Research Article - (2022) Volume 13, Issue 10
Abstract
PANI-ZnO nano composite was synthesized by both electrochemical and chemical synthesis methods. Chemically synthesized nanomaterials were prepared via in-situ oxidation polymerization method. Then, the synthesized nanomaterial’s: Polyaniline (PANI), ZnO and PANI-ZnO were characterized by Ultraviolet- Visible (UV-Vis), SEM and Fourier Transform Infrared spectroscopy (FT-IR). The UV-Vis spectrum of PANI-ZnO nano composite was studied to investigate the optical properties. After addition of ZnO particle to the PANI, the polymer shows red shift of π to π* transition. The Scanning Electron Microscope (SEM) shows PANI nano fibers distributed on the surface of ZnO. Moreover, Fourier Transform Infrared spectroscopy (FT-IR) spectra show that chemical bond and stretching assigned to different peaks. Polyaniline- Zinc Oxide/Glass Carbon Electrode (PANI-ZnO/ GCE) fabricated electrode was prepared via layer by layer electrochemical polymerization of aniline on the surface of Glass Carbon Electrode. Multilayered electro active PANI-ZnO/GCE composite was synthesized by electrochemical polymerization of aniline on GCE followed by coating with 5 μL ZnO nanoparticle suspension. The fabricated ZnO-PANI/GCE was again polymerized with aniline to produce PANI-ZnO/ GCE composite films. Then, electrochemical behavior of paracetamol at PANI-ZnO composite film fabricated glass carbon electrode was characterized by Cyclic Voltammetry (CV). The fabricated electrochemical sensor shows good analytical performance for paracetamol with a detection limit 7.50 × 10−7 M; the sensitivity of 4.84 with linear concentration of paracetamol within the range of 1-80 μM by employing deferential pulse voltammetry.
Keywords
Electrochemical sensor, Polyaniline (PANI) nanofibers, Polyaniline-Zinc Oxide/Glass Carbon Eectrode (PANI-ZnO/GCE), Nanocomposite
Introduction
In recent years, electrochemical sensors based on conducting polymer-metal oxide composite materials, have attracted much interest of researchers. Conducting polymers such as Polyaniline, polyethylene, polythiophene, polypyrrole and their derivatives can be utilized as active components in light emitting diodes, electrochemical cells, solar energy converting and photovoltaic devices, batteries, super capacitors and chemical sensors. This is due to their conductivity, light weight, mechanical flexibility and chemical stability (Ebrahim S, et al., 2014). Among conducting polymers, Polyaniline (PANI) is the most important because of its superior environmental and thermal stabilities, high electrical conductivity, oxidative properties, ease of preparation and low fabrication cost (Gerard M, et al., 2002).
PANI has three different fundamental oxidation forms: Leucomeraldine (fully reduced), Emeraldine Base (EB: Half oxidized) and pernigraniline (fully oxidized). The only electrically conducting is Emeraldine Salt form (ES: Half oxidized), which is the protonated form of PANI-EB (Masoumi V, et al., 2014). Polyaniline was first described in the mid-19th century by Henry Lethe, who investigated electrochemical and chemical oxidation products of aniline in acidic media. He noted that reduced form was colorless but the oxidized forms were deep blue (Inzelt G, 2012). The electrochemically polymerized PANI was uniformly deposited on the graphite electrode, the deposit was three dimensional and porous suitable for biomolecule immobilization (Gvozdenović MM, et al., 2011).
Several research works have already been made to prepare PANI nano composites by chemical and electrochemical preparation methods, using nanostructured metal oxides, in order to benefit from their unique electro catalytic properties. Owing to the synergistic effect, nano composites have usually higher electrical conductivity than individual nanostructured metal oxides. Recently, several groups reported the Polyaniline supported metal oxide nano composite prepared by in-situ polymerization methods (Alves KG, et al., 2012).
Nanostructured Zinc Oxide (ZnO) has unique properties like high isoelectric point, transparent n-type semiconductor with direct wide band gap (3.37 eV), biocompatibility, nontoxicity, high chemical stability, and high electron transfer capability (Alam M, et al., 2013). Carbon Dots (CDs)/ZnO/PANI modified ITO (Indium Tin Oxide) showed the largest current signal due to the cooperation of CDs/ZnO/PANI conductive composites. The reason is assigned to that the ZnO nano rod and CDs approaches the critical value which accounts for the increase in conductivity of composites. The developed electrochemical biosensor also showed good selectivity and was successfully used to detect E. coliO157:H7 in water samples with a detection limit of 1.3 × 10−18 M, indicating a promising method for sensitive electrochemical detection of pathogens (Pangajam A, et al., 2020). Wang P, et al., 2009 reported a novel method of designing catechol biosensors using Polyphenol Oxidase (PPO) and PANI with glutaraldehyde as cross-linker. A linear range of 0.2-80 μmol dm−3 was obtained with a short response time and good stability. Tan Y, et al., 2010 fabricated catechol biosensors based on the immobilization of PPO into PANI. A linear range of 1.25-150 μmol dm−3 was measured with a good reproducibility with 3.1% relative standard deviation. Fabrication of PANI nano composites with a number of electro active nanomaterial enhance electrochemistry by increasing surface area and promising stability for efficient, sensitive, and durable biosensor designs. Feng X, et al., 2013 fabricated PANI-modified GCE-based dopamine sensors, which showed high catalytic activity in electrochemical oxidation of dopamine.
In the present work, we report synthesis of ZnO nanoparticles and fabrication of PANI-ZnO nano composite on Glass Carbon Electrode (GCE) via layer by layer electrochemical polymerization of aniline for the detection of paracetamol. We have motive to fabricate this electrochemical sensor via layer by layer electrochemical polymerization of aniline for detection of paracetamol because there is no published report for this work. The prepared PANI, ZnO and PANI-ZnO nano composites were characterized by FT-IR, UV-Vis and Scanning Electron Microscopy (SEM).
Materials and Methods
Materials
Zn(NO3)2.6H2O (99.0% purity), NaOH ( ≥ 98% purity), Aniline, Ammonium Peroxodisulfate (APS) sodium dihydrogen phosphate (98%), disodium hydrogen phosphate (98%), hydrochloric acid (35%-37%), potassium Nitrate (99%), paracetamol, argon gas (99.9%), potassium ferricyanide (99%) purchased from India and distilled water.
Synthesis of ZnO nanoparticles
ZnO nanoparticles were synthesized by using reported method (Juma AO and Matibini A, 2017). A precursor solution containing 0.05 M Zn-solution was first prepared by dissolving 2.9749 g of Zn(NO3)2.6H2O in 200 ml of distilled water. The solution was magnetically stirred for 15 min at 80°C on a hot plate. In a separate beaker, 2.0 M NaOH solutions were prepared by dissolving 12 g of NaOH pellets in 250 ml distilled water. The solution was then magnetically stirred for 10 min at room temperature. 40 ml of the NaOH solution was then added into Zn-solution while stirring. A white precipitate started forming immediately. The white precipitate turned the whole solution white. The mixture was continuously stirred for a further 30 min at 80°C and then left to cool down to room temperature. The precipitate was harvested through filtration, washed several times with distilled water and left to dry in open air over night. The chemical reaction involved can be described by:
Zn(NO3)2+2NaOH ➝ Zn(OH)2+2NaNO3 1
Zn(OH)2 ➝ ZnO⭣+H2O (at low pH)
Chemical synthesis of PANI and PANI-ZnO composite
Polyaniline was chemically synthesized using the literature procedure (Alam M, et al., 2013). 20 mL of distilled aniline was added to 250 mL 1 M HCl in round bottom flask at 27°C stirred for 30 minutes and subsequently 125 mL of 1 M APS solution was drop wise added. After the addition of APS, stirring of the reaction mixture was continuously carried out up to 4 hours at 0°C-4°C, resulting in thick green solution kept for 24 hours. The precipitate was washed with 1 M HCl to remove oligomers. It was then dried in vacuum oven at 60°C for 24 hours to obtain green colored PANI (Emeraldine).
Polyaniline/ZnO nanocomposites were prepared by in-situoxidation polymerization method (Yerawar GR, 2012). 2 g of ZnO was added to 20 mL of aniline prepared in aqueous hydrochloric acid (1 M) and stirred for half an hour, then allow settling down for another half an hour. To this solution, Ammonium Persulphate (APS) as an oxidant prepared in aqueous hydrochloric acid (1 M) was added drop-wise for half an hour under constant stirring at 4°C. The monomer to oxidizing agent ratio was kept at 1:1.25. Stirring was continue for eight hours, the resulting dark green mixture was kept overnight and then filter. The precipitated polymer was washed with distilled water until the filtrate was colorless, then with acetone and methanol to remove excess initiator, monomer and oligomer. Finally, the polymer was dried in air for about a day and then in an oven at 80°C for 15 hours.
Electrochemical characterization of chemically synthesized PANI and PANI/ZnO nano composites were carried out using Cyclic Voltammetry. Before drop coating as synthesized nano materials the bare GCE was polished with 0.05 μM, alumina powder, rinsed with distilled water followed by ultra-sonication with and deionized water and ethanol respectively, and dried at room temperature. 2 mg of PANI dissolved in 2 mL of ethanol and ultrasonicated. Then approximately 5 μL of the above suspension was then drop-coated onto the surface of a bare glassy carbon electrode and dried at room temperature. The electrochemical characterizations of PANI/GCE was performed in 1 M HCl in the potential range was from -0.5 V to +1.1 V and scan rates 30 40,50,60,70 and 80 mV s-1. Similarly for 2 mg of PANI/ ZnO was dissolved in 2 mL of ethanol followed sonication were drop-coated on the bare electrode to obtain PANI/ZnO/GCE. The characterization was similar condition as PANI/GCE.
Electrochemical synthesis of multilayered electro active Polyaniline-ZnO composite films fabricated electrode
The potentiodynamic electrochemical polymerization of ANI (0.1 M) in 1 M HCl aqueous solution was carried out in one compartment cell with Glassy Carbon (GC) as working electrode, platinum wire counter electrode and Ag/AgCl (KCl) as reference electrode. Prior polymerization the bare GC working electrode was polished with 0.05 μM alumina powder to get shiny surface and rinsed with distilled water followed by ultra-sonication with ethanol and deionized water respectively, dried at room temperature. Before electrochemical polymerization solution containing monomer was purged with argon and overflow of argon was maintained during electro polymerization. Electro polymerization was performed by 10 potential cycles carried out within the potential range -0.5 to 1.2 V. Thus formed PANI polymer on GCE surface was washed with deionized water before characterization by CV. After 10 cycles of electro polymerization of PANI in 1 M HCl containing 0.1 M ANI, 5 μL of ZnO suspension was drop coated on PANI/GCE surface and dried for 30 min. Then, the electrode was transferred back into aniline solution and additional electrochemical polymerization was carried out by 5 potentiodynamic cycles with the same scan rate of 50 mV s-1, and within the same potential range of -0.5 to 1.2 V.
Structural, morphological and electrochemical analysis
The structure and morphology of produced nano composites were characterized by FT-IR and Scanning Electron Microscopy (SEM). FT-IR spectroscopy measured on Spectrum 65 FT-IR (PerkinElmer) in the range 4000-400 cm-1 using KBr pellets to assign functional groups of PANI and PANI-ZnO were conducted at Addis Ababa Science and Technology University. The as synthesized samples were also characterized by SEM. All electrochemical measurements were conducted by Bass 100 three electrode systems at Haramaya University.
Optimization of PAN-ZnO/GCE as electrochemical sensor
The effect of pH on the oxidation peak current of paracetamol was studied in the presence of supporting electrolyte (0.1 M phosphate buffer solution and 0.1 mol L−1 KCl) in the range of 4.0 to 8.0. Concentration of paracetamol was optimized with different concentration range (1, 5, 10, 20, 30, 40, 50, 60, 80 and 100 μM).
Results and Discussion
Structural characterization
Scanning Electron Microscope (SEM): The morphology of the as-prepared PANI and PANI-ZnO composite were characterized using Scanning Electron Microscope (SEM). Figure 1a shows the surface morphology of the as-synthesized Polyaniline with separation of particles and not highly affected by agglomeration. The morphology of Polyaniline has changed with the introduction of ZnO nanostructures in Polyaniline. Figure 1b shows effective interaction of ZnO nanostructures with Polyaniline matrix. It gave clear indication about the particle separation; the particles are separated smoothly and not highly affected by agglomeration.
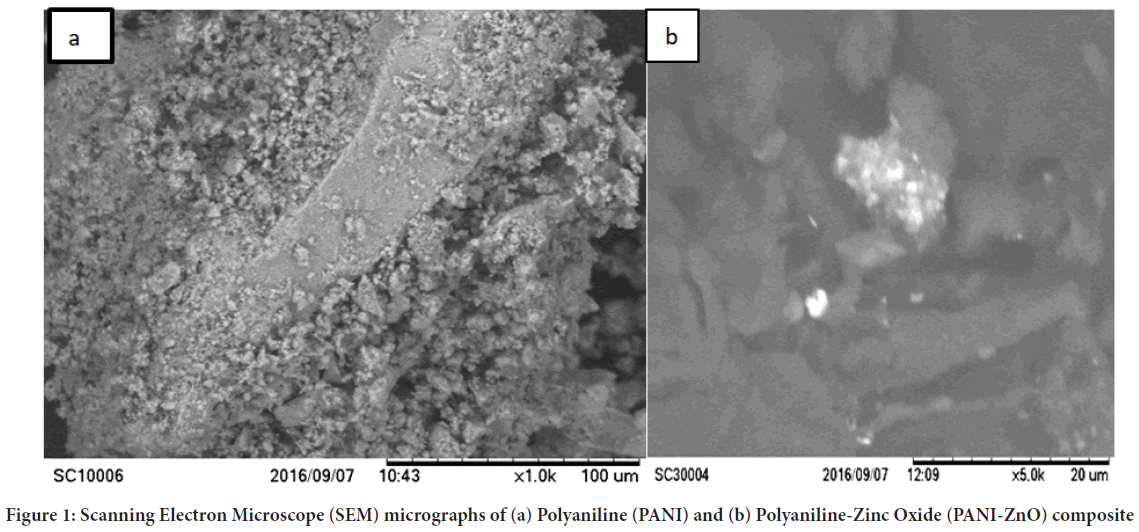
Figure 1: Scanning Electron Microscope (SEM) micrographs of (a) Polyaniline (PANI) and (b) Polyaniline-Zinc Oxide (PANI-ZnO) composite
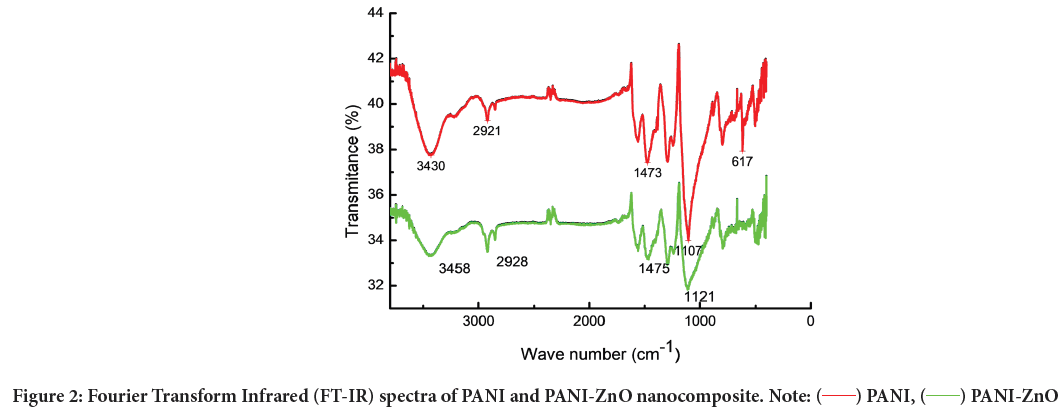
Fourier Transform Infrared (FT-IR) spectroscopy: As synthesized materials PANI and PANI-ZnO were characterized using FT-IR technique to indicate functional groups. The peaks at 1473 cm−1 and 1107 cm−1 show presence of quinonoid and benzenoid rings respectively Figure 2. The peaks at 1473 cm−1 and 1107 cm−1 are assigned to C–C ring asymmetric and symmetric stretching vibrations. The peak at 2921 cm−1 corresponds to C–H stretching. FT-IR spectrum of PANI-ZnO nanocomposite exhibit new distinct absorption peaks at 3458 cm−1, 2928 cm−1, 1475 cm−1 and 1121 cm−1 assigned to the presence of ZnO nanoparticles in the aniline polymer matrix. The absorption peaks in FT-IR spectra of PANI-ZnO composite film were found to shift to higher wave number when compared with pure PANI nanocomposite. The peak at 3435 cm-1 attributed due to interaction between ZnO and PANI by formation of hydrogen bonding between H–N and oxygen of ZnO.
Figure 2: Fourier Transform Infrared (FT-IR) spectra of PANI and PANI-ZnO nanocomposite. Note: ( ) PANI, ( ) PANI-ZnO
Ultraviolet−Visible (UV-Vis) optical spectroscopy and band gap study: UV-Visible spectra of ZnO, PANI and PANI-ZnO samples were obtained by using Diffuse Reflectance Spectroscopy (DRS) with BaSO4 as reference. The band gap can be estimated by Kubelka-Munk functions versus photon energy given by
(F(r)hv)2 vs. hv 2
F(r)=K/S 3
K=(1-R)2 4
S=(2R) 5
Where, hv is photo energy with v is frequency and h is Planks constant F(r) is Kubelka Munk function K is molar absorption coefficient R is reflectance data from the DRS data and S is the scattering factor
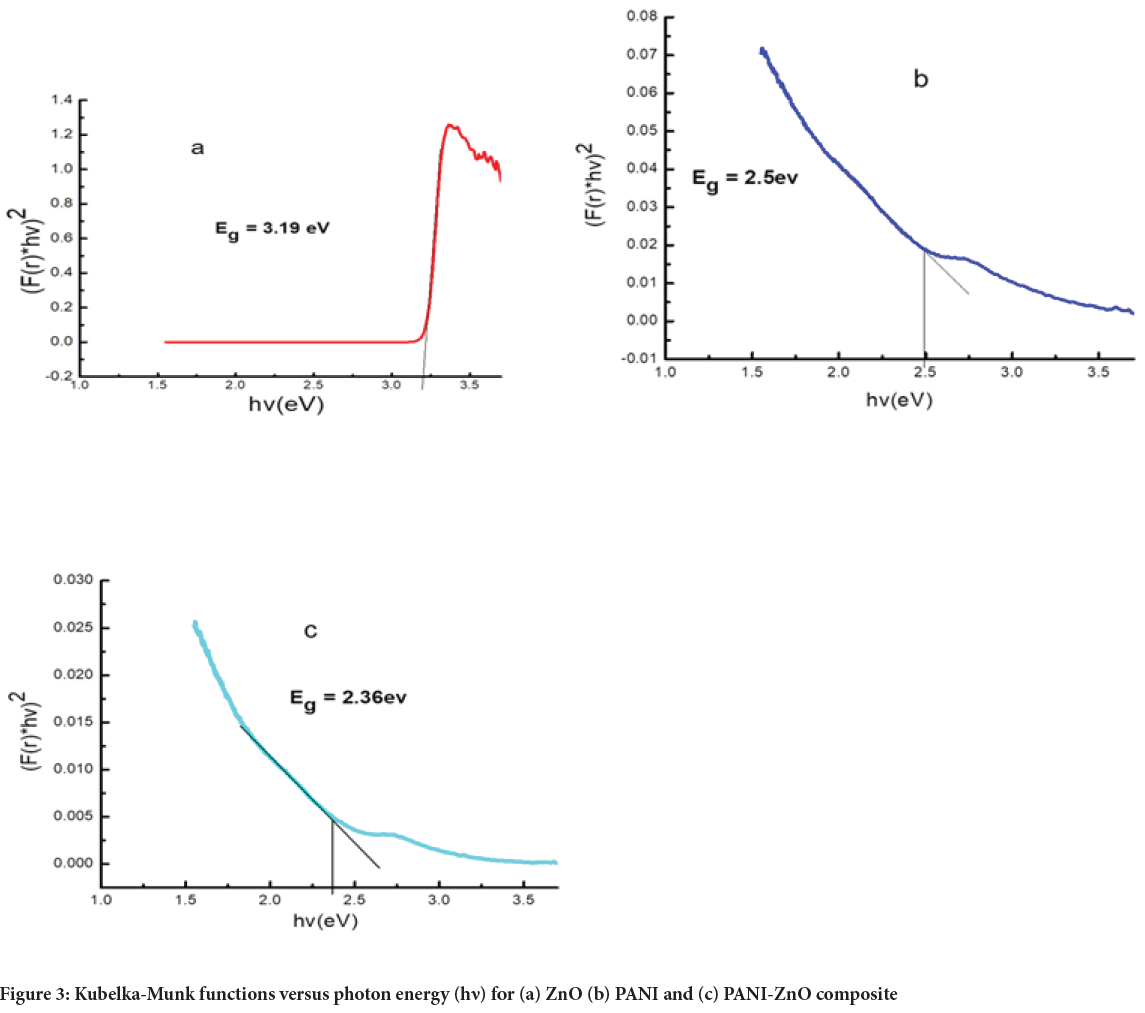
The optical band gap energy (E g) values were determined by transferring UV-Vis data into the absorbance scale by Kubelka-Munk analysis. The optical band gap graphs for ZnO, PANI and PANI-ZnO are sketched in Figures 3a-3c. The calculated E gof ZnO, PANI and PANI-ZnO were 3.19, 2.5 and 2.36 eV respectively. The band gap energy (2.50 eV) in the case of PANI is the same as some previous works (Farag AA, et al., 2010; Ziadan KM, 2005).
Figure 3: Kubelka-Munk functions versus photon energy (h?) for (a) ZnO (b) PANI and (c) PANI-ZnO composite
The band gap of PANI was decreased when ZnO nanoparticles added were added, what is due to the interaction between PANI and ZnO, causing changes in electron density of the Polyaniline chain (Kant S, et al., 2013). PANI-ZnO nano composite shows lower E gthan PANI alone. The reduced optical band gap energy of PANI-ZnO composite suggests an improve ment in the electrical and the optical properties.
Electrochemical polymerization and characterization of multilayered electro active Polyaniline-ZnO composite films fabricated electrode
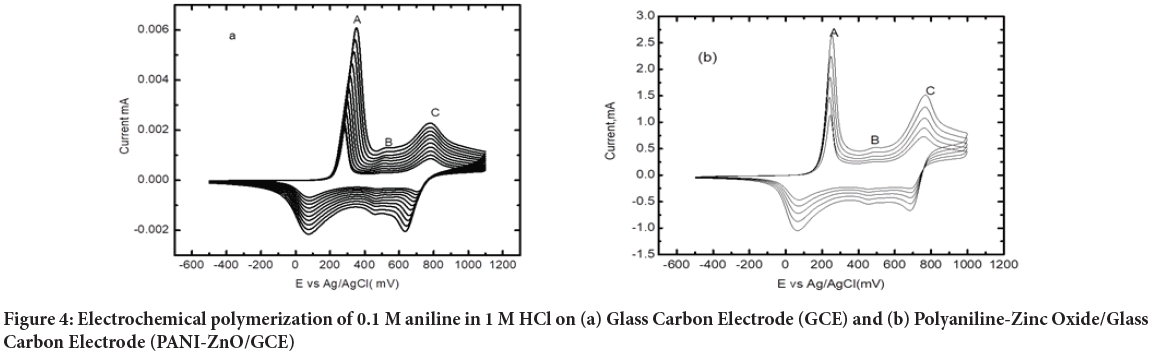
Cyclic Voltammograms recorded in the course of electro polymerization of ANI in 0.1 M HCl aqueous solution on GC electrode in the potential range -0.5 V to 1.2 V for 10 cycles at the scan rate of 50 mV s-1 is shown in Figure 4a. The first cycle is characterized by an irreversible oxidation of aniline during the forward scan. When the potential is switched in the reverse scan three cathodic waves appeared. As read from the last polymerization cycle; the three anodic peaks and three cathodic peaks occurred at 0.356, 0.538 and 0.783 V vs.Ag/AgCl and three cathodic peaks 0.076, 0.456 and 0.631 V for the first, second, and third waves respectively.
After drop coating of 5 μL ZnO suspension on PANI/GCE and dried for 30 min, aniline was polymerized on PANI-ZnO/GCE for 5 cycles at the same potential and scan rate. Figure 4b shows three redox peak and radical formation in first cycle polymerization, this shows that aniline monomers fully polymerized. The three redox peaks shows different fundamental oxidation forms of aniline: Leucomeraldine (fully reduced), Emeraldine Base (EB: Half oxidized) and pernigraniline (fully oxidized). The only electrically conducting is Emeraldine Salt form (ES: Half oxidized), which is the protonated form of PANI-EB.
Figure 4: Electrochemical polymerization of 0.1 M aniline in 1 M HCl on (a) Glass Carbon Electrode (GCE) and (b) Polyaniline-Zinc Oxide/Glass Carbon Electrode (PANI-ZnO/GCE)
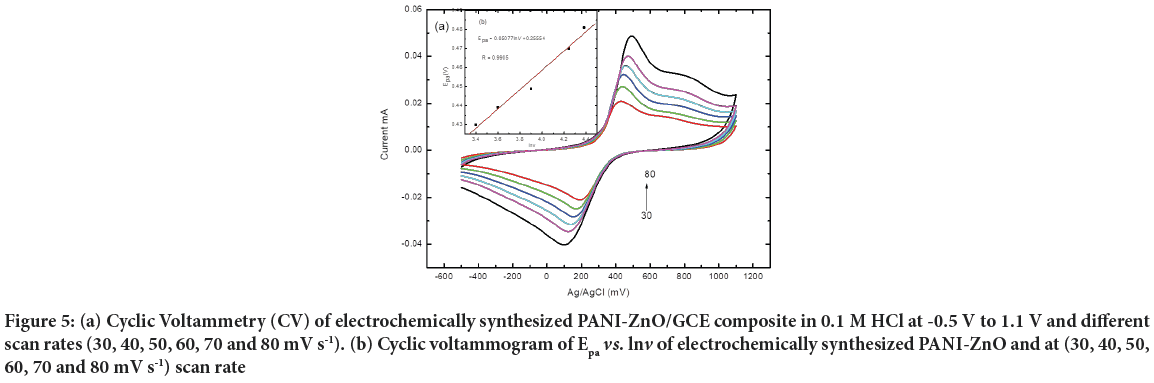
The fabricated PANI-ZnO/GCE electrode was characterized in 0.1 M HCl aqueous solution at potential range -0.5 to 1.1 V and 50 mV s−1 scan rate. The electrode characterized at different scan rates at the same potential. Figure 5ashow peak of current increases as scan rate increases; this indicates PANI-ZnO nano composite has good catalytic activities between GCE and 0.1 M HCl electrolyte.
The relationship between oxidation peak potential (Epa) and natural logarithm of scan rate (lnv) is also shown in Figure 5b. It can be seen that plotting of the Epa vs. lnv of PANI-ZnO/GCE produce a straight line at with equation and linear regression by using redox peak.
Figure 5: (a) Cyclic Voltammetry (CV) of electrochemically synthesized PANI-ZnO/GCE composite in 0.1 M HCl at -0.5 V to 1.1 V and different scan rates (30, 40, 50, 60, 70 and 80 mV s-1). (b) Cyclic voltammogram of Epa vs. lnv of electrochemically synthesized PANI-ZnO and at (30, 40, 50, 60, 70 and 80 mV s-1) scan rate
Electrochemical properties of chemically synthesized PANI/ GCE and PANI-ZnO/GCE
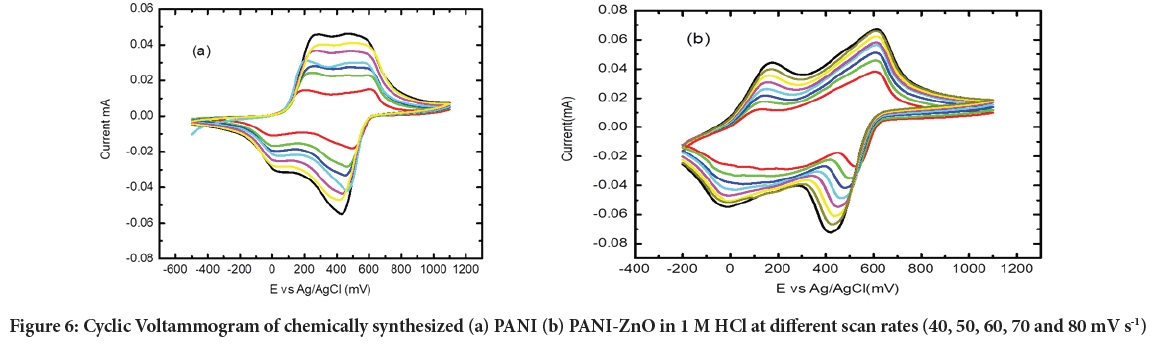
The nano composites PANI and PANI-ZnO were produced by in-situoxidation polymerization method. Each sonicated nano materials PANI and PANI-ZnO, 5 μL was drop coated on Glass Carbon Electrode (GCE) to produce PANI/GCE and PANI-ZnO/GCE respectively. The synthesized electrodes were characterized in 1 M HCl by applying Cyclic Voltammetry within the potential range of -500 to 1100 mV, and scan rate changing from 40 to 100 mV s-1. Figure 6a and b showed two anodic and two cathodic peaks as were also observed for electrochemically formed film. Two separate redox peaks correspond to two redox processes of PANI normally found in acid conditions, i.e., transition between fully reduced Leucoemeraldine state (LM) and half-oxidized Emeraldine state (EM), and transition between EM state and fully oxidized Pernigraniline state (PE). There is a linear relationship observed between the scan rate and peak current of the material.
Figure 6: Cyclic Voltammogram of chemically synthesized (a) PANI (b) PANI-ZnO in 1 M HCl at different scan rates (40, 50, 60, 70 and 80 mV s-1)
The response of PANI/GCE and PANI-ZnO/GCE electrodes showed change in scan rate. The peak current increases as the scan rate are increased. PANI-ZnO/GCE fabricated electrode in Figure 6b shows small peak to peak separation than PANI/GCE fabricated electrode in Figure 6a. The small peak to peak separation of PANI-ZnO reveals that a nano composite film was electro active, conducting and confined to the surface of the electrode.
Cyclic Voltammetric study of paracetamol behavior on multilayered electro active Polyaniline-ZnO composite films
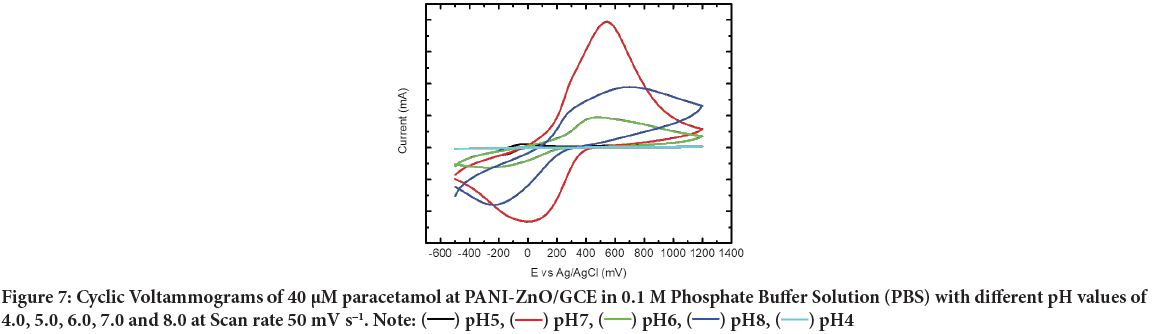
Effect of pH: The effect of solution pH on the redox reaction of paracetamol at the chemically synthesized PANI-ZnO/GCE was investigated in the range of pH 4.0-8.0. Solution pH also affects the performance of peak current and oxidation mechanism, so we examined the influence of pH on the electrochemical response of the electrodes. The relationship between chemical oxidation efficiency and pH of the solution was obtained by CV; the results are shown in Figure 7. The oxidation peak current increased from pH 4.0 to 7.0, the peak current reaching a maximum at pH 7.0. Further increasing the pH of the buffer solution caused the oxidation peak current to decrease. Furthermore, within the pH range of 4.0 to 7.0, the oxidation peak potential increased gradually, whereas from 7.0 to 8.0 it decreased, indicating low electron transfer rate.
Figure 7: Cyclic Voltammograms of 40 µM paracetamol at PANI-ZnO/GCE in 0.1 M Phosphate Buffer Solution (PBS) with different pH values of
4.0, 5.0, 6.0, 7.0 and 8.0 at Scan rate 50 mV s-1. Note:  pH5,
pH5,  pH7,
pH7,  pH6,
pH6,  pH8,
pH8,  pH4
pH4
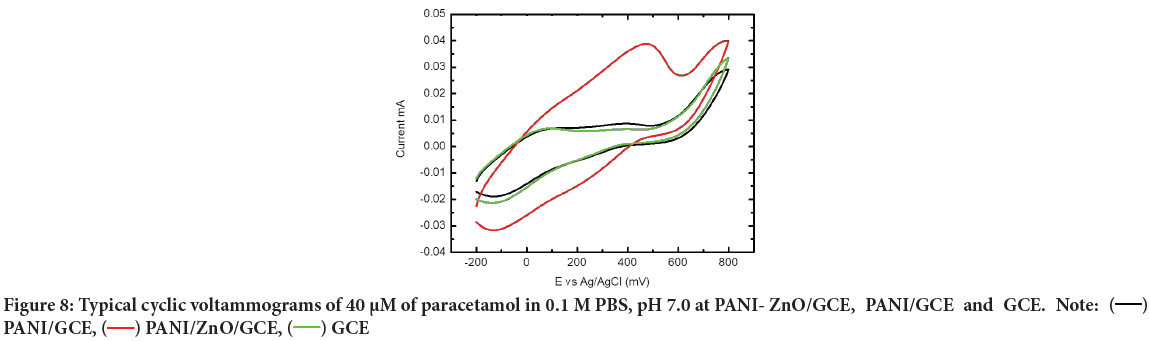
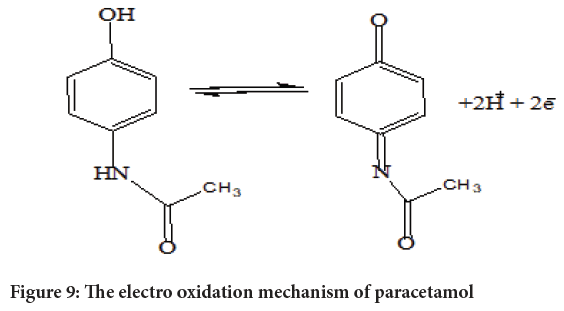
Cyclic Voltammetry (CV) of paracetamol at different electrodes: The electrochemical behavior of 40 μM of Paracetamol at the bare GCE, PANI/ GCE, and PANI-ZnO/GCE was investigated in phosphate buffer of pH 7.0 for comparison by using Cyclic Voltammetry and the results were shown in Figure 8. It can be seen the oxidation peak of paracetamol appears at the GCE, PANI/GCE, and PANI-ZnO/GCE with a potential range of -0.2 V to 0.8 V. PANI-ZnO/GCE electrode exhibits high anodic peak of current and voltammetric response revealing a catalytic property compared to the PANI/GCE and GCE electrodes in paracetamol solution. This may be due to good incorporation of ZnO nanoparticles and PANI-films which recognized to the large surface area and good conductivity of PANI-ZnO modified electrode. The increasing of anodic peak current of GCE<PANI/ GCE<PANI-ZnO/GCE electrodes show positive synergetic effect of synthesized nano composites. Due to thus catalytic property the fabricated PANI-ZnO/GCE uses for paracetamol detection. Electrochemical oxidation processes paracetamol shown in below schematic chemical reaction (Figure 9).
Figure 8: Typical cyclic voltammograms of 40 µM of paracetamol in 0.1 M PBS, pH 7.0 at PANI- ZnO/GCE, PANI/GCE and GCE. Note:  PANI/GCE,
PANI/GCE,  PANI/ZnO/GCE,
PANI/ZnO/GCE,  GCE
GCE
Figure 9: The electro oxidation mechanism of paracetamol
Electrochemical detection of paracetamol using Differential Pulse Voltammogram (DPV)
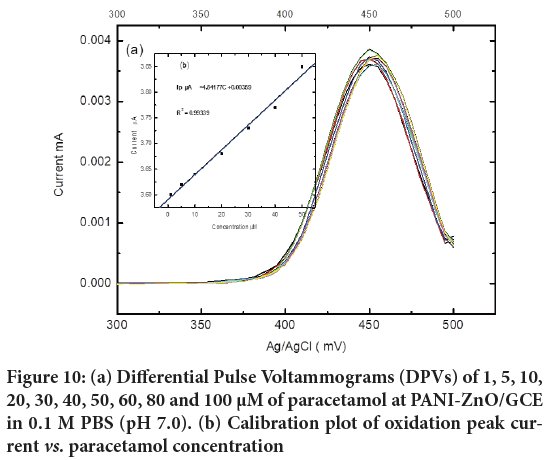
The voltammetric determination of paracetamol was carried out using a three electrode system in 0.1 M Phosphate Buffer Solution (PBS) (pH 7.0) and Differential Pulse Voltammetry at the PANI-ZnO/GCE as working electrode. The Differential Pulse Voltammograms (DPVs) of various concentrations of Paracetamol (1, 5, 10, 20, 30, 40, 50, 60, 80 and 100 μM) are illustrated in Figure 10 under the optimum experimental conditions of pH 7.0. The peak currents increase linearly against the concentration of paracetamol within the range of 1-80 μM. The calibration curve for paracetamol shows linear segment with the linear regression equation of Ip/mA=4.841 C+0.00359 (R=0.99339), the detection limit (S/N=3) is estimated to be 7.5 × 10−7 M and the sensitivity is 4.84. The synthesized PANI-ZnO/GCE is high sensitive for detection of Paracetamol at lower concentration at pH 7.0.
Figure 10: (a) Differential Pulse Voltammograms (DPVs) of 1, 5, 10, 20, 30, 40, 50, 60, 80 and 100 µM of paracetamol at PANI-ZnO/GCE in 0.1 M PBS (pH 7.0). (b) Calibration plot of oxidation peak current vs. paracetamol concentration
Conclusion
PANI-ZnO nanocomposite was synthesized and used as electrochemical sensing catalyst films on glassy carbon electrode for paracetamol. The films were developed chemically and electrochemically on glass carbon electrode using Cyclic Voltammetric method. The Cyclic Voltammetry shows that chemically synthesized PANI-ZnO/GCE were more electro active than PANI/GCE and GCE due to high surface area of PANI-ZnO. The PANI-ZnO/GCE shows larger estimated electron transfer rate constant 1.3 s-1 in 0.1 M HCl electrolytic solution. PANI-ZnO/GCE synthesized electrode based electrochemical sensor shows low detection limit and high sensitivity for determination of paracetamol. The produced PANI-ZnO nanocomposite may create a new approach for developing Polyaniline supported-metal oxide based electrochemical sensors and biosensors.
Acknowledgements
The authors would like to acknowledge Department of Chemistry; University of Haramaya for covering the Cyclic Voltammetry and Differential Pulse Voltammetry facility. Authors also acknowledge the Wolaita Sodo University Research office for financial support.
References
- Ebrahim S, El-Raey R, Hefnawy A, Ibrahim H, Soliman M, Abdel-Fattah TM. Electrochemical sensor based on polyaniline nanofibers/single wall carbon nanotubes composite for detection of malathion. Synth Met. 2014; 190: 13-19.
- Gerard M, Chaubey A, Malhotra BD. Application of conducting polymers to biosensors. Biosens Bioelectron. 2002; 17(5): 345-359.
[Crossref] [Google Scholar] [Pubmed]
- Masoumi V, Mohammadi A, Amini M, Khoshayand MR, Dinarvand R. Electrochemical synthesis and characterization of solid-phase microextraction fibers using conductive polymers: Application in extraction of benzaldehyde from aqueous solution. J Solid State Electrochem. 2014; 18(6): 1763-1771.
- Inzelt G. Conducting polymers: A new era in electrochemistry. Springer Science and Business Media. 2012.
- Gvozdenovic MM, Jugovic BZ, Bezbradica DI, Antov MG, Kneževic-Jugovic ZD, Grgur BN. Electrochemical determination of glucose using polyaniline electrode modified by glucose oxidase. Food Chem. 2011; 124(1): 396-400.
- Alves KG, Felix JF, de Melo EF, dos Santos CG, Andrade CA, de Melo CP. Characterization of ZnO/Polyaniline nanocomposites prepared by using surfactant solutions as polymerization media. J Appl Polym Sci. 2012; 125(S1): E141-E147.
- Alam M, Alandis NM, Ansari AA, Shaik MR. Optical and electrical studies of polyaniline/ZnO nanocomposite. J Nanomater. 2013.
- Pangajam A, Theyagarajan K, Dinakaran K. Highly sensitive electrochemical detection of E. coli O157: H7 using conductive carbon dot/ZnO nanorod/PANI composite electrode. Sens Bio-Sens Res. 2020; 29: 100317.
- Wang P, Liu M, Kan J. Amperometric phenol biosensor based on polyaniline. Sens Actuators B: Chem. 2009; 140(2): 577-584.
- Tan Y, Guo X, Zhang J, Kan J. Amperometric catechol biosensor based on polyaniline-polyphenol oxidase. Biosens Bioelectron. 2010; 25(7): 1681-1687.
[Crossref] [Google Scholar] [Pubmed]
- Feng X, Zhang Y, Yan Z, Chen N, Ma Y, Liu X, et al. Self-degradable template synthesis of polyaniline nanotubes and their high performance in the detection of dopamine. J Mater Chem A. 2013; 1(34): 9775-9780.
- Juma AO, Matibini A. Synthesis and structural analysis of ZnO-NiO mixed oxide nanocomposite prepared by homogeneous precipitation. Ceram Int. 2017; 43(17): 15424-15430.
- Yerawar GR. Characterization of chemically synthesized polyaniline-zinc oxide nanocomposites. Der Pharma Chem. 2012; 4(3): 1288-1291.
- Farag AA, Ashery A, Rafea MA. Optical dispersion and electronic transition characterizations of spin coated polyaniline thin films. Synth Met. 2010; 160(1-2): 156-161.
- Ziadan KM. The Electronic transitions of polyaniline doped with p-toluene Sulfonic acid. Journal of Kerbala University. 2005; 1: 67-74.
- Kant S, Kalia S, Kumar A. A novel nanocomposite of polyaniline and Fe0.01Ni0.01Zn0.98O: Photocatalytic, electrical and antibacterial properties. J Alloys Compd. 2013; 578: 249-256.
Author Info
Samuel Chufamo*, Bezabih Kelita, Alemu Lelago and Almaz KemalCitation: Chufamo S: Multilayered Electroactive Polyaniline-ZnO Composite Films Fabricated Electrode for Voltammetric Determination of Paracetamol
Received: 15-Sep-2022 Accepted: 10-Oct-2022 Published: 17-Oct-2022, DOI: 10.31858/0975-8453.13.10.694-700
Copyright: This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
ARTICLE TOOLS
- Dental Development between Assisted Reproductive Therapy (Art) and Natural Conceived Children: A Comparative Pilot Study Norzaiti Mohd Kenali, Naimah Hasanah Mohd Fathil, Norbasyirah Bohari, Ahmad Faisal Ismail, Roszaman Ramli SRP. 2020; 11(1): 01-06 » doi: 10.5530/srp.2020.1.01
- Psychometric properties of the World Health Organization Quality of life instrument, short form: Validity in the Vietnamese healthcare context Trung Quang Vo*, Bao Tran Thuy Tran, Ngan Thuy Nguyen, Tram ThiHuyen Nguyen, Thuy Phan Chung Tran SRP. 2020; 11(1): 14-22 » doi: 10.5530/srp.2019.1.3
- A Review of Pharmacoeconomics: the key to “Healthcare for All” Hasamnis AA, Patil SS, Shaik Imam, Narendiran K SRP. 2019; 10(1): s40-s42 » doi: 10.5530/srp.2019.1s.21
- Deuterium Depleted Water as an Adjuvant in Treatment of Cancer Anton Syroeshkin, Olga Levitskaya, Elena Uspenskaya, Tatiana Pleteneva, Daria Romaykina, Daria Ermakova SRP. 2019; 10(1): 112-117 » doi: 10.5530/srp.2019.1.19
- Dental Development between Assisted Reproductive Therapy (Art) and Natural Conceived Children: A Comparative Pilot Study Norzaiti Mohd Kenali, Naimah Hasanah Mohd Fathil, Norbasyirah Bohari, Ahmad Faisal Ismail, Roszaman Ramli SRP. 2020; 11(1): 01-06 » doi: 10.5530/srp.2020.1.01
- Manilkara zapota (L.) Royen Fruit Peel: A Phytochemical and Pharmacological Review Karle Pravin P, Dhawale Shashikant C SRP. 2019; 10(1): 11-14 » doi: 0.5530/srp.2019.1.2
- Pharmacognostic and Phytopharmacological Overview on Bombax ceiba Pankaj Haribhau Chaudhary, Mukund Ganeshrao Tawar SRP. 2019; 10(1): 20-25 » doi: 10.5530/srp.2019.1.4
- A Review of Pharmacoeconomics: the key to “Healthcare for All” Hasamnis AA, Patil SS, Shaik Imam, Narendiran K SRP. 2019; 10(1): s40-s42 » doi: 10.5530/srp.2019.1s.21
- A Prospective Review on Phyto-Pharmacological Aspects of Andrographis paniculata Govindraj Akilandeswari, Arumugam Vijaya Anand, Palanisamy Sampathkumar, Puthamohan Vinayaga Moorthi, Basavaraju Preethi SRP. 2019; 10(1): 15-19 » doi: 10.5530/srp.2019.1.3