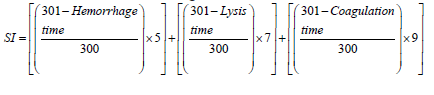Research Article - (2024) Volume 15, Issue 6
Abstract
This study focused on the development and characterization of Fluconazole (FLZ) loaded Nano Lipid Carriers (NLCs) based mucoadhesive gel to increase retention time on applied region to treat Vulvovaginal Candidiasis (VVC). FLZ is administered orally or topically to treat VVC, oral route has a cardiac adverse effect known as FLZ-induced Torsade de pointes. Patients with heart conditions and pregnant women are at risk of receiving orally FLZ, hence topical drug delivery is preferable. Nanostructured lipid carriers were formed in this study using a solvent-diffusion approach followed by high-speed homogenization. For the development of nanostructured lipid carriers Generally Recognized As Safe (GRAS) excipients were selected and optimised such as Compritol-888-ATO, oleic acid and tween-80. NLCs were characterised by particle size, Polydispersity Index (PDI), zeta potential, entrapment efficiency%, total drug content, in vitro release for 8 h and results were found to be 234.6 nm, 0.188, -8.25 mV, 78.99% ± 1.92%, 100.04% ± 0.46% and 84.22% ± 0.94% respectively and also examined for shape and morphology through transmission electron microscopy images. Optimized nanostructured lipid carriers were incorporated in gel by using Carbopol® 974P as gelling agent and was evaluated for pH, total drug content, spreadability and mucoadhesion which were found to be 4.2, 100.65% ± 1.166%, 7.8 cm ± 3.85 cm and 3534.53 ± 4.71 dyne/cm2 respectively. Rheological flow of the gel was found to be pseudoplastic. in vitro drug releases, ex vivo permeation, tissue-deposition and antifungal activity against Candida albicans were all compared to commercial formulation. Hen’s Egg Test-Chorioallantoic Membrane (HET-CAM) assay of gel was performed for irritation and was confirmed that formulation is non-irritant.
Keywords
Mucoadhesion, Vulvovaginal candidiasis, Fluconazole, NLCs, Solvent diffusion, Candida albicans, Mucoadhesive vaginal gel, Novel vaginal drug delivery
Introduction
VVC is a prevalent and distressing disease that affects the women worldwide, regardless of their age or social standing. The 2nd most common cause of vaginal infections are bacterial vaginosis (Lema VM, 2017). The primary pathogens for VVC are Candida albicans and other Candida species. VVC is a multifactorial infectious disease that induces pathologic inflammation in the lower female reproductive system, afflicting over 75% of all women at some point in their lives (Willems HM, et al., 2020; Sobel JD, 1997). VVC is characterized by white clumpy discharge, burning, redness with itching in vulva and vagina along with dyspareunia (Yano J, et al., 2019). Recurrent Vulvovaginal Candidiasis (RVVC) (>3 episodes/ year) affects nearly 8% of women worldwide (Willems HM, et al., 2020). Antimycotic-agents are prescribed orally or topically for 1-7 days or longer period to treat VVC. To avoid disadvantages of oral route, topical drug delivery is preferred (Takalkar D and Desai N, 2018). This route requires lower dosage compared to oral delivery and offers the advantage of targeted drug delivery to specifically desired location. Dosage forms for this route are gel, ointment, vaginal rings, tampons, vaginal tablets, vaginal capsules, pessaries and foams but the conventional vaginal dosage has short residence time. In this work novel VDDS have been developed with the intention of releasing the drug over a specific time period. Nanoparticle delivery systems have different strategies including in situ gel, drug-loaded nanoparticles, liposomes and cyclodextrin complexes (de Pereira ARR and Bruschi ML, 2012). FLZ is a bis-triazole antifungal agent with fluorine substitution, whose mechanism involves interfering with conversion of lanosterol to ergosterol by binding to fungal cytochrome p450 and causing membrane disruption (Pasko MT, et al., 1990). It has antifungal activity against Aspergillus spp., Blastomyces dermatitidis, Candida spp., Coccidioides immitis, Cryptococcus neoformans, Histoplasma capsulatum and Paracoccidioides brasiliensis (Pasko MT, et al., 1990).
Patients at risk of developing ventricular arrhythmias are often associated with oral route. However, FLZ-induced torsade de pointe which is characterized by QT interval prolongation poses a risk in patients with cardiac dysfunction and during pregnancy. Therefore topical drug delivery appears as a preferable alternative for such individuals (Tholakanahalli VN, et al., 2001; Pham CP, et al., 2006). In the recent years, researchers have focused more on the lipid nanoparticles Solid Lipid Nanoparticles (SLNs), NLCs, liposomes, ectosomes, etc., of antifungal drugs in order to avoid side effects and improve efficacy (Fernandes AV, et al., 2020).
SLNs has some disadvantages such as low drug loading capacity and stability related issues which include drug expulsion after polymeric transition and particle aggregation during storage due to composition of the solid matrix used in SLNs. NLCs overcome these issues by incorporating both liquid lipid and solid lipid components (Naseri N, et al., 2015).
In this work, NLCs based mucoadhesive gel was prepared to improve the therapeutic efficacy of drug and prolong the residence time of the formulation at the site of administration (intra-vaginal). Compared to creams and ointments, vaginal gels exhibit better vaginal secretion miscibility (Takalkar D and Desai N, 2018).
Materials and Methods
FLZ was received as a sample from Aarti Pharma Bhandup (Mumbai, India), while Compritol® 888 ATO, Labrafil® M 1944 CS, Gelucire® 44/14, Gelucire® 50/13, GelotTM 64, Precirol® ATO 5, LabrafacTM and Capryol® 90 were obtained as samples from Gattefosse India Pvt. Ltd., India). Marketed gel, FlucosTM was purchased from Oaknet Healthcare. Methanol AR and oleic acid were purchased from SD Fine Chemicals, Ltd., (Mumbai, India). Porcilian stomach mucine was purchased from Sigma Aldrich.
Dialysis bags and molecular cutoff 12-14 kD were procured from Hi- Media, India. Sabouraud dextrose agar and broth medium were purchased from HiMedia (Maharashtra, India). Sodium chloride, stearic acid, tween 20, glycerol, potassium hydroxide, acetone AR, Polyethylene Glycol (PEG) 400 was purchased from Loba Chemie Pvt. Ltd., tween 80 was procured from Merk Life Sciences, Pvt. Ltd., India. Lactic acid purchased from Amrut industrial product chemical house, India.
Analytical method development
The Ultraviolent Visible (UV) spectrophotometric method was developed and validated by using double beam UV visible spectrophotometer (model V-530, Jasco Inc., Japan) in methanol AR Simulated Vaginal Fluid (SVF) (pH 4.2). According to Owen DH and Katz DF, 1999, SVF was prepared where pH of vagina in vaginal candidiasis is below 4.5 (Abidin IZ, et al., 2020; Medscape, 2024). So, pH of SVF was adjusted to 4.2 pH with the help of glacial acetic acid. Compositions of SVF are shown in Table 1.
| Composition in distilled water | Quantity of ingredients (mg/ml) | |
|---|---|---|
| Sodium chloride | 3.51 | |
| Potassium hydroxide | 1.4 | |
| Lactic acid | 2 | |
| Acetic acid | 1 | |
| Glycerol | 0.16 | |
| Calcium hydroxide | 0.22 | |
| Bovine serum albumin | 0.02 | |
| Urea | 0.4 | |
| Glucose | 5 | |
| pH | 4.2 | |
Table 1: Simulated Vaginal Fluid (SVF) composition
Selection of lipids, surfactants and method for the preparation of NLCs
Screening of solid lipids: Different type of solid lipids such as stearic acid, Compritol® 888 ATO, Apifil®, GelotTM 64, Imwitor® 491, Precirol® ATO 5, Gelucire 44/14, GMS and Gelucire® 50/13 were screened for their solubilizing capacity to FLZ. For dissolve 10 mg of FLZ in molten solid lipid, a procedure was followed. Initially 10 mg of various solid lipids were individually heated on a water bath at a temperature 5°C-7°C higher than their respective melting points. Once the solid lipid completely melted 10 mg of FLZ was added to the molten lipid. If the FLZ did not dissolve completely, additional increments of 10 mg of solid lipid were added and the temperature was maintained 5°C-7°C above the melting point until complete solubilization of FLZ was achieved. The test tubes were visually examined for clarity. After FLZ completely dissolved in molten solid lipid that amount of solid lipid noted (Takalkar D and Desai N, 2018).
Screening of liquid lipids and surfactants: An excess of drug was added individually to 1 ml of liquid lipids such as oleic acid, Capryol® 90, Captex®, Sefsol, LabrafacTM, Miglyol® 812 and Labrafil® M 1944 CS and surfactants such as tween 80 and 20. The mixture mix with cyclomixer (CM-101 Plus, Mumbai, India) and shaken for 72 h in water bath shaker. Then centrifuged and supernatant was diluted with methanol AR and was analyzed by UV spectrophotometer (V-1900, Shimadzu, Japan) at specific wavelength (λmax of 261.5 nm) (Takalkar D and Desai N, 2018).
Optimization of composition of lipid blend: Ratio of solid lipid:liquid lipid was selected by miscibility test. Based on the solubility studies, miscibility tests were performed with various ratios of optimized solid lipids and liquid lipids (5:5-9:1). The lipid ratios were heated to temperature 5°C-7°C above the melting point of the selected solid lipid on watch glass. The blend was cooled until complete solidification occurred. After blend solidified, a filter paper was pressed onto it. If oil stains were observed on the filter paper it indicated that the solid lipid and liquid lipid compositions were immiscible. On the other hand, if no stains were present on the filter paper, then it indicated that the ratio of solid lipid and liquid lipid was miscible. FLZ was incorporated after optimizing blend. Lipid blend having maximum solubility of drug was selected for drug loading in formulation (Rangaraj N, et al., 2020).
Preparation and characterization of NLCs of FLZ
Development of nanostructured lipid carrier of FLZ: Drug loaded NLCs prepared using solvent-diffusion method followed by High shear homogenizer (Luo Y, et al., 2015; Hu FQ, et al., 2005; Esmaeili F, et al., 2008; Naik JB, et al., 2012). 0.5% of FLZ and required amount of solid lipid and liquid lipid completely dissolved in containing equal amount of acetone and ethanol (1:1 v/v) which was then heated at 5°C-7°C above the melting point of solid lipids. Clear organic phase was seen then mixed with aqueous phase containing emulsifiers at same temperature on magnetic stirrer (1MLH, Remi, Mumbai, India) at 1200 revolutions per minute (rpm) and homogenize (IKA T 10 basic ULTRA-TURRAX®, Germany) for 10 min at 18,000 rpm.
hitosan coating was applied on NLCs vesicle. A chitosan solution (0.05%- 0.25%) was prepared in distilled water with a pH of 4.2 adjusted using glacial acetic acid used as the aqueous phase in the formulation. The preparation procedures were carried out by the same steps as described previously.
NLCs batches were optimised on basis of particle size, Polydispersity Index (PDI) and EE%. Particle size and PDI were measured on Beckman Coulter particle size analyser (N5 submicron particle size analyser, Japan). Particle size, PDI and zeta potential of optimised formulation was measured with the help of Malvern Zetasizer (ZS90, Malvern, United Kingdom (UK)).
Optimization of concentration of surfactant: Surfactant was chosen based on the solubility of drug as well as the method of preparation of NLCs. To achieve physically stable dispersion the drug has optimal solubility in surfactant and partitioning between the lipid phase and aqueous surfactant phase. NLCs were prepared by solvent diffusion method followed by high shear homogenizer where previously optimized lipid blend was kept constant while various concentration of surfactant was assessed for optimization of surfactant. The prepared NLCs formulation was analysed using various parameters including particle size, PDI, zeta potential and encapsulation efficiency. Concentration at which surfactant gives desirable results that concentration was selected for preparation of NLCs (Kaithwas V, et al., 2017).
Characterization of NLCs of FLZ loaded NLCs
Visual morphology: Color and odor of the formulation were assessed through visual inspection.
Particle size, PDI and zeta potential measurements: Malvern Zetasizer ZS90 (Malvern, UK) was used to measure particle size, PDI and zeta potential. All the samples were diluted 10 times in double distilled water before being analyzed in disposable polystyrene cells.
Entrapment Efficiency (EE): FLZ EE in NLCs was determined using an indirect method (Takalkar D and Desai N, 2018). 1 ml of NLCs dispersion were placed in ultracentrifuge tube and subjected to centrifugation in an ultracentrifuge (Biofuge Stratos highspeed table top centrifuge) at 26,000 g for 40 min at 4°C. Clear supernatant by appropriate dilution with methanol AR was analyzed by UV spectrophotometer (V-1900, Shimadzu, Japan) at fixed wavelength (λmax 261.5 nm).
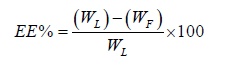
Where, WL=total amount of FLZ added WF=amount of FLZ present in the supernatant.
Total drug content: 1 ml of the prepared NLCs dispersion was taken (n=3) and dissolved in 10 ml of methanol AR sonicating for 20 min (2 cycles for 10 min) and analyzed by UV spectrophotometry (V-1900, Shimadzu, Japan) at fixed wavelength (λmax of 261.5 nm). The placebo formulation prepared similarly and used as blank (Takalkar D and Desai N, 2018).
in vitro drug release studies: A dialysis bags (Molecular cutoff 12-14 kD, Himedia, India) previously hydrated in SVF pH 4.2 was filled with 10 g of Nano particle Formulation (NF) 8, NF11 formulation and FLZ solution containing 50 mg of drug and placed in 100 ml of SVF pH 4.2 separately at 37°C ± 0.5°C and stirred at 50 rpm on magnetic stirrer. Aliquots taken at 15, 30, 60, 120, 180, 240, 300, 360, 420 and 480 minutes intervals and analyzed using UV spectrophotometer (V-1900, Shimadzu, Japan) at wavelength (λmax 261 nm) (Takalkar D and Desai N, 2018).
Transmission Electron Microscopy (TEM): The NLCs dispersion was diluted with double distilled water then few drops were placed on a copper grid (3 mm) using a micropipette. Allow to dry for about 10 minutes under an infrared lamp. The sample-loaded grid placed on a probe and bombarded with electrons accelerated to 120 kV. The particle size of the sample was analyzed and its structure was observed on computer screen (Takalkar D and Desai N, 2018).
Preparation and characterization of NLCs loaded gel of FLZ: Mucoadhesive polymers like Carbopol 971 P and Carbopol 974 P were tested as gelling polymers for vaginal delivery by incorporating them in NLCs dispersions to produce stable gels and hydrating for 8 hours the nano lipid vaginal gels were neutralized with triethanolamine to pH 4.2 (Takalkar D and Desai N, 2018).
Characterization of nano structural lipid carrier-based FLZ gel visual morphology: Colour and odor appearance by visual inspection was carried out.
Differential Scanning Calorimetry (DSC): The DSC thermogram of pure FLZ, excipient, physical mixture of drug, excipients and optimized gel formulation were obtained using DSC 1 STARe system (Mettler, Toledo, Switzerland). 10 mg of Sample was weighed and filled in a DSC pan and sealed properly. This pan was placed in DSC instrument along with a reference pan and heated from 30°C-300°C. The nitrogen gas is purged at the rate of 80 ml/min during an experiment to maintain an inert environment and endotherm was recorded (Nitsure A, et al., 2020).
pH and drug content: pH was measured by digital pH meter (Universal Enterprises, Mumbai, India) to determine total drug content weighed 1 g amounts of gel were extracted in triplicate by using methanol AR in water bath sonicating for 30-40 minutes. The drug content was determined by by UV spectrophotometer (V-1900, Shimadzu, Japan) at fixed wavelength (λmax 261.5 nm) after appropriate dilutions. The placebo formulation prepared similarly and used as blank (Takalkar D and Desai N, 2018).
Viscosity, spreadability and mucoadhesion strength: The viscosity determined by using a Brookfield viscometer LVT model (Brookfield Engineering Labs, Inc, USA) at room temperature, dial readings were taken with spindle numbers LV 4 0.3,0.6,1.5,3,6,12 and 30 rpm, respectively. The viscosity was calculated in centipoises and a rheogram of shear rate (rpm) vs. viscosity was plotted (Takalkar D and Desai N, 2018).
Spreadability of the gel determine by parallel-plate method on first glass plate marked circle of 1 cm diameter and 0.5 g NLCs loaded gel formulation put in it and second glass plate placed for 5 minutes over it contain weight of 500 g on the upper glass plate and allowed to rest. The spreading of the gels result in increase in diameter and increased dimeter noted (ShahKA, et al., 2007).
Modified two pan balances used to determine mucoadhesion of gel formulation. On left hand side of balance Teflon blocks having 3.8 cm in diameter kept inside the glass container at bottom which pleased below hanging cylinder. The two sides of pan were balanced using calibrated weights.
Preparation of mucin film for mucoadhesion: On a glass coverslip a mucin film was made by spreading 20 μl solution of 3% w/v porcine mucin in SVF on a perfectly horizontal surface. The film was air-dried before being employed in an adhesion test.
Measurement of adhesion force: During measurement the mucin films were hydrated with a drop of SVF pH 4.2. The cover slips were attached to the Teflon blocks with mucin containing sides facing each other using double sided adhesive tape. The test sample of gel was placed between the two cover slips on the left pan of the balance. Gels was allowed to adhere to the mucin film. After this incrementally weight added in right hand side pan of balance until separation of two mucin surface present at left hand side of balance and the mucoadhesion force was calculated as
F= (W×g )
Where, F is the mucoadhesion force (mN), W is the minimum weight required to break the mucoadhesive bond and g is the acceleration due to gravity (cm/s2)
in vitro drug release: A dialysis bags (molecular cutoff 12-14 kD, Himedia, India) previously hydrated in SVF pH 4.2 was filled with 10 g of NF8G, NF11G and marketed formulation separately containing 50 mg of drug and placed in 100 ml of SVF (pH4.2) at 37 ± 0.5°C and stirred 50 rpm on magnetic stirrer. Aliquots were taken at 15,30,60,120,180,240,300,360,420 and 480 minutes of intervals and analyzed using by UV spectrophotometer (V-1900, Shimadzu, Japan) at fixed wavelength (λmax 261 nm) (Takalkar D and Desai N, 2018).
ex vivo permeation and tissue deposition studies using porcine vaginal mucosa: ex vivo permeation studies on porcine vaginal mucosa were conducted. Vaginal mucosa was obtained from a nearby slaughterhouse and porcine tissues were separated. The surgical scissors were used to remove the majority of the adhering cartilaginous tissue from the mucosa without damaging the mucosa. During transportation, the vaginal mucosa was preserved in SVF pH 4.2 and was used within 2 h of the animals was slaughtered.
Franz diffusion cells were used in the ex vivo permeation studies. An appropriate size of vaginal tissue was cut and placed between the donor and receptor compartments of the cells. The drug permeation from a 1 g of gel containing 5 mg FLZ was measured in 20 ML of SVF pH 4.2 at 37°C ± 0.5°C and stirred at 50 rpm on magnetic stirrer. Aliquots were taken at 15, 30, 60, 120, 180, 240, 300, 360, 420 and 480 minutes intervals and analyzed using by UV spectrophotometer (V-1900, Shimadzu, Japan) at fixed wavelength (λmax 261 nm). The data was plotted as cumulative drug permeated through the porcine vaginal mucosa vs. time (Takalkar D and Desai N, 2018).
Tissue deposition study: At the end of 8 h the gel formulation present on vaginal tissue was collected in a container by using spatula and the tissue was washed with filtered distilled water and separated. Then, it was cut into fine pieces and extracted into methanol AR with sonication. Filtered through a 0.45 μm Millipore filter, diluted appropriately and was analyzed for drug content by by UV spectrophotometer (V-1900, Shimadzu, Japan) at fixed wavelength (λmax 261nm) (Takalkar D and Desai N, 2018).
in vitro antifungal activity: Colony Forming Units (CFU) per ml was calculated with help of viable cell count method. Using the cup plate method, the antifungal activity of optimized NLCs loaded FLZ gel, marketed gel, drug solution in PEG 400 and blank NLCs loaded gel was measured. For this study Candida albicans ATCC 10231 fungal strain was used on Sabouraud dextrose agar medium. The growth medium was seeded with C. albicans and wells of diameter 5 mm aseptically punched. Appropriately diluted samples containing the same volume of drug and formulations were placed in the wells with 45 μg of FLZ is present in each well. The plates were allowed to stand for complete diffusion of solution after that plate were incubated at 25°C for 48 hours. The zones of inhibition produced were measured in mm and the in vitro antifungal activity was determined (Takalkar D and Desai N, 2018).
Irritation study
Hen’s Egg Test-Chorioallantoic Membrane (HET-CAM) assay for vaginal irritation: CAM is a full tissue with arteries, veins and capillaries that responds to injury with an inflammatory mechanism.
Eggs and incubation conditions: Fresh fertile chicken eggs are used in the study (not more than 7 days). When eggs were brought into the lab, they were examined for checking shell damage and damaged eggs were discarded. Eggs which are not damaged were incubated for 8 days on automatic rotation at 37.8°C ± 0.3°C at 58% ± 2% relative humidity. To confirm embryo formation eggs were thoroughly examined on the 8th day with Light Emitting Diode (LED) light. Non-viable and non-embryonic eggs were discarded the eggs were incubated for another day under the same conditions mention above but without rotation (Palmeira-de-Oliveira R, et al., 2018).
Hen’s Egg Test-Chorioallantoic Membrane (HET-CAM) assay: After day 9, the eggs were removed from the incubator placed eggs such way that wider part facing upward direction. The internal membrane was exposed after the shell was opened with a scalpel and tweezers. A test sample of 0.3 ml was applied to the CAM (n=3). The entire CAM was then observed by observing three endpoints for 5 minutes.
The end points were observed at which hemorrhage (vessel bleeding), lysis (vessels disintegration) and coagulation (intra and extra-vascular protein denaturation) occur.
These endpoints were evaluated using two different analysis methods Irritability Scores (IS) A and B explain in (Table 2) (Palmeira-de-Oli - veira R, et al., 2018). Following test groups were used, 0.9% w/v sodium chloride in distilled water (negative control), 0.1 N sodium hydroxide in distilled water (positive control) and NF8G formulation.
|
IS analysis (method A) |
IS analysis (method B) |
|||
|---|---|---|---|---|---|
| Irritation response | 0-9: Non-severe irritant | 0-0.9: Non-irritant | |||
| >9-21: Severe irritant | 1-4.9: Slight irritant | ||||
| 5-8.9: Moderate irritant | |||||
| 9-21: Severe irritant | |||||
| IS calculation | According to the following scheme, the sum of the scores assigned at each time point to the occurrence of the corresponding effect | Calculation of the IS was carried out using following equation-
|
|||
| Endpoint | IS score | ||||
| 0.5 min | 2 min | 5 min | |||
| Lysis | 5 | 00:00 | 1 | ||
| Hemorrhage | 7 | 00:00 | 3 | ||
| Coagulation | 9 | 00:00 | 5 | ||
Table 2: Protocol for calculation of IS
Stability study: Goal of the stability study is to show how the quality of a drug substance or drug product changes over time and in response to changes in environmental conditions such as temperature, humidity and light. NF8G formulations were packed in collapse tubes stored at room temperature and refrigerator for the period of three months. The samples were analyzed at the end of each month (Helal DA, et al., 2012) as per International Council for Harmonisation (ICH) guidelines Q1C. Parameters such as appearance, total drug contain, viscosity, spreadability, mucoadhesive strength and pH of the samples were examined.
Results and Discussion
Analytical method development
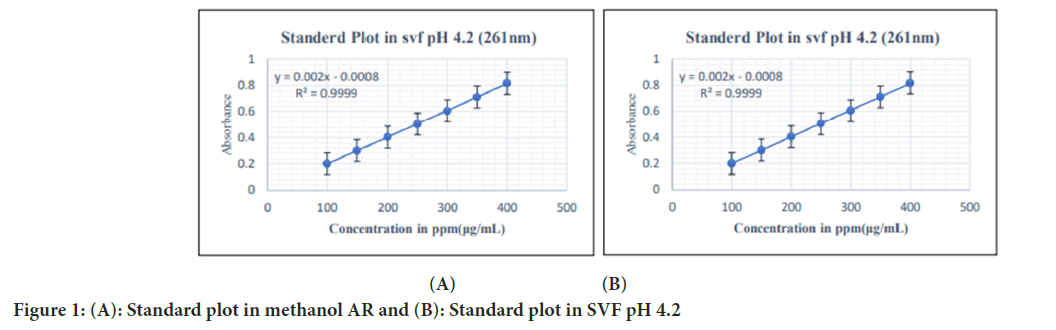
According to ICH Q2 (R2) guidelines, we validated analytical methods which were developed for quantification of FLZ using UV spectroscopy in methanol AR and SVF at pH 4.2 In methanol AR Regression coefficient (R2) was found to be 0.9994 with linearity range of 100-400 ppm shown in Figure 1a. In SVF pH 4.2, R2 and linearity range was found to be 0.9999 and 100-400 ppm, respectively. Standard plot in SVF pH 4.2 is shown in Figure 1b.
Figure 1: (A): Standard plot in methanol AR and (B): Standard plot in SVF pH 4.2
Selection of lipids, surfactants and method for the preparation of NLCs including the screening of solid lipids was carried out. FLZ exhibits the maximum solubility in steric acid, Gelot® 64 and Compritol® 888 ATO. The amount of solid lipid required to dissolve 10 mg of FLZ shown in (Figures 2a and 2b).
Figure 2: (A): Screening of solid lipids and (B): Screening of liquid lipid and surfactants
Further, liquid lipid and surfactants were screened. In comparison to other examined liquid lipid FLZ exhibits the maximum solubility in oleic acid and Caproyl® 90 and low in Tween 80. The system was more stable while using of Tween 80 than another surfactant. Saturation solubility of a FLZ in various liquid lipid and surfactant.
The composition of lipid blend was optimized. Ratio of solid lipid:liquid lipid was selected by miscibility test. Various solid lipid and liquid lipid composition and their miscibility is shown in Table 3. Among this optimized ratio of solid lipid: liquid lipid was found to be Compritol® 888 ATO:oleic acid was 8:2 which having high drug solubility than other miscible ratios.
| Solid lipid:liquid lipid | Ratio | Miscibility |
|---|---|---|
| Stearic acid:oleic acid | 07:03 | No |
| 08:02 | Yes | |
| 09:01 | Yes | |
| Stearic acid:Capryol® 90 | 07:03 | No |
| 08:02 | No | |
| 09:01 | Yes | |
| Compritol® 888 ATO:oleic acid | 07:03 | No |
| 08:02 | Yes | |
| 09:01 | Yes | |
| Compritol® 888 ATO: Capryol® 90 | 07:03 | No |
| 08:02 | Yes | |
| 09:01 | Yes |
Table 3: Solid lipid and liquid lipid composition and their miscibility
Subsequently, the nanostructured lipid carrier of FLZ was developed by using 8:2 ratio of Compritol® 888 ATO:oleic acid and 2%-2.5% w/w tween 80 placebo batches with solvent diffusion method followed by high-speed homogenization. During placebo formulation batches process parameter was optimized such as magnetic stirring rate (rpm) and time (min) as well as homogenization rate (rpm) and time (min) optimised by analysing particle size and PDI. Optimized process parameter used for formulation of drug loading NLCs.
NLCs of FLZ developed by solvent diffusion method followed by high shear homogenization various formulation shown in Table 4. According to partial size, PDI and % EE the formulation NF 8 and NF11 are optimized.
| S no. | NLCs formulations | Solid Lipid (SL) (%W/W) | Liquid Lipid (LL) (%W/W) | Ratio (SL: LL) | Surfactant (%W/W) | Chitosan pH 4.2 (%W/W) | Particle size (nm) | PDI | %EE (mean ± %RSD) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | NF1 | Steric acid (1.8%) | Olic acid | 8:2 | Tween 80 (2%) | 0.10% | 636.3 | 1.03 | 79.9±1.22 |
| 2 | NF2 | Steric acid | Olic acid | 8:2 | Tween 80 (2.5%) | - | 169.7 | 0.4 | 70.35±0.82 |
| 3 | NF3 | Steric acid | Olic acid | 8:2 | Tween 80 (2%) | 0.20% | 297.4 | 0.7 | 79.96±1.41 |
| 4 | NF4 | Steric acid | Olic acid | 8:2 | Tween 80 (2%) | - | 221.2 | 0.43 | 79.99±1.23 |
| 5 | NF5 | Steric acid | Capreol® 90 | 9:1 | Tween 80 (2%) | - | 234.4 | 0.75 | 70.59±2.04 |
| 6 | NF6 | Compritol® 888 ATO | Olic acid | 8:2 | Tween 80 (1.5%) | - | 374.3 | 0.74 | 80.9±1.75 |
| 7 | NF7 | Compritol® 888 ATO | Olic acid | 8:2 | Tween 80 (2.5%) | - | 315.6 | 0.4 | 68.32±1.21 |
| 8 | NF8 | Compritol® 888 ATO | Olic acid | 8:2 | Tween 80 (2%) | - | 202 | 0.3 | 78.99±1.92 |
| 9 | NF9 | Compritol® 888 ATO | Olic acid | 8:2 | Tween 20 (1.5%) | - | 200 | 0.15 | 64.58±1.41 |
| 10 | NF10 | Compritol® 888 ATO | Olic acid | 8:2 | Tween 20 (2%) | - | 276.6 | 0.07 | 71.44±0.32 |
| 11 | NF11 | Compritol® 888 ATO | Olic acid | 8:2 | Tween 80 (2%) | 0.10% | 281 | 0.34 | 80.53±0.48 |
| 12 | NF12 | Compritol® 888 ATO | Olic acid | 8:2 | Tween 80 (2%) | 0.20% | 331.9 | 0.5 | 84.35±1.58 |
Table 4: Various NLCs formulation
Characterization of optimized NLCs formulation of FLZ visual morphology denoted milky white dispersion with no any unpleasant odor.
Particle size, PDI and Zeta potential measurements
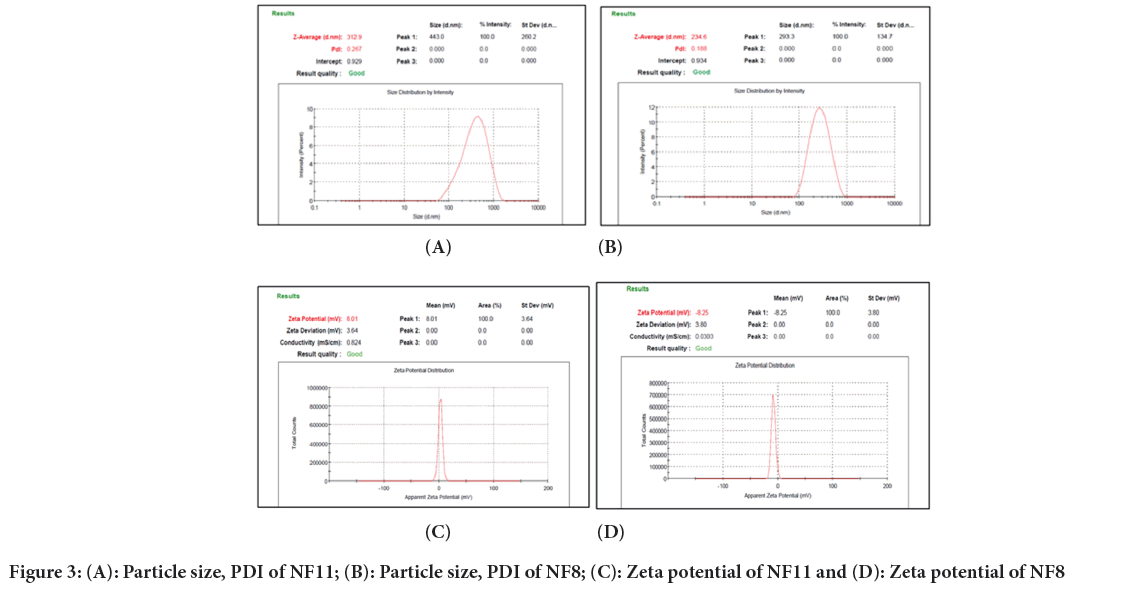
The average particle size of chitosan coated Nano lipid Formulation (NF11) and NF8 (uncoated) formulations were found to be 312.9 nm and 234.6 nm, respectively indicating narrow particle size distribution. In NF11 formulation, particle size was larger than particle size of NF8 formulation as well as surface charge is positive due chitosan coating on NLCs. Coating of chitosan on NLCs improves its mucodhesion on vaginal epithelium. Chitosan binds to mucous membrane by various mechanisms among which most commonly bind through Hydrogen (H) bonding with mucin glycoprotein due to the presence of Hydroxyl (-OH) and Amine (-NH2) groups where other is electrostatic interaction between positively charged chitosan amines and negatively charged mucin sialic acid residue which is assumed to be a factor in binding (Chatterjee B, et al., 2017). PDI of NF11 and NF8 formulations was found to be 0.267 and 0.188 respectively and it shows homogeneity of a system. An average particle size and PDI of NF11 and NF8 formulation is shown in Figures 3a and 3b respectively. Zeta potential of NF11 and NF8 formulations was found to be +8.01 mV and -8.25 mV respectively shown in Figures 3c and 3d respectively.
Figure 3: (A): Particle size, PDI of NF11; (B): Particle size, PDI of NF8; (C): Zeta potential of NF11 and (D): Zeta potential of NF8
Entrapment Efficiency (EE)%
Average FLZ entrapment in the developed NF11 formulation was found to be 82.74 ± 2.1% and NF8 formulation was found to be 78.99 ± 1.92%.
Total drug content of NF11 formulation was found to be 99.89 ± 0.7% while NF8 formulation was found to be 100.04% ± 0.46%. NF11 formulation entrapment is higher than NF8 formulation, due to the polymer coating (chitosan) on it.
in vitro drug release studies of NLCs formulation indicated an initial rapid release due to the presence of unentrapped drug outside the lipid core after this it shows sustained release within 8 h. NF11 and NF8 formulation was found to be 73.19% ± 1.01% and 84.22% ± 0.94% respectively over the period of 8 h. Drug release from drug solution was found 98.16% ± 0.62% over the period of 6 h. Graphical representation of the in vitro drug release of NLCs shown in Figure 4. NF11 formulation having less drug release than NF8 over the period of 8 h due to coating on it which is effect on drug release from lipid core of NLCs. Uncoated FLZ loaded NLCs (NF8) was selected as optimized batch as per results its drug release is more than chitosan coating (NF11) formulation within 8 h of period
Figure 4: In vitro drug release of D-solution, uncoated Nano particle Formulation (NF8) and chitosan coated Nano particle Formulation (NF11)
Note: ( ): D-solution; (
): D-solution; ( ): NF8 and (
): NF8 and ( ): NF11
): NF11
To understand the morphology (size and shape) of nanoparticles TEM studies were performed on NF8 formulation. The diameter of the nanoparticles was found to be below 200 nm as shown in Figure 5. TEM analysis revealed that the particles were uniform and spherical with no aggregation. This means that the particles in NLCs were uniformly distributed. The results were found to be consistent with the zeta-sizer. TEM images of NF8 formulation indicate that there was no evidence of drug precipitation and spherical nano lipid vessels are seen in images. According to all above observation NF8 formulation of NLCs had shown optimal results so it selected for incorporation of gelling agent.
Figure 5: TEM images of NF8 formulation
NLCs loaded FLZ gel were prepared and their characterization studies were observed. Between Carbopol 974P NF (USP/NF Monograph Carbomer 934P) and Carbopol 971P NF (USP/NF Monograph Carbomer 941) Carbopol 974P NF selected as gelling agent because it is, highly cross- linked polymer and high viscosity system formed.
NLCs loaded gel (NF8G gel contain NF8 NLCs formulation and NF11G gel contain NF11 NLCs formulation) were prepared using Carbopol 974P (1.25% w/w) as mucoadhesive polymer to improve the residence time of the formulation in the vaginal cavity selection of Carbopol 974P NF concentration is shown in Table 5.
| Concentration (% w/w) (pH 4.2) | Consistency |
|---|---|
| 0.5 | Low |
| 0.75 | Moderate |
| 1 | Moderate |
| 1.12 | High |
Table 5: Selection of carbopol concentration
Similarly, the visual morphology and characterization study of the nano structural lipid carrier-based gel was also studied where we observed clear milky white gel without any lumps and unpleasant odor.
DSC thermogram of drug, excipient, physical mixture and optimized gel is shown in Figure 6. In FLZ thermogram sharp endothermic peak shown at 139.68°C and in thermogram of optimized formulation, one broad peak shown all excipient are compatible with each other. The absence of a drug peak in the DSC thermogram of NF8G gel formulation indicated drug entrapment in the lipid matrix which is amorphous in nature; pH of NF11G and NF8G formulations was found to be 4.2 which mimic to environment of vaginal pH in fungal infection and drug content was found to be 99.74% ± 0.26% and 100.65% ± 1.166% respectively.
Figure 6: DSC image of (A): Fluconazole ATO; (B): Competerol 888; (C): Physical mixture and (D): NF8G formulation
Viscosity, spreadability and mucoadhesive strength
Viscosity of NF8G and NF11G was measured by using Brookfield viscometer LVT model; viscosity is shown in Figure 7. Concave nature of the rheogram indicated that the gel flow was pseudoplastic and it is observed that this system was shear thinning system because as shear rate (rpm) increases viscosity decreases. At 6 rpm viscosity of NF11G formulation was found to be 82333.33 ± 1.855 Centipoise (cPs) and viscosity of NF8G formulation was found to be it 75666.67 ± 0.76 CPs was observed. As compared to viscosity of uncoated NLCs loaded (NF8G) gel the viscosity of chitosan coated NLCs loaded (NFG11G) gel was comparatively more. The diameter increased by spreading of the gel using parallel-plate method was found to be 7.8 cm ± 3.85 cm.
Figure 7: Viscosity of NF8G and NF11G formulation
Note: ( ): NF8G and (
): NF8G and ( ): NF11G
): NF11G
Mucoadhesive strength of NF8G and marketed formulations were determined by using a modified two pan balance apparatus to be (3534.53 ± 4.71) dyne/cm2 and (2864.86 ± 1.75) dyne/cm2 respectively which was 1.2 times more than that of the marketed gel.
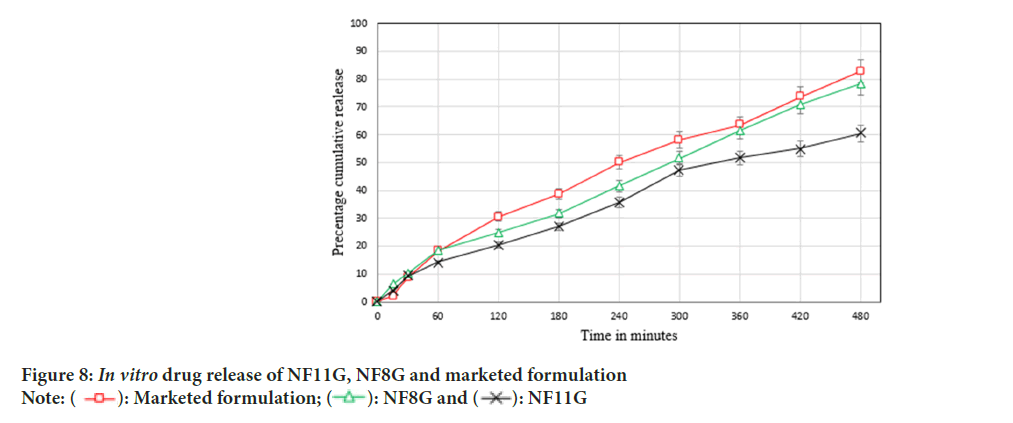
in vitro drug release of NF11G formulation, NF8G formulation and marketed formulation was found to be 60.56% ± 0.46%, 78.38% ± 0.81% and 80.14% ± 0.49%, respectively over the period of 8 hours. Graphical representation of the in vitro drug release shown in Figure 8. When chitosan coated NLCs are loaded in gelling agent (NF11G) the cumulative release of drug decreases due to crosslinking of gelling agent and chitosan. Compritol® 888 ATO used as a solid lipid in lipid blend and itself give sustained release of drug so slow drug release seen in NF11G gel formulation as compare to NF8G gel formulation. Uncoated NLCs loaded gel formulation (NF8G) has sufficient drug release over the period of 8 h. So NF8G gel formulation was selected as optimized gel formulation.
Figure 8: In vitro drug release of NF11G, NF8G and marketed formulation
Note: ( ): Marketed formulation; (
): Marketed formulation; ( ): NF8G and (
): NF8G and ( ): NF11G
): NF11G
Drug release kinetics
The drug release kinetics of FLZ from an optimized NF8G gel formulation was found to be zero order (R2=0.991) mathematical model of in vitro release data of the NF8G gel formulation is shown in (Table 6).
| Release kinetics | R2 |
|---|---|
| Zero order | 0.991 |
| Higuchi model | 0.979 |
| Hixson-Crowell model | 0.976 |
| First order | 0.9714 |
| Kors-peppas model | 0.809 |
Table 6: Release kinetics of optimized gel, NF8G
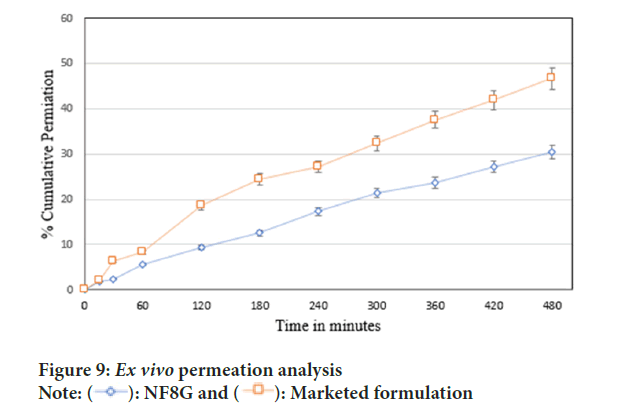
ex vivo permeation study as shown in Figure 9, denoted the cumulative percentage of drug permeated through porcine vaginal mucosa of marketed formulation and NF8G gel formulation was found to be 44.98% ± 0.55% and 30% ± 1.61% respectively in 8 hours of period.
Figure 9: Ex vivo permeation analysis
Note: ( ): NF8G and (
): NF8G and ( ): Marketed formulation
): Marketed formulation
We have further studied the amount of FLZ deposited in porcine vaginal mucosa was found to be 32.24% ± 1.71% in marketed preparation and optimized gel formulation showed 42.04% ± 1.12% indicating higher drug deposition in case of marketed formulation it was due to NF8G gel was NLCs based gel and marketed formulation gel does not contain NLCs. Higher drug deposition gives potential of localized effect it is beneficial to treating vaginal infections.
in vitro antifungal activity was done by using the cup plate method. Different test samples such as NF8G gel formulation, FLZ solution in polyethylene glycol 400, blank NF8G gel formulation and marketed FLZ gel formulation was evaluated for determination of zones of inhibition (n=2). The diameters of the zones of inhibition of FLZ loaded optimized NF8G formulation were found to be 29 mm ± 1.24 mm. Dimeter of FLZ solution and marketed FLZ gel was found to be 28 mm ± 2.41 mm and 30 mm ± 1.52 mm respectively. There is no zone of inhibition by blank NF8G gel formulation. Zone of inhibition of optimized gel, FLZ solution, blank vaginal gel and marketed FLZ gel is shown in Figure 10. Zone of inhibition is slightly more in case of NF8G gel formulation as compare to marketed formulation due to in NF8G formulation contain NLCs which lipid core made up by solid lipid and liquid lipid. Oleic acid was used as a liquid lipid according to literature (Fatima I, et al., 2022) oleic acid increases antifungal activity because oleic acid has fixed-bend C=C bonds, it can occupy a larger cross-section when entering the fungal membrane.
Figure 10: In vitro antifungal activity of the formulation
Irritation study
Hen’s eggs test-chorioallantoic membrane (HET-CAM) test was used to assess vaginal irritation. This is an alternative way to animal testing for determining vaginal-irritancy of ingredients used in formulation. The CAM is a full tissue with arteries, veins and capillaries that responds to injury with an inflammatory mechanism. Irritation Score (IS) of test group by anlysis method A and B, as shown in Table 7.
| Endpoint | IS score for negative control (0.9% NaCl) | IS score for positive control (0.1 N NaOH) | IS score for NF8G | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Method A | Method B | Method A | Method B | Method A | Method B | |||||
| 0.5 min | 2 min | 5 min | 0.5 min | 2 min | 5 min | 0.5 min | 2 min | 5 min | - | |
| Lysis | - | - | SI=0.023 | 5 | - | SI=16.55 | - | - | 1 | SI=0.04 |
| Haemorrhage | - | - | - | 7 | - | - | - | - | 3 | - |
| Coagulation | - | - | - | - | 7 | - | - | - | - | - |
| Total IS score | 1 non severe irritant | Non-irritant | 19 severe irritant | Severe irritant | 4 Non severe irritant | Non-irritant | ||||
Table 7: IS of negative, positive controls and optimised gel
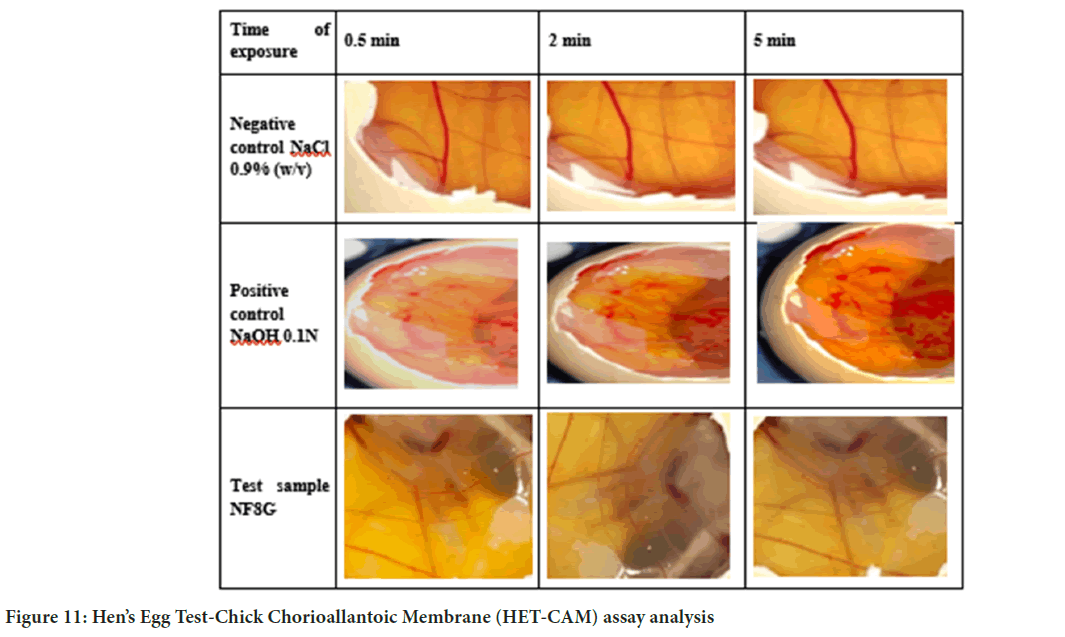
The negative control 0.9% Sodium Chloride (NaCl) does not show any hemorrhage, blood clotting but slightly damage vessel. The images displayed in Figure 11. conclude that the negative control solution does not show any irritancy within 5 minutes test period. 0.1 N Sodium Hydroxide (NaOH) solution on CAM membrane it started hemorrhage in blood vessels of CAM membrane irritation score by method A was found to be 19 which is indication of severe irritant and irritation score by method B was found to be 16.55 which shows severe irritant; HET-CAM image has been displayed in Figure 11.
Figure 11: Hen’s Egg Test-Chick Chorioallantoic Membrane (HET-CAM) assay analysis
Third set of eggs were used for NF8G gel formulation. On the CAM membrane it show slight lysis and hemmorage at the end of 5 minutes. IS by method A was found to be 4 which is indication of non-severe irritant and IS by method B was found to be 0.04 which was shown non-irritant. HET-CAM image displayed in Figure 11. Blood vessel were not ruptured and no excessive hemorrhage on CAM membrane was observed within the end of 5 min. This indicates that the optimised formulation of gel was nonirritant to the HET-CAM membrane and safe to use for the vaginal drug delivery.
Stability studies should be performed to ensure that quality, safety and efficacy of product are maintained over the duration of its shelf life. It was found to be stable within the period of three months.
Conclusion
In this work FLZ, NLCs were prepared by using solvent diffusion method followed by high share homogenization and it is good method for formation of NLCs having optimum particle size and PDI. Optimized NLCs batch having spherical vesicles are seen in TEM images. That optimized NLCs are incorporate in a gel. Two types of NLCs were prepared in this work first was chitosan coated NLCs (NF11) and another was uncoated NLCs (NF8). The intention behind applying a chitosan coating to the NLCs was that to get much adhesion to vaginal mucosa but final formulation was NLCs loaded gel and mucoadhesive gelling agent is used to formed NLCs based gel. Therefore, it is not necessary to give chitosan coat to NLCs for mucoadhesion because gelling agent also contributes significantly to enhanced adhesion. When chitosan coat given to NLCs and that that NLCs incorporate in gel the drug release decreases due to cross linking of gelling agent and chitosan coat present on NLCs thus, to achieve the highest level of drug release from the gel within 8 hours period chitosan coating to NLCs avoided. Drug release is maximum in that uncoated NLCs (NF8) within 8 hours so that uncoated NLCs formulation was optimized and used for formation of gel.
According to characterization it is conclude that surfactant concentration is inversely proportional to an EE%, Compritol® 888 ATO is used for sustained release and with oleic acid its activity increases also give good sustain release of drug. It is also seen that combination in lipid blend is increase solubility of FLZ as compere to separate solid lipid and liquid lipid it helps to carry sufficient amount of drug and according to drug release data shown above cross linking of gelling agent also effect on release of drug from gel. NLCs formulation NF8 show good result so that batch is optimized.
Optimized uncoated NLCs was used to formation of intravaginal gel by using Carbopol 974P which is muchoadhesive polymerase formulation of NLCs based gel it having good drug continent also more mucoadhesion and spreadability than marketed formulation pH of gel formulation was found to be 4.2 which is mimic to vaginal pH in fungal infection. Antifungal activity and tissue deposition of gel formulation was more than marketed formulation so it results indicate NLCs based gel having good effect than marketed gel. Also gel formulation was found stable in 3 months.
Acknowledgement
The authors are thankful to Aarti Pharma Bhandup (Mumbai, India) for providing the sample of FLZ with certificate of analysis. The authors also thank Icon Labs (Mumbai, India) and Narsee Monjee Institute of Management Studies (NMIMS) for carrying out the TEM and DSC, respectively of the formulations. The authors duly acknowledge the M K Rangnekar Memorial laboratory, Mumbai (India) for antifungal assay.
References
- Lema VM. Recurrent vulvo-vaginal candidiasis: Diagnostic and management challenges in a developing country context. Obstet Gynecol Int J. 2017; 7(5): 260.
- Willems HM, Ahmed SS, Liu J, Xu Z, Peters BM. Vulvovaginal candidiasis: A current understanding and burning questions. J Fungi. 2020; 6(1): 27.
[Crossref] [Google Scholar] [Pubmed]
- Sobel JD. Vaginitis. N Engl J Med. 1997; 337(26): 1896-903.
- Yano J, Sobel JD, Nyirjesy P, Sobel R, Williams VL, Yu Q, et al. Current patient perspectives of vulvovaginal candidiasis: Incidence, symptoms, management and post-treatment outcomes. BMC Women Health. 2019; 19: 1-9.
[Crossref] [Google Scholar] [Pubmed]
- Takalkar D, Desai N. Nanolipid gel of an antimycotic drug for treating vulvovaginal candidiasis-development and evaluation. AAPS PharmSciTech. 2018; 19: 1297-1307.
[Crossref] [Google Scholar] [Pubmed]
- de Pereira ARR, Bruschi ML. Vaginal mucoadhesive drug delivery systems. Drug Dev Ind Pharm. 2012; 38(6): 643-652.
[Crossref] [Google Scholar] [Pubmed]
- Pasko MT, Piscitelli SC, van Slooten AD. Fluconazole: A new triazole antifungal agent. DICP. 1990; 24(9): 860-867.
[Crossref] [Google Scholar] [Pubmed]
- Tholakanahalli VN, Potti A, Hanley JF, Merliss AD. Fluconazole-induced torsade de pointes. Ann Pharmacother. 2001; 35(4): 432-434.
[Crossref] [Google Scholar] [Pubmed]
- Pham CP, de Feiter PW, van der Kuy PH, van Mook WN. Long QTc interval and torsade de pointes caused by fluconazole. Ann Pharmacother. 2006; 40(7-8): 1456-1461.
[Crossref] [Google Scholar] [Pubmed]
- Fernandes AV, Pydi CR, Verma R, Jose J, Kumar L. Design, preparation and in vitrocharacterizations of fluconazole loaded nanostructured lipid carriers. Braz J Pharm Sci. 2020; 56: e18069.
- Naseri N, Valizadeh H, Zakeri-Milani P. Solid lipid nanoparticles and nanostructured lipid carriers: Structure, preparation and application. Adv Pharm Bull. 2015; 5(3): 305.
[Crossref] [Google Scholar] [Pubmed]
- Owen DH, Katz DF. A vaginal fluid simulant. Contraception. 1999; 59(2): 91-95.
[Crossref] [Google Scholar] [Pubmed]
- Abidin IZ, Rezoagli E, Simonassi-Paiva B, Fehrenbach GW, Masterson K, Pogue R, et al. A bilayer vaginal tablet for the localized delivery of disulfiram and 5-fluorouracil to the cervix. Pharmaceutics. 2020; 12(12): 1185.
[Crossref] [Google Scholar] [Pubmed]
- Vaginitis. Medscape. 2024.
- Rangaraj N, Pailla SR, Shah S, Prajapati S, Sampathi S. QbD aided development of ibrutinib-loaded nanostructured lipid carriers aimed for lymphatic targeting: Evaluation using chylomicron flow blocking approach. Drug Deliv Transl Res. 2020; 10: 1476-1494.
[Crossref] [Google Scholar] [Pubmed]
- Luo Y, Teng Z, Li Y, Wang Q. Solid lipid nanoparticles for oral drug delivery: Chitosan coating improves stability, controlled delivery, mucoadhesion and cellular uptake. Carbohydr Polym. 2015; 122: 221-229.
[Crossref] [Google Scholar] [Pubmed]
- Hu FQ, Jiang SP, Du YZ, Yuan H, Ye YQ, Zeng S. Preparation and characterization of stearic acid nanostructured lipid carriers by solvent diffusion method in an aqueous system. Colloids Surf B Biointerfaces. 2005; 45(3-4): 167-173.
[Crossref] [Google Scholar] [Pubmed]
- Esmaeili F, Atyabi F, Dinarvand R. Preparation and characterization of estradiol-loaded PLGA nanoparticles using homogenization-solvent diffusion method. 2008; 196-202.
- Naik JB, Lokhande AB, Mishra S, Kulkarni RD. Development of sustained release micro/nanoparticles using different solvent emulsification technique: A review. Int J Pharm Bio Sci. 2012; 3(4): 573-590.
- Kaithwas V, Dora CP, Kushwah V, Jain S. Nanostructured lipid carriers of olmesartan medoxomil with enhanced oral bioavailability. Colloids Surf B Biointerfaces. 2017; 154: 10-20.
[Crossref] [Google Scholar] [Pubmed]
- Nitsure A, Patel D, Wairkar S. Improved processability of ethambutol hydrochloride by spherical agglomeration. Pharm Dev Technol. 2020; 25(3): 376-384.
[Crossref] [Google Scholar] [Pubmed]
- Shah KA, Date AA, Joshi MD, Patravale VB. Solid Lipid Nanoparticles (SLN) of tretinoin: Potential in topical delivery. Int J Pharm. 2007; 345(1-2): 163-171.
[Crossref] [Google Scholar] [Pubmed]
- Palmeira-de-Oliveira R, Machado RM, Martinez-de-Oliveira J, Palmeira-de-Oliveira A. Testing vaginal irritation with the Hen’s egg test-chorioallantoic membrane assay. ALTEX. 2018; 35(4): 495-503.
[Crossref] [Google Scholar] [Pubmed]
- Helal DA, El-Rhman DA, Abdel-Halim SA, El-Nabarawi MA. Formulation and evaluation of fluconazole topical gel. Int J Pharm Pharm Sci. 2012; 4(5): 176-183.
- Chatterjee B, Amalina N, Sengupta P, Mandal UK. Mucoadhesive polymers and their mode of action: A recent update. J Appl Pharm Sci. 2017; 7(5): 195-203.
- Fatima I, Rasul A, Shah S, Saadullah M, Islam N, Khames A, et al. Novasomes as nano-vesicular carriers to enhance topical delivery of fluconazole: A new approach to treat fungal infections. Molecules. 2022; 27(9): 2936.
[Crossref] [Google Scholar] [Pubmed]
Author Info
Shubhangi Aher* and Munjaji Balasaheb PitaleCitation: Aher S: Mucoadhesive Nanostructured Lipid Carriers Based Gel to Treat Vulvovaginal Candidiasis
Received: 03-Jun-2024 Accepted: 19-Jun-2024 Published: 26-Jun-2024, DOI: 10.31858/0975-8453.15.6.194-204
Copyright: This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
ARTICLE TOOLS
- Dental Development between Assisted Reproductive Therapy (Art) and Natural Conceived Children: A Comparative Pilot Study Norzaiti Mohd Kenali, Naimah Hasanah Mohd Fathil, Norbasyirah Bohari, Ahmad Faisal Ismail, Roszaman Ramli SRP. 2020; 11(1): 01-06 » doi: 10.5530/srp.2020.1.01
- Psychometric properties of the World Health Organization Quality of life instrument, short form: Validity in the Vietnamese healthcare context Trung Quang Vo*, Bao Tran Thuy Tran, Ngan Thuy Nguyen, Tram ThiHuyen Nguyen, Thuy Phan Chung Tran SRP. 2020; 11(1): 14-22 » doi: 10.5530/srp.2019.1.3
- A Review of Pharmacoeconomics: the key to “Healthcare for All” Hasamnis AA, Patil SS, Shaik Imam, Narendiran K SRP. 2019; 10(1): s40-s42 » doi: 10.5530/srp.2019.1s.21
- Deuterium Depleted Water as an Adjuvant in Treatment of Cancer Anton Syroeshkin, Olga Levitskaya, Elena Uspenskaya, Tatiana Pleteneva, Daria Romaykina, Daria Ermakova SRP. 2019; 10(1): 112-117 » doi: 10.5530/srp.2019.1.19
- Dental Development between Assisted Reproductive Therapy (Art) and Natural Conceived Children: A Comparative Pilot Study Norzaiti Mohd Kenali, Naimah Hasanah Mohd Fathil, Norbasyirah Bohari, Ahmad Faisal Ismail, Roszaman Ramli SRP. 2020; 11(1): 01-06 » doi: 10.5530/srp.2020.1.01
- Manilkara zapota (L.) Royen Fruit Peel: A Phytochemical and Pharmacological Review Karle Pravin P, Dhawale Shashikant C SRP. 2019; 10(1): 11-14 » doi: 0.5530/srp.2019.1.2
- Pharmacognostic and Phytopharmacological Overview on Bombax ceiba Pankaj Haribhau Chaudhary, Mukund Ganeshrao Tawar SRP. 2019; 10(1): 20-25 » doi: 10.5530/srp.2019.1.4
- A Review of Pharmacoeconomics: the key to “Healthcare for All” Hasamnis AA, Patil SS, Shaik Imam, Narendiran K SRP. 2019; 10(1): s40-s42 » doi: 10.5530/srp.2019.1s.21
- A Prospective Review on Phyto-Pharmacological Aspects of Andrographis paniculata Govindraj Akilandeswari, Arumugam Vijaya Anand, Palanisamy Sampathkumar, Puthamohan Vinayaga Moorthi, Basavaraju Preethi SRP. 2019; 10(1): 15-19 » doi: 10.5530/srp.2019.1.3