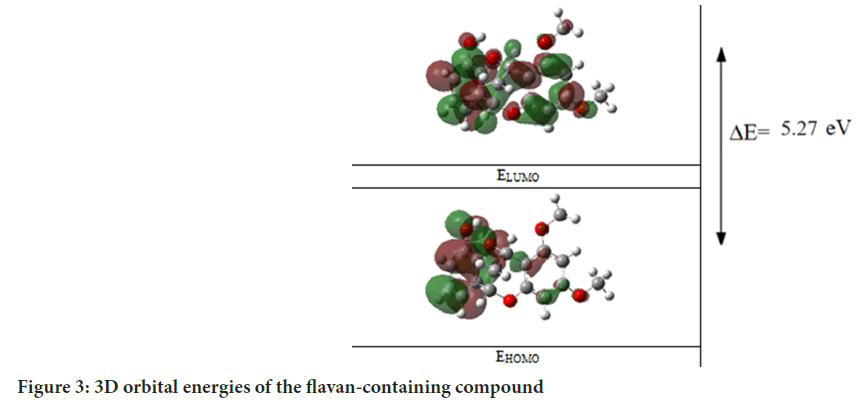
Figure 3: 3D orbital energies of the flavan-containing compound
3D representation vividly illustrates the atomic-level arrangement and electron distribution of the molecule (Figure 3). The color coding in these graphics enables a detailed examination of the electrical properties of various chemical groups and functional regions within the flavan compound. Furthermore, this visual analysis method aids in better understanding the nature of molecular interactions and chemical bonds, thereby providing a solid theoretical foundation for understanding potential effects on the biological activities of the compound.
This study, by comprehensively analyzing the structural and electronic properties of the flavan-containing compound at the molecular level, serves as an important reference point for future pharmacological and therapeutic research endeavors.
Molecular Electrostatic Potential surfaces (MEPs)
MEP (Gümüş HP, et al., 2014) is a useful method for describing the reactivity of molecular behaviors, structural activity, and hydrogen bonding. The 3D molecular electrostatic potential maps of the structures optimized using the B3LYP/6-311++G(d,p) method for the benzothiazole derivative molecule are depicted in Figure 4.
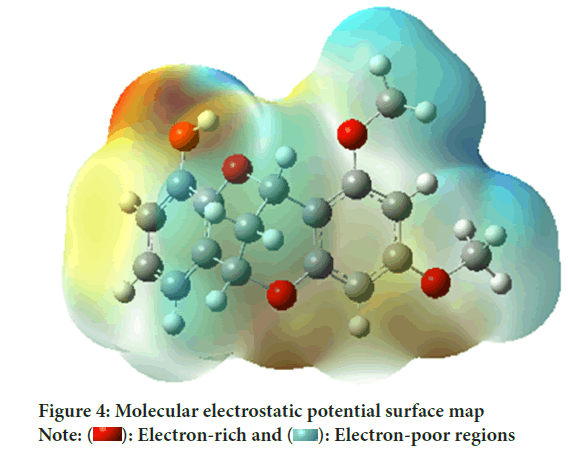
Figure 4: Molecular electrostatic potential surface map
Note: ( ): Electron-rich and (
): Electron-rich and (  ): Electron-poor regions
): Electron-poor regions
Analysis of the electron density of the molecule reveals a wide distribution. MEP maps show the size, shape and electrostatic properties of the molecule in detail. When the figure was analyzed, it was determined that the regions with the highest electron density for the neutral form were generally gathered around the oxygen atoms, while the regions with the lowest electron density were generally around the C-H bonds.
MEP maps provide an important tool for understanding the biological effects of the molecule. These maps can help predict the molecule’s interactions and potential reactivity. For example, electron-rich regions may indicate regions where the molecule is active in chemical reactions, while electron-poor regions may indicate regions that may be less active in reactions. Such analyzes provide an important source of information for understanding how the flavan-containing compound interacts with biological systems and potentially its health effects.
The widely used Gaussian package program in computational chemistry was employed to determine the molecular structure and energy of the compound containing flavan, C17H16O5 DFT calculations provided by this program utilized the Becke’s three-parameter hybrid exchange-correlation functional combined with B3LYP method and the 6-311++G(d,p) basis set. These settings facilitated the optimization of the molecular structure of the flavan-containing compound, thereby identifying the stable states of molecular systems.
Furthermore, the calculations allowed for a detailed examination of important molecular properties such as molecular orbital energies, molecular characteristics, and molecular electrostatic potential surface maps. Particularly, molecular orbital energies and electron distributions play critical role in understanding the biological activities of compounds containing flavan. Such analyses provide important insights into evaluating the potential effects of the compound on cancer or other diseased cells.
Conclusion
The compound containing flavan is recognized for its antioxidant properties, which are known to mitigate the oxidative damage associated with aging caused by free radicals. This attribute suggests potential benefits in reducing cardiovascular risks. Our study marks a significant advancement in exploring the anti-tumor properties of flavan-containing molecules. The findings support the hypothesis that this compound could serve as a potential drug candidate for combating free radical species within cells. These comprehensive molecular-level analyses finds the way for further investigations into the utilization of flavan-containing compounds in pharmacological and therapeutic applications.
References
- Bostan SZ, Karadeniz T. Relationships between growth strengths and flavans in apple and cherry saplings with different development rates. 2006: 32-36.
- Tranfic M, Halambek J, Cetina M, Jukic M. Synthesis, X-ray and spectroscopic analysis of 2-hydrazino-6-methyl-4-(methoxymethyl)-5-nitropyridine-3-carbonitrile. J Mol Structure. 2011; 1001(1-3): 145-151.
- Dragovich PS, Prins TJ, Zhou R, Johnson TO, Brown EL, Maldonado FC, et al. Structure-based design, synthesis, and biological evaluation of irreversible human rhinovirus 3C protease inhibitors. Part 7: Structure-activity studies of bicyclic 2-pyridone-containing peptidomimetics. Bioorg Med Chem Lett. 2002; 12(5): 733-738.
[Crossref] [Google Scholar] [Pubmed]
- Li Q, Mitscher LA, Shen LL. The 2-pyridone antibacterial agents: Bacterial topoisomerase inhibitors. Med Res Rev. 2000; 20(4): 231-293.
[Crossref] [Google Scholar] [Pubmed]
- Frish MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, et al. Gaussian 09 revision A. 1, Gaussian Inc. Wallingford CT, 2009.
- Dennington R, Keith T, Millam J. GaussView, version 5. 2009.
- Becke AD. Density-functional thermochemistry. I. The effect of the exchange-only gradient correction. J Chem Phys. 1992; 96(3): 2155-2160.
- Lee C, Yang W, Parr RG. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys Rev B Condens Matter. 1988; 37(2): 785-789.
[Crossref] [Google Scholar] [Pubmed]
- Cui YM, Wang H, Liu QR, Han M, Lu Y, Zhao CQ. Flavans from Iris tenuifolia and their effects on ß-amyloid aggregation and neural stem cells proliferation in vitro. Bioorg Med Chem Lett. 2011; 21(15): 4400-4403.
[Crossref] [Google Scholar] [Pubmed]
- Francesca M, Paolo P, Gabriele T, Anselmo C, Caleb D, Cristina D, et al. The effects of different levels of sports activity on health-related quality of life and lifestyle habits in high school Italian students. Eur J Pediatr. 2024; 183(9): 4041-4048.
[Crossref] [Google Scholar] [Pubmed]
- Gümüs HP, Tamer Ö, Avci D, Atalay Y. Quantum chemical calculations on the geometrical, conformational, spectroscopic and nonlinear optical parameters of 5-(2-Chloroethyl)-2, 4-dichloro-6-methylpyrimidine. Spectrochim Acta A Mol Biomol Spectrosc. 2014; 129: 219-226.
[Crossref] [Google Scholar] [Pubmed]
Author Info
Hacer Gumuş*Citation: Gumuş H: Investigating the Molecular Structure of 3’-Hydroxy-5,7-Dimethoxy-4-O-2’-Cycloflavan Molecule for the Risk of Chronic Cardiovascular Diseases
Received: 06-Aug-2024 Accepted: 22-Aug-2024 Published: 29-Aug-2024, DOI: 10.31858/0975-8453.15.8.273-276
Copyright: This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
ARTICLE TOOLS
- Dental Development between Assisted Reproductive Therapy (Art) and Natural Conceived Children: A Comparative Pilot Study Norzaiti Mohd Kenali, Naimah Hasanah Mohd Fathil, Norbasyirah Bohari, Ahmad Faisal Ismail, Roszaman Ramli SRP. 2020; 11(1): 01-06 » doi: 10.5530/srp.2020.1.01
- Psychometric properties of the World Health Organization Quality of life instrument, short form: Validity in the Vietnamese healthcare context Trung Quang Vo*, Bao Tran Thuy Tran, Ngan Thuy Nguyen, Tram ThiHuyen Nguyen, Thuy Phan Chung Tran SRP. 2020; 11(1): 14-22 » doi: 10.5530/srp.2019.1.3
- A Review of Pharmacoeconomics: the key to “Healthcare for All” Hasamnis AA, Patil SS, Shaik Imam, Narendiran K SRP. 2019; 10(1): s40-s42 » doi: 10.5530/srp.2019.1s.21
- Deuterium Depleted Water as an Adjuvant in Treatment of Cancer Anton Syroeshkin, Olga Levitskaya, Elena Uspenskaya, Tatiana Pleteneva, Daria Romaykina, Daria Ermakova SRP. 2019; 10(1): 112-117 » doi: 10.5530/srp.2019.1.19
- Dental Development between Assisted Reproductive Therapy (Art) and Natural Conceived Children: A Comparative Pilot Study Norzaiti Mohd Kenali, Naimah Hasanah Mohd Fathil, Norbasyirah Bohari, Ahmad Faisal Ismail, Roszaman Ramli SRP. 2020; 11(1): 01-06 » doi: 10.5530/srp.2020.1.01
- Manilkara zapota (L.) Royen Fruit Peel: A Phytochemical and Pharmacological Review Karle Pravin P, Dhawale Shashikant C SRP. 2019; 10(1): 11-14 » doi: 0.5530/srp.2019.1.2
- Pharmacognostic and Phytopharmacological Overview on Bombax ceiba Pankaj Haribhau Chaudhary, Mukund Ganeshrao Tawar SRP. 2019; 10(1): 20-25 » doi: 10.5530/srp.2019.1.4
- A Review of Pharmacoeconomics: the key to “Healthcare for All” Hasamnis AA, Patil SS, Shaik Imam, Narendiran K SRP. 2019; 10(1): s40-s42 » doi: 10.5530/srp.2019.1s.21
- A Prospective Review on Phyto-Pharmacological Aspects of Andrographis paniculata Govindraj Akilandeswari, Arumugam Vijaya Anand, Palanisamy Sampathkumar, Puthamohan Vinayaga Moorthi, Basavaraju Preethi SRP. 2019; 10(1): 15-19 » doi: 10.5530/srp.2019.1.3






