Research Article - (2022) Volume 13, Issue 1
Nanosponges as Emerging Carriers for Drug Delivery
Shiwangi Singh* and Monika KAbstract
Objective: The recent advancement in nanotechnology has led nanosponges to emerge as a suitable carrier for the delivery of both hydrophilic and lipophilic drugs. These are the tiny sponges that circulate throughout the body to achieve the desired dose of a drug in the specific organ of human bodies in a carefully controlled manner. They have higher drug loading capabilities as compared to other nanocarriers.
Methods: Nanosponges can also serve as a potent transporter for enzymes, proteins, and antibodies. This review attempts to elaborate on nanosponge’s merits and demerits, mechanism, composition, preparation, characterization, and their potential applications as delivery systems.
Conclusion: Nanosponges are a novel class of drug delivery systems as they treat both hydrophilic and hydrophobic drugs by forming inclusion and non-inclusion complexes. This nano technology provides beneficial effects that improve stability, reduce side effects and improve flexibility.
Keywords
Nanosponges, Hydrophilic and lipophilic medications, Gastro-retentive drug delivery system (GRDDS)
Introduction
Nanosponges, as the name suggests, lies in the modern category of drug carriers which comprises of minute particles with a diameter ranging up to a few nanometers. These minute particles work well with both hydrophilic and lipophilic drug substances. They can help in expanding the safety of poorly water-soluble drug substance or particles (Bolmal UB, et al., 2013). Nanosponges are tiny sponges as shown in Figure 1 that can travel around the body to pass a specific site and then join to target site to release the drug in a controlled and predictable manner. Nanosponge is water-soluble. This doesn’t mean the particles of the drug disintegrate in water but it means that nanosponge particles can combine with water and work it as a transport fluid, for example, to be inserted. Most other forms of nanoparticle delivery systems must be using several chemical transports, but they have lesser effects. The efficacy of nanosponge depends on its particle size. Earlier the particle size is predicted to be in the range of 150-400 nm, but most recently, the researchers have improved nanosponge with a particle size of 50 nm (Dhanalakshmi S, et al., 2020). Initially, the nanosponge drug delivery system was developed only as a topical delivery system, but by the 21st century, nanosponges could be administered orally as well as by intravenous route (Yadav GV and Panchory HP, 2013). The most common route of administration for systemic action is oral route. It is possible that at least 90% of all the drugs can be given by oral route (Streubel A, et al., 2006). Dosage forms that can be maintained in the stomach for longer duration are called GRDDS. GRDDS can enhance the controlled delivery of drugs that have an absorption chance by continuously releasing the drug for an extended period of time before it passes its absorption site (Sowmya B, et al., 2019). Drugs that are already absorbed into the gastrointestinal tract and those that have a short life span are quickly removed from the circulatory system because of the need for regular dosing. To control this problem, drug delivery systems that provide long-term plasma drug exposure thereby reduce the frequency of dosage. Gastroretentive drug delivery systems improve duration of dosing and therefore improve patient compliance. The presence of the drug in the form of a solution is essential for the drug to enter. However, if the dissolution of the drug is not correct, the time required for the drug to be excreted inside the stomach will be greater and the time to travel becomes more critical, which may affect the absorption of the drug. Therefore, the dose of the administration of such drugs should be kept periodically (Garg S and Sharma S, 2003).
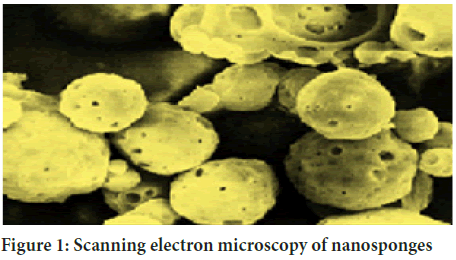
Figure 1: Scanning electron microscopy of nanosponges
Merits of nanosponges
• Increase the aqueous solubility of the poorly water-soluble drug
• They have the ability to produce a predictable/controlled drug manner
• They are non-irritating and non-toxic in nature
• Reduce dosing frequency
• Better patient compliance (Thakre AR, et al., 2016; Ahmed RZ, et al., 2013). Demerits of nanosponges
• The main disadvantage of these nanosponges is their ability to include only small molecules
• Depend just on loading limits (Trotta F, et al., 2012).
Mechanism of drug release from nanosponges
Nanosponges constitute three-dimensional structure of cross-linking polymer. The entrapment efficiency and solubilizing efficiency of nanosponges can be changed according to how much cross-linking polymer is being added to formulation. The toroidal shape of nanosponges allows them to have a cavity inside the structure which can fit various types of drug molecules. This because of such type of structure they can act as carriers for various types of drugs drug carriers, as long as the active compound is having compatibility with geometry and polarity of cavity the drug will release at target site. To find out when these active compounds will be delivered, the structure of nanosponge plays a crucial role which can be modified to depending on requirement of drug release. Several ligands or carriers can also be attached on to the surface of the nanosponge to target the molecules to various sites in body (Sadhasivam J, et al., 2020) (Table 1).
| S. No | Composition of nanosponges | Example | Reference |
|---|---|---|---|
| 1. | Polymer | Β-cyclodextrine | (Gharakhloo M, et al., 2020; Haimhoffer Á, et al., 2019) |
| 2-hydroxypropyl- Methyl- β- cyclodextrine | |||
| Methyl-β-cyclodextrine | |||
| β-cyclodextrine | |||
| Hyper crosslinked polystyrene | (Davankov VA, 1997) | ||
| Ethyl cellulose | (Penjuri SC, et al., 2016) | ||
| Polyvinyl alcohol | (Abbas N, et al., 2018) | ||
| 2. | Crosslinker | Diphenyl carbonate | (Omar SM, et al., 2020) |
| Carbonyldiimidazole | (Deshmukh K, et al., 2016) | ||
| Pyromellitic anhydride | (Rafati N, et al., 2019) | ||
| Diisocyanates | (Bachkar BA, et al., 2015) |
Table 1: Composition of nanosponges
Role of polymer and cross-linker
The choice of polymer can influence the composition along with the formation of nanosponges. They should have a property to bind with the specific ligands and the selection of cross-linking agent can be depending upon the polymer structure as well as the drug which is formulated.
Materials and Methods
Nanosponges prepared from hyper-cross linked β-cyclodextrins
β-cyclodextrin nanosponges are prepared by using cross-linker in a round bottom flask with the polymer which are added and shaken to complete the dissolution. Afterwards, cross-linker is added and the solution is allowed for 4 hours at 1000°C. When polymerization is complete, add some de-ionised water to remove the cross-linking agent. Finally, the rest of the products are removed by Soxhlet extraction by ethanol. Then, the product is dried at 600°C in oven (Lala R, et al., 2011).
Ultrasound-assisted synthesis
Nanosponges were obtained by converting polymers through crosslinkers when there was no solvent under sonication. In this way the nanosponges are obtained and will be circular and uniform in size. Di-phenyl carbonate or pyromellitic anhydride was used as a cross-linker. The amount of cyclodextrin anhydrous was added to di-phenyl carbonate at 90°C where the mixture was formed for five hours. Then, the product was filled in mortar and purified with Soxhlet extract with ethanol to remove impurities. The product was obtained and stored at 25°C until further use (Shivani S and Poladi KK, 2015).
Emulsion-solvent diffusion method
Nanosponges can be prepared by using different amount of co-polymer. The dispersed phase containing co-polymer and the drug is dissolved in cross-linker and then slightly added co-polymer in aqueous phase. Then, the reaction mixture is stirred at 1000 rpm for 2 hrs in magnetic stirrer. The nanosponges formed and dried in hot air oven at 40°C for 24 hrs. The dried nanosponges are stored in vacuum desiccators for the removal of residual solvents (Bolmal UB, et al., 2013).
Results and Discussion
Quasi-emulsion solvent diffusion
This phase is prepared by using the co-polymer and added to a suitable solvent. Drug is also included and dissolved under ultrasonication at 35°C. Then, the polymer is added which acts as emulsifying agent. The mixture is stirred for 3 hours at 1000-2000 rpm and dried in hot air oven for 12 hours at 40°C (Selvamuthukumar S, et al., 2012).
Loading of drug into nanosponges
In this method the nanosponges are first treated to obtain particle size less than 500 nm. Nanosponges are suspended in water and sonicated to avoid the presence of aggregates and after that the suspension is centrifuged to obtain a colloidal fraction. The liquid is separated and dried the sample through a freeze-drying process (Selvamuthukumar S, et al., 2012). The aqueous suspension of nanosponge is prepared and dispersed the large amount of the drug and the suspension is maintained at constant stirring for specific time required for complexation. After complexation, the undissolved drug separated from the dissolved drug by the process of centrifugation. Then solid crystals are obtained by the solvent evaporation or by freeze drying method (Swaminathan S, et al., 2010; Bolmal UB, et al., 2013).
Characterization and evaluation of nanosponges
Microscopy studies: Scanning electron microscopy and transmission electron microscopy can be used to analyze the morphology and further geology of nanosponges. Crystallization state of the crude material and the product detected under electron microscopy which shows the formation of embedded structures (Challa R, et al., 2005; Bolmal UB, et al., 2013).
Particle size and polydispersity: It can be determined by the Dynamic Light Scattering Instrument (DLSI) process equipped with particle sizing software. With this process the width of the index and the Poly-Dispersity Index (PDI) can also be determined. PDI is the measure of the width or variation within the distribution of particle size. The low PDI value consists of monodisperse samples, while the high PDI value indicates the wide particle size distribution and polydisperse nature of the sample. It can be calculated by the following equation-
PDI=Δd/davg
Where, Δd is the width of distribution and davg is the average particle size (nm) in particle size (Moura FC and Lago RM, 2009).
Thin layer chromatography: In thin layer chromatography, the Rf value of a drug substance decrease to a considerable range. It also helps in determining the formation of complex between the nanosponges and the drugs (Patel Ek and Oswal RJ, 2012).
Loading efficiency: It can be determined by the limited dose of the drug loaded into nanosponges by the process of UV spectrophotometer and High-performance liquid chromatography. The efficiency of nanosponges can be calculated by the following equation.
Loading efficiency=Amount of drug loaded in nanosponge/Theoretical drug loaded × 100 (Singh R, et al., 2010).
Solubility studies: The effect of nanosponges and solubility of drug was analysed by a complex model of phase solubility method described by Higuchi Connors (Osmani RA, et al., 2019; Shivani S and Poladi KK, 2015). These studies evaluate drugs pH solubilisation profile within the complex structure of nanosponges (Shringirishi M, et al., 2014).
Thermo-analytical methods: It determines whether the drug undergoes certain changes before the thermal degradation of the nanosponge. The process of drug substance can melt, evaporate, decompose or change polymorphic. Drug modification shows a complex structure. According to the thermo-analytical technique, a thermogram obtained by differential thermal analysis and differential scanning calorimetry can be detected to increase, shift and appearance of new peaks or disappearance of any peaks (Maravajhala V, et al., 2012) (Table 2).
| S. No | Author name | Drug used | Nanosponge ingredients | Result outcomes | Ref. No. |
|---|---|---|---|---|---|
| 1 | Kiran Deshmukh et al. Biomed Pharmacoether. 2016 Dec. | Antibacterial and Antihypocalcemic drugs | β-Cyclodextrin Nanosponges | The result was concluded as a promising anti-bacterial protein transporter and to prevent calcium depletion in the case of antibiotic hypercalcaemic. | (Singh P, et al., 2018) |
| 2 | Parbeen Singh et al. Carbohydr Polym. 2018 | Doxorubicin | Cyclodextrin nanosponges | Cellular uptake of nanosponges was detected and improved after the conversion of cholesterol hydrogen succinate (CHS). | (Hayiyana Z, et al., 2016) |
| 3 | Zikhona Hayiyana et al. Curr Pharm Des. 2016 | Ocular drugs | Hydrophilic cyclodextrin based nanosponges | The result was studied to improve corneal penetration and drug solubility. | (Chen Y, et al., 2019) |
| 4 | Yijie Chen et al. ACS Nano. 2019 | Organophosphates | Cloaked oil nanosponges | Oil nanosponges serves as a prototype of multimodal detoxification compound has been studied. | (Ye H, et al., 2020) |
| 6 | Hao Ye et al. Biomaterials. 2020 | Doxorubicin and Indocyanine green | Exosomes | NSK (nanosponges and nanokillers) can be a promising nanomedicine for future clinical interventions for metastasis breast cancer. | (Kumar S, et al., 2018) |
| 7 | Sunil Kumar et al. Pharmaceutics. 2018 | Antibacterial, Antifungal, Antioxidant, Anti-inflammatory, Immunomodulatory and Antitumor elements. | Cyclodextrin nanosponges | The installation of Babchi oil oil in nanosponges was bought with an active transporter frame until solvency, image stability, and its oil life alongside the benefits. | (Kamble M, et al., 2019) |
| 9 | Monica R P Rao et al. AAPS PharmSciTech. 2018 | Rilpivirine | Cyclodextrin-nanosponges | The result examines uncovered conceivable method of capture of rilpivirine inside β-CD space. | (Rao MR, et al., 2018) |
| 10 | Hongwang Wang et al. Nanomedicine. 2017 | Anticancer drugs | Peptide nanosponge | The composition of novel nanosponges was examined clear dissolvable and subsequent atomic (MD) re-engineering. | (Wang H, et al., 2017) |
| 11 | Qingli Huang et al. Spectrochim Acta A Mol Biomol Spectrosc. 2018 | Pazufloxacin mesylate | Ag nanosponges | Pazufloxacin mesilate (PM) were recognized helpfully utilizing these uniform nanosponges as SERS substrates. | (Huang Q, et al., 2018) |
| 12 | Maria Tannous et al. Methods Mol Bio. 2021 | Antibacterial, anticancer, antiviral drugs | Cyclodextrin nanosponges | cyclodextrin nanosponges" (CDNSs), pull in incredible consideration from scientists for tackling significant bioavailability issues, for example, deficient solvency, poor disintegration rate, and limited strength of certain specialists, just as expanding their viability and diminishing undesirable results. | (Tannous M, et al., 2021) |
| 13 | Diego F Suarez et al. J Photochem Photobiol B. 2017 Dec. | Doxycycline and Zno nanoparticles | Antibacterial nanosponges | The result was showed that ZnO-NPs filled with DOX have productive UV photocatalytic action against bacterial delicate decay contaminations. | (Suárez DF, et al., 2017) |
| 14 | Phillip S Coburn et al. mSphere. 2019 | Intraocular drugs | biomimetic erythrocyte-derived nanosponge | Biomimetic nanosponges kill pore-framing poisons from these visual microbes and help in saving retinal capacity. | (Coburn PS, et al., 2019) |
| 15 | Yijie Chen et al. Small. 2019 Feb. | Antibacterial drugs | Biomimetic nanosponges | The results provide a systematic review of RBC-NS (red blood cells nanosponges) for the treatment of severe MRSA infections (methicillin-resistant Staphylococcus aureus) such as MRSA bacteremia and MRSA-induced sepsis. | (Chen Y, et al., 2019) |
| 16 | Ute Distler et al. ACS Nano. 2017. | Antibacterial drugs | Biomimetic nanosponges | It has been shown that nanosponges coated with a membrane in combination with many proteinomic substances can also be used as effective "fishing aids" for the detection of hazardous substances specific to a particular cell type. | (Distler U and Tenzer S, 2017) |
| 17 | Monica Argenziano et al. Oncotarget. 2018 | Anticancer drugs | Glutathione/pH-responsive nanosponges | It shows that GSH/pH-NS are a proficient instrument for the controlled transfer of SLs to increase critical starvation and may increase the therapeutic efficacy of these compounds. | (Argenziano M, et al., 2018) |
| 18 | Yue Zhang et al. ACS Nano.2017 | Antibacterial drugs | Colloidal gel nanosponge | The nanosponge colloidal gel framework is promising as an injectable application for correction applications for example, antivirulence treatment that is close to viral infections. | (Zhang Y, et al., 2017) |
| 19 | Nilesh Kumar Dhakar et al. Pharmaceutics. 2019 | Resveratrol and Oxyresveratrol | β-cyclodextrin nanosponges | The high solubilization of nanosponges filled with resveratrol- and oxyresveratrol leads to a higher cell-reinforcing action compared to drug particles alone. | (Dhakar NK, et al., 2019) |
| 20 | Antonella Di Vincenzo et al. Beilstein J Org Chem. 2019. | Polyamionazides mixtures | Calixarene based nanosponges | The ideal responsivity to pH varieties of the nanosponges acquired was confirmed by methods for ingestion tests on a bunch of natural toxin model particles. | (Di Vincenzo A, et al., 2019) |
| 21 | Nausicaa Clemente et al. Front Pharmacol. 2019 | Paclitaxel | Pyromellitic nanosponges | It showed that our new PTX (paclitaxel) nanoformulation can react to significant issues identified with paclitaxel treatment, bringing down the counter tumour successful dosages and expanding the adequacy in hindering melanoma development in vivo. | (Clemente N, et al., 2019) |
| 22 | Jing Wang et al. ACS Nano. 2019 | Anticancer drugs | DNA zyme nanosponges | The present DNA zyme NS framework could be designed with more remedial arrangements and specialists and was foreseen to show remarkable guarantee and adaptability for applications in biomedicine and bioengineering. | (Wang J, et al., 2019) |
| 23 | Yijie Chen et al. Adv Healthc Mater. 2018 Jul. | Antibacterial drugs | Biomimetic nanosponges | It demonstrates the wide range of efficacy and high performance of hRBC nanosponges (red blood cells) as a novel anti-haemolytic drug platform from various types of viruses. | (Chen Y, et al., 2018) |
| 24 | Atul P Sherje et al. J Mater Sci Med. 2019. | Paliperidone | β-cyclodextrin based nanosponges | Cyclodextrin-based nanosponges talk about a novel way of developing solvency and improving the dispersion of selected PLP (paliperidone) drugs. | (Sherje AP, et al., 2019) |
| 25 | Monica Ferro et al. Beilstein J Org Chem. 2017 | Ibuprofen | Cyclodextrin nanosponges | It obtained from different NMR solid state models incorporates data from powder X-beam diffraction profiles. | (Ferro M, et al., 2017) |
| 26 | F Caldera et al. Carbohydr Polym. 2018. | Doxorubicin | Cyclic nigerosyl-1-6-nigerose (CNN) nanosponges | CNN-nanosponges may promise biocompatible nanocarriers for supported delivery of doxorubicin and anticipated inhibitory system in malignancy medicines. | (Caldera F, et al., 2018) |
| 27 | Francesco Trotta et al. Chempluschem. 2016 May. | Doxorubicin | β-cyclodextrin nanosponges | The cleavage of di-sulfide bridges allows the targeted release of cancer-fighting drugs into glutathione-rich cells that resist cells. | (Trotta F, et al., 2016) |
| 28 | Monica R P Rao et al. AAPS PharmSciTech. 2017 Jul. | Efavirenz | β-cyclodextrin nanosponges | Nanosponge properties have been found to have twice the oral administration of efavirenz compared to simple drugs. | (Rao MR and Shirsath C, 2017) |
| 29 | Michael Appell et al. Toxins (Basel). 2012 Feb. | Ochratoxin A | β-cyclodextrin-polyurethane polymer | These results suggest cyclodextrin nanosponge materials are suitable to reduce levels of ochratoxin A from spiked aqueous solutions and red wine samples. | (Appell M and Jackson MA, 2012) |
| 30 | Ilaria Simionato et al. Food Chem Toxicol. 2019 Oct. | Antimicrobial drug | Cyclodextrin nanosponges | The results described herein encourage the use of cyclodextrin nanosponges as encapsulating agents for active food packaging applications. | (Simionato I, et al., 2019) |
| 31 | Yacine Nait Bachir et al. Drug Dev Ind Pharm. 2019 Feb. | Salvia Officinalis essential oil | β-cyclodextrin nanosponges | Salvia officinalis is a basic nanoemulsion oil based on β-cyclodextrin-naphthalene dicarboxylic nanosponges that bring the highest potency and promising use in the drug industry. | (Nait Bachir Y, et al., 2019) |
| 32 | Francesco Trotta et al. Expert Opin Drug Deliv. 2016 Dec. | L-Dopa | Cyclodextrin nanosponges | MIP-NS exhibits a prolonged released profile that is slower and longer than non-labeled nanosponges. No L-DOPA-induced degradation in MIP-NS was observed after prolonged storage at room temperature. | (Trotta F, et al., 2016) |
| 33 | Pravin K Shende et al. Colloids Surf B Biointerfaces. 2015. | Meloxicam | β-cyclodextrin-based nanosponges | Nanosponges based on β-cyclodextrin talk about a novel approach to the controlled arrival of meloxicam to detect and reduce effects. | (Shende PK, et al., 2015) |
| 34 | Mohamed F Zidan et al. Drug Dev Ind Pharm. 2018 Aug. | Atorvastatin calcium | Cyclodextrin nanosponges | It has been confirmed that AC-NS integration will be an effective way to improve oral availability and vivo function of AC. | (Zidan MF, et al., 2018) |
| 35 | Casimiro Luca Gigliotti et al. Drug Deliv. 2017 Nov. | Camptothecin | β-cyclodextrin-nanosponges. | CN-CPT significantly impaired development, vascular permeability and the use of orthotopic ATC xenografts vascularization in SCID/beige mice without significant toxic effects on vivo. | (Gigliotti CL, et al., 2017) |
| 36 | Nesa Rafati et al. J Microencapsul. 2019 Dec. | Curcumin herbal remedies | Cyclodextrin nanosponges | Cytotoxicity test results did not show cell toxicity in a healthy cell line, while it was toxic compared to cancer cells. | (Rafati N, et al., 2019) |
| 37 | Francesco Trotta et al. Chempluschem. 2016 May. | Doxorubicin | Cyclodextrin-based nanosponges | The activity of this GSH (glutathione) reaction has been demonstrated using a few tumor cells and doxorubicin as a model anticancer drug. The arrival of the drugs was consistent with the content of GSH in tumor cells. | (Mihailiasa M, et al., 2016) |
| 38 | Manuela Mihailiasa et al. Carbohydr Polym. 2016. | Melatonin | β-cyclodextrin nanosponges | The result of the union is a 3-D structure allowed, in which melatonin atoms are made harder. | (Ataee-Esfahani H, et al., 2011) |
| 39 | Hamed Ataee-Esfahani et al. Chem Commun (Camb). 2011 | Silica particles | Pt spheres | It was shown that this technique improves the electrocatalytic performance of Pt catalysts by making electroactive species more accessible to the entire Pt surface. | (Torne SJ, et al., 2010) |
| 40 | Martina Daga et al. Free Radic Biol Med. 2016 Aug. | Doxorubicin | glutathione-responsive cyclodextrin nanosponges(GSH-NS) | It was demonstrated that GSH-NS inhibited human tumour growth in xenograft studies. It may be a viable carrier for future drug delivery aplications. | (Alongi J, et al., 2011) |
Table 2: Nanosponges driven research in formulation development
Infra-red spectroscopy: It can be used to study the interactions between nanosponges and drug molecules in a solid state. If there is a complex formation between drug mutations and nanosponge IR and if a fraction of drug molecules is subjected to a pressure of less than 25% bands and can be assigned to enclose a portion of other molecules that are easily marked with multiple nanosponges. This process is generally unsuitable for obtaining installed properties and is less specific than other methods (Deshmukh K, et al., 2016) (Table 3).
| Drugs | Nanosponges vehicle | Indication | Reference |
|---|---|---|---|
| Paclitaxel | β-cyclodextrin | Cancer | (Minelli R, et al., 2011) |
| Tamoxifen | β-cyclodextrin | Breast cancer | (Sharma R and Pathak K, 2011) |
| Camptothecin | β-cyclodextrin | Cancer | (Swaminathan S, et al., 2007) |
| Econazole nitrate | Ethyl cellulose, polyvinyl alcohol | Antifungal | (Aynie I, et al., 1999) |
| Itraconazole | β-cyclodextrin and copolyvidonum | Antifungal | (Ansari KA, et al., 2011) |
| Antisense | Sodium alginate | Cancer therapy | (Ansari KA, et al., 2011) |
| Resveratrol | β-cyclodextrin | Inflammation, cardiovascular diseases, dermatitis, gonorrhoea | (Aynie I, et al., 1999) |
Table 3: Nanosponges based marketed formulation
Conclusion
Nanosponges are a novel class of drug delivery systems as they treat both hydrophilic and hydrophobic drugs by forming inclusion and non-inclusion complexes. They can deliver drugs through a different route such as oral, topical and parenteral. This nano technology provides beneficial effects that improve stability, reduce side effects and improve flexibility. In the field of drug delivery, potential applications are available for cosmetics, biomedicine, agro-chemistry and catalysis. The drug carrier delivered by nanosponges can be shown to be safe and effective and the pharmaceutical industry will benefit greatly if medical studies prove that they can be used by humans.
Scope of the Study
The field of nanosponges continues to grow interest with major discoveries as well as new scientific challenges. Nanosponges play a role in the various fields of drug delivery systems like oral, topical, intravenous and immuno-suppressant. Nanosponge’s particle can also play role in the targeted drug delivery system which is effective via lungs, liver and spleen. Some techniques can also be used to identify nanosponges at disease sites like Crohn’s disease, auto-immune disease and cancer which are affected in different organs or tissues. Nowadays, nanosponges are also used in gastro-retentive drug delivery systems.
References
- Bolmal UB, Manvi FV, Kotha R, Palla SS, Paladugu A, Reddy KR. Recent advances in nanosponges as drug delivery system. Int J Pharm Sci Nanotechnol. 2013; 6(1): 1934-1944.
- Dhanalakshmi S, Harikrishnan N, Tanisha BA, Pooja G, Yuvarani L, Begum AS, et al. A perspective view on nanosponge drug delivery system. Drug Invent Today. 2020; 14(3).
- Yadav GV, Panchory HP. Nanosponges: A boon to the targeted drug delivery system. J drug deliv ther. 2013; 3(4): 151-155.
- Streubel A, Siepmann J, Bodmeier R. Drug delivery to the upper small intestine window using gastroretentive technologies. Curr Opin Pharmacol. 2006; 6(5): 501-508.
[Crossref] [Google scholar] [Pubmed]
- Sowmya B, Arvapalli S, Gupta AV. A review on gastroretentive drug delivery system. World J Pharm Life Sci. 2019; 5(4): 101-110.
- Garg S, Sharma S. Gastroretentive drug delivery systems. Business Briefing: Pharmatech. 2003; 5(2).
- Thakre AR, Gholse YN, Kasliwal RH. Nanosponges: A novel approach of drug delivery system. J Med Pharm Allied Sci. 2016; 78(92): 78.
- Ahmed RZ, Patil G, Zaheer Z. Nanosponges-a completely new nano-horizon: Pharmaceutical applications and recent advances. Drug Dev Ind Pharm. 2013; 39(9): 1263-1272.
[Crossref] [Google scholar] [Pubmed]
- Trotta F, Zanetti M, Cavalli R. Cyclodextrin-based nanosponges as drug carriers. Beilstein J Org Chem. 2012; 8(1): 2091-2099.
[Crossref] [Google scholar] [Pubmed]
- Sadhasivam J, Sugumaran A, Narayanaswamy D. Nano sponges: A potential drug delivery approach. Res J Pharm Technol. 2020; 13(7): 3442-3448.
- Gharakhloo M, Sadjadi S, Rezaeetabar M, Askari F, Rahimi A. Cyclodextrin‐based nanosponges for improving solubility and sustainable release of curcumin. Chemistry Select. 2020; 5(5): 1734-1738.
- Haimhoffer Á, Rusznyák Á, Réti-Nagy K, Vasvári G, Váradi J, Vecsernyes J, et al. Cyclodextrins in drug delivery systems and their effects on biological barriers. Scientia Pharmaceutica. 2019; 87(4): 33.
- Davankov VA, Timofeeva GI, Ilyin MM, Tsyurupa MP. Formation of regular clusters through self‐association of intramolecularly hypercrosslinked polystyrene‐type nanosponges. J Polym Sci A Polym Chem. 1997; 35(17): 3847-3852.
- Penjuri SC, Ravouru N, Damineni S, Bns S, Poreddy SR. Formulation and evaluation of lansoprazole loaded nanosponges. Turk J Pharm Sci. 2016; 13(3): 304-310.
- Abbas N, Irfan M, Hussain A, Arshad MS, Hussain SZ, Latif S, et al. Development and evaluation of scaffold-based nanosponge formulation for controlled drug delivery of naproxen and ibuprofen. Trop J Pharm Res. 2018; 17(8): 1465-1474.
- Omar SM, Ibrahim F, Ismail A. Formulation and evaluation of cyclodextrin-based nanosponges of griseofulvin as pediatric oral liquid dosage form for enhancing bioavailability and masking bitter taste. Saudi Pharm J. 2020; 28(3): 349-361.
[Crossref] [Google scholar] [Pubmed]
- Deshmukh K, Tanwar YS, Sharma S, Shende P, Cavalli R. Functionalized nanosponges for controlled antibacterial and antihypocalcemic actions. Biomed Pharmacother. 2016; 84: 485-494.
[Crossref] [Google scholar] [Pubmed]
- Rafati N, Zarrabi A, Caldera F, Trotta F, Ghias N. Pyromellitic dianhydride crosslinked cyclodextrin nanosponges for curcumin-controlled release; formulation, physicochemical characterization and cytotoxicity investigations. J Microencapsul. 2019; 36(8): 715-727.
[Crossref] [Google scholar] [Pubmed]
- Bachkar BA, Gadhe LT, Battase P, Mahajan N, Wagh R, Talele S. Nanosponges: A potential nanocarrier for targeted drug delivery. World J Pharm Res. 2015; 4(3): 751-768.
- Lala R, Thorat A, Gargote C. Current trends in β-cyclodextrin based drug delivery systems. Int J Res Ayur Pharm. 2011; 2(5): 1520-1526.
- Shivani S, Poladi KK. Nanosponges-novel emerging drug delivery system: A review. Int J Pharm Sci Res. 2015; 6(2): 529.
- Bolmal UB, Manvi FV, Kotha R, Palla SS, Paladugu A, Reddy KR. Recent advances in nanosponges as drug delivery system. Int J Pharm Sci Nanotechnol. 2013; 6(1): 1934-1944.
- Selvamuthukumar S, Anandam S, Krishnamoorthy K, Rajappan M. Nanosponges: A novel class of drug delivery system-review. J Pharm Pharm Sci. 2012; 15(1): 103-111.
[Crossref] [Google scholar] [Pubmed]
- Swaminathan S, Pastero L, Serpe L, Trotta F, Vavia P, Aquilano D, et al. Cyclodextrin-based nanosponges encapsulating camptothecin: Physicochemical characterization, stability and cytotoxicity. Eur J Pharm Biopharm. 2010; 74(2): 193-201.
[Crossref] [Google scholar] [Pubmed]
- Challa R, Ahuja A, Ali J, Khar RK. Cyclodextrins in drug delivery: An updated review. Aaps Pharmscitech. 2005; 6(2): E329-357.
[Crossref] [Google scholar] [Pubmed]
- Bolmal UB, Manvi FV, Kotha R, Palla SS, Paladugu A, Reddy KR. Recent advances in nanosponges as drug delivery system. Int Journal of Pharm Sci-Nanotechnol. 2013; 6(1): 1934-1944.
- Moura FC, Lago RM. Catalytic growth of carbon nanotubes and nanofibers on vermiculite to produce floatable hydrophobic “nanosponges” for oil spill remediation. Appl Catal B. 2009; 90(3-4): 436-440.
- Patel EK, Oswal RJ. Nanosponge and micro sponges: A novel drug delivery system. Int J Res Pharm Sci. 2012; 2(2): 2281-2781.
- Singh R, Bharti N, Madan J, Hiremath SN. Characterization of cyclodextrin inclusion complexes: A review. J Pharm Sci Technol. 2010; 2(3): 171-183.
- Osmani RA, Thirumaleshwar S, Bhosale RR, Kulkarni PK. The nanosponges: An innovative drug delivery system. Asian J Pharm Clin Res. 2019; 12(7).
- Shringirishi M, Prajapati SK, Mahor A, Alok S, Yadav P, Verma A. Nanosponges: Apotential nanocarrier for novel drug delivery-a review. Asian Pac J Trop Dis. 2014; 4: S519-526.
- Maravajhala V, Papishetty S, Bandlapalli S. Nanotechnology in development of drug delivery system. Int J Pharm Sci. 2012; 3(1): 84.
- Deshmukh K, Tanwar YS, Sharma S, Shende P, Cavalli R. Functionalized nanosponges for controlled antibacterial and antihypocalcemic actions. Biomed Pharmacother. 2016; 84: 485-494.
- Singh P, Ren X, Guo T, Wu L, Shakya S, He, Y, et al. Biofunctionalization of β-cyclodextrin nanosponges using cholesterol. Carbohydr Polym. 2018; 190: 23-30.
- Hayiyana Z, Choonara Y, Makgotloe A, du Toit L, Kumar P, Pillay V. Ester-based hydrophilic cyclodextrin nanosponges for topical ocular drug delivery. Curr Pharm Des. 2016; 22(46): 6988-6997.
[Crossref] [Google scholar] [Pubmed]
- Chen Y, Zhang Y, Zhuang J, Lee JH, Wang L, Feng RH, et al. Cell-membrane-cloaked oil nanosponges enable dual-modal detoxification. ACS Nano. 2019; 13(6): 7209-7215.
[Crossref] [Google scholar] [Pubmed]
- Ye H, Wang K, Lu Q, Zhao J, Wang M, Kan Q, et al. Nanosponges of circulating tumor-derived exosomes for breast cancer metastasis inhibition. Biomaterials. 2020; 242: 119932.
[Crossref] [Google scholar] [Pubmed]
- Kumar S, Trotta F, Rao R. Encapsulation of babchi oil in cyclodextrin-based nanosponges: Physicochemical characterization, photodegradation, and in vitro cytotoxicity studies. Pharmaceutics. 2018; 10(4): 169.
[Crossref] [Google scholar] [Pubmed]
- Kamble M, Zaheer Z, Mokale S, Zainuddin R. Formulation optimization and biopharmaceutical evaluation of imatinib mesylate loaded β-cyclodextrin nanosponges. Pharm Nanotechnol. 2019; 7(5): 343-361.
[Crossref] [Google scholar] [Pubmed]
- Rao MR, Chaudhari J, Trotta F, Caldera F. Investigation of cyclodextrin-based nanosponges for solubility and bioavailability enhancement of rilpivirine. AAPS Pharm Sci Tech. 2018; 19(5): 2358-2369.
[Crossref] [Google scholar] [Pubmed]
- Wang H, Yapa AS, Kariyawasam NL, Shrestha TB, Kalubowilage M, Wendel SO, et al. Rationally designed peptide nanosponges for cell-based cancer therapy. Nanomed: Nanotechnol Biol Med. 2017; 13(8): 2555-2564.
[Crossref] [Google scholar] [Pubmed]
- Huang Q, Wei W, Wang L, Chen H, Li T, Zhu X, et al. Synthesis of uniform Ag nanosponges and its SERS application. Spectrochim Acta A Mol Biomol. 2018; 201: 300-305.
- Tannous M, Caldera F, Hoti G, Dianzani U, Cavalli R, Trotta F. Drug-encapsulated cyclodextrin nanosponges: Supramolecules in drug discovery and drug delivery. Springer. 2021: 247-283.
- Suárez DF, Monteiro AP, Ferreira DC, Brandão FD, Krambrock K, et al. Efficient antibacterial nanosponges based on ZnO nanoparticles and doxycycline. J Photochem Photobiol. 2017; 177: 85-94.
[Crossref] [Google scholar] [Pubmed]
- Coburn PS, Miller FC, LaGrow AL, Land C, Mursalin H, Livingston E, et al. Disarming pore-forming toxins with biomimetic nanosponges in intraocular infections. Msphere. 2019; 4(3).
[Crossref] [Google scholar] [Pubmed]
- Chen Y, Zhang Y, Chen M, Zhuang J, Fang RH, Gao W, et al. Biomimetic nanosponges suppress in vivo lethality induced by the whole secreted proteins of pathogenic bacteria. Small. 2019; 15(6): 1804994.
[Crossref] [Google scholar] [Pubmed]
- Distler U, Tenzer S. Tools for pathogen proteomics: Fishing with biomimetic nanosponges. ACS Nano. 2017; 11(12): 11768-11772.
- Argenziano M, Lombardi C, Ferrara B, Trotta F, Caldera F, Blangetti M, et al. Glutathione/pH-responsive nanosponges enhance strigolactone delivery to prostate cancer cells. Oncotarget. 2018; 9(88): 35813.
[Crossref] [Google scholar] [Pubmed]
- Zhang Y, Gao W, Chen Y, Escajadillo T, Ungerleider J. Self-assembled colloidal gel using cell membrane-coated nanosponges as building blocks. ACS Nano. 2017; 11(12): 11923-11930.
[Crossref] [Google scholar] [Pubmed]
- Dhakar NK, Matencio A, Caldera F, Argenziano M, Cavalli R, Dianzari C, et al. Comparative evaluation of solubility, cytotoxicity and photostability studies of resveratrol and oxyresveratrol loaded nanosponges. Pharmaceutics. 2019; 11(10): 545.
[Crossref] [Google scholar] [Pubmed]
- di Vincenzo A, Piccionello AP, Spinella A, Martino DC, Russo M. Polyaminoazide mixtures for the synthesis of pH-responsive calixarene nanosponges. Beilstein J Org Chem. 2019; 15(1): 633-641.
- Clemente N, Argenziano M, Gigliotti CL, Ferrara B, Boggio E, Chiochetti A, et al. Paclitaxel-loaded nanosponges inhibit growth and angiogenesis in melanoma cell models. Frontiers in Pharmacology. 2019; 10: 776.
[Crossref] [Google scholar] [Pubmed]
- Wang J, Wang H, Wang H, He S, Li R, Deng Z, et al. Nonviolent self-catabolic DNAzyme nanosponges for smart anticancer drug delivery. ACS Nano. 2019; 13(5): 5852-5863.
[Crossref] [Google scholar] [Pubmed]
- Chen Y, Chen M, Zhang Y, Lee JH, Escajadillo T, Gong H, et al. Broad‐spectrum neutralization of pore‐forming toxins with human erythrocyte membrane‐coated nanosponges. Advanced Healthcare Materials. 2018; 7(13): 1701366.
[Crossref] [Google scholar] [Pubmed]
- Sherje AP, Surve A, Shende P. CDI cross-linked β-cyclodextrin nanosponges of paliperidone: synthesis and physicochemical characterization. J Mater Sci Mater Med. 2019; 30(6): 1-7.
[Crossref] [Google scholar] [Pubmed]
- Ferro M, Castiglione F, Pastori N, Punta C, Melone L, Panzeri W, et al. Dynamics and interactions of ibuprofen in cyclodextrin nanosponges by solid-state NMR spectroscopy. Beilstein J Org Chem. 2017; 13(1): 182-194.
[Crossref] [Google scholar] [Pubmed]
- Caldera F, Argenziano M, Trotta F, Dianzani C, Gigliotti L, Tannous M, et al. Cyclic nigerosyl-1, 6-nigerose-based nanosponges: An innovative pH and time-controlled nanocarrier for improving cancer treatment. Carbohydr Polym. 2018; 194: 111-121.
[Crossref] [Google scholar] [Pubmed]
- Trotta F, Caldera F, Dianzani C, Argenziano M, Barrera G, Cavalli R. Glutathione bioresponsive cyclodextrin nanosponges. Chem Plus Chem. 2016; 81(5): 439-443.
[Crossref] [Google scholar] [Pubmed]
- Rao MR, Shirsath C. Enhancement of bioavailability of non-nucleoside reverse transciptase inhibitor using nanosponges. AAPS Pharm Sci Tech. 2017; 18(5): 1728-38.
[Crossref] [Google scholar] [Pubmed]
- Appell M, Jackson MA. Sorption of ochratoxin A from aqueous solutions using β-cyclodextrin-polyurethane polymer. Toxins. 2012; 4(2): 98-109.
[Crossref] [Google scholar] [Pubmed]
- Simionato I, Domingues FC, Nerín C, Silva F. Encapsulation of cinnamon oil in cyclodextrin nanosponges and their potential use for antimicrobial food packaging. Food Chem Toxicol. 2019; 132: 110647.
[Crossref] [Google scholar] [Pubmed]
- Nait Bachir Y, Nait Bachir R, Hadj-Ziane-Zafour A. Nanodispersions stabilized by β-cyclodextrin nanosponges: Application for simultaneous enhancement of bioactivity and stability of sage essential oil. Drug Dev Ind Pharm. 2019; 45(2): 333-347.
[Crossref] [Google scholar] [Pubmed]
- Trotta F, Caldera F, Cavalli R, Soster M, Riedo C, Biasizzo M, et al. Molecularly imprinted cyclodextrin nanosponges for the controlled delivery of L-DOPA: Perspectives for the treatment of Parkinson’s disease. Expert Opin Drug Deliv. 2016; 13(12): 1671-1680.
[Crossref] [Google scholar] [Pubmed]
- Shende PK, Gaud RS, Bakal R, Patil D. Effect of inclusion complexation of meloxicam with β-cyclodextrin-and β-cyclodextrin-based nanosponges on solubility, in vitro release and stability studies. Colloids Surf B. 2015; 136: 105-110.
[Crossref] [Google scholar] [Pubmed]
- Zidan MF, Ibrahim HM, Afouna MI, Ibrahim EA. In vitro and in vivo evaluation of cyclodextrin-based nanosponges for enhancing oral bioavailability of atorvastatin calcium. Drug Development and Industrial Pharmacy. 2018; 44(8): 1243-1253.
[Crossref] [Google scholar] [Pubmed]
- Gigliotti CL, Ferrara B, Occhipinti S, Boggio E, Barrera G, Pizzimenti S, et al. Enhanced cytotoxic effect of camptothecin nanosponges in anaplastic thyroid cancer cells in vitro and in vivo on orthotopic xenograft tumors. Drug Deliv. 2017; 24(1): 670-680.
[Crossref] [Google scholar] [Pubmed]
- Rafati N, Zarrabi A, Caldera F, Trotta F, Ghias N. Pyromellitic dianhydride crosslinked cyclodextrin nanosponges for curcumin-controlled release; formulation, physicochemical characterization and cytotoxicity investigations. J Microencapsul. 2019; 36(8): 715-727.
[Crossref] [Google scholar] [Pubmed]
- Mihailiasa M, Caldera F, Li J, Peila R, Ferri A, Trotta F. Preparation of functionalized cotton fabrics by means of melatonin loaded β-cyclodextrin nanosponges. Carbohydr Polym. 2016; 142: 24-30.
[Crossref] [Google scholar] [Pubmed]
- Ataee-Esfahani H, Nemoto Y, Wang L, Yamauchi Y. Rational synthesis of Pt spheres with hollow interior and nanosponge shell using silica particles as template. Chem Comm. 2011; 47(13): 3885-3387.
- Torne SJ, Ansari KA, Vavia PR, Trotta F, Cavalli R. Enhanced oral paclitaxel bioavailability after administration of paclitaxel-loaded nanosponges. Drug Deliv. 2010; 17(6): 419-425.
[Crossref] [Google scholar] [Pubmed]
- Alongi J, Poskovic M, Frache A, Trotta F. Role of β-cyclodextrin nanosponges in polypropylene photooxidation. Carbohydr Polym. 2011; 86(1): 127-135.
- Minelli R, Cavalli R, Fantozzi R, Dianzani C, Pettazzoni P, Ellis L, et al. Antitumor activity of nanosponge-encapsulated camptotechin in human prostate tumors. Am Assoc Cancer Res. 2011.
- Sharma R, Pathak K. Polymeric nanosponges as an alternative carrier for improved retention of econazole nitrate onto the skin through topical hydrogel formulation. Pharm Dev Technol. 2011; 16(4): 367-376.
[Crossref] [Google scholar] [Pubmed]
- Swaminathan S, Vavia PR, Trotta F, Torne S. Formulation of betacyclodextrin based nanosponges of itraconazole. J Incl Phenom Macrocycl Chem. 2007; 57(1): 89-94.
- Aynie I, Vauthier C, Chacun H, Fattal E, Couvreur P. Spongelike alginate nanoparticles as a new potential system for the delivery of antisense oligonucleotides. Antisense Nucleic Acid Drug Dev. 1999; 9(3): 301-312.
[Crossref] [Google scholar] [Pubmed]
- Ansari KA, Vavia PR, Trotta F, Cavalli R. Cyclodextrin-based nanosponges for delivery of resveratrol: In vitro characterisation, stability, cytotoxicity and permeation study. Aaps Pharmscitech. 2011; 12(1): 279-286.
[Crossref] [Google scholar] [Pubmed]
Author Info
Shiwangi Singh* and Monika KCitation: Singh S: Nanosponges as Emerging Carriers for Drug Delivery
Received: 15-Dec-2021 Accepted: 29-Dec-2021 Published: 05-Dec-2022, DOI: 10.31858/0975-8453.13.1.55-62
Copyright: This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
ARTICLE TOOLS
- Dental Development between Assisted Reproductive Therapy (Art) and Natural Conceived Children: A Comparative Pilot Study Norzaiti Mohd Kenali, Naimah Hasanah Mohd Fathil, Norbasyirah Bohari, Ahmad Faisal Ismail, Roszaman Ramli SRP. 2020; 11(1): 01-06 » doi: 10.5530/srp.2020.1.01
- Psychometric properties of the World Health Organization Quality of life instrument, short form: Validity in the Vietnamese healthcare context Trung Quang Vo*, Bao Tran Thuy Tran, Ngan Thuy Nguyen, Tram ThiHuyen Nguyen, Thuy Phan Chung Tran SRP. 2020; 11(1): 14-22 » doi: 10.5530/srp.2019.1.3
- A Review of Pharmacoeconomics: the key to “Healthcare for All” Hasamnis AA, Patil SS, Shaik Imam, Narendiran K SRP. 2019; 10(1): s40-s42 » doi: 10.5530/srp.2019.1s.21
- Deuterium Depleted Water as an Adjuvant in Treatment of Cancer Anton Syroeshkin, Olga Levitskaya, Elena Uspenskaya, Tatiana Pleteneva, Daria Romaykina, Daria Ermakova SRP. 2019; 10(1): 112-117 » doi: 10.5530/srp.2019.1.19
- Dental Development between Assisted Reproductive Therapy (Art) and Natural Conceived Children: A Comparative Pilot Study Norzaiti Mohd Kenali, Naimah Hasanah Mohd Fathil, Norbasyirah Bohari, Ahmad Faisal Ismail, Roszaman Ramli SRP. 2020; 11(1): 01-06 » doi: 10.5530/srp.2020.1.01
- Manilkara zapota (L.) Royen Fruit Peel: A Phytochemical and Pharmacological Review Karle Pravin P, Dhawale Shashikant C SRP. 2019; 10(1): 11-14 » doi: 0.5530/srp.2019.1.2
- Pharmacognostic and Phytopharmacological Overview on Bombax ceiba Pankaj Haribhau Chaudhary, Mukund Ganeshrao Tawar SRP. 2019; 10(1): 20-25 » doi: 10.5530/srp.2019.1.4
- A Review of Pharmacoeconomics: the key to “Healthcare for All” Hasamnis AA, Patil SS, Shaik Imam, Narendiran K SRP. 2019; 10(1): s40-s42 » doi: 10.5530/srp.2019.1s.21
- A Prospective Review on Phyto-Pharmacological Aspects of Andrographis paniculata Govindraj Akilandeswari, Arumugam Vijaya Anand, Palanisamy Sampathkumar, Puthamohan Vinayaga Moorthi, Basavaraju Preethi SRP. 2019; 10(1): 15-19 » doi: 10.5530/srp.2019.1.3






