Review Article - (2023) Volume 14, Issue 7
Abstract
Nanotechnology is widely the area of science and technology. It gives the study of phenomena of the dimensions in the nanometre scale utilized to design, production, characterization, structures, devices, and application of materials. However, cancer is 2nd most common disease, causing death and poor quality of life globally. Early diagnosis holds a key to the improvement of the technology revolution in cancer detection and its treatment. Nanotechnology has been expanded to help the treatment of cancer therapy at a very tiny level. The cell particulate of small sizes of nanoparticles helps them locate and kill the cancer cells precisely. In addition, nanoparticle systems have been shown to play a role in cancer drug resistance. For cancer diagnosis, nanomaterials are applied such as quantum dots, and gold nanoparticles as well as biomarker screening which is used at the molecular level. Numerous studies have been carried out to explore the tumor targeting of the design of nanoparticle- based drug delivery systems in cancer therapy. Given that, several studies have examined different forms of nanomaterials such as polymers, antibodies, and liposomes with the conclusion that a combination of these nanomaterials in cancer drug design can achieve a balance between reducing toxicity and increasing the efficacy of drugs. Despite that, different medicines have been found to treat cancer therapy which is targeting cancer cells with different forms of nanomaterials. Furthermore, nanotechnology has enhanced chemotherapy and reduced the adverse effects of drugs targeting cancer cells. In this review, the roles of nanoparticles for drug delivery in radiotherapy, and immunotherapy and describe the targeting as well as the function in reversing drug mechanisms.
Keywords
Nanotechnology, Nanoparticles, Nanomaterials, Cancer therapy
Introduction
Cancer is an extremely complicated illness that has no cure, involving apoptosis resistance, uncontrolled cell proliferation, changes in cellular signalling, tissue invasion, metastasis, and angiogenesis. Cancer can start anywhere in the body and is composed of three trillion cells, which can split into one another in a process known as cell division. To make new cells, human cells generally grow and multiply. When cells are injured, they die and are replaced by new ones. According to 2018 data on worldwide cancer incidence, mortality, and prevalence, the number of new malignancies is predicted to grow by 18.1 million, with 9.6 cancer-related deaths. According to the WHO, there would be more than 19.3 million new cancer diagnoses and 10 million deaths from cancer in 2020. After 2030, it is expected that 30 million people would die from cancer each year.
Cancer-causing factors include smoking, pollution, an improper diet, microorganism infection, radiation, and hormones, which are becoming more widespread in developing nations. Any of these characteristics can cause damaged genetics, or DNA, to lead to cancer. Individual cells that have developed and can replicate at tremendous rates outnumber all healthy functional cells, culminating in death.
Nanotechnology involves the development and use of a chemical, physical system at sizes ranging from individual molecules (1-100 nm) or atoms to submicron dimensions, as well as the incorporation of the generated nanomaterials into larger systems. It understands the biosystem using nanoscale principles and methodologies, and it is emerging with current biology and medicine to develop more nanoscale materials that can be utilized in biological systems (Nikolova M, et al., 2020).
Chemotherapies can be targeted directly and selectively to malignant cells using nanotechnology. Nanotechnology-based diagnostic technologies are being developed as prospective real-time, cost-effective, and convenient methods for cancer diagnosis (Jaishree V and Gupta PD, 2012). For effective cancer diagnosis, nanoparticles are being utilized to capture cancer biomarkers such as exosomes, circulating tumors, circulating DNA, and cancer-associated proteins. Because of these properties, nanoparticle surfaces can be densely coated with antibodies, peptides, aptamers, small molecules, and moieties to detect specific cancer cells or toxins.
Chemotherapy, radiation, surgery, and a combination of treatments are currently the most prevalent cancer diagnoses. However, these techniques have major downsides, including severe side effects and toxicity. To avoid any unfavourable reactions, the drug’s local concentration at a spot must be high but the tissue concentration must be low. Modern medicine aims to maximize drug pharmacological efficacy while minimizing the risk of side effects. The use of nanotechnology in cancer diagnosis has the potential to overcome the limitations of traditional approaches (Jena S, et al., 2022). The amount of drug required for a therapeutic effect can be deemed low when using nanotechnology (Jin C, et al., 2020).
Furthermore, Quantum Dots (QDs) and gold nanoparticles are used to diagnose cancer at the molecular level (Fang M, et al., 2012). Biomarker discovery and other molecular diagnostic tools based on these nanoparticles can precisely and efficiently diagnose cancers. Nanotechnology therapies, such as nanoscale drug delivery, will ensure that cancerous tissues are targeted specifically while reducing problems. The various uses of nanotechnology have been recapitulated in Table 1.
| Diagnostic use | Therapeutic use |
|---|---|
| Dendrimers | Liposomes |
| Nanoshells | Carbon nanotubes |
| Gold nanoparticles | Polymeric micelles, Quantum Dots (QDs) |
Table 1: Uses of nanotechnology
Literature Review
Nanotechnology in cancer therapy
The advancement of nanotechnology is predicated on the use of tiny molecular structures and particles as medication delivery methods. Nano-carriers like liposomes, micelles, dendritic macromolecules, quantum dots, and carbon nanotubes have all been mainly used to treat cancer (Torchilin VP, 2005).
Liposomes: Liposomes, the first enclosed micro phospholipid bilayer nanosystem, were authorized in 1965. Liposomes are spherical vesicles comprised mostly of uni-lamellar or multi-lamellar phospholipids, with sizes ranging from 20 nm to more than 1 um. Liposomes are composed of a hydrophilic core and a hydrophobic phospholipid bilayer. This structure allows for the trapping of both hydrophilic and hydrophobic medicines, depending on the drug’s pharmacokinetic characteristics. The characteristic shape of liposomes encapsulates hydrophilic medications inside their aqueous core and hydrophobic drugs within the lipid bilayer. Drug enclosed within the core chamber of a liposome is shielded from environmental degradation throughout human bloodstream circulation (Bozzuto G and Molinari A, 2015) (Figure 1).
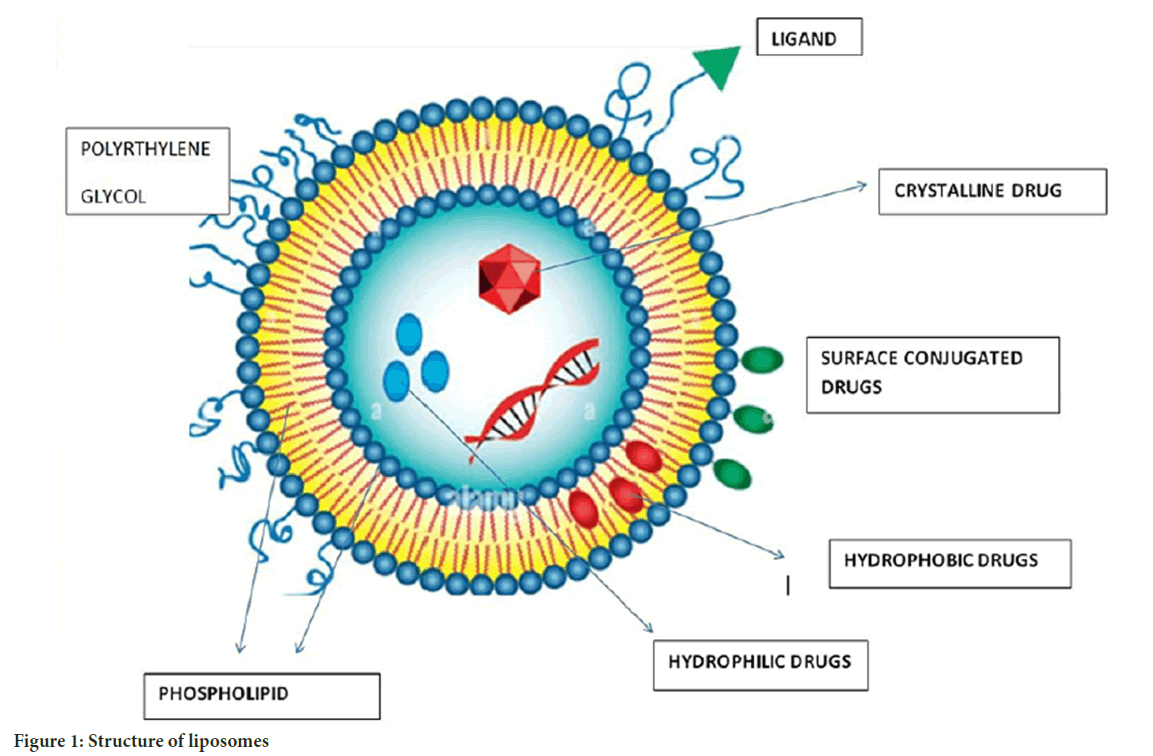
Figure 1: Structure of liposomes
Drug loading and controlled release of liposomes are additional significant considerations that must be resolved in the design of liposome nanocarriers (Jani P, et al., 2020). Bioavailability impacts drug effectiveness in cancer treatment. Doxorubicin (DOX) liposome has poorer bioavailability than free DOX, indicating that enhancing bioavailability should be considered while designing liposomes. Liposomes’ main applications are co-delivery and controlled release (Samad A, et al., 2007; Allen TM and Cullis PR, 2013). To achieve active targeting, liposome-bound antibodies target tumor-specific antigens and then carry medicines to the tumor. Chemical drugs, metals, gene agents, and other chemotherapeutic agents have been combined. One of the patterns of cancer occurrence is the overactivation of signalling pathways, and drugs targeting these signalling pathways are used. To increase efficacy, scientists loaded a novel PEGylated liposomal with ncl-240 and cobimetinib, both of which are small-molecule inhibitors of the Phosphoinositide 3-Kinase/mammalian Target of Rapamycin (PI3K/mTOR) pathway and the Mitogen-Activated Protein Kinase/Extracellular signal-Regulated protein Kinase (MAPK/ERK) pathway. The results demonstrated that synergistic effects increased the cytotoxic impact. In advanced solid tumors, a new liposome-encapsulated nanocarrier loaded with irinotecan and floxuridine demonstrated improved effectiveness (Saxena SK, et al., 2020). The delicate structure of a new liposome with several layers allowed it to successfully load up to 3500 siRNA molecules in a single bilayer and codelivery of DOX, resulting in improved DOX effectiveness and tumor mass shrinkage in breast cancer therapy. Liposomal formulations of Adriamycin, for example, have demonstrated significant therapeutic improvement in the treatment of metastatic ovarian cancer (Saraf S, et al., 2020). Liposomes help to overcome multidrug resistance. Drugs such as cytarabine, doxorubicin, daunorubicin, irinotecan, mitoxantrone, and paclitaxel are used with liposome delivery for cancer diagnosis.
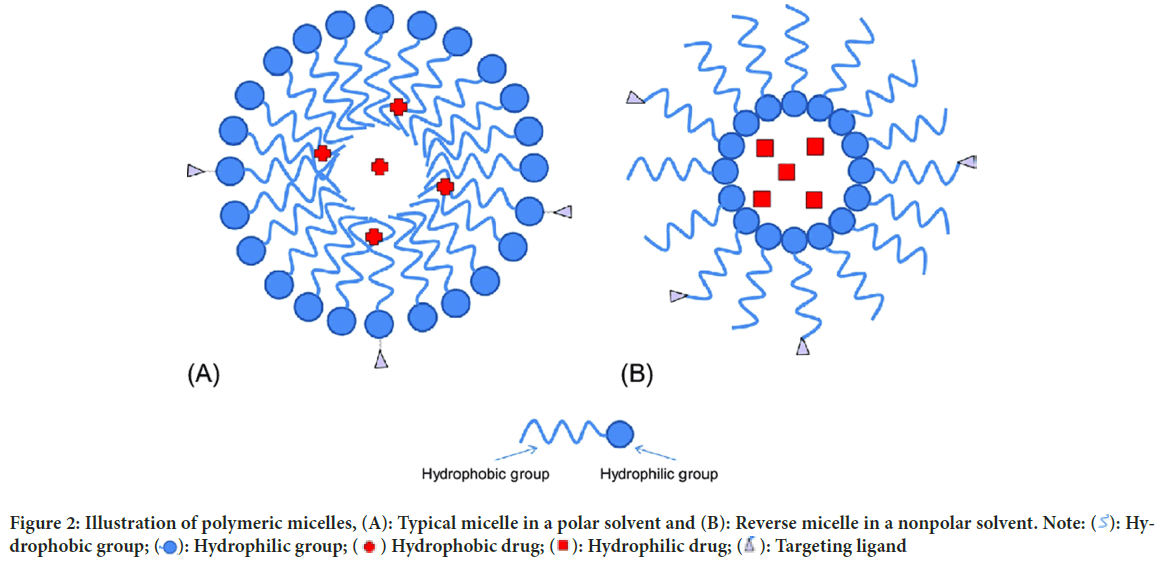
Polymeric micelles: Micelles are self-structured spherical particles with a hydrophilic covering shell and a hydrophobic core floating in an aqueous medium with a diameter of 10-100 nm. Hydrophobic medications can be contained in the core of the micelle (Figure 2). In active tumor cell targeting, a range of compounds that may bind to receptors, such as aptamers, peptides, antibodies, polysaccharides, and folic acid, are employed to coat the surface of the micelle.
Figure 2: Illustration of polymeric micelles, (A): Typical micelle in a polar solvent and (B): Reverse micelle in a nonpolar solvent. Note:  : Hydrophobic group;
: Hydrophobic group;  : Hydrophilic group;
: Hydrophilic group;  Hydrophobic drug;
Hydrophobic drug;  : Hydrophilic drug;
: Hydrophilic drug; 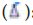 : Targeting ligand
: Targeting ligand
Polymeric micelles have recently been widely utilized in the creation of nanotechnology-based cancer drugs because of their high prospective benefits for patient care (Majumder N, et al., 2020). For example, an adriamycin conjugated nanomaterial was employed to treat different forms of cancer and showed promising therapeutic results. Unfortunately, it had several negative effects, including toxicity and cardiac issues, which limited its usage. Doxil (a liposomal version of doxorubicin), which is less related to cardiotoxicity in patients and hence may give a safer nanomaterial synthesis technique for researchers in the future, overcomes such issues (Bharali DJ, Mousa SA, 2010; Thakor AS and Gambhir SS, 2013). Paclitaxel is a potent microtubule growth inhibitor; however, it is poorly soluble, resulting in rapid drug aggregation and capillary embolisms. By encapsulating such medications in micelles, their solubility can be increased to 0.0015-2 mg/ml. Polymeric micelles are currently being studied for their potential application in nano therapy (Masood F, 2016).
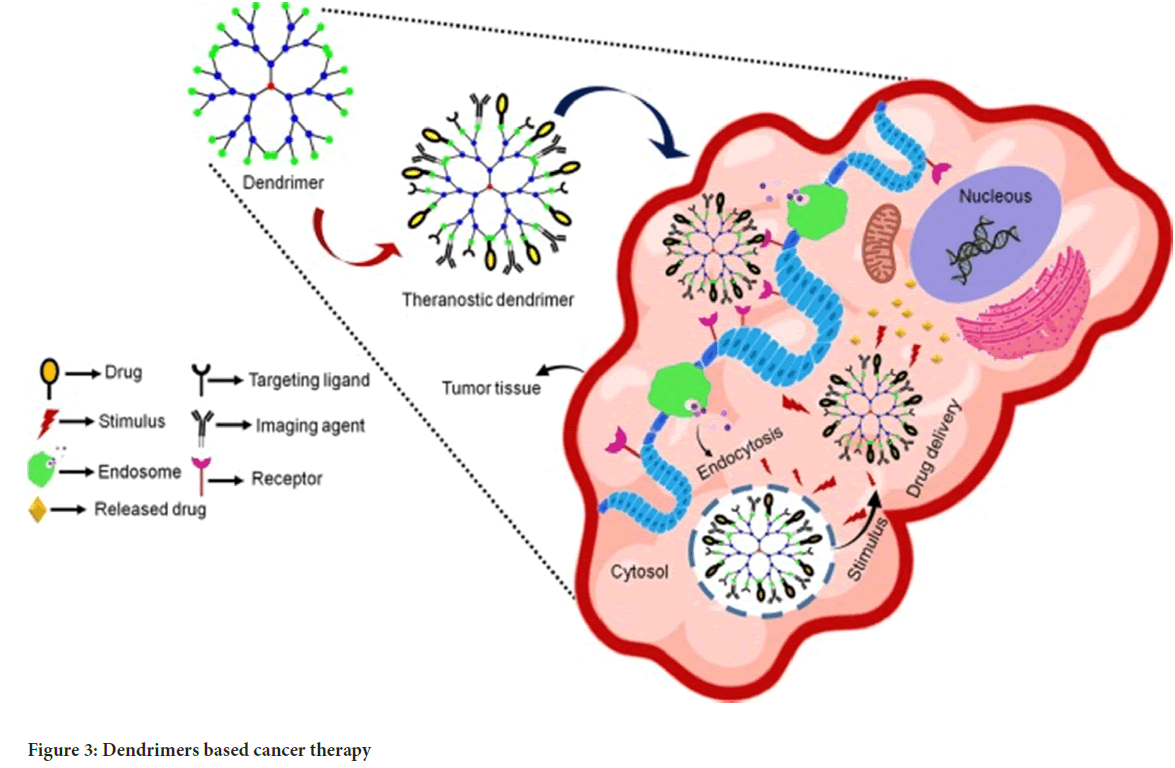
Dendrimers: They are unique nano architectures with distinguishing features such as a three-dimensional spherical form, a monodispersed uni-micellar nature, and a nanometric size range. Several molecules including polyacrylamide, polyglycerol-succinic acid, polylysine, polyglycerine, poly 2, 2 bis (hydroxymethyl) propionic acid, and melamine are commonly used to form dendrimers. Dendrimer biocompatibility has been used to administer strong drugs such as doxorubicin. Its nanostructure attaches ligands to the surfaces of cancerous cells. Dendrimers have been thoroughly researched for their potential to target and administer cancer therapies as well as magnetic resonance imaging contrast agents (Sharma AK, et al., 2017) (Figure 3).
Figure 3: Dendrimers based cancer therapy
Various researches have additionally demonstrated that cancer cells with high folate receptor expression may generate foils from dendritic molecules coupled to folate. Dendrimers also have the capacity to attach to DNA, as demonstrated by the DNA-polyamides clustered DNA-Poly(amidoamine) (DNAPAMAM), making them extremely efficient at killing cancer cells that express the folate receptor. Thus, it also worked as an anchor for high-affinity targeting molecules to be attached to tumour cells.
Quantum dots: Quantum Dots (QDs) are microscopic particles or nanocrystals of semiconductor materials that range in size from 2 to 10 nm. The intermediate electron property of QDs, which lies between a mass semiconductor and a discrete atom, is provided by the ratio of the height of the surface to the volume of these particles. Moreover, QD immunostaining is more accurate than standard immunochemical approaches at low protein expression levels and in a low context. QD immunostaining is a possible method in cancer diagnostics for detecting numerous tumour biomarkers, such as a cell protein or other components of a heterogeneous tumour sample. Quantum dots may congregate in certain areas of the body and transport medications to those areas (Peng CW and Li Y, 2010).
The potential of QDs to concentrate in a single internal organ gives them a realistic solution to untargeted medication delivery, as well as a possible way to prevent chemotherapy side effects. The most recent progress in surface modification of QDs, which may be used to target tumours and mix with biomolecules such as peptides and antibodies in vivo, can be utilized to target tumours and enable their prospective uses in cancer imaging and therapy. High-sensitivity quantum dot probes for multicolor fluorescence imaging of cancer cells in vivo have been described, and they may also be utilized to identify the ovarian cancer marker-Cancer Antigen 125 (CA125) in many types of tissues such as; fixed cells, tissue sections, and xenograft (Chinen AB, et al., 2015).
Carbon nanotubes: Carbon Nanotubes (CNTs) are hollow cylinders made from carbon derived from burnt graphite. They have particular physical and chemical properties that make them appealing candidates for biomolecule carriers and drug delivery transporters (Guo D, et al., 2013). They have a unique function in the transport of anticancer medicines with tiny molecular sizes. Carbon nanotubes also have the ability to absorb light in the Near-Infrared (NIR) range, enabling the nanotubes to heat up due to the thermal effect and therefore targeting tumour cells. Mukherjee A and Bhattacharyya S, 2020 created a medication carrier system out of Multi-Walled Carbon Nanotubes (MWCNTs) and the anticancer drug 10-Hydroxycamptothecin (HCPT). They used hydrophilic diamine trimethylene glycol as a spacer between MWCNTs and HCPT. Because of the appropriateness of carbon nanotubes for cancer treatment, medicines such as paclitaxel are assembled with them and delivered both in vitro and in vivo .
Nanotechnology in cancer diagnosis
Although nanoparticles have not yet been utilized in cancer diagnosis, they are being used in a variety of medical screening assays. Gold nanoparticles are a popular ingredient in home test strips. One notable advantage of employing nanoparticles for cancer detection is that they have a high surface area to volume ratio when compared to bigger equivalents. Nanotechnology applications in diagnostics include the identification of extracellular biomarkers for cancer and in vivo imaging (Zhang Y, et al., 2019; Sim S and Wong NK, 2021).
Detection of biomarkers: Nanodevices have been explored for their ability to detect blood indicators as well as toxicity to surrounding healthy tissues. Cancer-related circulating tumour cells, associated proteins or cell surface proteins, carbohydrates or circulating tumour nucleic acids, and tumour-shed exosomes are examples of biomarkers (Kher C and Kumar S, 2022). Even while it is generally recognized that these biomarkers aid in the early detection of cancer, they also aid in the monitoring of therapy and recurrence. They have disadvantages such as low concentrations in bodily fluids, differences in amounts and timings between individuals, and challenging prospective investigations.
Imagining using nanotechnology: Nanotechnology utilizes nanoprobes that preferentially aggregate in tumour cells via passive or active targeting (Xu JJ, et al., 2014). The interactions of nanoparticles with blood proteins, their clearance by the reticuloendothelial system, and tumour targeting are all problems. Passive targeting implies a tendency for collecting nanoparticles in solid tumours as a result of extravasation from blood arteries. This is made possible by the tumor’s defective angiogenesis, in which the new blood vessels lack tight connections in their endothelial cells, allowing nanoparticles up to 150 nm in size to leak out, resulting in a preferential concentration of nanoparticles in the tumour tissue. This is known as improved permeability and retention. The identification of nanoparticles by tumour cell surface receptors is referred to as active targeting (Seleci M, et al., 2017). This will improve the sensitivity of tumour detection in vivo. Active targeting surpasses passive targeting in terms of early cancer detection (Dessale M, et al., 2022).
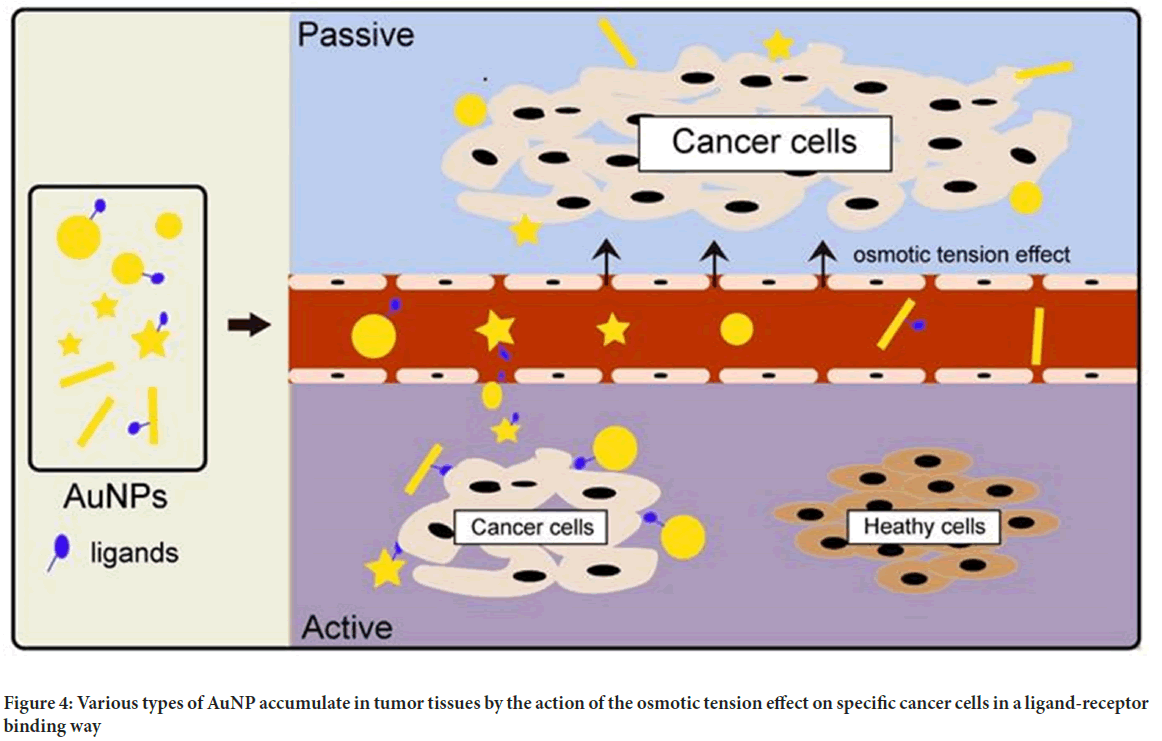
Gold nanoparticles: Given their tiny size, outstanding biocompatibility, and high atomic number, gold nanoparticles (AuNPs) are an excellent contrast material. According to the investigation, AuNPs target cells in both active and passive ways. The passive targeting mechanism is guided by a collection of gold nanoparticles to improve imaging due to the permeability tension effect in tumour tissues (Figure 4). Active targeting, on the other hand, is achieved by pairing AuNPs with tumor-specific targeted medicines such as Epidermal Growth Factor Receptor (EGFR) monoclonal antibodies. As the energy exceeds 80 keV, the mass attenuation rate of gold increases faster than that of other elements such as iodine, indicating a larger potential for gold nanoparticles (Zhou W, et al., 2015; Alam F, et al., 2015).
Figure 4: Various types of AuNP accumulate in tumor tissues by the action of the osmotic tension effect on specific cancer cells in a ligand-receptor binding way
Choi YE, et al., 2010 combined AuNPs with liver cancer cells and discovered that the clusters of liver cancer cells in the gold nanocomposite group were substantially stronger than those in the liver cancer cells alone using X-ray imaging. These discoveries have significant implications for early detection, as the technology may identify cancers as tiny as a few millimetres in diameter in the body.
Discussion and Conclusion
Throughout the years, nanotechnology has shown a lot of promise in cancer therapy. Nanomaterials have helped to enhance cancer detection and therapy by improving pharmacokinetic and pharmacodynamic qualities. Because of their specificity, nanotechnology enables targeted medicine delivery in damaged tissues with minimal systemic toxicity.
Nevertheless, like with other therapeutic approaches, nanotechnology is not without toxicity and comes with a few obstacles with its usage, such as systemic and specific organ toxicities, creating delays in clinical applications. Nanomaterials are highly versatile, with several benefits that can enhance cancer therapies and diagnostics. Because of their small size and unique binding capabilities, they are particularly valuable as drug-delivery devices.
In recent clinical practice, drugs such as doxorubicin, daunorubicin, mitoxantrone, paclitaxel, cytarabine, irinotecan, and amphotericin B are conjugated with liposomes for delivery. Doxorubicin, cytarabine, vincristine, daunorubicin, mitoxantrone, and paclitaxel are essential components of cancer treatment. Particles such as nanoshells, dendrimers, and gold nanoparticles are now used in cancer diagnostics for imaging and detection of tumour markers.
In vivo imaging, innovative treatments, and better drug delivery technologies will be used by pharmaceutical firms in their new commercial applications. When employed to treat brain tumours, neuro-electronic interfaces, and cell healing technologies may alter medicine and the biological sciences in the future.
References
- Nikolova M, Slavchov R, Nikolova G. Nanotechnology in medicine. Drug Discovery and Evaluation: Methods in Clinical Pharmacology. 2020: 533-546.
- Jaishree V, Gupta PD. Nanotechnology: A revolution in cancer diagnosis. Indian J Clin Biochem. 2012; 27(3): 214-220.
[Crossref] [Google Scholar] [Pubmed]
- Jena S, Mohanty S, Ojha M, Subham K, Jha S. Nanotechnology: An emerging field in protein aggregation and cancer therapeutics. Bio-Nano Interface. 2022: 177-207.
- Jin C, Wang K, Oppong-Gyebi A, Hu J. Application of nanotechnology in cancer diagnosis and therapy-a mini-review. Int J Med Sci. 2020; 17(18): 2964-2973.
[Crossref] [Google Scholar] [Pubmed]
- Fang M, Peng CW, Pang DW, Li Y. Quantum dots for cancer research: Current status, remaining issues, and future perspectives. Cancer Biol Med. 2012; 9(3): 151-163.
[Crossref] [Google Scholar] [Pubmed]
- Torchilin VP. Recent advances with liposomes as pharmaceutical carriers. Nat Rev Drug Discov. 2005; 4(2): 145-160.
[Crossref] [Google Scholar] [Pubmed]
- Bozzuto G, Molinari A. Liposomes as nanomedical devices. Int J Nanomedicine. 2015; 10: 975-999.
[Crossref] [Google Scholar] [Pubmed]
- Jani P, Subramanian S, Korde A, Rathod L, Sawant KK. Theranostic nanocarriers in cancer: Dual capabilities on a single platform. Functional Bionanomaterials. 2020: 293-312.
- Samad A, Sultana Y, Aqil M. Liposomal drug delivery systems: An update review. Curr Drug Deliv. 2007; 4(4): 297-305.
[Crossref] [Google Scholar] [Pubmed]
- Allen TM, Cullis PR. Liposomal drug delivery systems: From concept to clinical applications. Adv Drug Deliv Rev. 2013; 65(1): 36-48.
[Crossref] [Google Scholar] [Pubmed]
- Saxena SK, Nyodu R, Kumar S, Maurya VK. Current advances in nanotechnology and medicine. NanoBioMedicine. 2020: 3-16.
- Saraf S, Jain A, Tiwari A, Verma A, Panda PK, Jain SK. Advances in liposomal drug delivery to cancer: An overview. J Drug Deliv Sci Technol. 2020; 56: 101549.
- Majumder N, G Das N, Das SK. Polymeric micelles for anticancer drug delivery. Ther Deliv. 2020; 11(10): 613-635.
[Crossref] [Google Scholar] [Pubmed]
- Bharali DJ, Mousa SA. Emerging nanomedicines for early cancer detection and improved treatment: Current perspective and future promise. Pharmacol Ther. 2010; 128(2): 324-335.
[Crossref] [Google Scholar] [Pubmed]
- Thakor AS, Gambhir SS. Nanooncology: The future of cancer diagnosis and therapy. CA Cancer J Clin. 2013; 63(6): 395-418.
[Crossref] [Google Scholar] [Pubmed]
- Masood F. Polymeric nanoparticles for targeted drug delivery system for cancer therapy. Mater Sci Eng C Mater Biol Appl. 2016; 60: 569-578.
[Crossref] [Google Scholar] [Pubmed]
- Sharma AK, Gothwal A, Kesharwani P, Alsaab H, Iyer AK, Gupta U. Dendrimer nanoarchitectures for cancer diagnosis and anticancer drug delivery. Drug Discov Today. 2017; 22(2): 314-326.
[Crossref] [Google Scholar] [Pubmed]
- Peng CW, Li Y. Application of quantum dots-based biotechnology in cancer diagnosis: Current status and future perspectives. J Nanomater. 2010; 2010: 1-6.
- Chinen AB, Guan CM, Ferrer JR, Barnaby SN, Merkel TJ, Mirkin CA. Nanoparticle probes for the detection of cancer biomarkers, cells, and tissues by fluorescence. Chem Rev. 2015; 115(19): 10530-10574.
[Crossref] [Google Scholar] [Pubmed]
- Guo D, Xie G, Luo J. Mechanical properties of nanoparticles: Basics and applications. J Phys D: Appl Phys. 2013; 47(1): 013001.
- Mukherjee A, Bhattacharyya S. Nanotechnology in medicine. Biotechnology Business-Concept to Delivery. 2020: 57-64.
- Zhang Y, Li M, Gao X, Chen Y, Liu T. Nanotechnology in cancer diagnosis: Progress, challenges and opportunities. J Hematol Oncol. 2019; 12(1): 1-3.
[Crossref] [Google Scholar] [Pubmed]
- Sim S, Wong NK. Nanotechnology and its use in imaging and drug delivery. Biomed Rep. 2021; 14(5): 1-9.
[Crossref] [Google Scholar] [Pubmed]
- Kher C, Kumar S. The application of nanotechnology and nanomaterials in cancer diagnosis and treatment: A review. Cureus. 2022; 14(9).
[Crossref] [Google Scholar] [Pubmed]
- Xu JJ, Zhao WW, Song S, Fan C, Chen HY. Functional nanoprobes for ultrasensitive detection of biomolecules: An update. Chem Soc Rev. 2014;43(5):1601-11.
[Crossref] [Google Scholar] [Pubmed]
- Seleci M, Ag Seleci D, Scheper T, Stahl F. Theranostic liposome-nanoparticle hybrids for drug delivery and bioimaging. Int J Mol Sci. 2017; 18(7): 1415.
[Crossref] [Google Scholar] [Pubmed]
- Dessale M, Mengistu G, Mengist HM. Nanotechnology: A promising approach for cancer diagnosis, therapeutics and theragnosis. Int J Nanomedicine. 2022; 17: 3735-3749.
[Crossref] [Google Scholar] [Pubmed]
- Zhou W, Gao X, Liu D, Chen X. Gold nanoparticles for in vitro diagnostics. Chem Rev. 2015; 115(19): 10575-10636.
[Crossref] [Google Scholar] [Pubmed]
- Alam F, Naim M, Aziz M, Yadav N. Unique roles of nanotechnology in medicine and cancer-II. Indian J Cancer. 2015; 52(1): 1-9.
[Crossref] [Google Scholar] [Pubmed]
- Choi YE, Kwak JW, Park JW. Nanotechnology for early cancer detection. Sensors. 2010; 10(1): 428-455.
[Crossref] [Google Scholar] [Pubmed]
Author Info
Vyasa Mudra*Citation: Mudra V: Nanotechnology in Cancer Diagnosis: Current Perspectives
Received: 12-Jun-2023 Accepted: 07-Jul-2023 Published: 14-Jul-2023, DOI: 10.31858/0975-8453.14.7.441-446
Copyright: This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
ARTICLE TOOLS
- Dental Development between Assisted Reproductive Therapy (Art) and Natural Conceived Children: A Comparative Pilot Study Norzaiti Mohd Kenali, Naimah Hasanah Mohd Fathil, Norbasyirah Bohari, Ahmad Faisal Ismail, Roszaman Ramli SRP. 2020; 11(1): 01-06 » doi: 10.5530/srp.2020.1.01
- Psychometric properties of the World Health Organization Quality of life instrument, short form: Validity in the Vietnamese healthcare context Trung Quang Vo*, Bao Tran Thuy Tran, Ngan Thuy Nguyen, Tram ThiHuyen Nguyen, Thuy Phan Chung Tran SRP. 2020; 11(1): 14-22 » doi: 10.5530/srp.2019.1.3
- A Review of Pharmacoeconomics: the key to “Healthcare for All” Hasamnis AA, Patil SS, Shaik Imam, Narendiran K SRP. 2019; 10(1): s40-s42 » doi: 10.5530/srp.2019.1s.21
- Deuterium Depleted Water as an Adjuvant in Treatment of Cancer Anton Syroeshkin, Olga Levitskaya, Elena Uspenskaya, Tatiana Pleteneva, Daria Romaykina, Daria Ermakova SRP. 2019; 10(1): 112-117 » doi: 10.5530/srp.2019.1.19
- Dental Development between Assisted Reproductive Therapy (Art) and Natural Conceived Children: A Comparative Pilot Study Norzaiti Mohd Kenali, Naimah Hasanah Mohd Fathil, Norbasyirah Bohari, Ahmad Faisal Ismail, Roszaman Ramli SRP. 2020; 11(1): 01-06 » doi: 10.5530/srp.2020.1.01
- Manilkara zapota (L.) Royen Fruit Peel: A Phytochemical and Pharmacological Review Karle Pravin P, Dhawale Shashikant C SRP. 2019; 10(1): 11-14 » doi: 0.5530/srp.2019.1.2
- Pharmacognostic and Phytopharmacological Overview on Bombax ceiba Pankaj Haribhau Chaudhary, Mukund Ganeshrao Tawar SRP. 2019; 10(1): 20-25 » doi: 10.5530/srp.2019.1.4
- A Review of Pharmacoeconomics: the key to “Healthcare for All” Hasamnis AA, Patil SS, Shaik Imam, Narendiran K SRP. 2019; 10(1): s40-s42 » doi: 10.5530/srp.2019.1s.21
- A Prospective Review on Phyto-Pharmacological Aspects of Andrographis paniculata Govindraj Akilandeswari, Arumugam Vijaya Anand, Palanisamy Sampathkumar, Puthamohan Vinayaga Moorthi, Basavaraju Preethi SRP. 2019; 10(1): 15-19 » doi: 10.5530/srp.2019.1.3