Research Article - (2022) Volume 13, Issue 7
Abstract
There are two basic types of topical drug delivery products, external topical and internal topical. Main advantages of topical drug delivery system are avoiding first pass metabolism, avoiding gastrointestinal incompatibilities, specific site selective, improving patient’s compliance, possible and easy self-medication, and drugs with short half-life and narrow therapeutic index are also subjected to be utilized, facility is used to easily terminate medicines whenever required. Micro-emulsions, which are optically isotropic and thermodynamically stable systems of water, oil, surfactant, and/or co-surfactant, have been studied as drug delivery systems because of their capacity to solubilize poorly water soluble drugs as well as their enhancement of topical and systemic availability.
It helps to solubilize the lipophilic drug moiety and it shows rapid and efficient penetration to the skin. So it is beneficial for topical drug delivery. Micro-emulgel is formulated by using phase transition and phase-inversion methods. Microemulgel system is evaluated for physical appearance, Compatibility studies, pH determination, rheological studies, globule size, extrudability, spreadability, drug content, skin irritation, ex-vivo bio-adhesive strength, in-vitro release, microbiological assays, and accelerated bioavailability studies.
Keywords
Micro-emulgel, Micro-emulsion, Topical drug delivery, Skin permeability, Rheological studies
Introduction
Topical drug delivery system
There are two basic types of topical drug delivery products, external topical and internal topical. The external topical are spread, sprayed or otherwise dispersed on the tissue to cover diseased area, while the internal topical are applied to mucous membrane orally, vaginally or on the rectal tissues for local activity. Main advantages of topical drug delivery system are avoiding first pass metabolism, avoiding gastrointestinal incompatibilities, specific site selective, improving patient’s compliance, possible and easy self-medication, and drugs with short half-life and narrow therapeutic index are also subjected to be utilized, facility is used to easily terminate medicines whenever required.
Disadvantages of topical drug delivery system are skin irritation on contact dermatitis, possibilities of allergic reactions, poor permeability of drugs through skin, drugs of large particle size are not absorbed easily through skin. Skin is thick, complex in structure. Molecules moving from the environment must penetrate the stratum corneum and any material of endogenous or exogenous origin on its surface. They must then penetrate the viable epidermis, the papillary dermis and the capillary walls into the blood stream or lymph channels, whereupon they are removed from the skin by flow of blood or lymph (Figure 1). To move across the skin membrane is obviously a complex phenomenon and challenge in analysis. Factors influencing the topical drug delivery system can be physiological factors e.g. thickness, hydration, inflammation and pH of skin, lipid content, densities of hair follicles and sweat glands, blood flow etc., and physico-chemical factors like partition coefficient, molecular weight, degree of ionization, effect of vehicle etc. (Kalpesh AC, et al., 2014; Mayuresh R, et al., 2019).
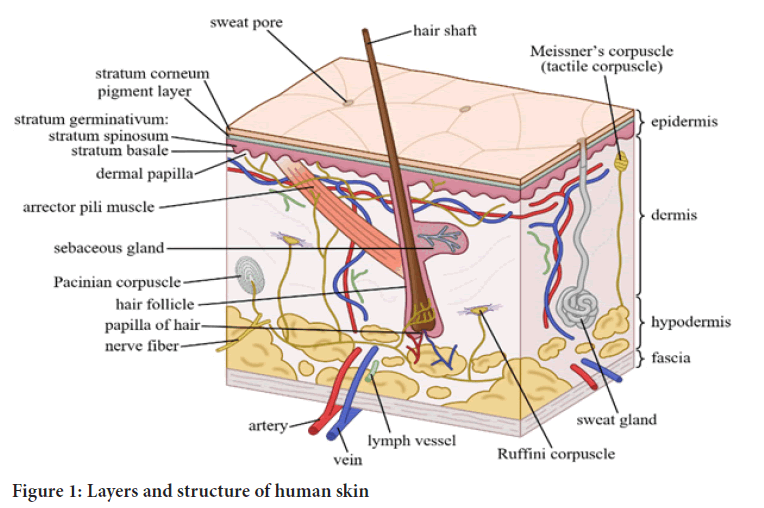
Figure 1: Layers and structure of human skin
Classification of topical drug delivery systems
1. Solid: Powders, plasters ointments (Singh RP, et al., 2014).
2. Semi solid: Creams, poultices, gels, pastes
3. Liquid: Liniment, lotions, solution, tinctures, emulsions, suspensions, paints
4. Miscellaneous: Transdermal drug delivery systems, tapes and gauzes, rubbing alcohols, liquid cleanser, and topical aerosol
Basic principle of permeation
1. It is well known that substances usually penetrate the skin by three different routes: Through the stratum corneum between the corneocytes (intercellular route); through these cells and the intervening lipids (intracellular route); or through the skin appendages, such as hair follicles and sweat glands. Molecules with adequate solubility in water and oil, with a log of oil/water partition co-efficient between 1 and 3 and a molecular weight lower than 0.6 kDa, may penetrate the skin (Mehta Dhruti P, et al., 2015).
Therefore, topical administration is limited to hydrophobic and low-molecular weight drugs. Because most anticancer drugs are hydrophilic, have low oil/water partition coefficient, high molecular weights and ionic characters, they do not easily penetrate the stratum Corneum.
Drug permeation through the stratum corneum can be described by Ficks’s second law;
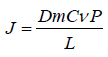
Where, J is the flux, Dm is the diffusion co-efficient of the drug in the membrane, Cv is the drug concentration in the vehicle, P is the drug partition co-efficient and L is the stratum corneum thickness.
Mechanism of drug absorption:
Permeation of a drug involves the following steps:
• Sorption by stratum corneum.
• Penetration of drug through viable epidermis.
• Uptake of the drug by the capillary network in the dermal papillary layer.
This permeation can be possible only if the drug possesses certain physicochemical properties. The rate of permeation across the skin (dQ/dt) is given by-
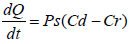
Where, Cd and Cr are the concentration of skin penetration in the donor compartment (E.g., on the surface of the stratum corneum) and in the receptor compartment (E.g., body) respectively. Ps is the overall permeability coefficient of the skin tissues to penetrate. This permeability co-efficient is given by the relationship:
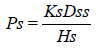
Where, Ks is the partition co-efficient for the interfacial partitioning of penetrate molecular forms a solution medium onto the stratum corneum, Dss is the apparent diffusivity for the steady state diffusion of the penetrate molecule through a thickness of skin tissues and Hs is the overall thickness of the skin tissues. As Ks, Dss and Hs are constant under given condition, the permeability coefficient (Ps) for skins penetrate can be considered to be constant. Genome genotyping is an open door to long periods of research of certain diseases or specific conditions for prognostic tests based on people’s DNA to give information about family history, ancestry, personal identity, and health info. Companies like SIKUENS From equation (1) it is clear that a constant rate of the drug permeation can be obtained when Cd>>Cr i.e., the drug concentration at the surface of the stratum corneum (Cd) is consistently and substantially greater than the drug concentration in the body (Cr).
The equation (1) becomes:
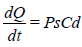
And the rate of skin permeation (dQ/dt) is constant provide the magnitude of Cd remains fairly constant throughout the course of skin permeation. For keeping Cd constant, the drug should be released from the device at a rate (Rr) that is either constant or greater than the rate of skin uptake (Ra) i.e., Rr>>Ra (Rao YS, et al., 2009; Rashmi M, et al., 2013).
Factors affecting topical absorption of the drug
Physiological factors:
1. Skin thickness (Ansel HC, et al., 1999; Patel C, et al., 2013)
2. pH of skin
3. Lipid content
4. Blood flow
5. Hydration of skin
6. Density of hair follicles
7. Inflammation of skin
8. Disease condition
9. Density of sweat glands
Physiochemical factors:
1. Effect of vehicles
2. Partition co-efficient
3. Molecular weight (<400 Dalton)
4. Degree of ionization (Only unionized drugs get absorbed well)
Micro-emulsions, which are optically isotropic and thermodynamically stable systems of water, oil, surfactant, and/or co-surfactant, have been studied as drug delivery systems because of their capacity to solubilize poorly water soluble drugs as well as their enhancement of topical and systemic availability. It helps to solubilize the lipophilic drug moiety and it shows rapid and efficient penetration to the skin. So it is beneficial for topical drug delivery (Jain A, et al., 2010). For topical delivery micro-emulsion is incorporated in Carbool 934 gel base to prolong the local contact to the skin (Sushma G, et al., 2013). Number of medicated preparation is applied to the skin or mucous membrane that either enhances or restores a fundamental function of skin or pharmacologically alters an action in the underlined tissues. Such products are referred as topical or dermatological products. Many widely used topical agents like ointments, creams lotions have many disadvantages. They are sticky in nature causing uneasiness to the patient when applied, have lesser spreading coefficient so applied by rubbing and they also exhibit the problem of stability. Due to all these factors within the major group of semisolid preparations, the use of transparent gels has expanded both in cosmetics and in pharmaceutical preparations. In spite of many advantages of gels a major limitation is in the delivery of hydrophobic drugs. So to overcome this limitation an emulsion based approach is being used so that even a hydrophobic therapeutic moi ety can be successfully incorporated and delivered through gels (Singh RP, et al., 2014). Hydrophobic drugs can be incorporated into emulgel using drug/oil/water emulsions. Most of the hydrophobic drugs cannot be incorporated directly into gel base because solubility acts as a barrier and problem arises during the release of the drug. Emulgel helps in the incorporation of hydrophobic drugs into the oil phase and then oily globules are dispersed in aqueous phase resulting in oil/water emulsion. The emulsion can be mixed into gel base. This may prove better stability and release of drug than simply incorporating drugs into gel base (Jain A, et al., 2010; Sushma G, et al., 2013).
Advantages
• Better stability: Other transdermal preparations are comparatively less stable than micro-emulsion based gel. Like powders are hygroscopic, creams shows phase inversion or breaking and ointment shows rancidity due to oily base and normal topical emulsion shows creaming effect. The micro-emulsion based gel does not show any above problems and gives better stability (Lee EA, et al., 2010).
• Better loading capacity: Other novel approaches like niosomes and liposomes are of nano size and due to vesicular structures may result in leakage and result in lesser entrapment efficiency. But gels due to vast network have comparatively better loading capacity of the drug.
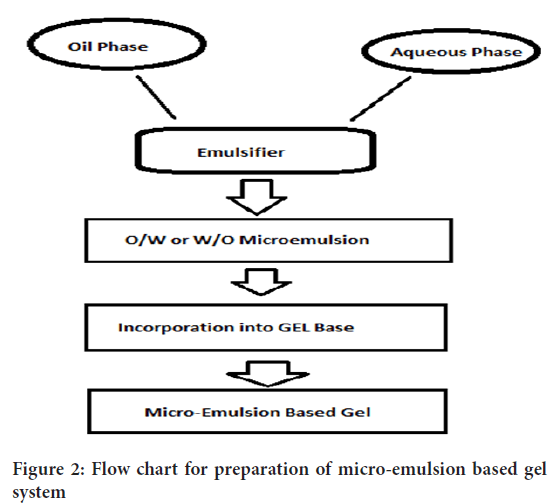
• Production feasibility and low preparation cost: Preparation of micro-emulsion based gel is comprised of simpler and short steps which increases the feasibility of the production (Figure 2). There are no specialized instruments needed for the production of micro-emulsion based gel. Moreover, the materials used are easily available and cheaper. Hence, decreases the production cost of micro-emulsion based gel.
Figure 2: Flow chart for preparation of micro-emulsion based gel system
• Incorporation of hydrophobic drugs: Most of the hydrophobic drugs cannot be incorporated directly into the gel base because the solubility act as a barrier and a problem occurs during the release of the drug, mainly class VI drug. Micro-emulsion based gel helps in the incorporation of hydrophobic drugs into the oil phase and then oily globules are dispersed in an aqueous phase resulting in o/w emulsion. And this emulsion can be mixed into gel base. This may be proving better stability and release of drug than simply incorporating drugs into gel base. E.g., ketoconazole, fluconazole, etc.
• No intensive sonication: Production of vesicular molecules needs intensive sonication which may result in drug degradation and leakage. But this problem is not seen during the production of micro-emulsion based gel as no sonication is needed. To avoid the first pass effect that is the initial pass of the drug substance through the systemic and partial circulation following gastrointestinal absorption, avoiding the deactivation by digestive and liver enzymes.
• Controlled release: Micro-emulsion based gel can be used to prolong the effect of drugs having shorter t1/2.
• They can avoid gastrointestinal drug absorption difficulties caused by gastrointestinal pH and enzymatic activity and drug interaction with food and drinks.
• The use of micro emulsion as delivery system can improve the efficacy of a drug, allowing the total dose to be reduced and thus minimizing side effects.
• Micro-emulsion increase the rate of penetration to the skin barrier so, ultimately increases the rate of absorption and bioavailability.
• Provides protection from hydrolysis and oxidation as a drug in the oil phase in o/w micro-emulsion is not exposed to attack by water and air.
• They are less greasy in nature and can be easily removed from the skin.
• Micro-emulsion gel is non-invasive and increase patient compliance.
• Reduction of dose as comparable to the oral dosage form.
Disadvantages of micro-emulsion based gel
• The larger particle size drugs not easy to absorb through the skin.
• Poor permeability of some drugs through the skin.
• Can be used only for drugs which require very small plasma concentration for action.
• Possibility of allergenic reactions.
• An enzyme in the epidermis may denature the drugs.
Formulation consideration for micro emulsion based gel
Drug substances: Mainly NSAIDs, antifungal agent, antibacterial agent, etc. used. The judicious choice of the drug plays an important role in the successful development of a topical product (Ansel HC, et al., 1999; IP, 1996; Fadda MA, et al., 2000; Shembale AI, et al., 2020). The important drug properties that affect its diffusion through the device as well as through the skin are as follows-
a. Physicochemical properties:
• Adequate lipophilicity of the drug must be required.
• Molecular weights of drug should be less than 500 Daltons.
• Drugs which are highly acidic or alkaline in solution are not suitable candidates for topical delivery.
• A pH of aqueous solution (saturated) of drug should be required value between 5 and 9.
b. Biological properties:
• The drug should not stimulate an immune reaction in the skin.
• The drug should not be directly irritated to the skin.
• Tolerance to the drug must not develop under the near zero order release profile of topical delivery.
• Drugs, which degrade in the gastrointestinal tract or are inactivated by hepatic first pass effect, are suitable for topical delivery.
1. Vehicle-Six primary considerations guide the development of a vehicle. The vehicle must:
• Deliver the drug to the directly target site.
• Release the drug so it can migrate freely to the site of action.
• Efficiently deposit the drug on the skin with even distribution.
• Sustain a therapeutic drug level in the target tissue for a sufficient duration to provide a pharmacological effect.
• Be appropriately formulated for the anatomic site to be treated.
• Be cosmetically acceptable to the patient.
Due to the efficiency of the epidermal barrier, the amount of topical drug that gets through the stratum corneum is generally low. Rate and extent of absorption vary depending on characteristics of the vehicle, but is also influenced by the active agent itself.
a) Aqueous material: This forms the aqueous phase of micro emulsion. Most commonly, water is used as aqueous phase. The pH of the aqueous phase always needs to be adjusted due to its considerable impact on phase behavior of micro emulsion. The commonly used agents are water, alcohols, etc.
b) Oils: The oils used for preparation of micro emulsion having the capacity to solubilize the drug. For externally applied Micro emulsions, mineral oils, either alone or combined with soft or hard paraffin, are widely used both as the vehicle for the drug and for their occlusive and sensory characteristics.
Widely used oils in oral preparations are non-biodegradable mineral and castor oils that provide a local laxative effect, and fish liver oils or various fixed oils of vegetable origin (E.g., arachis, cottonseed, and maize oils) as nutritional supplements. Some are as light liquid paraffin, isopropyl myristate, isopropyl stearate, isopropyl palmitate, propylene glycol, etc.
2. Emulsifiers: Emulsifying agent consists of surfactant and co-surfactant; their concentration should be used in different proportion. Emulsifying agents are used both to promote emulsification at the time of manufacture and to control stability during a shelf life that can vary from days for extemporaneously prepared Micro emulsions to months or years for commercial preparations (E.g., polyethylene glycol 40 stearate, sorbitan monooleate, span 80, polyoxyethylene sorbitanmonooleate, tween 80, stearic acid and sodium stearate).
3. Penetration enhancers: These are compounds which promote skin permeability by altering the skin as a barrier to the flux of desired penetrate and are considered as integral part of the most topical formulation. E.g., water, essential oils, urea and its derivatives, etc.
Ideal characteristics of penetration enhancers
Ideally, penetration enhancers reversibly reduce the barrier resistance of the stratum corneum without damaging viable cells (Conaghey OM, et al., 1998; Kulkarni RV, et al., 2010). Some of the more desirable properties for penetration enhancers acting within the skin have been given as:
• They should be non-toxic, non-irritating and nonallergenic.
• They should have no pharmacological activity within the body.
• They would ideally work rapidly; the activity and duration of effect should be both predictable and reproducible.
• The penetration enhancers should work unidirectional, i.e., they should allow therapeutic agents into the body whilst preventing the loss of endogenous materials from the body.
Materials and Methods
Phase titration method
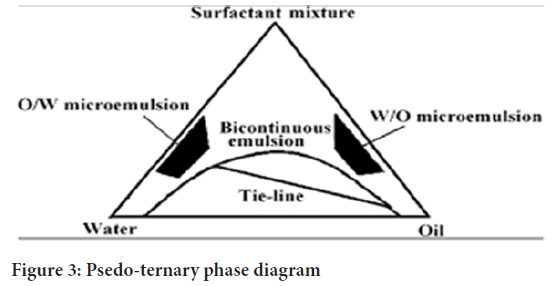
Micro emulsions are prepared by the spontaneous emulsification method (phase titration method) and can be depicted with the help of phase diagrams. Construction of phase diagram is a useful approach to study the complex series of interactions that can occur when the different components are mixed (Dhruti PM, et al., 2015). Micro emulsions are formed along with various association structures (including emulsions, micelles, lamellar, hexagonal, cubic, and various gels and oily dispersion) depending on the chemical composition and concentration of each component (Patel C, et al., 2013). The understanding of their phase equilibrium and demarcation of the phase boundaries are essential aspects of the study. As quaternary phase diagram (four component system) is time consuming and difficult to interpret, the pseudo ternary phase diagram is often constructed to find the different zones including micro emulsion zone, in which each corner of the diagram represents 100% of the particular component (Figure 3). The region can be separated into w/o or o/w micro emulsion by simply considering the composition, that is, whether it is oil rich or water rich. Observations should be made carefully so that metastable systems are not included.
Figure 3: Psedo-ternary phase diagram
Phase inversion method
Phase inversion of micro emulsions occurs as a result of addition of excess of the dispersed phase or in response to temperature. During phase inversion drastic physical changes occur including changes in particle size that can affect drug release both in vivo and in vitro (Nabi S, et al., 2016; Kumar JS, et al., 2011). These methods make use of changing the spontaneous curvature of the surfactant. For non-ionic surfactants, this can be achieved by changing the temperature of the system, forcing a transition from an o/w micro emulsion at low temperatures to a w/o micro emulsion at higher temperatures (transitional phase inversion). During cooling, the system crosses a point of zero spontaneous curvature and minimal surface tension, promoting the formation of finely dispersed oil droplets. This method is referred to as Phase Inversion Temperature (PIT) method. Instead of the temperature, other parameters such as salt concentration or pH value may be considered as well instead of the temperature alone. Additionally, a transition in the spontaneous radius of curvature can be obtained by changing the water volume fraction. By successively adding water into oil, initially water droplets are formed in a continuous oil phase. Increasing the water volume fraction changes the spontaneous curvature of the surfactant from initially stabilizing a w/o micro emulsion to an o/w micro emulsion at the inversion locus. Short-chain surfactants form flexible monolayer at the o/w interface resulting in a discontinuous micro emulsion at the inversion.
Method of preparation of micro-emulsion based gel:
Step 1: Formulation of micro emulsion either O/W or W/O.
Step 2: Formulation of gel base.
Step 3: Incorporation of micro emulsion into gel base with continuous stirring.
Results and Discussion
Characterization
1. Physical appearance: The prepared micro-emulsion based gel formulations were inspected visually for their color, consistency and phase separation (Lachman L and Lieberman HA, 1990).
2. Compatibility studies by FTIR: Compatibility study of drug with the excipients was determined by FTIR Spectroscopy. Sample preparation involved mixing the sample with potassium bromide, triturating in glass mortar and finally placing in the sample holder. IR spectra of pure drug, drug-oil phase, drug surfactant, drug-co-surfactant, were taken. By this analysis, it was clear that there were no changes in the main peaks of drug in IR spectra, confirming no physical interactions between the excipients and the drug. If any changes, IR spectra conclude that any interaction (Vyas SP and Khar RK, 2002; Gennaro AR, 1995).
3. pH determination: A 10% dispersion of formulation was prepared in distilled water and pH was determined using pH meter which was prior standardized with standard buffers of pH 4 and pH 7.
4. Rheological studies: The viscosity of the different micro emulsion based gel formulations is determined at 25°C using a cone and plate viscometer with spindle 52, and connected to a thermostatically controlled circulating water bath (Ansel HC, et al., 1999).
5. Globule size and its distribution in micro emulsion gel: The average globule size of the micro emulsion was determined in triplicate by Zetasizer Nano ZS. Measurements were carried at an angle of 90°C at 25°C. Micro emulsion was diluted with double distilled water to ensure that the light scattering intensity was within the instrument’s sensitivity range. All the measurement was carried out at 25°C (IP, 1996).
6. Extrudability study of topical micro emulsion based gel: It is usual empirical test to measure the force required to extrude the material from the tube. The method adopted for evaluating micro emulsion based gel formulation for extrudability. And it is based upon the quantity in percentage of gel and gel extruded from aluminium collapsible tube on application of weight in grams required to extrude at least 0.5 cm ribbon of micro emulsion based gel in 10 seconds. More quantity extruded better is extrudability. The measurement of extrudability of each formulation is in triplicate and the average values are presented. The extrudability is then calculated by using the following formula.
Extrudability (gm/cm2)=(Applied weight to extrude micro emulsion based gel from the tube)/Area
7. Spreadability: The spreading co-efficient (Spreadability) of the formulations was determined using an apparatus described by Jain et al. The apparatus consisted of two glass slides (7.5 × 2.5 cm), one of which was fixed onto the wooden board and the other was movable, tied to a thread which passed over a pulley, carrying a weight. Formulation (1 g) was placed between the two glass slides. Weight (100 g) was allowed to rest on the upper slide for 1 to 2 minutes to expel the entrapped air between the slides and to provide a uniform film of the formulation. The weight was removed, and the top slide was subjected to a pull obtained by attaching 30 g weight over the pulley. The time (sec) required for moving slide to travel a premarked distance (6.5 cm) was noted and expressed as spreadability. Spreadability is calculated by using the following formula
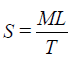
Where, M=Weight tied to upper slide
L=Length of glass slide
T=Time taken to separate the slides
8. Swelling index: To determine the swelling index of prepared topical micro emulsion based gel, 1 gm of gel is taken on porous aluminum foil and then placed separately in a 50 ml beaker containing 10 ml 0.1 N NaOH. Then samples were removed from beakers at different time intervals and put it in a dry place for some time after it reweighed. Swelling index is calculated as follows:
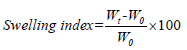
Where, (SW)%=Equilibrium percent swelling
Wt=Weight of swollen micro emulsion based gel after time t
Wo=Original weight of micro emulsion based gel at zero time
9. Drug content determination: Take 1 gm of micro emulsion based gel and mix it in a suitable solvent. Filter it to obtain clear solution. Determine its absorbance by using UV-Visible spectrophotometer. Standard plot of drug is prepared in the same solvent. Concentration and drug content can be determined by using the same standard plot by putting the value of absorbance. Drug content calculated as given formula:
Drug content=Concentration × Dilution factor × Volume taken × Conversion factor
10. Skin irritation test: The skin irritation study was conducted in accordance with the approval of the Animal Ethical Committee, using white male rabbits (n=3) as test animals. The hair of rabbits on dorsal side was shaved with electrical shaver and about 4 gm sample of the test article was then applied to each site (two site per rabbit) by introduction under a double gauze layer to an area of skin approximately 1” × 1” (2.54 × 2.54 cm2). The gellified emulsion is applied on the skin of rabbits. The animals were returned to their cages. After 24 hour exposure, the gellified emulsion is removed. The test sites were wiped with tap water to remove any residual gel. The development of erythema/edema was monitored for 3 days by visual observation.
11. Ex-vivo bioadhesive strength measurement of topical micro emulsion gel: (Mice shaven skin): The modified method is used for the measurement of bioadhesive strength. The fresh skin is cut into pieces and washed with 0.1 N NaOH. Two pieces of skin were tied to the two glass slides separately from that one glass slide is fixed on the wooden piece and another piece is tied with the balance on right hand side. The right and left pans were balanced by adding extra weight on the left-hand pan. 1 gm of topical micro emulsion based gel is placed between these two slides containing hairless skin pieces, and extra weight from the left pan is removed to sandwich the two pieces of skin and some pressure is applied to remove the presence of air. The balance is kept in this position for 5 minutes. Weight is added slowly at 200 mg/min to the left-hand pan until the patch detached from the skin surface. The weight (gram force) required to detach the micro emulsion based gel from the skin surface gave the measure of bioadhesive strength. The bioadhesive strength is calculated by using the following formula:
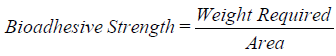
12. In-vitro release/permeation studies: The in-vitro permeation rates of prepared micro emulsion based gel were determined to evaluate the effects of the formulation factors. The diffusion experiments were performed using Franz diffusion cells fabricated locally with dialysis membrane pore size-0.2 mm at 37 ± 0.1°C. The beaker was filled with 200 ml of phosphate buffer pH 7.4 which acts as receptor fluid. The receptor fluid was constantly stirred by externally driven magnetic beads. Accurately 1 gm of micro emulsion based gel was placed in the cylindrical hollow tube one end of which sealed by dialysis membrane pore size-0.2 mm. It acts as donor compartment. The aliquots 10 ml were collected at suitable time intervals of 30 min up to 6 h. An equal volume of the fresh phosphate buffer was immediately replenished after each sampling. The sample was analyzed by UV-Visible spectrophotometer at a suitable wavelength after appropriate dilutions with suitable solvent. Cumulative corrections were made to obtain the total amount of drug release at each time interval.
13. Microbiological assay: Ditch plate technique was used. It is a technique used for evaluation of bacteriostatic or fungistatic acivity of a compound. It is mainly applied for semisolid formulations. Previously prepared Sabouraud’s agar dried plates were used. Three grams of the gellified emulsion are placed in a ditch cut in the plate. Freshly prepared culture loops are streaked across the agar at a right angle from the ditch to the edge of the plate. After incubation for 18 to 24 hours at 25°C, the fungal growth was observed and the percentage inhibition was measured as follows:
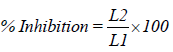
Where,
L1=total length of the streaked culture
L2=length of inhibition
14. Accelerated stability studies of gellified emulsion: Stability studies were performed according to ICH guidelines. The formulations were stored in hot air oven at 37 ± 2 ºC, 45 ± 2 ºC and 60 ± 2 ºC for a period of 3 months. The samples were analyzed for drug content every two weeks by UV-Visible spectrophotometer. Stability study was carried out by measuring the change in pH of gel at regular interval of time (Madan J, et al., 2011).
Conclusion
Microemulgel system evaluated and subjected for various tests proves to help to solubilize the lipophilic drug moiety showing rapid and efficient penetration into the skin. Thus micro-emulsions, which are optically isotropic and thermodynamically stable systems of water, oil, surfactant, and/ or co-surfactant, have been accepted as best drug delivery systems due to their capacity to solubilize poorly water soluble drugs as well as their enhancement of topical and systemic availability.
References
- Kalpesh AC, Paun JS, Soniwala MM, Chavada JR, Mori NB. Micro-emulsion based emulgel: A novel topical drug delivery system. Asian Pac J Trop Dis. 2014; 4(Suppl 1): S27-S32.
- Singh RP, Parpani S, Narke S, Chavan R. Emulgel: A recent approach for topical drug delivery system. Asian J Pharm Res Dev. 2014; 22: 112-23.
- Mayuresh R, Sachinkumar PV, Rukari TG. Emulgel: A modern tool for topical drug delivery. World J Pharm Res. 2019; 8(4): 586-594.
- Dhruti PM, Hemendrasinh RJ, Dhiren SP, Chainesh SN. A review on microemulsion based gel: A recent approach for topical drug delivery system. Res J Pharm and Tech. 2015; 8(2): 118-126.
- Rao YS, Deepthi KS, Chowdary KP. Micro-emulsions: A novel drug carrier system. Int J Drug Deliv Technol. 2009; 1(2): 39-41.
- Rashmi M, Garg, R, Kumar S, Gupta GD. Topical gel: A review. Canada: Official publication of Pharmainfo.net. 2013.
- Ansel HC, Allen LV, Popovich NG. Pharmaceutical dosage forms and drug delivery systems. 1999.
- Patel C, Tyagi S, Gupta A, Shrma P, Potdar M. Emulgel: A combination of emulsion and gel. Drug Discov Ther. 2013; 1(6): 57-61.
- Lee EA, Balakrishnan P, Song CK, Choi JH, Noh GY, Park CG, et al. Micro-emulsion-based hydrogel formulation of itraconazole for topical delivery. J Pharm Investig. 2010; 40(5): 305-311.
- Jain A, Gautam SP, Jain S. Development and characterization of ketoconazole microemulsion based gel for topical drug delivery. Der Pharmacia Sinica. 2010; 1(3): 221-231.
- Sushma G, Pravallika T, Sri BR, Priyanka P, Priya PV, Sharma JVC. Emulgels: A novel approach to topical drug delivery. Int J Pharm Sci Rev Res. 2013; 2(1): 134-148.
- Ansel HC, Allen LV, Popovich NG. Pharmaceutical dosage forms and drug delivery systems. Lippincott Williams and Wilkins. 1999.
- Indian pharmacopoeia commission Ghaziabad. Indian Pharmacopoeia (IP). 1996.
- Fadda MA, Blanco MJ, Baroli B, Quintela MAL, Charro MBD. Microemulsions for topical delivery of 8-methoxsalen. J Control Release. 2000; 69: 209-218.
[Crossref] [Google Scholar] [Pubmed]
- Shembale AI, Borale DK, Lohiya RT. Useful penetration enhancers for transdermal drug delivery: A review. Int J Pharm Res Dev. 2010; 2(5): 1-5.
- Conaghey OM, Corish J, Corrigan OI. Iontophoretically assisted in-vitro membrane transport of nicotine from a hydrogel containing ion exchange resin. Int J Pharm. 1998; 170: 225-237.
- Kulkarni RV, Sreedhar V, Mutalik S, Setty CM, Biswanath S. Interpenetrating network hydrogel membrane of sodium alginate and poly (vinyl alcohol) for controlled release of Prazocine hydrochloride through skin. Int J Biol Macromol. 2010; 47: 520-527.
[Crossref] [Google Scholar] [Pubmed]
- Nabi S, Shakeel F, Talegaonkar S. Formulation development and optimization using nanoemulsion technique: A technical note. AAPS Pharm Sci Tech. 2007; 8: 1-6.
[Crossref] [Google Scholar] [Pubmed]
- Kumar JS, Sanjay D, Roopa K. Micro emulsions potential carrier for improved drug delivery. Int Pharm Sci. 2011; 1(2): 25-31.
- Lachman L, Lieberman HA. The theory and practice of industrial pharmacy. Varghese Publishing house. 1990: 534.
- Vyas SP, Khar RK. Controlled drug delivery. Vallabh Prakashan. 2002: 416-417.
- Gennaro AR. Remington: The science and practice of pharmacy. Easton, Mack Publishing Company. 1995.
- Madan J, Dangi M, Banode S. Emulsion based drug delivery system. Indian J Nov Drug Deliv. 2011; 3(1): 28. [Crossref]
Author Info
Sagar Kasar*Citation: Kasar S: Micro-Emulgel Technique: A Novel Approach for Topical Delivery of Drug
Received: 30-Jun-2022 Accepted: 22-Jul-2022 Published: 29-Jul-2022, DOI: 10.31858/0975-8453.13.7.451-456
Copyright: This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
ARTICLE TOOLS
- Dental Development between Assisted Reproductive Therapy (Art) and Natural Conceived Children: A Comparative Pilot Study Norzaiti Mohd Kenali, Naimah Hasanah Mohd Fathil, Norbasyirah Bohari, Ahmad Faisal Ismail, Roszaman Ramli SRP. 2020; 11(1): 01-06 » doi: 10.5530/srp.2020.1.01
- Psychometric properties of the World Health Organization Quality of life instrument, short form: Validity in the Vietnamese healthcare context Trung Quang Vo*, Bao Tran Thuy Tran, Ngan Thuy Nguyen, Tram ThiHuyen Nguyen, Thuy Phan Chung Tran SRP. 2020; 11(1): 14-22 » doi: 10.5530/srp.2019.1.3
- A Review of Pharmacoeconomics: the key to “Healthcare for All” Hasamnis AA, Patil SS, Shaik Imam, Narendiran K SRP. 2019; 10(1): s40-s42 » doi: 10.5530/srp.2019.1s.21
- Deuterium Depleted Water as an Adjuvant in Treatment of Cancer Anton Syroeshkin, Olga Levitskaya, Elena Uspenskaya, Tatiana Pleteneva, Daria Romaykina, Daria Ermakova SRP. 2019; 10(1): 112-117 » doi: 10.5530/srp.2019.1.19
- Dental Development between Assisted Reproductive Therapy (Art) and Natural Conceived Children: A Comparative Pilot Study Norzaiti Mohd Kenali, Naimah Hasanah Mohd Fathil, Norbasyirah Bohari, Ahmad Faisal Ismail, Roszaman Ramli SRP. 2020; 11(1): 01-06 » doi: 10.5530/srp.2020.1.01
- Manilkara zapota (L.) Royen Fruit Peel: A Phytochemical and Pharmacological Review Karle Pravin P, Dhawale Shashikant C SRP. 2019; 10(1): 11-14 » doi: 0.5530/srp.2019.1.2
- Pharmacognostic and Phytopharmacological Overview on Bombax ceiba Pankaj Haribhau Chaudhary, Mukund Ganeshrao Tawar SRP. 2019; 10(1): 20-25 » doi: 10.5530/srp.2019.1.4
- A Review of Pharmacoeconomics: the key to “Healthcare for All” Hasamnis AA, Patil SS, Shaik Imam, Narendiran K SRP. 2019; 10(1): s40-s42 » doi: 10.5530/srp.2019.1s.21
- A Prospective Review on Phyto-Pharmacological Aspects of Andrographis paniculata Govindraj Akilandeswari, Arumugam Vijaya Anand, Palanisamy Sampathkumar, Puthamohan Vinayaga Moorthi, Basavaraju Preethi SRP. 2019; 10(1): 15-19 » doi: 10.5530/srp.2019.1.3