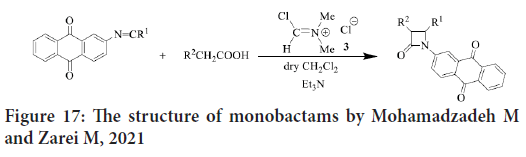Review - (2022) Volume 13, Issue 3
Monobactams as Anticancers: A Review Study
Ahmed AJ Mahmood*Abstract
Cancer is a heterogeneous disease which categories as the second-leading cause of death worldwide. It is fatal to 42% of diagnosed patient every year. The ideal anticancer agents needed to be powerful, well tolerated in patients, with low side effect and high selectivity. Antibiotics on the other hand are efficient, well tolerated, with very low level of toxicity. Therefore, they continue to be the most important goal for new drugs invention. 2-Azetidinones (β-lactams) are most marketing drugs than any other antibiotics. The β-lactams provide unlimited choices of biological activities, and they efficaciously used as anticancer agent’s delivery system directly to the tumor sites as pro-drugs. These records indicate that the designation and synthesis of new monobactams will be favorable zone for improvement anticancer studying researches.
Keywords
Monobactams, β-lactams, 2-Azetidinones, Anticancer
Introduction
Cancer is a mixed disease and considered as the major threats for human health in 20th and 21th century, it classified as the 2nd trigger of death worldwide. Cancer described as the malignant cell progression that at the last leads to change the regular physiological activities (Kuhn D, et al., 2004). Antimalignant agent investigation concentrates on reducing tumor cell growing rate and initiation of apoptosis in malignant cells. Programmed cellular death (apoptosis) is described as the capability of a cell to undertake a step-by-step self-suicide program without disturbing adjacent cells (Kuhn D, et al., 2004; Dong J, et al., 2019). Initiation of such apoptotic action in malignant cells is very necessary in cancer management process (Dong J, et al., 2019).
The percentage of death in cancer diagnosed patient are high and it approximately about 42%. There for there is an urgent need to introduce new and safe anticancer agents, and this consider as an objective for medical research (Sharipova RR, et al., 2019). However, nowadays the cancer treatments are almost as hazardous as the disease itself caused by incapability of the currently existing chemotherapeutic agents to target the cancer cells away from normal cells (Vento S and Cainelli F, 2003). Another drawback of cancer treatments is the critical unwanted effects of the anticancer agents as they weakening the immunity system cause by neutrophil cells decline (Vento S and Cainelli F, 2003; Tikhomirov AS, et al., 2018). The ideal anticancer agents needed to be powerful, well tolerated in patients, with low side effect and high tumor cells selectivity.
The innovation of natural and synthetic antibiotics is one of the most significant medical discoveries in human history (Mehta PD, et al., 2010). Antibiotics are efficient, mostly well tolerated in patients, with very low level of toxicity. This is why the antibiotics continue to be the main aim for drug invention (Basu S and Banik BK, 2017). 2-Azetidinones (β-lactams) are the most famous antibacterial compounds and they are most marketing antibiotics drugs (Basu S and Banik BK, 2017; Zhou P, et al., 2018). The β-Lactams category including penicillins, cephalosporins, carbapenems, and monobactams, all with a rigid ring structure (Figure 1) (Zhou P, et al., 2018).
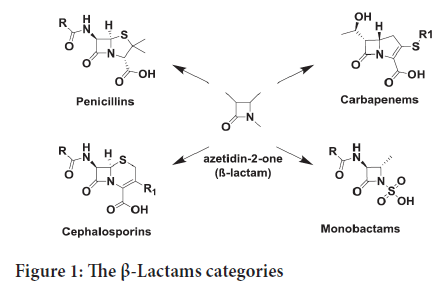
Figure 1: The β-Lactams categories
Their antimicrobial action is a result of inhibiting the peptidoglycan enzyme and thus inducing cell wall damage (Borazjani N, et al., 2019). Around year 1981, couple of teams belongs to the Squibbs and Takeda Laboratories define and isolate the initial N-thiolated monobactams from natural resources. These monobactams were the first with the N-sulfonic acid substituent that attached directly to the nitrogen atom. They possess a flexible monocyclic structure.
Literature Review
Nowadays, β-lactam compounds offer an extensive range of biological activities (Hammersley D and Signy M, 2017). The new drug with monobactam moiety Ezetimibe selectively inhibits intestinal cholesterol absorption (Hammersley D and Signy M, 2017; Chen D, et al., 2008). Moreover, many studies indicate that several antibiotics are qualified to inhibit the growth of tumor cells. In addition to many antitumor antibiotics approved nowadays for cancer treatment, they interacted with DNA leading to inhibit tumor cell growth (Chen D, et al., 2008; Tripodi F, et al., 2018). Recently, a new class of N-thiolated monobactams was used to prompt apoptosis powerfully in specific manner in many lines of tumor cells but they do not do so in non-transformed cell lines (Tripodi F, et al., 2018; Banik I, et al., 2003). These records indicate that the designation and synthesis of new monobactams will be favorable zone for improvement anticancer researches (Banik I, et al., 2003).
The monobactams structural moiety has attracted a great deal of attention owing to their extensive biological activities (Trivedi AR, et al., 2012; Pathak RB, et al., 2012; Kumar A, et al., 2007). In addition, many research indicating that these monobactams used in efficaciously manner as pro- drugs to deliver the anticancer agents directly to the tumor sites (Ojima I and Delaloge F, 1997; Hodge M, et al., 2009; Mahmood AA, et al., 2021).
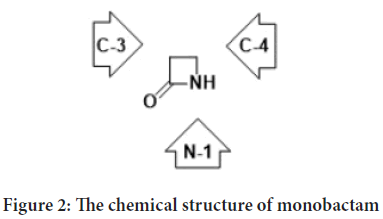
Monobactam is a four-membered cyclic amide, at the second position of the ring there is an oxo group, with various substitutions on the amide nitrogen (N-1), on the carbon neighboring to the carbonyl group (C-3), and on the carbon next to the nitrogen (C-4) (Figure 2) (Kuhn D, et al., 2004; El-Shorbagi AN and Chaudhary S, 2019).
Figure 2: The chemical structure of monobactam
The monobactams reactivity can control, by varying the character of the electron withdrawing substituent (Kuhn D, et al., 2004). From the mechanistic view, monobactam moiety acts as an electrophilic nucleus targeting serine-containing enzymes (El-Shorbagi AN and Chaudhary S, 2019).
Monocyclic β-lactams were diagnosed to have an anti-mycobacterial, anti-protozoal, anti-fungal, anti-HIV, anti-inflammatory, anti-tumor, anti-parkinsonianic, anti-diabetic and anti-depressant effects. They have been reported as cholesterol absorption inhibitors, vasopressin V1a antagonists, tryptase, chymase and thrombin inhibitors, human leukocyte elastase and carbonic anhydrase inhibitors, N-acytlethanolamine acid amidase inhibitors and also inhibitors of dengue and West Nile virus NS2B-NS3 protease (Trivedi AR, et al., 2012; Pathak RB, et al., 2012; Kumar A, et al., 2007; Jain AK, et al., 2012; Galletti P and Giacomini D, 2011; Decuyper L, et al., 2018; Dražić T, et al., 2019).
In recent years Tripodi F, et al. mention the ant malignant effect of 1,4-Diaryl-2-azetidinones caused by the stimulation of Adenosine Monophosphate Activated Protein Kinase (AMPK) leading to initiate an apoptotic response in colorectal cancer cell lines (Tripodi F, et al., 2012; Valtorta S, et al., 2014). Also they indicated these monobactams specifically for the Small Bowel Adenocarcinoma (SBA) and also for the Colorectal Cancer Diseases (CRC), as they all sharing comparable pathophysiology (Koch V, et al., 2019; Carr M, et al., 2010). In another study 1, 4-diarylazetidin-2-ones were present potent antiproliferative action against MCF-7 (Michigan Cancer Foundation-7), MDA-MB-231 (51-year-old caucasian female with a metastatic mammary adenocarcinoma) human breast carcinoma cell lines (Koch V, et al., 2019; Banik BK, 2014; Chavan AA and Pai NR, 2007). Other monobactams act as effective antimalignant agents as they provoke DNA destruction and inhibit the replication of the Jurkat T cells DNA (Kamath A and Ojima I, 2012).
Synthesis of monobactams
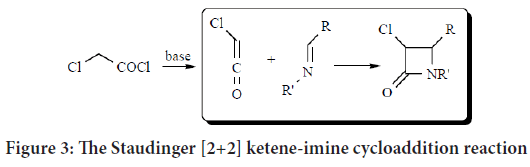
2-azetidinone (monobactams) compounds are synthesized according to [2+2] ketene-imine cycloaddition (Staudinger cycloaddition reaction) (Tanaka T, et al., 2007). In 1907, this reaction was first reported and defined as a [2+2] cycloaddition involving both a ketene (formed from acid chloride) and suitably substituted imines under basic conditions. This reaction stays as the most commonly used method for its flexibility and efficiency. The required imines were prepared by the reaction of the required substituted benzaldehydes and the aromatic amines (Figure 3) (Tanaka T, et al., 2007; Mahmood AA, 2021; Singh GS, 2003; Lima LM, et al., 2020; Söderström TS, et al., 2002).
Figure 3: The Staudinger [2+2] ketene-imine cycloaddition reaction
Monobactams as anticancers
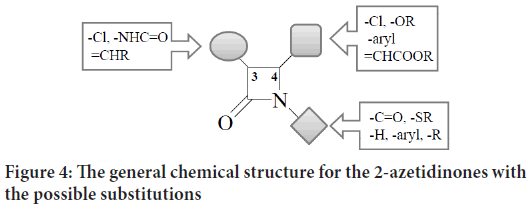
The general chemical structure for the 2-azetidinones with the possible substitutions on each monobactam corner is clarified in Figure 4:
Figure 4: The general chemical structure for the 2-azetidinones with the possible substitutions
In early 2000, some studies indicate that the N-thiolated β-lactams capable to induce apoptotic reaction in tumor cell by causing DNA destruction and without affecting the normal body cells (Kazi A, et al., 2004; Cainelli G, et al., 2003). They found that the N-methyl-thio group is very important for such apoptosis-inducing activity (Cainelli G, et al., 2003). Another study indicates that 4-alkylidene-β-lactams could be as inhibiter for matrix Metalloproteinases-2, and Metalloproteinases-9 (MMP) (Chin JR, et al., 1985), both were a classes of mammalian proteases, which playing a very critical role in cancer propagation by promoting neovascularization needed for tumor growing (Hojilla CV, et al., 2003). MMPs activated specifically in malignant cells, and not in normal cells (Chin JR, et al., 1985; Wilhelm SM, et al., 1987; Malig TC, et al., 2018).
Banik I, et al. described a class of poly aromatic imine β-lactams (the 4-alkylideneazetidin-2-ones). Many of these compounds were verified in vitro for their preventing action against MMP-2 and -9 and human leukocyte elastase (LE) (Zhuang C, et al., 2017). They conclude that monobactams bearing phenanthrene and chrysene substituents dedicate excellent activity. While naphthalene, anthracene, and pyrene substituents cause no cytotoxic activities (Zhuang C, et al., 2017).
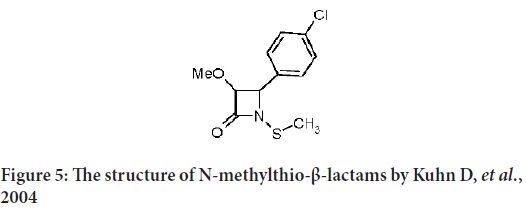
Kuhn D, et al. describe some of synthetic N-methylthio-β-lactams that provoke apoptosis in many tumor cell lines as the breast cancer (MCF-7 and MDA-MB-231) human leukemia Jurkat T cells, head-and-neck (Human Prostatic Carcinoma cell line-13 (PCI-13)) and Prostate Carcinoma cell line (PC-3 and DU-145) (Frezza M, et al., 2008). They finally conclude several key points that to be considered in upcoming purpose and synthesis of monobactams as an anticancer (Figure 5):
Figure 5: The structure of N-methylthio-β-lactams by Kuhn D, et al., 2004
1) The N-methyl-thio group must maintain as it is without any addition.
2) All substitutions on ortho, meta and para of the phenyl ring retain the apoptotic effect, and the most potent are on the ortho position.
3) The bigger groups in the ortho position will cause the powerful apoptotic-inducing activity.
Frezza M, et al. studied the effects of C3 and N1 substitutions on β-lactams moiety for their apoptotic effect with Structure-Activity Relationship (SAR). They mentioned couple of β-lactam compounds, both bearing a branched-chain moiety on C3, they exhibit a potent apoptosis-inducing activity. They concluded that any substituents on the N1 or C3 atoms of the monobactam will further potentiate such an effect (Arya N, et al., 2014).
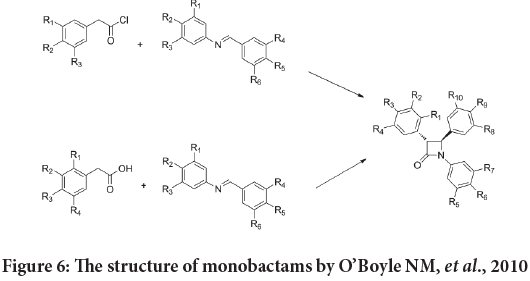
Based on the studies, that mentions the anticancer activity of the combretastatin analogues with OH and OMe groups at C-3 (Sun L, et al., 2004; Carr M, et al., 2010; Meegan MJ, et al., 2008). Also the potent anticancer action of monobactams compounds that contain methyl group at C-3 (Martelli G, et al., 2020; Pettit GR, et al., 1987; O’Boyle NM, et al., 2010) studied the anti-proliferative action of a naval synthesized series of inflexible analogues of natural combretastatin which bearing the 1,4-diaryl-2-azetidinone (monobactams) instead of the regular ethylene link existing in the natural combretastatin stilbene compounds. Moreover, these new compounds substituted with an aryl ring at position 3 on monobactam moiety. Also the 3,4,5-trimethoxyphenyl moiety introduced at the N-1 atom and the 4-methoxyphenyl ring is hosted at the C-4 atom (Figure 6) (Cerić H, et al., 2010).
Figure 6: The structure of monobactams by O’Boyle NM, et al., 2010
First, these compounds were estimated for their anticancer effect against the MCF-7 human breast cancer cell line, and then the most powerful agents were tested versus the MDA-MB-231 cell line.
They indicate that the highest effective compounds in this groups were establish in those with tiny, polar substituents such as (4-fluorophenyl), (4-hydroxyphenyl), (3-hydroxyphenyl), and (4-aminophenyl). The SAR studies indicate that the replacement of the β-lactam carbonyl group with the thione resulted in a decrease of the antiproliferative activity (Cerić H, et al., 2010).
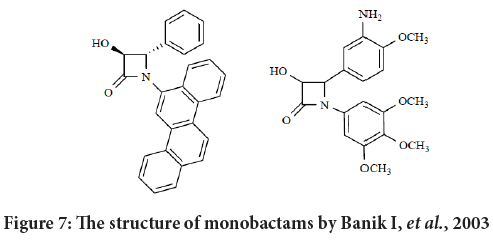
Galletti and Giacomini in their review reported that Banik and its colleague established a new series of N-polyaromatic-azetidinones with antitumor actions. Their SAR study exposed that the aromatic structural requirement for cytotoxicity must contain three aromatic rings as a minimum in an rangy configuration (compounds with chrysene substituents established activity, while naphthalene, anthracene or pyrene substituents with no activities (Banik BK, et al., 2010; Tripodi F, et al., 2012). Their results revels that the inhibition of the growth are not equal against all tumor lines, suggesting that there are specificity in the targeting process. In addition, the existence of a OH group on C3 enhanced the activity or even it may switch inactive compound into active ones (Figure 7) (Banik BK, et al., 2010).
Figure 7: The structure of monobactams by Banik I, et al., 2003
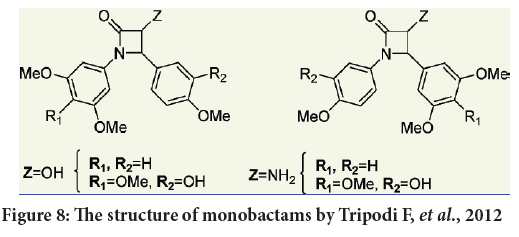
Tripodi F, et al. considered four series of compounds. One with a 3,4,5-trimethoxyphenyl moiety at position 1 accompanied by a 4-methoxy-3-hydroxyphenyl moiety at 4 position of the monolactam ring and also a OH or HH2 group at position number 3. The other series with 3,5-dimethoxyphenyl moiety accompanied by a 4-methoxyphenyl group at position number 4 of the monobactam ring. The third and fourth series were with the inverted aryl moieties of the first and second series respectively (Figure 8) (Petrovski G, et al., 2011). All the synthesized products evaluated against HeLa cells (cervical adenocarcinoma), and also against the breast cancer cell line MCF-7, as well as against several colon cancer cell lines (SW48, SW480, SW620, T84) and finally against the neuroblastoma cell line (SK- N-BE) in order to estimate their antiproliferative activities (Petrovski G, et al., 2011; Kondratyuk TP, et al., 2011).
Figure 8: The structure of monobactams by Tripodi F, et al., 2012
The results indicate that the water solubility of the tested compounds were enhanced by the presence of a OH group at position number 3 of the monobactam ring (Kondratyuk TP, et al., 2011; Meegan MJ, et al., 2001). Also compounds bearing the 3,4,5-trimethoxy or 3,5-dimethoxyphenyl substituents at position 1 (N atom) of monobactam ring represent efficient activity comparing to those with polymethoxylated substituents at C-4 position (Kondratyuk TP, et al., 2011).
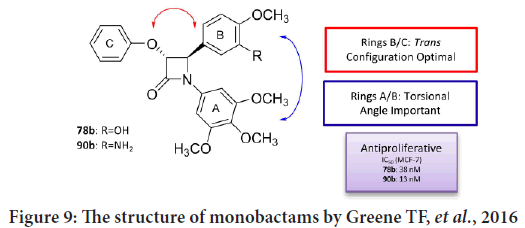
Greene TF, et al. studied the SAR for a new family of 3-phenoxy-1,4-diary-lazetidin-2-ones (Olazaran FE, et al., 2017; Fu DJ, et al., 2017; Barrett I, et al., 2010), that finally led to synthesize a group of active anticancer compounds involving trans-4-(3-hydroxy-4-methoxyphenyl)-3-phenoxy- 1-(3,4,5-trimethoxyphenyl)azetidin-2-one and trans-4-(3-amino-4- methoxyphenyl)-3-phenoxy-1-(3,4,5 trimethoxyphenyl) azetidin-2-one (Barrett I, et al., 2010). The anticancer activities were tested against MCF-7 breast cancer cells (targeting tubulin polymerization process) (Barrett I, et al., 2010; Greene TF, et al., 2016). Their results indicate that these compounds targeted tubulin effectively, and that the monobactams synchronize within the colchicine-binding site of the β-tubulin. In addition, and by studying the X-ray crystallography for a number of chosen compounds, the results indicating that the torsional angle among the aryl rings at N-1 and C-4 of the monobactams considered to be essential for effective anticancer effects (Figure 9) (Barrett I, et al., 2010; Deep A, et al., 2016).
Figure 9: The structure of monobactams by Greene TF, et al., 2016
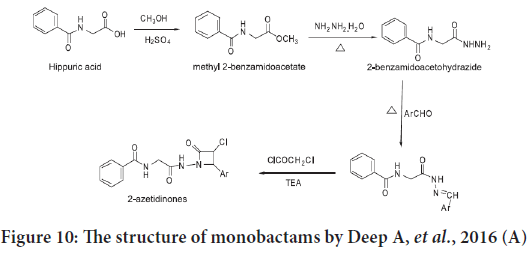
Deep A, et al. synthesized another series of monobactams derivatives in 2016 from hippuric acid (Figure 10), they assessed the in vitro anticancer activities for these compounds against an estrogen receptor positive human breast adenocarcinoma MCF-7 (ATCC HTB-22) cancer cell line. The results showed a regular anticancer potential possessed by these com pounds, and the best compound was N-[2-(3-chloro-2-oxo-4-styrylazeti din-1-ylamino)-2-oxoethyl] benzamide (Deep A, et al., 2016).
Figure 10: The structure of monobactams by Deep A, et al., 2016 (A)
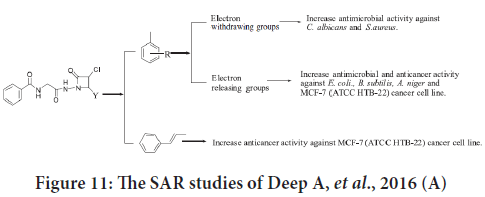
The SAR studies indicate that the existence of electron releasing groups on benzylidene portion will improve the anticancer activity. Cinnamaldehyde derivatives were categorized as the most effective compounds (Deep A, et al., 2016). These findings summarized in Figure 11.
Figure 11: The SAR studies of Deep A, et al., 2016 (A)
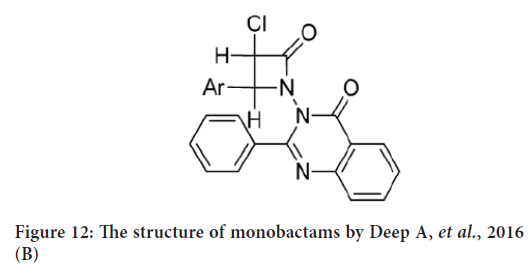
Another study for Deep A, et al. (2016) in which they synthesized an other monabactam series from hydrazone and chloroacetyl chloride in dimethylformamide (Figure 12). The anticancer activity of synthesized compounds was tested against human breast cancer cell line (MCF-7). Their results indicated that the synthesized compounds exposed weak anticancer activity against breast cancer cell line (MCF-7) comparing to standard drug (Deep A, et al., 2016).
Figure 12: The structure of monobactams by Deep A, et al., 2016 (B)
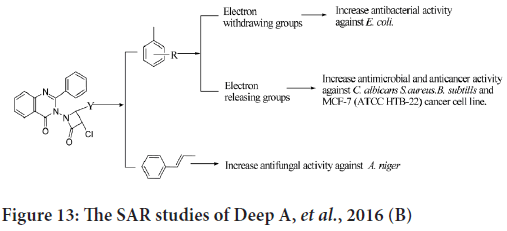
The SAR studies indicate that the presence of electron releasing groups will improve the anticancer activity against human breast cancer cell line (MCF-7) (Figure 13) (Deep A, et al., 2016).
Figure 13: The SAR studies of Deep A, et al., 2016 (B)
Also in another research, Deep A, et al. design and synthesis a new series of 4-thiazolidinone derivatives, and evaluate their in vitro antimicrobial and anticancer activities. The anticancer study results revealed that N-(2- (5-(4-hydroxybenzylidene)-2-(4-methoxyphenyl)-4-oxothiazolidin-3- ylamino)-2-oxoeth-yl) benzamide was the most powerful anticancer agent (Zhang Y, 2010).
Geesala R, et al. synthesized a new monobactams by introducing allyl moieties in steed of aromatic ring on the N1 atom of 1,4-diarylazetidin-2-ones, indicating that these allyl (isothiocyanate and disulfide compounds) will give a potent anticancer effect (Geesala R, et al., 2016).
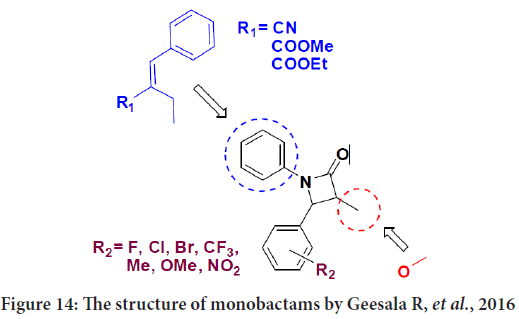
The synthesized monobactams were tested by the 3-(4,5-Dimethylthiazol-2-Yl)-2,5-Diphenyltetrazolium Bromide (MTT) cell viability assay against human breast cancer cell lines MCF-7 (Estrogen Receptor+(ER+)/ Progesterone Receptor+(PR+)/Human epidermal growth factor receptor 2-(Her2-), Luminal type) and MDA MB-231 (ER-/PR-/Her2-, Basal type) and also against non-transformed normal human Mammary Epithelial Cells (MEpiC) (Meenakshisundaram S, et al., 2016). The results represent that when fluorine located at the p-position of 3-methoxy-2-oxo-4- phenylazetidin will be more active than chlorine or bromine atoms at the same location in the phenyl acrylonitrile derivatives. In addition, the presence of methyl moiety at the p-position on the same derivatives was noticed to enhance the antiproliferative activity. It is worth noting that when the methyl moiety located on the m-position and also when the second and fifth location occupied by methoxy groups and chlorine located on the p-position of these derivatives the activity will decrease. Also they mention that the methyl-phenyl acrylate compounds of 3-methoxy-2-oxo-4- phenylazetidin bearing either nitro or tri-fluoro methyl group or chlorine atom at m-position were ineffective (Figure 14) (Meenakshisundaram S, et al., 2016).
Figure 14: The structure of monobactams by Geesala R, et al., 2016
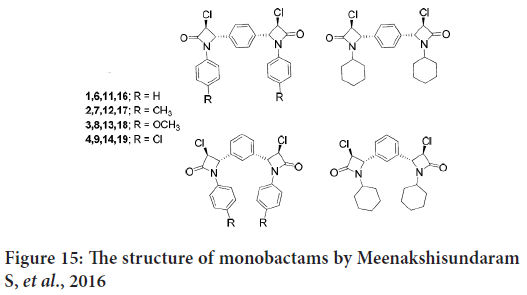
In another study, Meenakshisundaram S, et al. synthesize and evaluate in vitro inhibitory activities of new monobactams group against three human cancer cell lines (cervical HeLa, renal ACHN and breast MDA-MB-231). In this study, the dimeric Schiff bases were prepared from terephalaldehyde and groups of amines (Kayarmar R, et al., 2017). Another group of Schiff bases were created by the reaction of the isophthalaldehyde with other amines. Then the monobactams were synthesized from these Schiff bases (Figure 15) (Kayarmar R, et al., 2017).
Figure 15: The structure of monobactams by Meenakshisundaram S, et al., 2016
The results indicates that the 1,3-positional isomers of 3-chloroazetidinones revealed strong anticancer activity, and also the 4-methyl derivative exhibited potent cytotoxic activity (Kayarmar R, et al., 2017).
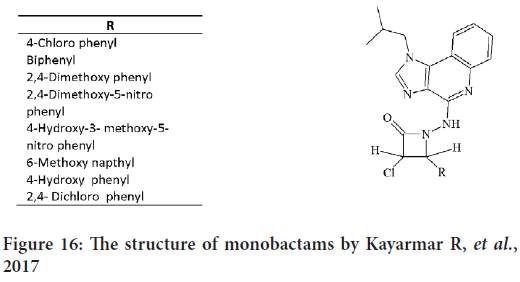
In 2017, a novel group of N-substituted monobactams were synthesized by Kayarmar R, et al. by the reaction of chloroacetyl chloride with 4-ary-lidene hydrazino1-isobutyl-1H-imidazo[4,5-c]quinolines compounds. Also by substitute imidazoquinoline at the 4-position they create a group of 3-chloro-4-arylsubstituted-1-[(1-isobutyl-1H-imidazo[4,5-c]quinolin- 4yl)amino] azetidin-2-ones (Figure 16), and then they examined the antimicrobial and anticancer activities of these compounds (Mohamadzadeh M and Zarei M, 2021).
Figure 16: The structure of monobactams by Kayarmar R, et al., 2017
The anticancer activities against the HeLa cancer cell line for the synthesized compounds were compared with cyclophosphamide as standard drug. Compounds with 4-OH phenyl and 2,4-Dichloro phenyl represent a highest anticancer activity against HeLa cells (Mohamadzadeh M and Zarei M, 2021).
In early 2020, Mohamadzadeh and Zarei synthesize new groups of monobactams having anthraquinone moiety with several substituents (Figure 17), and then they evaluate their antitumor activities. The results showed that many of these monobactams represent reasonable to significant antitumor activity against Prostate Carcinoma (PC3), colon carcinoma (HCT116), breast carcinoma (MCF7) and neuroblastoma (SK-N-MC) cell lines via MTT assay. Due to the structure similarity, the reference compound was Doxorubicin (Zhou P, et al., 2016).
Figure 17: The structure of monobactams by Mohamadzadeh M and Zarei M, 2021
The SAR study reveals that when 2-naphthoxy or methoxy groups substituted on C-3, there will be a higher activity than when substituted at N1, C2 or C4. Little activity also noticed with chloro, azido and phthalimido. Reasonable activity is shown by 3-thiolated or -sulfonated derivatives. In addition, cytotoxicity decreased with the replacement of methylthio by methylsufonyl. The potent cytotoxicity against all cell lines were noticed when 2-azetidinoneanthraquinone hybrids substituted at location 4 by 4-(dimethylamino) phenyl moiety. They concluded that monobactams could be used as antitumor because it’s low toxicity and their potent anticancer activities (Zhou P, et al., 2016).
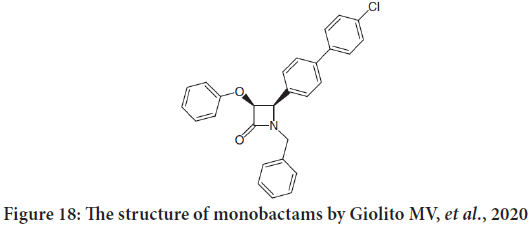
Another study done by Giolito MV, et al. and they described the in vitro activity estimation of 35 novel chemical compounds against two negative murinemammary adenocarcinoma tumors. The selection involves two compounds which are β-lactams: PGC22i (penicillin) and CIT171B3 (monobactam) (Figure 18). Both penicillin and monobactams are having antitumor activity (Nam NH, 2003; Giolito MV, et al., 2020).
Figure 18: The structure of monobactams by Giolito MV, et al., 2020
They found that the monobactam compound cause promotion of apoptosis and lower clonogenic capacity. They also decreased the migratory capacity of tumor cells. The results propose the possibility of their future utilization as antitumor and/or antimetastatic agents (Leite TH, et al., 2020).
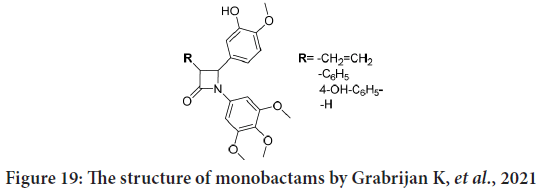
Grabrijan K, et al. in their patent overview mentioned the patent from 2011 (WO201107321) in which the authors designate synthetic monbactams derivatives of combretastatin as anti-cancer agents (Grabrijan K, et al., 2021). The rigid beta lactam ring catches the compound in the preferred stereochemistry. The synthesized compounds strongly look like combrestatine, with trimethoxybenzyl at N-1 position, and 2-methoxyphenol at C-4 position. Different groups were presented on C-3 position (Figure 19). The anticancer effect was tested against MCF-7 breast cancer cell line. The reported IC50 values for all compounds were in nanomolar range. In addition, some compound was shown to inhibit the growth of human chronic myeloid leukaemia K562 cells and human promyelocytic leukaemia HL-60 cells, their IC50 were in the nanomolar or sub-nanomolar scope. They conclude that the possibility of using of such compounds as anti-cancer agents would inhibit angiogenesis and metastases (Patil SA, et al., 2021).
Figure 19: The structure of monobactams by Grabrijan K, et al., 2021
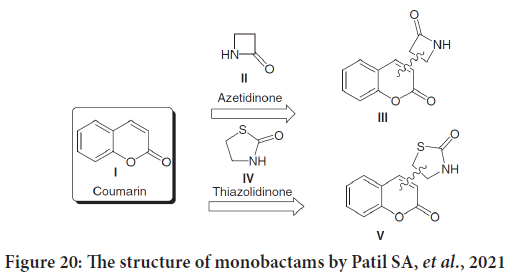
Recently, Patil SA, et al. in their coumarins review study mentioned many bioactive coumarins holding azetidinone and thiazolidinone moieties, which found with a wide range of therapeutic characteristics, including anticancer activities. Azetidinone moieties were added to the positions three, four, six and seven of the coumarin skeleton (Figure 20) (Keri RS, et al., 2010).
Figure 20: The structure of monobactams by Patil SA, et al., 2021
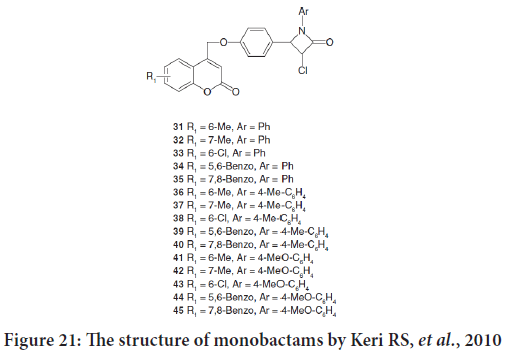
The review mention the synthesis of a new series 3-chloro-4-[4-(2-oxo- 2Hchromen-4-ylmethoxy)phenyl]-1-phenylazetidin-2-ones by Keri RS, et al.. Compounds 33, 36, 38, 40 and 43 exposed powerful anticancer activity against Artemia salina (Figure 21).
Figure 21: The structure of monobactams by Keri RS, et al., 2010
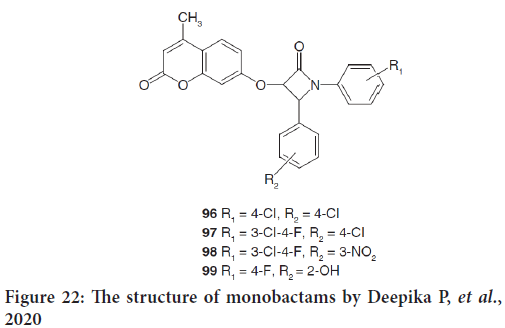
In the same review, another study by Deepika P, et al. mentioned the design and synthesis of new azetidin-2-one coumarin derivatives (96-99) as anticancer drugs (Figure 22) (Deepika P, et al., 2020). The docking scores indicated that out of all compounds docked, 96 had the powerfull binding affinity with protein 1XJD. Furthermore, all coumarin azetidinones exhibited good cytotoxic effects against HeLa cancer cell lines. They indicate that these series could be considered to be future leads for anticancer therapy (Głowacka IE, et al., 2021).
Figure 22: The structure of monobactams by Deepika P, et al., 2020
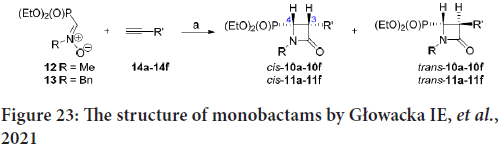
A new family of N-substituted 3-aryl-4-(diethoxyphosphoryl)azetidin- 2-ones was synthesized by Głowacka IE, et al. by reacting the N-methyl- or N-benzyl- (diethyoxyphosphoryl)nitrone (compounds 12 and 13) with the 14a-14f aryl alkynes (Figure 23) (Fram RJ, et al., 1986).
Figure 23: The structure of monobactams by Głowacka IE, et al., 2021
The 50% cytostatic Inhibitory Concentration (IC50) was estimated against 9 cancerous cell lines: Capan-1 (pancreatic adenocarcinoma), Hap1 (chronic myeloid leukemia), HCT-116 (Colorectal Carcinoma), DND- 41 (acute lymphoblastic leukemia), HL-60 (acute myeloid leukemia), NCI-H460 (lung carcinoma), MM.1S (multiple myeloma), K-562 (chronic myeloid leukemia), normal retina (non-cancerous) cells (Human Telomerase Reverse Transcriptase Retinal Pigment Epithelial-1 (hTERT RPE-1)), as well as Z-138 non-Hodgkin lymphoma. Some monobactams represent reasonable cytostatic effect concerning Capan-1, Hap1 and HCT-116 cells (Fram RJ, et al., 1986).
Also Głowacka IE, et al. mentioned number of compounds and studies that resemble their work as synthesis of 4-(2-chlorophenyl)-3-methoxy-1- (methylthio)azetidin-2-one which stop proliferation and provoke apoptosis in human solid cell lines, like prostate and breast (Chimento A, et al., 2013). Similarly, 1,4-diaryl-3-chloroazetidin-2-ones which documented as effective compounds against human breast cancer (Smith DM, et al., 2002). Another group of 1,4-diarylazetidinone with methoxyphenyl moiety were synthesized and then evaluated for their cytotoxicity effect (Smith SR, et al., 2014; Malebari AM, et al., 2020). Many of them showed antiproliferative effect against MCF-7 and MDA-MB-231 human breast carcinoma cells with nanomolar value. Furthermore, the fluorinated compounds revealed substantial antitumor effects against HT-29 colon cancer cells (Smith SR, et al., 2014; Malebari AM, et al., 2020).
CONCLUSION
Cancer considered as one of the major hazards to human health. It described as the malignant cells evolution that finally ended with the interference with ordinary physiological works. Anticancer drug investigation concentrates on reserve of tumor cell development and induction of apoptosis in the malignant cells, without disturbing adjacent cells. However, the low selectively and the serious chemotherapeutic drugs side effects are the major drawbacks of the current antitumor agents. Therefor there is an urgent need to develop appropriate (safe and selective) chemotherapeutic drugs.
Antibiotics are efficient, mostly well tolerated in patients and have very low level of toxicity. Thus, it may be an attractive target for antitumor agent’s invention. There are now lots of antitumor antibiotics accepted as anticancer agents. In addition to that, and by varying the character of the electron withdrawing substituent of the monobactams, there is an excellent chance get a safe and effective antitumor monobactams. These records indicate that the designation and synthesis of monobactams represent a talented area for promoting improvement in anticancer fields.
References
- Kuhn D, Coates C, Daniel K, Chen D, Bhuiyan M, Kazi A, et al. Beta-lactams and their potential use as novel anticancer chemotherapeutics drugs. Front Biosci. 2004; 9: 2605-2617.
[Crossref] [Google Scholar] [Pubmed]
- Dong J, Huang G, Zhang Q, Wang Z, Cui J, Wu Y, et al. Development of benzochalcone derivatives as selective CYP1B1 inhibitors and anticancer agents. Med Chem Comm. 2019; 10(9): 1606-1614.
[Crossref] [Google Scholar] [Pubmed]
- Sharipova RR, Belenok MG, Garifullin BF, Sapunova AS, Voloshina AD, Andreeva OV, et al. Synthesis and anti-cancer activities of glycosides and glycoconjugates of diterpenoid isosteviol. Med Chem Comm. 2019; 10(8): 1488-1498.
[Crossref] [Google Scholar] [Pubmed]
- Vento S, Cainelli F. Infections in patients with cancer undergoing chemotherapy: Aetiology, prevention, and treatment. Lancet Oncol. 2003; 4(10): 595-604.
[Crossref] [Google Scholar] [Pubmed]
- Tikhomirov AS, Shtil AA, Shchekotikhin AE. Advances in the discovery of anthraquinone-based anticancer agents. Recent Pat Anticancer Drug Discov. 2018; 13(2): 159-183.
[Crossref] [Google Scholar] [Pubmed]
- Mehta PD, Sengar NP, Pathak AK. 2-Azetidinone-a new profile of various pharmacological activities. Eur J Med Chem. 2010; 45(12): 5541-5560.
[Crossref] [Google Scholar] [Pubmed]
- Basu S, Banik BK. Beta-lactams: Novel synthetic pathways and applications. Springer. 2017.
- Zhou P, Liang Y, Zhang H, Jiang H, Feng K, Xu P, et al. Design, synthesis, biological evaluation and cocrystal structures with tubulin of chiral β-lactam bridged combretastatin A-4 analogues as potent antitumor agents. Eur J Med Chem. 2018; 144: 817-842.
[Crossref] [Google Scholar] [Pubmed]
- Borazjani N, Sepehri S, Behzadi M, Jarrahpour A, Rad JA, Sasanipour M, et al. Three-component synthesis of chromeno β-lactam hybrids for inflammation and cancer screening. Eur J Med Chem. 2019; 179: 389-403.
[Crossref] [Google Scholar] [Pubmed]
- Hammersley D, Signy M. Ezetimibe: An update on its clinical usefulness in specific patient groups. Ther Adv Chronic Dis. 2017; 8(1): 4-11.
[Crossref] [Google Scholar] [Pubmed]
- Chen D, Falsetti SC, Frezza M, Milacic V, Kazi A, Cui QC, et al. Anti-tumor activity of N-thiolated β-lactam antibiotics. Cancer Lett. 2008; 268(1): 63-69.
[Crossref] [Google Scholar] [Pubmed]
- Tripodi F, Dapiaggi F, Orsini F, Pagliarin R, Sello G, Coccetti P. Synthesis and biological evaluation of new 3-amino-2-azetidinone derivatives as anti-colorectal cancer agents. Med Chem Comm. 2018; 9(5): 843-852.
[Crossref] [Google Scholar] [Pubmed]
- Banik I, Becker FF, Banik BK. Stereoselective synthesis of β-lactams with polyaromatic imines: Entry to new and novel anticancer agents. J Med Chem. 2003; 46(1): 12-15.
[Crossref] [Google Scholar] [Pubmed]
- Trivedi AR, Desai JM, Dholariya BH, Dodiya DK, Shah VH. Synthesis and antimicrobial evaluation of novel benzo [b] thiophenes comprising β-lactam nucleus. Med Chem Res. 2012; 21(7): 1471-1479.
- Pathak RB, Chovatia PT, Parekh HH. Synthesis, antitubercular and antimicrobial evaluation of 3-(4-chlorophenyl)-4-substituted pyrazole derivatives. Bioorg Med Chem Lett. 2012; 22(15): 5129-5133.
[Crossref] [Google Scholar] [Pubmed]
- Kumar A, Rajput CS, Bhati SK. Synthesis of 3-[4′-(p-chlorophenyl)-thiazol-2′-yl]-2-[(substituted azetidinone/thiazolidinone)-aminomethyl]-6-bromoquinazolin-4-ones as anti-inflammatory agent. Bioorgan Med Chem. 2007; 15(8): 3089-3096. [Crossref]
[Google Scholar] [Pubmed]
- Ojima I, Delaloge F. Asymmetric synthesis of building-blocks for peptides and peptidomimetics by means of the β-lactam synthon method. Chem Soc Rev. 1997; 26(5): 377-386.
- Hodge M, Chen QH, Bane S, Sharma S, Loew M, Banerjee A, et al. Synthesis and bioactivity of a side chain bridged paclitaxel: A test of the T-Taxol conformation. Bioorg Med Chem Lett. 2009; 19(10): 2884-2887.
[Crossref] [Google Scholar] [Pubmed]
- Mahmood AA, Al-Iraqi MA, Abachi FT. Design, synthesis and anti-β-lactamase activity for new monobactam compounds. Materials Today: Proceedings. 2021; 42: 1860-1866.
- El-Shorbagi AN, Chaudhary S. Monobactams: A unique natural scaffold of four-membered ring skeleton, recent development to clinically overcome infections by multidrug-resistant microbes. Lett Drug Des Discov. 2019; 16(12): 1305-1320.
- Jain AK, Vaidya A, Ravichandran V, Kashaw SK, Agrawal RK. Recent developments and biological activities of thiazolidinone derivatives: A review. Bioorg Med Chem. 2012; 20(11): 3378-3395.
[Crossref] [Google Scholar] [Pubmed]
- Galletti P, Giacomini D. Monocyclic β-lactams: New structures for new biological activities. Curr Med Chem. 2011; 18(28): 4265-4283.
[Crossref] [Google Scholar] [Pubmed]
- Decuyper L, Jukič M, Sosič I, Žula A, D'hooghe M, Gobec S. Antibacterial and β‐lactamase inhibitory activity of monocyclic β‐Lactams. Med Res Rev. 2018; 38(2): 426-503.
[Crossref] [Google Scholar] [Pubmed]
- Dražić T, Kopf S, Corridan J, Leuthold MM, Bertosa B, Klein CD. Peptide-β-lactam inhibitors of dengue and west nile virus NS2B-NS3 protease display two distinct binding modes. J Med Chem. 2019; 63(1): 140-156.
[Crossref] [Google Scholar] [Pubmed]
- Tripodi F, Pagliarin R, Fumagalli G, Bigi A, Fusi P, Orsini F, et al. Synthesis and biological evaluation of 1, 4-diaryl-2-azetidinones as specific anticancer agents: Activation of adenosine monophosphate activated protein kinase and induction of apoptosis. J Med Chem. 2012; 55(5): 2112-2124.
[Crossref] [Google Scholar] [Pubmed]
- Valtorta S, Nicolini G, Tripodi F, Meregalli C, Cavaletti G, Avezza F, et al. A novel AMPK activator reduces glucose uptake and inhibits tumor progression in a mouse xenograft model of colorectal cancer. Invest New Drugs. 2014; 32(6): 1123-1133.
[Crossref] [Google Scholar] [Pubmed]
- Koch V, Lorion MM, Barde E, Bräse S, Cossy J. Cobalt-catalyzed α-arylation of substituted α-halogeno β-lactams. Org Lett. 2019; 21(16): 6241-6244.
[Crossref] [Google Scholar] [Pubmed]
- Carr M, Greene LM, Knox AJ, Lloyd DG, Zisterer DM, Meegan MJ. Lead identification of conformationally restricted β-lactam type combretastatin analogues: Synthesis, antiproliferative activity and tubulin targeting effects. Eur J Med Chem. 2010; 45(12): 5752-5766.
[Crossref] [Google Scholar] [Pubmed]
- Banik BK. A personal perspective on medicinal and pharmaceutical chemistry. Front Chem. 2014; 2: 8.
[Crossref] [Google Scholar] [Pubmed]
- Chavan AA, Pai NR. Synthesis and biological activity of N-substituted-3-chloro-2-azetidinones. Molecules. 2007; 12(11): 2467-2477.
[Crossref] [Google Scholar] [Pubmed]
- Kamath A, Ojima I. Advances in the chemistry of β-lactam and its medicinal applications. Tetrahedron. 2012; 68(52): 10640.
[Crossref] [Google Scholar] [Pubmed]
- Tanaka T, Muto T, Maruoka H, Imajo S, Fukami H, Tomimori Y, et al. Identification of 6-substituted 4-arylsulfonyl-1, 4-diazepane-2, 5-diones as a novel scaffold for human chymase inhibitors. Bioorg Med Chem Lett. 2007; 17(12): 3431-3434.
[Crossref] [Google Scholar] [Pubmed]
- Mahmood AA. Synthesis, antioxidant and antimicrobial activities for new 4, 4'-methylenedianiline amide compounds. Egypt J Chem. 2021; 64(12): 2-3.
- Singh GS. Recent progress in the synthesis and chemistry of azetidinones. Tetrahedron. 2003; 59(39): 7631-7649.
- Lima LM, da Silva BN, Barbosa G, Barreiro EJ. β-lactam antibiotics: An overview from a medicinal chemistry perspective. Eur J Med Chem. 2020; 112829.
[Crossref] [Google Scholar] [Pubmed]
- Söderström TS, Poukkula M, Holmström TH, Heiskanen KM, Eriksson JE. Mitogen-activated protein kinase/extracellular signal-regulated kinase signaling in activated T cells abrogates TRAIL-induced apoptosis upstream of the mitochondrial amplification loop and caspase-8. J Immunol. 2002; 169(6): 2851-2860.
[Crossref] [Google Scholar] [Pubmed]
- Kazi A, Hill R, Long TE, Kuhn DJ, Turos E, Dou QP. Novel N-thiolated β-lactam antibiotics selectively induce apoptosis in human tumor and transformed, but not normal or nontransformed, cells. Biochem Pharmacol. 2004; 67(2): 365-374.
[Crossref] [Google Scholar] [Pubmed]
- Cainelli G, Galletti P, Garbisa S, Giacomini D, Sartor L, Quintavalla A. 4-Alkylidene-azetidin-2-ones: Novel inhibitors of leukocyte elastase and gelatinase. Bioorg Med Chem. 2003; 11(24): 5391-5399.
[Crossref] [Google Scholar] [Pubmed]
- Chin JR, Murphy G, Werb Z. Stromelysin, a connective tissue-degrading metalloendopeptidase secreted by stimulated rabbit synovial fibroblasts in parallel with collagenase, biosynthesis, isolation, characterization, and substrates. J Biol Chem. 1985; 260(22): 12367-12376.
[Crossref] [Google Scholar] [Pubmed]
- Hojilla CV, Mohammed FF, Khokha R. Matrix metalloproteinases and their tissue inhibitors direct cell fate during cancer development. Br J Cancer. 2003; 89(10): 1817-1821.
[Crossref] [Google Scholar] [Pubmed]
- Wilhelm SM, Collier IE, Kronberger A, Eisen AZ, Marmer BL, Grant GA, et al. Human skin fibroblast stromelysin: Structure, glycosylation, substrate specificity, and differential expression in normal and tumorigenic cells. Proc Natl Acad Sci USA. 1987; 84(19): 6725-6729.
[Crossref] [Google Scholar] [Pubmed]
- Malig TC, Yu D, Hein JE. A revised mechanism for the kinugasa reaction. J Am Chem Soc. 2018; 140(29): 9167-9173.
[Crossref] [Google Scholar] [Pubmed]
- Zhuang C, Zhang W, Sheng C, Zhang W, Xing C, Miao Z. Chalcone: A privileged structure in medicinal chemistry. Chem Rev. 2017; 117(12): 7762-7810.
[Crossref] [Google Scholar] [Pubmed]
- Frezza M, Garay J, Chen D, Cui C, Turos E, Dou QP. Induction of tumor cell apoptosis by a novel class of N-thiolated β-lactam antibiotics with structural modifications at N1 and C3 of the lactam ring. Int J Mol Med. 2008; 21(6): 689-695.
[Crossref] [Google Scholar] [Pubmed]
- Arya N, Jagdale AY, Patil TA, Yeramwar SS, Holikatti SS, Dwivedi J, et al. The chemistry and biological potential of azetidin-2-ones. Eur J Med Chem. 2014; 74: 619-656.
[Crossref] [Google Scholar] [Pubmed]
- Sun L, Vasilevich NI, Fuselier JA, Hocart SJ, Coy DH. Examination of the 1, 4-disubstituted azetidinone ring system as a template for combretastatin A-4 conformationally restricted analogue design. Bioorg Med Chem Lett. 2004; 14(9): 2041-2046.
[Crossref] [Google Scholar] [Pubmed]
- Carr M, Greene LM, Knox AJ, Lloyd DG, Zisterer DM, Meegan MJ. Lead identification of conformationally restricted β-lactam type combretastatin analogues: Synthesis, antiproliferative activity and tubulin targeting effects. Eur J Med Chem. 2010; 45(12): 5752-5766.
[Crossref] [Google Scholar] [Pubmed]
- Meegan MJ, Carr M, Knox AJ, Zisterer DM, Lloyd DG. β-Lactam type molecular scaffolds for antiproliferative activity: Synthesis and cytotoxic effects in breast cancer cells. J Enzyme Inhib Med Chem. 2008; 23(5): 668-685.
[Crossref] [Google Scholar] [Pubmed]
- Martelli G, Bloise N, Merlettini A, Bruni G, Visai L, Focarete ML, et al. Combining biologically active β-lactams integrin agonists with poly (l-lactic acid) nanofibers: Enhancement of human mesenchymal stem cell adhesion. Biomacromolecules. 2020; 21(3): 1157-1170.
[Crossref] [Google Scholar] [Pubmed]
- Pettit GR, Smith CR, Singh SB. Recent advances in the chemistry of plant antineoplastic constituents. Oxford University Press. 1987.
- O’Boyle NM, Carr M, Greene LM, Bergin O, Nathwani SM, mc Cabe T, et al. Synthesis and evaluation of azetidinone analogues of combretastatin A-4 as tubulin targeting agents. J Med Chem. 2010; 53(24): 8569-8584.
[Crossref] [Google Scholar] [Pubmed]
- Cerić H, Šindler-Kulyk M, Kovačević M, Perić M, Živković A. Azetidinone-isothiazolidinones: Stereoselective synthesis and antibacterial evaluation of new monocyclic beta-lactams. Bioorg Med Chem. 2010; 18(9): 3053-3058.
[Crossref] [Google Scholar] [Pubmed]
- Banik BK, Banik I, Becker FF. Asymmetric synthesis of anticancer β-lactams via Staudinger reaction: Utilization of chiral ketene from carbohydrate. Eur J Med Chem. 2010; 45(2): 846-848.
[Crossref] [Google Scholar] [Pubmed]
- Tripodi F, Pagliarin R, Fumagalli G, Bigi A, Fusi P, Orsini F, et al. Synthesis and biological evaluation of 1, 4-diaryl-2-azetidinones as specific anticancer agents: Activation of adenosine monophosphate activated protein kinase and induction of apoptosis. J Med Chem. 2012; 55(5): 2112-2124.
[Crossref] [Google Scholar] [Pubmed]
- Petrovski G, Gurusamy N, Das DK. Resveratrol in cardiovascular health and disease. Ann NY Acad Sci. 2011; 1215(1): 22-33.
[Crossref] [Google Scholar] [Pubmed]
- Kondratyuk TP, Park EJ, Marler LE, Ahn S, Yuan Y, Choi Y, et al. Resveratrol derivatives as promising chemopreventive agents with improved potency and selectivity. Mol Nutr Food Res. 2011; 55(8): 1249-1265.
[Crossref] [Google Scholar] [Pubmed]
- Meegan MJ, Hughes RB, Lloyd DG, Williams DC, Zisterer DM. Flexible estrogen receptor modulators: Design, synthesis, and antagonistic effects in human MCF-7 breast cancer cells. J Med Chem. 2001; 44(7): 1072-1084.
[Crossref] [Google Scholar] [Pubmed]
- Olazaran FE, Rivera G, Pérez-Vázquez AM, Morales-Reyes CM, Segura-Cabrera A, Balderas-Rentería I. Biological evaluation in vitro and in silico of azetidin-2-one derivatives as potential anticancer agents. ACS Med Chem Lett. 2017; 8(1): 32-37.
[Crossref] [Google Scholar] [Pubmed]
- Fu DJ, Fu L, Liu YC, Wang JW, Wang YQ, Han BK, et al. Structure-activity relationship studies of β-lactam-azide analogues as orally active antitumor agents targeting the tubulin colchicine site. Sci Rep. 2017; 7(1): 1-2.
[Crossref] [Google Scholar] [Pubmed]
- Barrett I, Carr M, O’Boyle N, Greene LM, Knox AJS, Lloyd DG, et al. Lead identification of conformationally restricted benzoxepin type combretastatin analogs: Synthesis, antiproliferative activity, and tubulin effects. J Enzyme Inhib Med Chemy. 2010; 25(2): 180-194.
[Crossref] [Google Scholar] [Pubmed]
- Greene TF, Wang S, Greene LM, Nathwani SM, Pollock JK, Malebari AM, et al. Synthesis and biochemical evaluation of 3-phenoxy-1, 4-diarylazetidin-2-ones as tubulin-targeting antitumor agents. J Med Chem. 2016; 59(1): 90-113.
[Crossref] [Google Scholar] [Pubmed]
- Deep A, Kumar P, Narasimhan B, Lim SM, Ramasamy K, Mishra RK, et al. 2-Azetidinone derivatives: Synthesis, antimicrobial, anticancer evaluation and QSAR studies. Acta Pol Pharm Drug Res. 2016; 73: 65-78.
[Google Scholar] [Pubmed]
- Deep A, Kumar P, Narasimhan B, Meng LS, Ramasamy K, Mishra RK, et al. Synthesis, antimicrobial and anticancer evaluation of 2-azetidinones clubbed with quinazolinone. Pharm Chem J. 2016; 50(1): 24-28.
[Crossref] [Google Scholar] [Pubmed]
- Zhang Y. Allyl isothiocyanate as a cancer chemopreventive phytochemical. Mol Nutr Food Res. 2010; 54(1): 127-135.
[Crossref] [Google Scholar] [Pubmed]
- Geesala R, Gangasani JK, Budde M, Balasubramanian S, Vaidya JR, Das A. 2-Azetidinones: Synthesis and biological evaluation as potential anti-breast cancer agents. Eur J Med Chem. 2016; 124: 544-558.
[Crossref] [Google Scholar] [Pubmed]
- Meenakshisundaram S, Manickam M, Vinayagam V. Synthesis, antibacterial and anticancer activity of novel bis-azetidinones. J Chem Pharm Res. 2016; 8(2): 733-742.
- Kayarmar R, Nagaraja GK, Naik P, Manjunatha H, Revanasiddappa BC, Arulmoli T. Synthesis and characterization of novel imidazoquinoline based 2-azetidinones as potent antimicrobial and anticancer agents. J Saudi Chem Soc. 2017; 21: 434-444.
- Mohamadzadeh M, Zarei M. Anticancer activity and evaluation of apoptotic genes expression of 2-azetidinones containing anthraquinone moiety. Mol Divers. 2021; 25(4): 2429-2439.
[Crossref] [Google Scholar] [Pubmed]
- Zhou P, Liu Y, Zhou L, Zhu K, Feng K, Zhang H, et al. Potent antitumor activities and structure basis of the chiral β-lactam bridged analogue of combretastatin A-4 binding to tubulin. J Med Chem. 2016; 59(22): 10329-10334.
[Crossref] [Google Scholar] [Pubmed]
- Nam NH. Combretastatin A-4 analogues as antimitotic antitumor agents. Curr Med Chem. 2003; 10(17): 1697-1722.
[Crossref] [Google Scholar] [Pubmed]
- Giolito MV, Camacho CM, Martinez-Amezaga M, Traficante CI, Giordano RA, Cornier PG, et al. Antitumor activity of new chemical compounds in triple negative mammary adenocarcinoma models. Future Sci OA. 2020; 6(3): 442.
[Crossref] [Google Scholar] [Pubmed]
- Leite TH, Saraiva MF, Pinheiro AC, de Souza MV. Monocyclic β-lactam: A Review on synthesis and potential biological activities of a multitarget core. Mini Rev Med Chem. 2020; 20(16): 1653-1682.
[Crossref] [Google Scholar] [Pubmed]
- Grabrijan K, Strašek N, Gobec S. Monocyclic beta-lactams for therapeutic uses: A patent overview (2010-2020). Expert Opin Ther Pat. 2021; 31(3): 247-266.
[Crossref] [Google Scholar] [Pubmed]
- Patil SA, Patil SA, Fariyike T, Marichev KO, Martinez HM, Bugarin A. Medicinal applications of coumarins bearing azetidinone and thiazolidinone moieties. Future Med Chem. 2021; 13(21): 1907-1934.
[Crossref] [Google Scholar] [Pubmed]
- Keri RS, Hosamani KM, Reddy HS, Shingalapur RV. Synthesis, invitro antimicrobial and cytotoxic studies of novel azetidinone derivatives. Arch Pharm. 2010; 343(4): 237-247.
[Crossref] [Google Scholar] [Pubmed]
- Deepika P, Dhanya P, Naseef PP. Docking investigation, synthesis and cytotoxic studies of coumarin substituted azetidinone derivatives. Res J Pharm Technol. 2020; 13(12): 6182-6185.
- Głowacka IE, Grabkowska-Drużyc M, Andrei G, Schols D, Snoeck R, Witek K, et al. Novel N-Substituted 3-Aryl-4-(diethoxyphosphoryl) azetidin-2-ones as antibiotic enhancers and antiviral agents in search for a successful treatment of complex infections. Int J Mol Sci. 2021; 22(15): 8032.
[Crossref] [Google Scholar] [Pubmed]
- Fram RJ. A comparison of the effects of cytosine arabinoside and beta-lactams on DNA synthesis and cellular proliferation. Cell Biol Toxicol. 1986; 2(4): 531-539.
[Crossref] [Google Scholar] [Pubmed]
- Chimento A, Sala M, Gomez-Monterrey IM, Musella S, Bertamino A, Caruso A, et al. Biological activity of 3-chloro-azetidin-2-one derivatives having interesting antiproliferative activity on human breast cancer cell lines. Biorg Med Chem Lett. 2013; 23(23): 6401-6405.
[Crossref] [Google Scholar] [Pubmed]
- Smith DM, Kazi A, Smith L, Long TE, Heldreth B, Turos E, et al. A novel β-lactam antibiotic activates tumor cell apoptotic program by inducing DNA damage. Mol Pharmacol. 2002; 61(6): 1348-1358.
[Crossref] [Google Scholar] [Pubmed]
- Smith SR, Douglas J, Prevet H, Shapland P, Slawin AM, Smith AD. Isothiourea-catalyzed asymmetric synthesis of β-lactams and β-amino esters from arylacetic acid derivatives and N-sulfonylaldimines. J Org Chem. 2014; 79(4): 1626-1639.
[Crossref] [Google Scholar] [Pubmed]
- Malebari AM, Fayne D, Nathwani SM, O’Connell F, Noorani S, Twamley B, et al. β-Lactams with antiproliferative and antiapoptotic activity in breast and chemoresistant colon cancer cells. Eur J Med Chem. 2020; 189: 112050.
[Crossref] [Google Scholar] [Pubmed]
Author Info
Ahmed AJ Mahmood*Citation: Mahmood AA: Monobactams as Anticancers: A Review Study
Received: 07-Feb-2022 Accepted: 22-Feb-2022 Published: 01-Mar-2022, DOI: 10.31858/0975-8453.13.3.196-204
Copyright: This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
ARTICLE TOOLS
- Dental Development between Assisted Reproductive Therapy (Art) and Natural Conceived Children: A Comparative Pilot Study Norzaiti Mohd Kenali, Naimah Hasanah Mohd Fathil, Norbasyirah Bohari, Ahmad Faisal Ismail, Roszaman Ramli SRP. 2020; 11(1): 01-06 » doi: 10.5530/srp.2020.1.01
- Psychometric properties of the World Health Organization Quality of life instrument, short form: Validity in the Vietnamese healthcare context Trung Quang Vo*, Bao Tran Thuy Tran, Ngan Thuy Nguyen, Tram ThiHuyen Nguyen, Thuy Phan Chung Tran SRP. 2020; 11(1): 14-22 » doi: 10.5530/srp.2019.1.3
- A Review of Pharmacoeconomics: the key to “Healthcare for All” Hasamnis AA, Patil SS, Shaik Imam, Narendiran K SRP. 2019; 10(1): s40-s42 » doi: 10.5530/srp.2019.1s.21
- Deuterium Depleted Water as an Adjuvant in Treatment of Cancer Anton Syroeshkin, Olga Levitskaya, Elena Uspenskaya, Tatiana Pleteneva, Daria Romaykina, Daria Ermakova SRP. 2019; 10(1): 112-117 » doi: 10.5530/srp.2019.1.19
- Dental Development between Assisted Reproductive Therapy (Art) and Natural Conceived Children: A Comparative Pilot Study Norzaiti Mohd Kenali, Naimah Hasanah Mohd Fathil, Norbasyirah Bohari, Ahmad Faisal Ismail, Roszaman Ramli SRP. 2020; 11(1): 01-06 » doi: 10.5530/srp.2020.1.01
- Manilkara zapota (L.) Royen Fruit Peel: A Phytochemical and Pharmacological Review Karle Pravin P, Dhawale Shashikant C SRP. 2019; 10(1): 11-14 » doi: 0.5530/srp.2019.1.2
- Pharmacognostic and Phytopharmacological Overview on Bombax ceiba Pankaj Haribhau Chaudhary, Mukund Ganeshrao Tawar SRP. 2019; 10(1): 20-25 » doi: 10.5530/srp.2019.1.4
- A Review of Pharmacoeconomics: the key to “Healthcare for All” Hasamnis AA, Patil SS, Shaik Imam, Narendiran K SRP. 2019; 10(1): s40-s42 » doi: 10.5530/srp.2019.1s.21
- A Prospective Review on Phyto-Pharmacological Aspects of Andrographis paniculata Govindraj Akilandeswari, Arumugam Vijaya Anand, Palanisamy Sampathkumar, Puthamohan Vinayaga Moorthi, Basavaraju Preethi SRP. 2019; 10(1): 15-19 » doi: 10.5530/srp.2019.1.3