Research Article - (2024) Volume 15, Issue 10
Modulating Starch Digestibility in Achieving Glycemic Control
Isiosio UE1* and Omoregie E2Abstract
Starch digestibility is an important indicator of the glycemic response of foods. It can be influenced by processing methods, such as the degree of gelatinization. This study examines the effect of gelatinization on starch digestibility as a measure of regulating glycemic response in vivo. Two commonly consumed foods in the southern part of Nigeria, Ugba Salad Ingredients (USI) and Ogi Koko Pudding and Akara (OKPA), were investigated for their Rapidly Digestible Starch (RDS), Slowly Digestible Starch (SDS) and Resistant Starch (RS) content at varying degrees of gelatinization. Based on the gelatinization properties low and high degree of gelatinization, two meals were prepared from each food sample.
The Glycemic Index (GI) of the low gelatinized USI sample was not significantly different p<0.05 from that of the high gelatinized sample. However, the GI of the low gelatinized OKPA was significantly lower p<0.05 than that of the high gelatinized sample. This data suggests that the degree of gelatinization is not a primary determinant of the high GI of USI.
Keywords
Gelatinization, Resistant Starch (RS), Starch digestibility, Glucometer, Rapidly Digestible Starch (RDS)
Introduction
Digestive properties have been utilized for the classification of carbohydrates in foods (Englyst HN, et al., 1992). Based on these characteristics, starches are categorized as rapidly digestible carbohydrates, slowly digestible carbohydrates and resistant starch. Rapidly digestible carbohydrates undergo hydrolysis within thirty minutes of in vitro digestion, whereas slowly digestible carbohydrates are hydrolyzed within 120 minutes (Rosin PM, et al., 2002). Resistant starch is not digested in the small intestines and instead undergoes fermentation to produce short-chain fatty acids (Lunn J and Buttriss JL, 2007; Dhital S, et al., 2010). (Nils Georg ASP, 1992) defined resistant starch as the aggregate of starch and its derivatives that evade absorption in the small intestine of healthy individuals due to physical in accessibility. This inaccessibility may arise from being enclosed within the food matrix RS1, encapsulated within starch granules RS2, or existing as retrograded starch RS3, which is generated during food preparation and manufacturing. Additionally, starch can undergo chemical modifications RS4 to render it impervious to enzymatic breakdown (Bindels LB, et al., 2015).
Consumption of foods with low levels of resistant starch and slowly digestible starch has been linked to an increase in metabolic disorders; thus, the ability to regulate the absorption of postprandial glucose is important in mitigating these complications (Bindels LB, et al., 2015).
The GI is used to regulate postprandial glucose levels by comparing the rate of glucose release from foods to a standard, typically glucose or white bread (Jenkins DJ, et al., 1981). The nature, processing method and granular properties of starch all play a role in determining the glycemic index. Diets rich in SDS and RS are associated with low GI foods (Onipe OO, et al.,2020; Englyst HN, et al., 1992).
Some research has reported that the content of resistant starch and slowly digestible starch can be altered by processing methods (Ludwig DS, 2003). Prolonged cooking of foods elevates the glycemic index; for example, Ludwig DS, 2003 noted that pasta cooked to a firm texture exhibits a lower GI compared to pasta that is cooked for an extended duration. This phenomenon is likely attributed to the extent of starch gelatinization. A heightened level of starch gelatinization has been linked to increased digestibility and higher GI values (Widanagamage RD, et al., 2013).
As the concept of GI gains traction in Nigeria, with local foods' glycemic indices being determined (Onimawo IA, et al., 2007; Osagie AU and Omoregie ES, 2011; Onipe OO, et al., 2020), there is a need to modify processing methods to reduce the glycemic indices of foods. This study examines the effect of degree of gelatinization on starch digestibility as a measure of regulating glycemia in vivo, using two commonly eaten foods: USI and OKPA.
Materials and Methods
Preparation of food samples
Cassava and bambara groundnut were purchased from Edjeovyanre Market in Oghara and Nsukka Market in Delta and Enugu states. The food samples were processed by a trained cook into commonly eaten forms, with the processing methods summarized in Table 1. The food samples were prepared under controlled temperatures to determine the degree of gelatinization and in vitro starch digestibility.
| Processed forms | Pre-processing | Processing methods |
|---|---|---|
| Usi | Cassava tubers were peeled, grinded, filtered and allowed to sediment | Sediment mixed with water and palm oil and heated while stirring in a saucepan till it gelatinizes |
| Okpa | Bambara groundnut seeds were oven dried at 60℃, removed and then grinded | Flour mixed with water, tomatoes, pepper, salt, palm oil, seasoning is added and paste is steamed in aluminium cups. |
Table 1: Processing and preparation of food samples.
Degree of gelatinization and in vitro starch digestibility
To assess varying degrees of gelatinization, suspensions of food samples 100 mg/mL were subjected to heating at temperatures ranging from 45°C to 100°C for durations between fifteen and 60 minutes. Changes in the moisture content of USI and OKPA during processing were monitored and the water-to-starch ratio of the food samples was analyzed to assess alterations in water content due to processing temperature.
In vitro starch digestibility
In vitro starch digestibility was determined according to the methodology described by (Kasote DM, et al., 2014). Fifty milligrams of food samples were incubated with 10 milliliters of HCl-KCl buffer pH 1.5 and 0.2 milliliters of 10% pepsin at 40°C for 1 hour with continuous agitation. The pH was then adjusted by bringing the volume to 25 milliliters with Tris-maleate buffer pH 6.9. Subsequently, 5 milliliters of pancreatic α-amylase solution 1 mg/5 mL in Tris-maleate buffer was introduced to the reaction mixture, which was allowed to incubate at 37°C with continuous agitation for 30 minutes. Samples of 1 milliliter were extracted at 30 minutes and 120 minutes from the reaction mixture and promptly immersed in boiling water for 5 minutes to halt the enzymatic reaction. Following this, each aliquot was treated with 1 milliliter of 0.4 M sodium acetate buffer pH 4.75 and 60 μl of amyloglucosidase 3,300 U/mL. The reaction mixture was then incubated at 60°C for 45 minutes with continuous agitation. The glucose released was quantified using a GOD/POD reagent assay kit and starch content was calculated as glucose mg % × 0.9. Hydrolysis at 30 and 120 minutes indicated the RDS and SDS, respectively.
RDS % =(G30×0.9 / W) ×100
SDS % =((G180 - G30) ×0.9 / W) ×100
Determination of resistant and total starch
Resistant starch, digestible starch and total starch content were determined according to the method by (McCleary BV and Monaghan DA, 2002) using a commercially available kit from Magazine International Ireland Ltd., Bray, Co. Wicklow, Ireland.
Serving sizes
Total starch was determined by (McCleary BV and Monaghan DA, 2002). Portions providing 50 g of carbohydrates were used for the feeding experiment.
Experimental design
Twenty subjects, consisting of twelve males and eight females, were selected from Western Delta University, Oghara, for the study. Inclusion criteria required that subjects be aged 18 years or older, healthy, non-obese, non-diabetic and without a family history of diabetes. All protocols for the experiment were approved by the ethical committee of western delta university, Oghara. Participants were invited for feeding on three days within a 2-week period. On test days, participants arrived at the laboratory at 8:00 AM after a fast of 10-12 hours. Details of the research were explained to the subjects, who then provided their consent. Measurements of height and weight were obtained and Body Mass Index (BMI) was calculated kg/m². Subjects were fed serving sizes of foods USI or OKPA providing 50 g of carbohydrates with different degrees of gelatinization on two occasions. On a different day, subjects were also fed 50 g of glucose.
Collection of capillary blood samples
Capillary blood samples were collected via finger prick from groups Gr, Go and Gs. Readings were taken at baseline (0), as well as at 15 minutes, 30 minutes, 45 minutes, 60 minutes and 120 minutes. A drop of blood from each sample was analyzed using a glucometer Accu-Chek Active Roche Diagnostic Laboratories.
Determination of glycemic index
The GI was determined as described by Jenkins DJ, et al., 1981. The increase in area under the curve for test food was divided by the increase in area under the curve for reference food and multiplied by 100.
Statistical analysis
Analyses were carried out in triplicates and results were expressed as mean ± standard deviation. Analysis of Variance (ANOVA) was used to determine significant variations among the samples, with values considered significantly different at P ≤ 0.05. Statistical analysis was performed using graphpad prism version 10 GraphPad Software Inc., USA.
Results and Discussion
The total and resistant starch content of USI and OKPA are presented in Table 2. The resistant starch content of uncooked USI was the highest among the food samples p<0.05. Processing of USI and OKPA resulted in significant p<0.05 differences between raw USI (US) and cooked USI as well as between raw OKPA and cooked OKPA. These findings align with various studies indicating that alterations in resistant starch content occur due to processing techniques (Kasote DM, et al., 2014).
| Total starch g/100 g | Digestible starch g/100 g | Resistant starch g/100 g | |
|---|---|---|---|
| US* | 85.02 ± 2.00 | 77.45 ± 1.52 | 7.56 ± 0.23 |
| Us | 86.99 ± 2.45 | 77.73 ± 1.87 | 10.26 ± 0.68 |
| Ok | 54.75 ± 2.35 | 49.45 ± 1.84 | 5.30 ± 0.78 |
| OK* | 61.02 ± 1.72 | 44.48 ± 1.02 | 6.54 ± 0.45 |
Note: Results are expressed as US*=Ugba Salad, OK*=Ogi Koko
Table 2: Total and resistant starch in cooked Usi and Okpa.
The reduction in resistant starch may be attributed to increased gelatinization, which renders the starch more susceptible to digestion by α-amylase. Additionally, the removal of seed coats from legumes has been proposed to diminish the RSI content of legumes (Isiosio UE, et al., 2015; Englyst HN, et al., 1992).
Starch digestibility
The values of in vitro starch digestibility for OKPA and USI at varying temperatures are presented in Table 3. The results revealed that an increase in the degree of gelatinization and time resulted in increased digestibility, which is consistent with findings.
| Sample | 45℃ | 50℃ | 60℃ | 75℃ | 100℃ | |
|---|---|---|---|---|---|---|
| Okpa | RDS | 21.08 ± 2.66 | 25.92 ± 4.02 | 29.60 ± 4.06 | 29.62 ± 1.59 | 30.14 ± 3.02 |
| SDS | 28.37 ± 1.51 | 23.53 ± 4.10 | 19.85 ± 0.16 | 19.83 ± 0.04 | 19.31 ± 0.57 | |
| Usi | RDS | 69.85 ± 6. 01 | 70.39 ± 6.11 | 73.42 ± 1.54 | 75.63 ± 4.9 | 69.49 ± 6.33 |
| SDS | 7.60 ± 1.42 | 7.06 ± 2.10 | 4.03 ± 0.94 | 1.82 ± 0.48 | 1.42 ± 0.30 |
Table 3: In vitro starch digestibility of Usi and Okpa g/100 g of processed samples.
The hydrolysis of starch in OKPA was temperature dependent, with the lowest RDS values recorded at 45°C and the highest at 100°C. The optimum degree of gelatinization for USI was found to be between 60°C and 75°C, where RDS reached its maximum. This result supports the common practice of cooking USI at mild temperatures. However, heating at temperatures above 75°C caused a reduction in RDS values, suggesting the formation of resistant starch (RS3) (Bennion M and ScheuleB, 2000).
(Magallanes PA, et al., 2017) suggested that the gelatinization and subsequent cooling of starch could lead to an increase in resistant starch due to the development of retrograded starch RS3 at temperatures higher than 75°C. OKPA starch exhibits a high gelatinization temperature, which supports the practice of cooking OKPA using steam. The higher gelatinization temperature for OKPA starch may be attributed to its more rigid granular structure (Singh N, et al., 2003). Another probable reason for this may be the high fat and protein content of OKPA; previous studies have confirmed that OKPA meals contain significant amounts of fat and protein. As noted by (Bennion M and ScheuleB, 2000), high fat and protein content can delay starch gelatinization.
Additionally, OKPA has a high SDS content, which aligns with results reported by (Sandhu KS and Lim ST, et al., 2008), indicating a high SDS content in legumes. Analyses were carried out in triplicates and values are expressed as mean ± Standard Error of the Mean (SEM).
The portion sizes of USI and OKPA meals administered to participants are detailed in Table 4. The serving size of USI 143.9 g, which contained 50 g of carbohydrates with a moisture content of 56.02 g/100 g, was designated as the low gelatinized USI sample (Figure 1). This particular sample exhibited a reduced moisture content and was prepared under mild heat conditions ranging from 45°C to 59°C. As reported by (Donmez D, et al., 2021), the presence of water and the application of specific temperatures are recognized factors that contribute to the degree of gelatinization in food products with higher moisture levels, temperature plays an important role in ensuring complete gelatinization of the starch present in the food items; hence, at higher temperatures, starch in USI and OKPA is believed to be completely gelatinized.
| Samples | Moisture content (g/100 g) | 50 grams of available carbohydrates |
|---|---|---|
| Usi (low gelatinized) | 56.02 ± 0.05 | 143.9 g |
| Usi (Highly gelatinized) | 70.45 ± 0.54 | 199.1 g |
| Okpa (low gelatinized) | 54.25 ± 0.11 | 203.7 g |
| Okpa (Highly gelatinized) | 70.50 ± 0.23 | 264.7 g |
Table 4: Serving size of food.
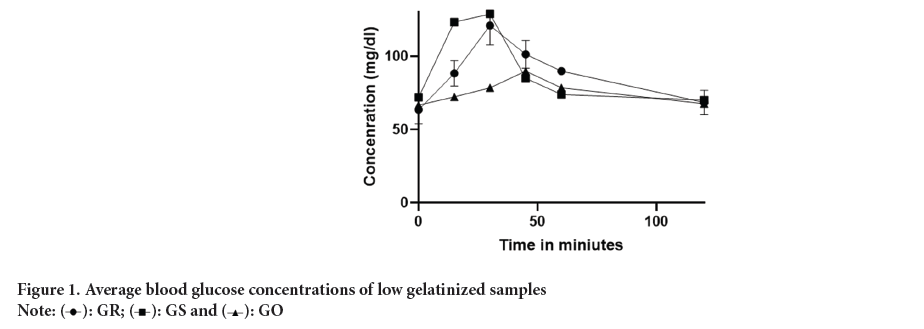
Figure 1: Average blood glucose concentrations of low gelatinized samples.
 .
.
The average glucose response of subjects fed on USI and OKPA diets, providing 50 g of available carbohydrates, is shown in Table 5. The glucose response for all groups peaked between 30 minutes and 60 minutes, after which there was a gradual decline in average glucose concentration. The results of the glycemic indices revealed that USI is a high GI food 93.4 and 95.9, which agrees with findings by (Osagie AU and Omoregie ES, 2011). As shown in Table 2, USI consists mainly of RDS thus, its high GI may again be attributed to this property. Several reports have indicated that alterations in the carbohydrate structure of food through gelatinization make it more susceptible to amylase digestion, resulting in an increased GI (Jimoh AK, et al., 1981). There was no significant p<0.05 difference in glycemic indices between low gelatinized and high gelatinized samples, suggesting that other factors are responsible for its glycemic properties (Figure 2 and Table 6).

Figure 2: Average blood glucose concentrations of highly gelatinized samples.
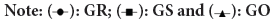 .
.
| Subjects | 0 mins | 15 mins | 30 mins | 45 mins | 60 mins | 120 mins |
|---|---|---|---|---|---|---|
| GR | 63.3 ± 9.5 | 88.2 ± 8.7 | 120.8 ± 13.1 | 101.2 ± 9.5 | 89.8 ± 2.0 | 68.4 ± 8.2 |
| Gs | 71.9 ± 9.4 | 123.2 ± 4.6 | 128.8 ± 5.0 | 84.9 ± 3.4 | 73.8 ± 6.6 | 70.0 ± 7.8 |
| Go | 66.6 ± 4.7 | 72.2 ± 3.0 | 84.6 ± 3.0 | 89.8 ± 3.8 | 78.4 ± 5.3 | 67.6 ± 1.4 |
Note: Results are expressed as Mean ± SEM n=20, GR= Blood glucose concentrations for reference food, GO= Blood glucose concentrations for Okpa meal. GS=Blood glucose concentrations for Usi meal
Table 5: Average glucose concentrations (mg/dl) of subjects fed with low gelatinized samples.
| Subjects | 0 mins | 15 mins | 30 mins | 45 mins | 60 mins | 120 mins |
|---|---|---|---|---|---|---|
| GR | 63.3 ± 9.5 | 88.2 ± 8.7 | 120.8 ± 13.1 | 101.2 ± 9.5 | 89.8 ± 2.0 | 68.4 ± 8.2 |
| Gs | 68.5 ± 3.2 | 82.7 ± 6.3 | 95.3 ± 3.5 | 90.1 ± 4.5 | 87.3 ± 2.2 | 78.8 ± 2.2 |
| Go | 72.2.2 ± 5.6 | 110.5± 2.4 | 122.3± 3.2 | 125.8±7.4 | 81.5±5.1 | 70.0±4.6 |
Note: Results are expressed as Mean ± SEM n=20, GR= Blood glucose concentrations for reference food, GO= Blood glucose concentrations for Okpa meal. GS=Blood glucose concentrations for Usi meal
Table 6: Average glucose concentrations (mg/dl) of subjects fed with high gelatinized samples.
In contrast, the GI of OKPA 37.9 and 44.8 is considered low, which may be attributed to the presence of SDS. This finding contrasts with results reported by (Onimawo IA, et al., 2007), where OKPA was associated with a higher glycemic index. The differences in GI reported in this study compared to Onimawo IA, et al., 2007 might be due to the different reference foods used; for instance, white bread was used as the reference food in their study while this research used glucose as its reference diet. A similar argument has been put forward by (Brand MJ and Foster PK, 2005), which reported a higher GI with white bread (Table 7).
| Samples | Low gelatinization | High gelatinization |
|---|---|---|
| Okpa | 37.9 ± 1.8 | 44.8 ± 0.9 |
| Usi | 93.4 ± 2.4 | 95.9 ± 1.2 |
Table 7: Glycemic indices of samples with different degree of gelatinization.
Conclusion
The degree of gelatinization does not affect the GI of USI; however, this factor can be utilized in the preparation of OKPA to further reduce its GI. OKPA meals can be beneficial in the dietary management of diabetics.
Ethical Committee Consent
This study is exempt (Protocol number IRB22-0196) while the Ethical committee of Western Delta University, Oghara gave ethical approval (approval number: HREC/PAN/2021/008/0410)
References
- Englyst HN, Kingman SM, Cummings JH. Classification and measurement of nutritionally important starch fractions. Eur J ClinNutr. 1992; 46: 33-50.
[Google Scholar] [PubMed]
- Rosin PM, Lajolo FM, Menezes EW. Measurement and characterization of dietary starches. J Food Compos Anal. 2002; 15(4): 367-377.
- Lunn J, Buttriss JL. Carbohydrates and dietary fiber. Nutrition Bulletin. 2007; 32(1): 21-64.
- Dhital S, Katawal SB, Shrestha AK. Formation of resistant starch during processing and storage of instant noodles. Int J Food Prop. 2010; 13(3): 454-463.
- Nils Georg ASP. Resistant starch proceeding from the second plenary meeting of EURESTA: European FLAIR concerted action no. 11 on physiological implications of the consumption of resistant starch in man. Eur J Clin Nutr. 1992; 46: 1-148.
[Google Scholar] [PubMed]
- Bindels LB, Walter J, Ramer Tait AE. Resistant starches for the management of metabolic diseases. Curr Opin Clin Nutr Metab Care. 2015; 18(6): 559-565.
[Crossref] [Google Scholar] [PubMed]
- Jenkins DJ, Wolever TM, Taylor RH, Barker H, Fielden H, Baldwin JM, et al. Glycemic index of foods: A physiological basis for carbohydrate exchange. Am J Clin Nutr. 1981; 34(3): 362-366.
- Onipe OO, Beswa D, Jideani AI. In vitro starch digestibility and glycemic index of fried dough and batter enriched with wheat and oat bran. Foods. 2020; 9(10): 1374.
[Crossref] [Google Scholar] [PubMed]
- Ludwig DS. Dietary glycemic index and the regulation of body weight. Lipids. 2003; 38(2): 117-121.
[Crossref] [Google Scholar] [PubMed]
- Widanagamage RD, Ekanayake S, Welihinda J. Effect of extent of gelatinization of starch on the glycemic responses of carbohydrate rich breakfast meals. Mal J Nutr. 2013.
- Onimawo IA, Ijeh I, Ukoha U, Nwachukwu GT. Determination of the glycemic index of steamed cakes using two different legumes bambara nut Vigna subterranean and cowpea Vigna unguiculata. Afr J Biochem Res. 2007; 1(7): 142-147.
- Osagie AU, Omoregie ES. The Nigeria high glycemic index starchy foods, obesity and the environment. Nig Q J Hosp Med. 2011; 21(4): 290-293. [Crossref]
[Google Scholar] [PubMed]
- Kasote DM, Nilegaonkar SS, Agte VV. Effect of different processing methods on resistant starch content and in vitro starch digestibility of some common Indian pulses. 2014.
- McCleary BV, Monaghan DA. Measurement of resistant starch. J AOAC Int. 2002; 85(3): 665-675.
[Crossref] [Google Scholar] [PubMed]
- Jenkins DJ, Wolever TM, Taylor RH, Barker H, Fielden H, Baldwin JM, et al. Glycemic index of foods: A physiological basis for carbohydrate exchange. Am J Clin Nutr. 1981; 34(3): 362-366.
- Isiosio UE, Isiosio IO, Emudianughe PT. Resistant starch and in vitro starch digestibility of five Nigerian foods. J Nat Sci. 2015; 5: 131-135.
- Bennion M, Scheule B. Introductory Foods 11th Edition. 2000.
- Magallanes PA, Flores PC, Bello LA. Starch structure influences its digestibility: A review. J Food Sci. 2017; 82(9): 2016-2023.
[Crossref] [Google Scholar] [PubMed]
- Singh N, Singh J, Kaur L, Sodhi NS, Gill BS. Morphological, thermal and rheological properties of starches from different botanical sources. Food Chem. 2003; 81(2): 219-231.
- Sandhu KS, Lim ST. Digestibility of legume starches as influenced by their physical and structural properties. Carbohydr Polym. 2008; 71(2): 245-252.
- Donmez D, Pinho L, Patel B, Desam P, Campanella OH. Characterization of starch-water interactions and their effects on two key functional properties: Starch gelatinization and retro gradation. Curr Opin Food Sci. 2021; 39: 103-109.
- Jimoh AK, Adediran OS, Adebisi SA, Biliaminu SA, Okesina AB. Effect of food processing on glycemic response to white yam Dioscorea rotunda meals. Am J Clin Nutr. 1981; 34(3): 362-366.
[Crossref] [Google Scholar] [PubMed]
- Onimawo IA, Ijeh I, Ukoha U, Nwachukwu GT. Determination of the glycemic index of steamed cakes using two different legumes bambara nut Vigna subterranean and cowpea Vigna unguiculata. Afr J Biochem Res. 2007; 1(7): 142-147.
- Brand MJ, Foster PK. The new glucose revolution low GI guide to diabetes: The only authoritative guide to managing diabetes using the glycemic index. Da Capo Press. 2005.
Author Info
Isiosio UE1* and Omoregie E22Department of Biochemistry, Faculty of Life Sciences, University of Benin, Benin City, Edo State, Nigeria
Citation: Isiosio UE, et al.: Modulating Starch Digestibility in Achieving Glycemic Control
Received: 04-Oct-2024 Accepted: 24-Oct-2024 Published: 31-Oct-2024, DOI: 10.31858/0975-8453.15.10.326-330
Copyright: This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
ARTICLE TOOLS
- Dental Development between Assisted Reproductive Therapy (Art) and Natural Conceived Children: A Comparative Pilot Study Norzaiti Mohd Kenali, Naimah Hasanah Mohd Fathil, Norbasyirah Bohari, Ahmad Faisal Ismail, Roszaman Ramli SRP. 2020; 11(1): 01-06 » doi: 10.5530/srp.2020.1.01
- Psychometric properties of the World Health Organization Quality of life instrument, short form: Validity in the Vietnamese healthcare context Trung Quang Vo*, Bao Tran Thuy Tran, Ngan Thuy Nguyen, Tram ThiHuyen Nguyen, Thuy Phan Chung Tran SRP. 2020; 11(1): 14-22 » doi: 10.5530/srp.2019.1.3
- A Review of Pharmacoeconomics: the key to “Healthcare for All” Hasamnis AA, Patil SS, Shaik Imam, Narendiran K SRP. 2019; 10(1): s40-s42 » doi: 10.5530/srp.2019.1s.21
- Deuterium Depleted Water as an Adjuvant in Treatment of Cancer Anton Syroeshkin, Olga Levitskaya, Elena Uspenskaya, Tatiana Pleteneva, Daria Romaykina, Daria Ermakova SRP. 2019; 10(1): 112-117 » doi: 10.5530/srp.2019.1.19
- Dental Development between Assisted Reproductive Therapy (Art) and Natural Conceived Children: A Comparative Pilot Study Norzaiti Mohd Kenali, Naimah Hasanah Mohd Fathil, Norbasyirah Bohari, Ahmad Faisal Ismail, Roszaman Ramli SRP. 2020; 11(1): 01-06 » doi: 10.5530/srp.2020.1.01
- Manilkara zapota (L.) Royen Fruit Peel: A Phytochemical and Pharmacological Review Karle Pravin P, Dhawale Shashikant C SRP. 2019; 10(1): 11-14 » doi: 0.5530/srp.2019.1.2
- Pharmacognostic and Phytopharmacological Overview on Bombax ceiba Pankaj Haribhau Chaudhary, Mukund Ganeshrao Tawar SRP. 2019; 10(1): 20-25 » doi: 10.5530/srp.2019.1.4
- A Review of Pharmacoeconomics: the key to “Healthcare for All” Hasamnis AA, Patil SS, Shaik Imam, Narendiran K SRP. 2019; 10(1): s40-s42 » doi: 10.5530/srp.2019.1s.21
- A Prospective Review on Phyto-Pharmacological Aspects of Andrographis paniculata Govindraj Akilandeswari, Arumugam Vijaya Anand, Palanisamy Sampathkumar, Puthamohan Vinayaga Moorthi, Basavaraju Preethi SRP. 2019; 10(1): 15-19 » doi: 10.5530/srp.2019.1.3






