Research Article - (2024) Volume 15, Issue 10
Network Pharmacology and Experimental Validation Reveal the Anti-Inflammatory Effects of Schefflera octophylla via Inhibition of the PI3K-AKT Pathway
Xiaoqin Zhou1, Bin Yang2, Yao Liang2 and Xiaobu Lan1*Abstract
Background: Ethnopharmacological relevance shows that Schefflera octophylla (Lour.) Harms is an indigenous plant and traditional Chinese medicine. WaiGan mixture II, which is a complex herbal preparation contains Schefflera octophylla as the main herb and is commonly employed in clinical practice to treat conditions associated with wind-heat colds, such as the resolution of heat, toxins and alleviation of throat symptoms. Although these uses of Schefflera octophylla highlight its potential as an anti-inflammatory agent, its efficacy and underlying mechanisms remain to be thoroughly explored.
As traditional Chinese medicines contain a multitude of ingredients, it can be challenging to determine the specific small molecular compounds responsible for their medicinal effects. Here, we aim to identify the active anti-inflammatory compounds and targets of Schefflera octophylla through network pharmacology, evaluate their efficacy using molecular docking techniques and investigate their anti-inflammatory effects and mechanisms using in vitro and in vivo experiments.
Materials and methods: Compound-disease-target-pathway network was established through network pharmacology to determine the potential anti-inflammatory mechanism pathways in Schefflera octophylla. The efficacy of the medication was assessed by injecting the toes of Kunming (KM) mice with carrageenan (an inflammatory agent) to induce swelling, before measuring the swelling inhibition rate and effects on serum Interleukin (IL)-6 and Malondialdehyde (MDA). Nitrogen Monoxide (NO) content of medicated Lipopolysaccharide (LPS)-induced RAW264.7 cells was measured using the Griess method, while the levels of IL-1 Beta (β), IL-6 and IL-10 secreted by RAW264.7 cells were measured using Enzyme Linked Immunosorbent Assay (ELISA). Western blot was used to analyze the effects of the medication on protein expression of the Phosphatidylinositol 3 Kinase/protein Kinase B (PI3K/Akt) pathway. In this study, aqueous and ethanol extracts of Schefflera octophylla were used as medication.
Results: Aqueous and ethanol extracts of Schefflera octophylla significantly reduced carrageenan-induced toe swelling, and decreased IL-6 and MDA levels. The medication also demonstrated potent anti-inflammatory properties in RAW264.7 cells by decreasing the levels of the LPS-induced inflammation-related factors NO, IL-6 and IL-1β, increasing the level of the anti-inflammatory cytokine IL-10 and decreasing LPS-induced phosphorylation of PI3K-Akt pathway proteins.
Conclusion: These results provide strong evidence for further investigation into the molecular mechanisms of Schefflera octophylla for treating acute inflammation from an anti-inflammatory perspective.
Keywords
Schefflera octophylla, Network pharmacology, molecular docking, anti-inflammation, Chinese medicine, PI3K-Akt pathway
Introduction
Inflammation is an adaptive response triggered by specific conditions and noxious stimuli, and representing one of the most essential and prominent protective responses of an organism (Medzhitov R, 2008; Kuprash DV and Nedospasov SA, 2016). The initiation of inflammation is a response to cellular injury or the presence of pathogens which is generally mediated by resident immune cells. An inflammatory response is characterized by the engagement of Pathogen Recognition Receptors (PRRs), such as Toll Like Receptors (TLRs), leading to the synthesis and release of pro-inflammatory cytokines, which activate downstream pro-inflammatory signaling pathways (Ala A, et al., 2003; Takeuchi O and Akira S, 2010; Feehan KT and Gilroy DW, 2019). The resolution of inflammation is a tightly regulated process that is driven by a complex set of mediators that regulate the cellular activities required to restore homeostasis and clear inflammatory cells from sites of infection or injury in the body (Gilroy D and de Maeyer R, 2015). However, in certain circumstances, the resolution process may become dysregulated leading to a state of chronic inflammation, which is characterized by persistent activation of immune cells and excessive secretion of chemokines, resulting in progressive tissue damage (Tang F, et al., 2018). Because inflammation is widely involved in the pathological process of many diseases, anti-inflammation is an attractive field of drug development. In the current clinical context, anti-inflammatory treatments comprise a range of widely prescribed drugs, including Non-Steroidal Anti-Inflammatory Drugs (NSAIDs), non-selective NSAIDs (nsNSAIDs) and selective Cyclooxygenase 2 NSAIDs (COXIBs), which are used for managing fever, pain and inflammation which are associated with diseases such as rheumatoid arthritis and osteoarthritis (Layton D, et al., 2008; Bacchi S, et al., 2012). Despite their efficacy, certain pharmaceuticals may possess limitations that restrict their widespread and sustained usage (Cunningham K, et al., 2020). For example, despite their broad therapeutic effects, NSAIDs that hinder Cyclooxygenase (COX) (Mizushima T, 2010) are associated with many serious side effects, including cardiovascular risk, gastrointestinal toxicity, hepatotoxicity, kidney damage, hypertension and other minor diseases (Harirforoosh S, et al., 2013; Patricio JP, et al., 2013; Arfe A, et al., 2016; Gunter BR, et al., 2017; Bindu S, et al., 2020). The quest for the discovery of safer and more effective anti-inflammatory agents continues to present a major challenge for the medical community. Chinese herbal medicine offers a potentialvenue to tackle the problem of drug safety, particularly with regard to anti-inflammatory drugs. The “Manifestation theory” of Chinese medicine, proposed by Cai SQ, et al., 2015, suggests that Chinese medicine achieves therapeutic effects while reducing toxicity through a combination of single-target superposition of various manifestation forms, multi-target synergistic effects and toxic dispersion effects. Traditional Chinese medicine is a valuable resource that has been critical to disease prevention and treatment since ancient times. Chinese herbal medicine has an overwhelming advantage for treating inflammation, with low cost and few side effects. Indeed, there is a growing body of evidence supporting the anti-inflammatory effects of traditional Chinese remedies such as Paeonia lactiflora (Bai Shao) (Zhang L and Wei W, 2020), Scutellaria baicalensis (Huangqin) (Jiang M, et al., 2020) and Forsythia (Lianqiao) (Chen L, et al., 2018), all of which exhibit significant efficacy in controlling inflammation.
Schefflera octophylla (Lour.) Harms, belonging to Araliaceae family is a frequently used plant in traditional ethnomedicine, with the bark, either fresh or dried, representing the primary source of its medicinal properties (Pang S, et al., 2016). The genus Schefflera has been traditionally used for its medicinal properties in China and its use has been documented in various authoritative sources, including the “Chinese Dictionary of Traditional Chinese Medicine,” the “Chinese Materia Medica,” and the “Xinhua Materia Medica Compendium.” These records indicate that the genus has not only been used for its analgesic and anti-inflammatory effects, but also for its anti-tumor and anti-viral properties (Xu J, et al., 2006). The medicinal preparation “WaiGan mixture II,” composed primarily of Schefflera octophylla, has demonstrated clinical efficacy in treating cold and flu symptoms and inflammation caused by influenza viruses (Wang Y, et al., 2006). Its use at the First People’s Hospital of Nanning for more than 40 years suggest that Schefflera octophylla has positive effect on alleviating cold- and flu-related symptoms, but its exact efficacy and underlying mechanisms warrant further investigation.
Over the past decade, molecular pharmacology has gained prominence in the field of pharmacology, leading to new avenues of research into the mechanisms of drug action, as well as uncovering innovative strategies for mediating biological systems using molecular probes, receptor signaling, drug disposition and targeting of biological systems. This advancement in molecular pharmacology has led to the development of innovative strategies for modulating biological systems and facilitated investigation into the biological processes underlying pharmacology and drug design (Tasneem S, et al., 2019). Here, we employed network pharmacology to investigate the anti-inflammatory mechanism of Schefflera octophylla and identify specific targets. The results of this research provide a foundation for promoting the application of local Chinese herbal medicines in China.
Materials and Methods
Network pharmacology analysis
Active ingredient screening and target prediction of Schefflera octophylla: After conducting a comprehensive study (Zhang H, 2014; Shuhong T, et al., 2015; Wang G, 2018) on the chemical constituents present in Schefflera octophylla, chemical data was obtained from the PubChem database (https://pubchem.ncbi.nlm.nih.gov/) (Kim S, et al., 2021) and active ingredient targets were screened using the SWISS Absorption, Distribution, Metabolism and Excretion (ADME) criteria (http://www.swissadme.ch/) (Daina A, et al., 2017), which considered high Gastrointestinal (GI) absorption and favorable drug-like properties. The potential targets of action for these active ingredients were then predicted using the SwissTargetPrediction database (http://www.swisstargetprediction.ch/) (Daina A, et al., 2019). This research established a library of chemical information on Schefflera octophylla and identified potential targets for further investigating its pharmacological properties.
Inflammatory disease target prediction: Online Mendelian Inheritance in Man (OMIM) (https://omim.org/), DisGeNET (https://www.disgenet.org/home/) (Piñero J, et al., 2020) and GeneCards (https://genecards.org/) databases were utilized to search the keyword “Inflammation” to obtain a comprehensive list of inflammation-related targets.
Common target analysis of components and diseases: Venny software version 2.1.0 (https://bioinfogp.cnb.csic.es/tools/venny/index.html) was used to identify the overlapping active ingredients and disease targets related to inflammation. The intersection of the active ingredients and disease targets resulted in a set of common target genes, which were then subjected to further analysis.
Protein-Protein Interaction (PPI) network construction and visualization: The intersecting target genes were analyzed using the Search Tool for the Retrieval of Interacting Genes (STRING) (https://cn.string-db.org/) (Szklarczyk D, et al., 2021) database to create a PPI network of the active ingredients and inflammation-related targets of Schefflera octophylla. The network was processed with the aid of Cytoscape software using the CytoNCA plug-in. Centrality values, including Betweenness Centrality (BC), Closeness Centrality (CC) and Degree Centrality (DC) were calculated using the network analyzer function where the core targets were ranked based on these values.
Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analyses: GO and KEGG pathway enrichment analyses were conducted on the common target genes using the Database for Annotation, Visualization and Integrated Discovery (DAVID) database (https://cn.string-db.org/) (Huang DW, et al., 2009; Sherman BT, et al., 2022). The results were visualized through correlation maps generated on a bioinformatics platform (https://www.bioinformatics.com.cn/).
Construction of the “active ingredient-target-disease-pathway” network: Cytoscape version 3.9.1 software was used to generate a graphical representation of the 20 most significant KEGG pathways and the interconnected network of active ingredients, targets and diseases (p<0.5).
Validation of molecular docking technology: Subsequently, molecular docking experiments were performed to validate the network pharmacology results. The active compounds were obtained in mol2 format from the PubChem database and the protein structures of Schefflera octophylla and the target proteins involved in inflammation were obtained from the Research Collaboratory for Structural Bioinformatics Protein Data Bank (RCSB PDB) database (https://www.rcsb.org/). These structures were processed using PyMOL software for hydrogenation and dehydration, before subjecting to docking simulations using AutoDock Vina. Finally, the binding energies were visualized using heat maps generated in Origin2021.
Medication preparation
In a multifunctional extraction tank, an ethanol extract (Concentration of 8.425 g/ml) of Schefflera octophylla was obtained by adding the bark (Guangxi, Batch No: 20201102) of the plant to 80% ethanol and concentrating it three times. The aqueous extract (Concentration of 10.42 g/ml) was obtained by extracting the plant with pure water.
Animal experiment
Male KM mice which were 6-7 weeks old were obtained from the Experimental Animal Center of Guangxi Medical University and were maintained in a controlled environment at a temperature of 25 ± 2°C and 50% ± 5% humidity, under a 12 h light/12 h dark cycle and with ad libitum access to food and water. The study was conducted in accordance with the guidelines approved by the Guangxi Medical University Laboratory Animal Centre (Grant No: SYXK2020-0004).
Determination of toe swelling, IL-6 and MDA levels in KM mice induced by carrageenan: The animals were divided into 8 groups, including the control group, dexamethasone group (10 mg/kg) and those treated with ethanol and aqueous extracts of Schefflera octophylla with different concentrations (2.5, 5 and 10 g/kg). In all cases, treatment was administered daily for 7 days. The inflammatory response was induced by injecting 2% carrageenan into the right foot of the mice and the swelling was calculated by subtracting the left foot weight from the right foot weight 4 h later.
The levels of IL-6 and the antioxidant indicator MDA were measured in the serum of inflamed mice using ELISA (Elabscience, Wuhan, China) and biochemical kits (Nanjing Jiancheng Bioengineering Institute), respectively.
Cellular experiments
RAW264.7 cells were obtained from the Chinese academy of sciences cell bank and were cultured in high-glucose medium (Gibco) with 10% fetal bovine serum at 37°C, 5% Carbon dioxide (CO2).
Effect of Schefflera octophylla on cell activity: The viability of RAW264.7 cells was evaluated following 24 h exposure to various concentrations of Schefflera octophylla ethanol and aqueous extracts (100 µg/ml, 200 µg/ml, 400 µg/ml, 600 µg/ml, 800 µg/ml, 1000 µg/ml and 2000 µg/ml) using the 3-(4,5-dimethylthiazolyl-2)-2,5-diphenyltetrazolium bromide (MTT) assay. IC10 values were calculated and analyzed using Statistical Package for Social Sciences (SPSS) version 23.0 software.
Effect of Schefflera octophylla on inflammatory factors and morphology of RAW264.7 cells: The RAW264.7 cells were seeded in 12-well plates at a cell density of 3 × 104 cells/well and then pretreated with Schefflera octophylla ethanol extract (75.5, 150 and 300 μg/ml) or aqueous extract (171.5, 343 and 686 μg/ml) for 1 h. Subsequently, the cells were challenged with Lipopolysaccharide (LPS) solution (1 µg/ml) for 24 h, before collecting the supernatant. NO content in the supernatant was determined using Griess reagent (Nanjing Jiancheng Bioengineering Institute) and the levels of IL-1β, IL-6 and IL-10 were quantified using ELISA kits (Elabscience, Wuhan, China).
Changes in the morphology were observed following successful induction of inflammation in RAW264.7 cells using LPS. The efficacy of anti-inflammatory drugs can be evaluated by their ability to restore the cells to their normal state. Therefore, the morphological assessment of RAW264.7 cells using light microscopy is a valuable tool for validating the in vitro inflammation model and determining the anti-inflammatory effects of Schefflera octophylla extract.
The cellular morphologies of the blank control, LPS and experimental groups were analyzed under a light microscope, with a field of view magnified 40 times to compare and contrast any differences. This analysis serves as a reliable method for evaluating the success of the in vitro inflammation model and the efficacy of Schefflera octophylla extract as an anti-inflammatory agent.
Western blot: RAW264.7 cells were incubated as described previously. Proteins were extracted and their concentration was determined using a Bicinchoninic Acid (BCA) assay. Subsequently, the proteins were subjected to denaturation by boiling, followed by electrophoresis in 10% Polyacrylamide Gel Electrophoresis (PAGE) gels and transfer to Polyvinylidene Difluoride (PVDF) membranes. The membranes were blocked with a blocking solution for 30 min and incubated with specific primary antibodies overnight. The primary antibodies used were as follows-
Phosphoinositide 3-Kinase (PI3K) (1:1000, #CST-4292), phosphorylated (p) PI3K (1:1000, AF3242) (Affinity Bioscience), Akt (1:1000, 10176-2-AP, Wuhan Proteintech), p-Akt (1:1000, 28731-1-AP, Wuhan Proteintech) and β-actin (1:1000, 20536-1-AP, Wuhan Proteintech). Following incubation, the membranes were washed and incubated with goat anti-rabbit IgG Heavy+Light (H+L) chains secondary antibody (1:10,000, SA5-35571, ThermoFisher Scientific). The next day, after washing three times using Tris-Buffered Saline with 0.1% Tween (TBST) (TBS was prepared by adding 1 ml of Tween 20 dissolved in pure water) for 5 min/time, the membranes were immersed in the corresponding secondary antibody for 1 h (on a shaker, at room temperature), before washing a further three times. Finally, the protein bands were detected using two-colored infrared imaging system (Li-COR, Odysse Clx, USA).
Results
Schefflera octophylla active ingredient screening and target prediction
The chemical composition of Schefflera octophylla was characterized through a comprehensive study (Zhang H, 2014; Shuhong T, et al., 2015; Wang G, 2018), and PubChem database was used to obtain the Canonical Simplified Molecular Input Line Entry System (SMILES) strings and Structured Data File (SDF) format files of the chemical ingredients. Summarization of these data was used to create a Schefflera octophylla composition library. ADME properties of the chemical ingredients were evaluated using the SWISS ADME screening tool, which resulted in the identification of seven major active ingredients (Table 1). Swiss TargetPrediction database was employed to predict the potential biological targets of these active ingredients, which lead to the identification of 291 targets after de-duplication.
| Chemical name in English | MOLID/ PubChem ID | SMILES |
|---|---|---|
| Asiatic acid | MOL007253 | CC1CCC2(CCC3(C(=CCC4C3(CCC5C4(CC(C(C5(C)CO)O)O)C)C)C2C1C)C)C(=O)O |
| Decanol | MOL003508 | CCCCCCCCCCO |
| Hexadecanoic acid | MOL000069 | CCCCCCCCCCCCCCCC(=O)O |
| Isovanillin | MOL001867 | COC1=C(C=C(C=C1)C=O)O |
| Vanillin | MOL000635 | COC1=C(C=CC(=C1)C=O)O |
| 2-hydroxy-4-(octyloxy)benzophenone | Compound CID: 129820667 | CCCCC(CCC)OC1=CC=CC(=C1O)C(=O)C2=CC=CC=C2 |
| (+)-balanophonin | Compound CID: 23252258 | COC1=CC(=CC2=C1OC(C2CO)C3=CC(=C(C=C3)O)OC)C=CC=O |
Table 1: Active ingredients of Schefflera octophylla.
Inflammatory disease target prediction
To identify disease targets related to inflammation, a comprehensive search was performed using OMIM, DisGeNET and GeneCards databases were used. The results were filtered to obtain 1318 de-duplicated targets, which were associated with inflammation-related diseases.
Common target analysis of components and diseases
The overlap between the active ingredients of Schefflera octophylla and targets related to inflammatory disease were analyzed using Venny 2.1.0, which revealed 98 shared targets, as depicted in Figure 1.
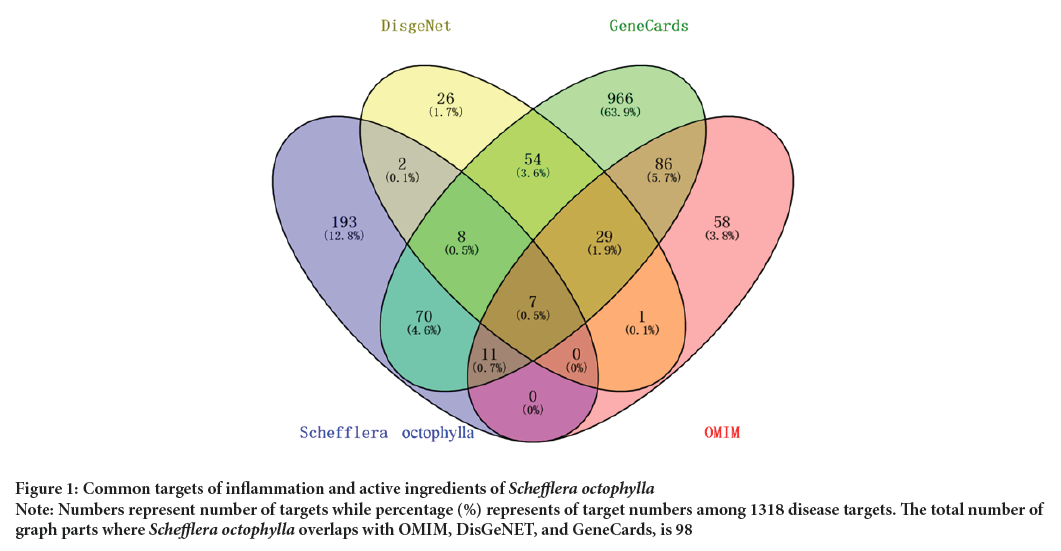
Figure 1: Common targets of inflammation and active ingredients of Schefflera octophylla.
Note: Numbers represent number of targets while percentage (%) represents of target numbers among 1318 disease targets. The total number of
graph parts where Schefflera octophylla overlaps with OMIM, DisGeNET, and GeneCards, is 98.
PPI network construction and core target screening
PPI network was constructed between the active ingredients of Schefflera octophylla and the targets of inflammatory diseases using the STRING database. This network was analyzed using the CytoNCA plugin and network analyzer function in Cytoscape to determine the centrality measures (BC, CC and DC) of each target gene. Based on these calculations, the core targets were identified and ranked. The size of the circle and darkness of the center in Figure 2, represent the proximity of a target to other proteins within the network.
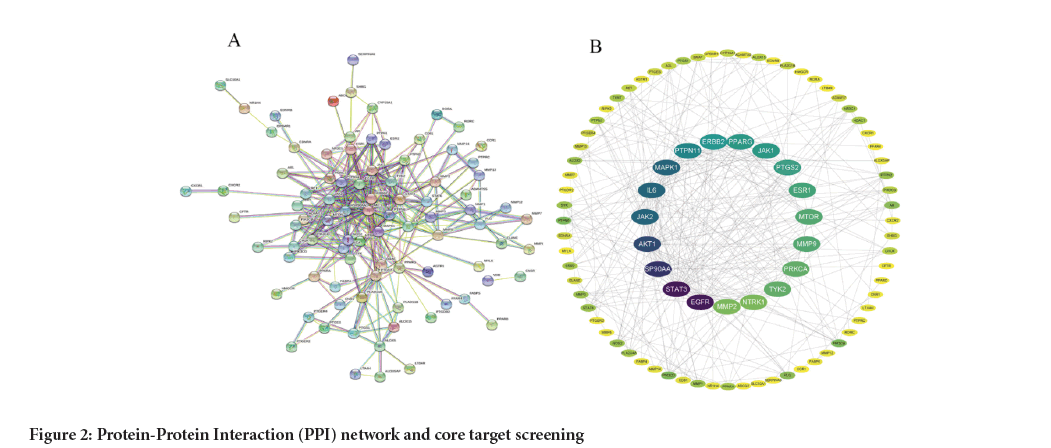
Figure 2: Protein-Protein Interaction (PPI) network and core target screening.
GO and KEGG pathway enrichment analysis
GO analysis was performed on the 98 common targets using DAVID software. The results were categorized into 434 Biological Process (BP), 103 Molecular Function (MF) and 47 Cellular Component (CC) categories and the top ten entries were selected and represented as a bar chart (Figure 3). KEGG pathway enrichment analysis was also performed, which revealed the involvement of 137 pathways in the 98 intersecting targets. The top 20 enriched pathways were visualized as a bubble map using p-value sorting.
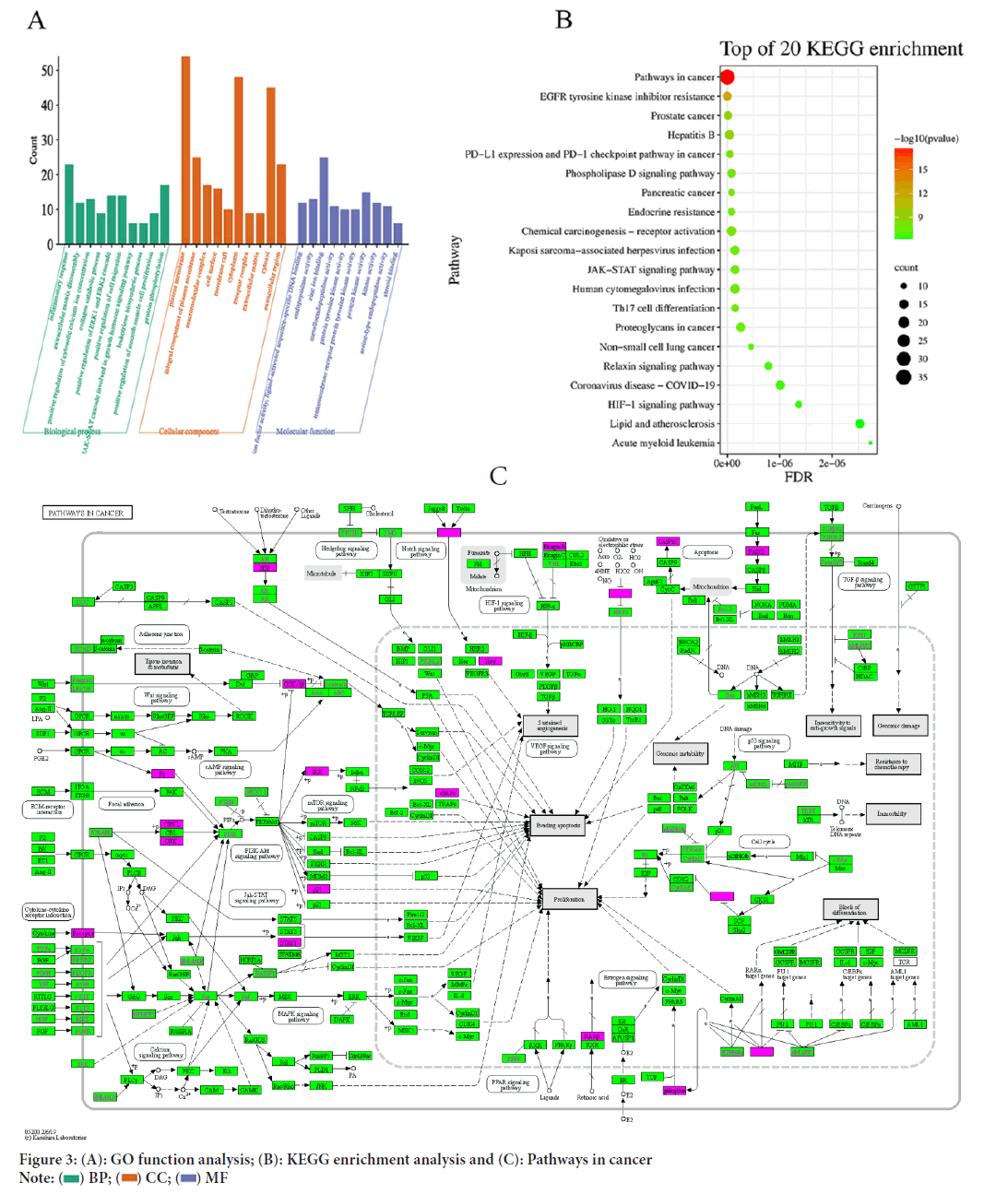
Figure 3: (A): GO function analysis; (B): KEGG enrichment analysis and (C): Pathways in cancer.

Construction of the active ingredient-target-disease-pathway network
Cytoscape 3.9.1 software was used to generate a network map of the top 20 KEGG pathways and their associated targets, drugs, active components and diseases (Figure 4).
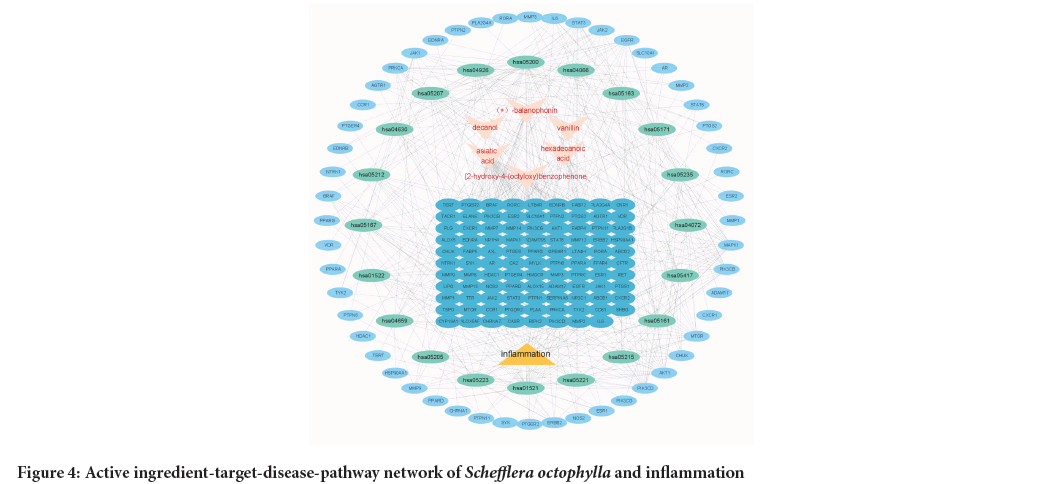
Figure 4: Active ingredient-target-disease-pathway network of Schefflera octophylla and inflammation.
Molecular docking results
The core targets screened through network pharmacology analysis included Epidermal Growth Factor Receptor (EGFR), Signal Transducer and Activator of Transcription 3 (STAT3), Heat Shock Protein 90 Alpha family class A member 1 (HSP90AA1), Threonine Kinase 1 (AKT1), Mitogen Activated Protein Kinase 1 (MAPK1), Janus Kinase 2 (JAK2), and IL6, all of which were subjected to molecular docking to evaluate the binding affinity between the active ingredient of Schefflera octophylla and the predicted target genes (Figure 5). The results showed strong binding capacities of the active ingredient toward the core targets with AKT1, EGFR and STAT3 being among the strongest targets, as demonstrated by the molecular docking simulations (Figure 6). These results provide a basis for the anti-inflammatory therapeutic activity of Schefflera octophylla and further validate the predictions made in this study; however, further investigation into the mechanism of action and experimental validation are necessary to fully understand its effects.
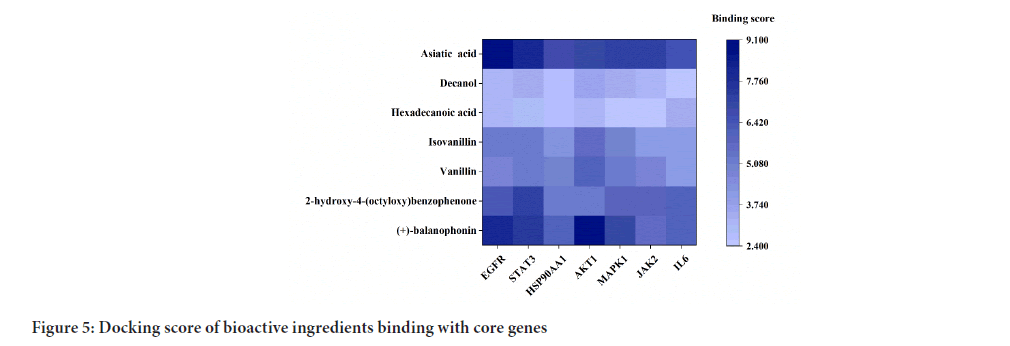
Figure 5: Docking score of bioactive ingredients binding with core genes.
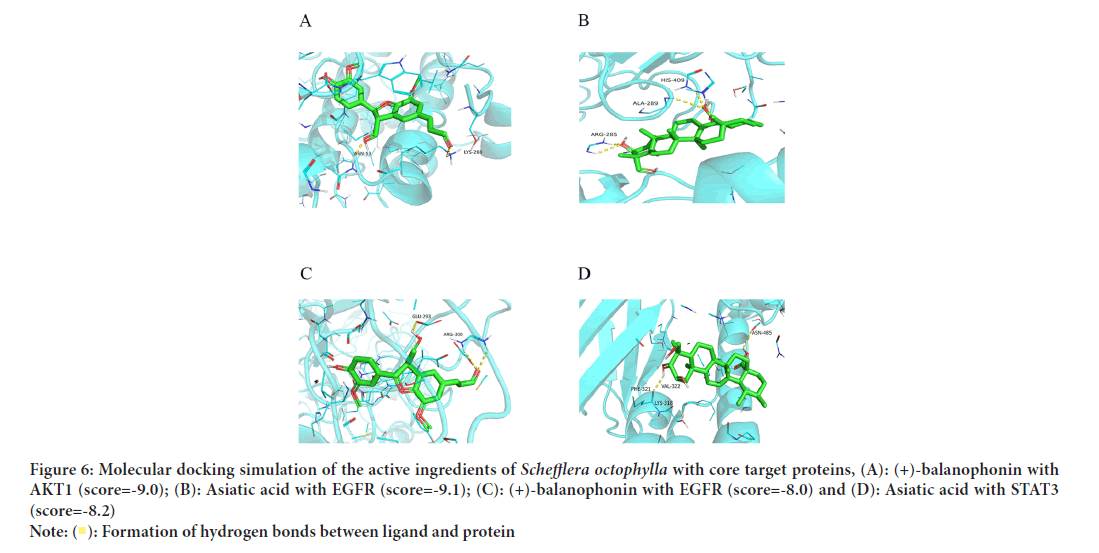
Figure 6: Molecular docking simulation of the active ingredients of Schefflera octophylla with core target proteins, (A): (+)-balanophonin with
AKT1 (score=-9.0); (B): Asiatic acid with EGFR (score=-9.1); (C): (+)-balanophonin with EGFR (score=-8.0) and (D): Asiatic acid with STAT3
(score=-8.2).
Effects of Schefflera octophylla on inflammatory KM mice
The carrageenan-induced toe swelling assay (Morris CJ, 2003) was applied to mice to assess the anti-inflammatory effects of drugs, with IL-6 (del Giudice M and Gangestad SW, 2018) being a commonly used marker of inflammation and MDA (Gaweł S, et al., 2004) serving as a marker of oxidative stress and antioxidant status. As depicted in (Figure 7), the results showed that the carrageenan-induced toe swelling in the model group was 44.09 mg, while dexamethasone treatment, high and medium doses of Schefflera octophylla ethanol and high doses of aqueous extracts significantly reduced toe swelling (p<0.05), indicating the anti-inflammatory properties of the treatments. Additionally, all treatments resulted in a significant decrease in IL-6 and MDA levels (p<0.05), indicating their anti-inflammatory and antioxidant effects. These results highlight the potential of ethanol and aqueous extracts of Schefflera octophylla as promising anti-inflammatory agents.
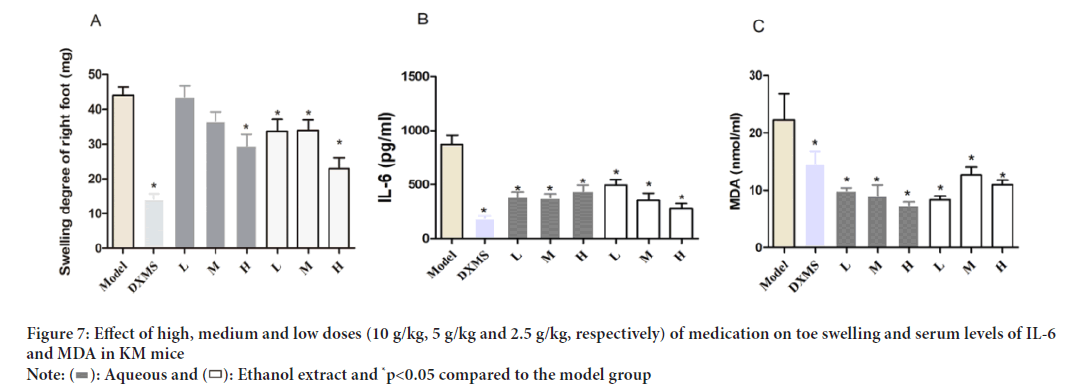
Figure 7: Effect of high, medium and low doses (10 g/kg, 5 g/kg and 2.5 g/kg, respectively) of medication on toe swelling and serum levels of IL-6
and MDA in KM mice.
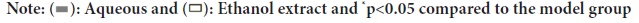
Effect of Schefflera octophylla on the content of inflammatory factors in RAW264.7 cells and their morphology
We next conducted in vitro experiments to validate the anti-inflammatory mechanism of Schefflera octophylla as predicted by network pharmacology. The levels of relevant cytokines secreted by LPS-stimulated RAW264.7 cells were measured and the cellular proteins were extracted for Western blotting. IC10 concentration of Schefflera octophylla ethanol and aqueous extracts were 149.935 μg/ml and 343.248 μg/ml, respectively, based on the MTT results. LPS-stimulated RAW264.7 cells produced significantly higher levels of NO compared to untreated cells, while treatment with ethanol and aqueous extracts of Schefflera octophylla significantly reduced the levels of NO (p<0.05). Similarly, the levels of IL-1β, IL-6 and IL-10 produced by LPS-stimulated RAW264.7 cells were significantly different from those produced by untreated cells (p<0.05), while the extracts inhibited the secretion of IL-6 and IL-1β and increased the level of IL-10. Compared to the model group, the treatment with Schefflera octophylla extract inhibited the secretion of IL-6 and IL-1β and increased the level of IL-10 (p<0.05). These findings demonstrate that both the aqueous and ethanol extracts of Schefflera octophylla have anti-inflammatory effects on LPS-induced inflammation in RAW264.7 cells (Figure 8).
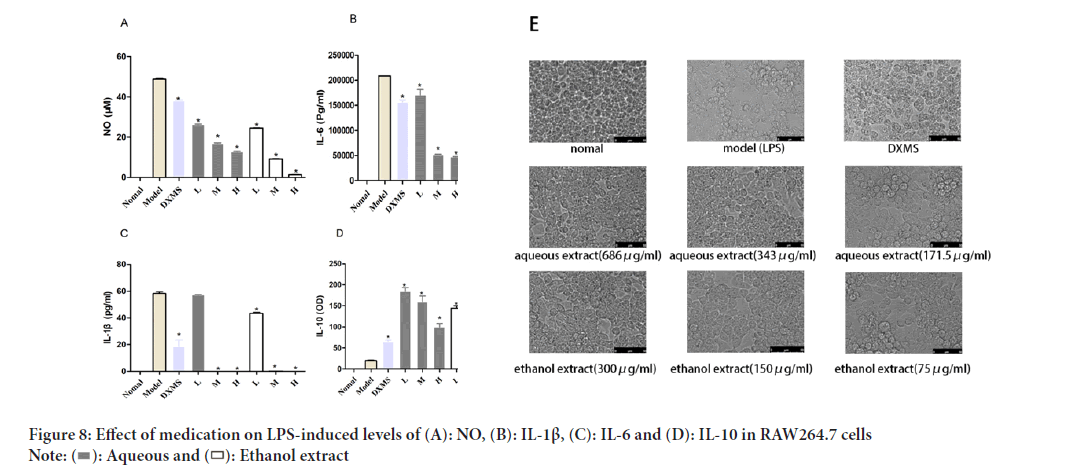
Figure 8: Effect of medication on LPS-induced levels of (A): NO, (B): IL-1β, (C): IL-6 and (D): IL-10 in RAW264.7 cells.
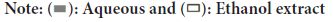
Further, we observed the morphology of RAW264.7 cells under an inverted microscope at 40X following different treatments. The control group exhibited normal cell morphology, with round or oval shapes, intact organelles and compact cell clusters. Upon LPS stimulation, the cells showed a differentiated state, with an expanded cell area and prominent branching. High and medium doses of Schefflera octophylla extracts, as well as LPS, induced a significant reduction in branching cells, returning most cells to a normal morphology. Similarly, low doses of the extracts and LPS resulted in a decrease in branching cells. The combination of dexamethasone and LPS effectively reduced branched cells and restored the normal morphology of most cells. Thus, these results suggest that 1 µg/ml of LPS can induce inflammation in RAW264.7 cells and that of the Schefflera octophylla extracts can effectively inhibit cell differentiation and alleviate the inflammatory response.
Effects of Schefflera octophylla on the PI3K/Akt signaling pathway in RAW264.7 cells
The anti-inflammatory effect of Schefflera octophylla was further investigated by examining its effect on the PI3K/Akt signaling pathway. The results showed that treatment with ethanol and aqueous extracts of Schefflera octophylla resulted in significant reduction of expression of phosphorylated PI3K and Akt (p-PI3K and p-Akt, respectively) compared to LPS-stimulated RAW264.7 cells (p<0.05) (Figures 9A-9C).
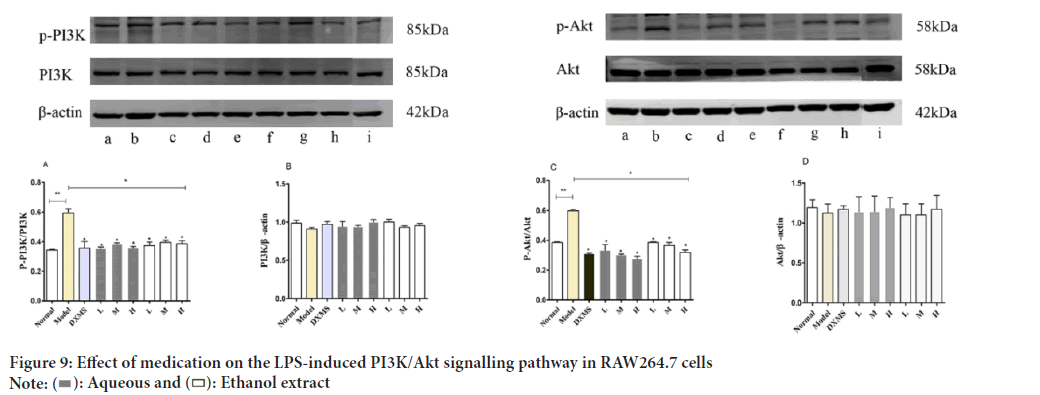
Figure 9: Effect of medication on the LPS-induced PI3K/Akt signalling pathway in RAW264.7 cells.
Discussion
The traditional Chinese herb Schefflera octophylla has a rich history of medicinal use, as documented in ancient medical texts. Previous research on Schefflera octophylla has focused on the isolation and characterization of its constituent components. For instance, Song M, et al., 2019; Sung TV and Adam G, 1991; Sung TV, et al., 1991; Sung TV, et al., 1991; Sung TV, et al., 1992; van Sung T and Adam G, 1992 conducted a study on the isolation of terpenoids in Schefflera J. R.Forst and G.Forst. Subsequently, Liu X, et al., 2019 established a method for the efficient extraction of triterpenoids in Schefflera octophylla. Studies examining the pharmacological properties of Schefflera octophylla have reported its antiviral, antioxidant (Li YL, et al., 2004; Zheng YJ, 2009), analgesic, and anti-inflammatory effects (Chen et al., 2015), which were evaluated using alcoholic extracts and active fractions of different polarities. The structure of the active components was also analyzed using spectroscopic data. However, there remains limited research on the underlying mechanism of action of Schefflera octophylla.
This study represents advancement in the exploration of the pharmacology and pharmacodynamics of Schefflera octophylla. Utilizing a network pharmacology approach, the previously identified active ingredients were integrated to predict the targets of action and molecular docking techniques which were employed to determine the specific pathways responsible for the anti-inflammatory effects of Schefflera octophylla. Experimentally, aqueous and ethanol extracts of Schefflera octophylla were compared to assess their efficacies in different treatment groups. The results indicate that the compounds of Schefflera octophylla impact multiple targets, with some instances of overlapping targets identified among different components. This finding suggests that the anti-inflammatory effects of Schefflera octophylla may be due to the synergistic actions of its compounds, which aligns with the “Manifestation theory” of Chinese medicine proposed by Cai SQ, et al., 2015 and others.
The compounds of Schefflera octophylla are known to affect multiple targets, including core targets such as AKT1, EGFR, and STAT3. Results obtained from molecular docking analysis verified that the active ingredients of Schefflera octophylla bind to these targets. The anti-inflammatory effects of Schefflera octophylla were confirmed through in vivo and in vitro experiments, which confirmed that the inhibition of various inflammatory cytokines was the primary mechanism. Moreover, KEGG enrichment analysis revealed that Schefflera octophylla primarily interferes with the onset and progression of inflammation through cancer signaling pathway, specifically PI3K/Akt, as well as through EGFR, JAK/STAT and other pathways.
The current study was conducted to investigate the anti-acute inflammatory effects of Schefflera octophylla. The results indicated that both aqueous and ethanol extracts of Schefflera octophylla inhibited toe swelling in animals, with more pronounced effect observed at higher doses. Additionally, the extracts down-regulated the inflammatory factor, IL-6 and reduced inflammatory peroxidation. The results of in vitro studies demonstrated that the extracts were equally effective in reducing inflammation in LPS-induced RAW264.7 cells, with high doses of the extracts demonstrating a significant reduction in inflammation and the state of cell differentiation. Overall, Schefflera octophylla shows promising anti-inflammatory properties, making it a potential candidate for future drug development.
PI3K, an upstream kinase of the serine/threonine protein kinase AKT (Song M, et al., 2019), promotes the release of pro-inflammatory cytokines and mediates the secretion of IL-6 through Nuclear Factor Kappa B (NFkB) activation downstream of AKT (Koorella C, et al., 2014). Studies have demonstrated that PI3K plays a regulatory role in certain innate immune responses (Hawkins PT and Stephens LR, 2015; Stark AK, et al., 2015), while AKT is central to immune regulation by down-regulating inhibitory signals and promoting reactivation of immune cells (Tang F, et al., 2018). The results of KEGG pathway enrichment analysis implicate that PI3K/Akt pathway as the most significant pathway involved in mediating anti-inflammatory activity, which was further validated through Western blot experiments.
Regarding the limitations of the study, the database-based analysis and screening represent only part of the approach and may not fully reflect the components, and targets that exert efficacy. Therefore, further research is needed to fully validate the specific anti-inflammatory pathways of Schefflera octophylla through in vivo and in vitro experiments.
Conclusion
In this study, we demonstrate the anti-inflammatory effects of Schefflera octophylla using both animal and cellular experiments. Both the aqueous and ethanol extracts of Schefflera octophylla showed potent anti-inflammatory properties. In vivo, Schefflera octophylla reduced carrageenan-induced toe swelling and decreased serum levels of IL-6 and MDA, while in vitro, Schefflera octophylla inhibited LPS-induced secretion of NO, IL-6, and IL-1β by RAW264.7 cells and up-regulated IL-10. The anti-inflammatory mechanism of Schefflera octophylla was further substantiated by the results of Western blotting, which showed decreased phosphorylation of PI3K and Akt, as predicted by network pharmacology. However, other potential anti-inflammatory mechanisms require further investigation in the future.
Author’s Contributions
Zhou Xiaoqin was involved in writing the original draft and performed the experiments. Lan Xiaobu contributed in formal analysis, data curation and analyzing the data. Liang Yao was included in data curation and performed the experiments. Yang Bin investigated, writing, reviewing and editing of the paper.
Acknowledgement
This research was supported by the Nanning Youth Science and Technology Innovation and Entrepreneurship Cultivation Project (Grant No: RC20180103) and The Fifth Affiliated Hospital of Guangxi Medical University.
Data Availability
The research presented in this study requires continued accumulation of data. The data that has been used is confidential.
Ethical Statement
The research was carried out in strict conformity with the recommendations of the Guide for the Care and Use of Laboratory Animals of the Ministry of Science and Technology of China. The protocol was approved by the Guangxi Medical University Laboratory Animal Centre (Guangxi, China).
References
- Medzhitov R. Origin and physiological roles of inflammation. Nature. 2008; 454(7203): 428-435.
[Crossref] [Google Scholar] [PubMed]
- Kuprash DV, Nedospasov SA. Molecular and cellular mechanisms of inflammation. Biochemistry (Moscow). 2016; 81: 1237-1239.
[Crossref] [Google Scholar] [PubMed]
- Ala A, Dhillon AP, Hodgson HJ. Role of cell adhesion molecules in leukocyte recruitment in the liver and gut. Int J Exp Pathol. 2003; 84(1): 1-6.
[Crossref] [Google Scholar] [PubMed]
- Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell. 2010; 140(6): 805-820.
[Crossref] [Google Scholar] [PubMed]
- Feehan KT, Gilroy DW. Is resolution the end of inflammation? Trends Mol Med. 2019; 25(3): 198-214.
[Crossref] [Google Scholar] [PubMed]
- Gilroy D, de Maeyer R. New insights into the resolution of inflammation. Semin Immunol. 2015; 27(3): 161-168.
[Crossref] [Google Scholar] [PubMed]
- Tang F, Wang Y, Hemmings BA, Rüegg C, Xue G. PKB/Akt-dependent regulation of inflammation in cancer. Semin Cancer Biol. 2018; 48: 62-69.
[Crossref] [Google Scholar] [PubMed]
- Layton D, Souverein PC, Heerdink ER, Shakir SA, Egberts AG. Prescriber adoption of newly approved selective COX‐2 inhibitors. Pharmacoepidemiol Drug Saf. 2008; 17(12): 1168-1174.
[Crossref] [Google Scholar] [PubMed]
- Bacchi S, Palumbo P, Sponta A, Coppolino MF. Clinical pharmacology of non-steroidal anti-inflammatory drugs: A review. Antiinflamm Antiallergy Agents Med Chem. 2012; 11(1): 52-64.
[Crossref] [Google Scholar] [PubMed]
- Cunningham K, Candelario DM, Angelo LB. Nonsteroidal anti-inflammatory drugs: Updates on dosage formulations and adverse effects. Orthop Nurs. 2020; 39(6): 408-413.
[Crossref] [Google Scholar] [PubMed]
- Mizushima T. Molecular mechanism for various pharmacological activities of NSAIDS. Pharmaceuticals. 2010; 3(5): 1614-1636.
[Crossref] [Google Scholar] [PubMed]
- Harirforoosh S, Asghar W, Jamali F. Adverse effects of non-steroidal antiinflammatory drugs: An update of gastrointestinal, cardiovascular and renal complications. J Pharm Pharm Sci. 2013; 16(5): 821-847.
[Crossref] [Google Scholar] [PubMed]
- Patricio JP, Barbosa JP, Ramos RM, Antunes NF, de Melo PC. Relative cardiovascular and gastrointestinal safety of non-selective non-steroidal anti-inflammatory drugsversus cyclo-oxygenase-2 inhibitors: Implications for clinical practice. Clin Drug Investig. 2013; 33: 167-183.
[Crossref] [Google Scholar] [PubMed]
- Arfe A, Scotti L, Varas-Lorenzo C, Nicotra F, Zambon A, Kollhorst B, et al. Non-steroidal anti-inflammatory drugs and risk of heart failure in four European countries: Nested case-control study. BMJ. 2016; 354.
[Crossref] [Google Scholar] [PubMed]
- Gunter BR, Butler KA, Wallace RL, Smith SM, Harirforoosh S. Non‐steroidal anti‐inflammatory drug‐induced cardiovascular adverse events: A meta‐analysis. J Clin Pharm Ther. 2017; 42(1): 27-38.
[Crossref] [Google Scholar] [PubMed]
- Bindu S, Mazumder S, Bandyopadhyay U. Non-Steroidal Anti-Inflammatory Drugs (NSAIDs) and organ damage: A current perspective. Biochem Pharmacol. 2020; 180: 114147.
[Crossref] [Google Scholar] [PubMed]
- Cai SQ, Wang X, Shang MY, Xu F, Liu GX. "Efficacy theory" may help to explain characteristic advantages of traditional Chinese medicines. Zhongguo Zhong Yao Za Zhi. 2015; 40(17): 3435-3443.
[Google Scholar] [PubMed]
- Zhang L, Wei W. Anti-inflammatory and immunoregulatory effects of paeoniflorin and total glucosides of paeony. Pharmacol Ther. 2020; 207: 107452.
[Crossref] [Google Scholar] [PubMed]
- Jiang M, Li Z, Zhu G. Immunological regulatory effect of flavonoid baicalin on innate immune toll-like receptors. Pharmacol Res. 2020; 158: 104890.
[Crossref] [Google Scholar] [PubMed]
- Chen L, Lin L, Dong Z, Zhang L, Du H. Comparison of neuroprotective effect of Forsythia suspensa leaf extract and forsythiaside, one of its metabolites. Nat Prod Res. 2018; 32(22): 2705-2708.
[Crossref] [Google Scholar] [PubMed]
- Pang S, Sun A, Wang GC, Xu R. Studies on the chemical constituents of Schefflera octophylla. J Chin Med Mat. 2016; 39(2); 334-336.
- Xu J, Peng X, Li W. Chemical constituents, pharmacology and clinical application of Schefflera. J Hunan Univ Chin Med. 2006; 5: 62-64.
- Wang Y, Huang S, Qin P, Wu S, Zhen H. Preparation and application of Chinese medicine mixture Ⅱ for exogenous disease. Guangxi Med J. 2006; 10: 1609-1610.
- Tasneem S, Liu B, Li B, Choudhary MI, Wang W. Molecular pharmacology of inflammation: Medicinal plants as anti-inflammatory agents. Pharmacol Res. 2019; 139: 126-140.
[Crossref] [Google Scholar] [PubMed]
- Zhang H. Studies on enrichment of total triterpene and chemical constituents of Schefflera octophylla. Guangdong Pharm Univ. 2014.
- Shuhong T, Fanlin Z, Yanfen C, ZhiBin S. Chemical constituents from roots of Schefflera octophylla. Chin Tradit Herbal Drugs. 2015; 46(21): 3151-3154.
- Wang G. Study on triterpenoids of Schefflera octophylla (Lour.) Harms and its main pharmacodynamics. Huaqiao Univ. 2018.
- Kim S, Chen J, Cheng T, Gindulyte A, He J, He S, et al. PubChem in 2021: New data content and improved web interfaces. Nucleic Acids Res. 2021; 49(D1): D1388-D1395.
[Crossref] [Google Scholar] [PubMed]
- Daina A, Michielin O, Zoete V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci Rep. 2017; 7(1): 42717.
[Crossref] [Google Scholar] [PubMed]
- Daina A, Michielin O, Zoete V. SwissTargetPrediction: Updated data and new features for efficient prediction of protein targets of small molecules. Nucleic Acids Res. 2019; 47(W1): W357-W364.
[Crossref] [Google Scholar] [PubMed]
- Piñero J, Ramírez-Anguita JM, Saüch-Pitarch J, Ronzano F, Centeno E, Sanz F, et al. The DisGeNET knowledge platform for disease genomics: 2019 update. Nucleic Acids Res. 2020; 48(D1): D845-D855.
[Crossref] [Google Scholar] [PubMed]
- Szklarczyk D, Gable AL, Nastou KC, Lyon D, Kirsch R, Pyysalo S, et al. The STRING database in 2021: Customizable protein-protein networks, and functional characterization of user-uploaded gene/measurement sets. Nucleic Acids Res. 2021; 49(D1): D605-D612.
[Crossref] [Google Scholar] [PubMed]
- Huang DW, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009; 4(1): 44-57.
[Crossref] [Google Scholar] [PubMed]
- Sherman BT, Hao M, Qiu J, Jiao X, Baseler MW, Lane HC, et al. DAVID: A web server for functional enrichment analysis and functional annotation of gene lists (2021 update). Nucleic Acids Res. 2022; 50(W1): W216-W221.
[Crossref] [Google Scholar] [PubMed]
- Morris CJ. Carrageenan-induced paw edema in the rat and mouse. Methods Mol Biol. 2003: 115-121.
[Crossref] [Google Scholar] [PubMed]
- del Giudice M, Gangestad SW. Rethinking IL-6 and CRP: Why they are more than inflammatory biomarkers, and why it matters. Brain Behav Immun. 2018; 70: 61-75.
[Crossref] [Google Scholar] [PubMed]
- Gaweł S, Wardas M, Niedworok E, Wardas P. Malondialdehyde (MDA) as a lipid peroxidation marker. Wiad Lek. 2004; 57(9-10): 453-455.
[Google Scholar] [PubMed]
- Song M, Bode AM, Dong Z, Lee MH. AKT as a therapeutic target for cancer. Cancer Res. 2019; 79(6): 1019-1031.
[Crossref] [Google Scholar] [PubMed]
- Sung TV, Adam G. A sulphated triterpenoid saponin from Schefflera octophylla. Phytochemistry. 1991; 30(8): 2717-2720.
[Crossref] [Google Scholar] [PubMed]
- Sung TV, Steglich W, Adam G. Triterpene glycosides from Schefflera octophylla. Phytochemistry. 1991; 30(7): 2349-2356.
[Crossref] [Google Scholar] [PubMed]
- Sung TV, Peter-Katalinic J, Adam G. A bidesmosidic triterpenoid saponin from Schefflera octophylla. Phytochemistry. 1991; 30(11): 3717-3720.
[Crossref] [Google Scholar] [PubMed]
- Sung TV, Lavaud C, Porzel A, Steglich W, Adam G. Triterpenoids and their glycosides from the bark of Schefflera octophylla. Phytochemistry. 1992; 31(1): 227-231.
[Crossref] [Google Scholar] [PubMed]
- van Sung T, Adam G. An acetylated bidesmosidic saponin from Schefflera octophylla. J Nat Prod. 1992; 55(4): 503-505.
[Crossref] [Google Scholar] [PubMed]
- Liu X, Niu Y, Liu J, Shi M, Xu R, Kang W. Efficient extraction of anti-inflammatory active ingredients from Schefflera octophylla leaves using ionic liquid-based ultrasonic-assisted extraction coupled with HPLC. Molecules. 2019; 24(16): 2942.
[Crossref] [Google Scholar] [PubMed]
- Li YL, Yeung CM, Chiu LC, Cen YZ, Ooi VE. Chemical composition and antiproliferative activity of essential oil from the leaves of a medicinal herb, Schefflera heptaphylla. Phytother Res. 2009; 23(1): 140-142.
[Crossref] [Google Scholar] [PubMed]
- Zheng YJ. Antioxidant activities of extracts from Schefflera. Chin J Trop Crops. 2009; 30: 500-504.
- Chen Y, Tao S, Zeng F, Xie L, Shen Z. Antinociceptive and anti-inflammatory activities of Schefflera octophylla extracts. J Ethnopharmacol. 2015; 171: 42-50.
[Crossref] [Google Scholar] [PubMed]
- Koorella C, Nair JR, Murray ME, Carlson LM, Watkins SK, Lee KP. Novel regulation of CD80/CD86-induced phosphatidylinositol 3-kinase signaling by NOTCH1 protein in interleukin-6 and indoleamine 2, 3-dioxygenase production by dendritic cells. J Biol Chem. 2014; 289(11): 7747-7762.
[Crossref] [Google Scholar] [PubMed]
- Hawkins PT, Stephens LR. PI3K signalling in inflammation. Biochim Biophys Acta. 2015; 1851(6): 882-897.
[Crossref] [Google Scholar] [PubMed]
- Stark AK, Sriskantharajah S, Hessel EM, Okkenhaug K. PI3K inhibitors in inflammation, autoimmunity and cancer. Curr Opin Pharmacol. 2015; 23: 82-91.
[Crossref] [Google Scholar] [PubMed]
Author Info
Xiaoqin Zhou1, Bin Yang2, Yao Liang2 and Xiaobu Lan1*2Department of Pharmacy, Guangxi Medical University, Nanning, Guangxi Province, China
Citation: Zhou X: Network Pharmacology and Experimental Validation Reveal the Anti-Inflammatory Effects of Schefflera octophylla via Inhibition of the PI3K-AKT Pathway
Received: 03-Oct-2024 Accepted: 23-Oct-2024 Published: 30-Oct-2024, DOI: 10.31858/0975-8453.15.10.311-320
Copyright: This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
ARTICLE TOOLS
- Dental Development between Assisted Reproductive Therapy (Art) and Natural Conceived Children: A Comparative Pilot Study Norzaiti Mohd Kenali, Naimah Hasanah Mohd Fathil, Norbasyirah Bohari, Ahmad Faisal Ismail, Roszaman Ramli SRP. 2020; 11(1): 01-06 » doi: 10.5530/srp.2020.1.01
- Psychometric properties of the World Health Organization Quality of life instrument, short form: Validity in the Vietnamese healthcare context Trung Quang Vo*, Bao Tran Thuy Tran, Ngan Thuy Nguyen, Tram ThiHuyen Nguyen, Thuy Phan Chung Tran SRP. 2020; 11(1): 14-22 » doi: 10.5530/srp.2019.1.3
- A Review of Pharmacoeconomics: the key to “Healthcare for All” Hasamnis AA, Patil SS, Shaik Imam, Narendiran K SRP. 2019; 10(1): s40-s42 » doi: 10.5530/srp.2019.1s.21
- Deuterium Depleted Water as an Adjuvant in Treatment of Cancer Anton Syroeshkin, Olga Levitskaya, Elena Uspenskaya, Tatiana Pleteneva, Daria Romaykina, Daria Ermakova SRP. 2019; 10(1): 112-117 » doi: 10.5530/srp.2019.1.19
- Dental Development between Assisted Reproductive Therapy (Art) and Natural Conceived Children: A Comparative Pilot Study Norzaiti Mohd Kenali, Naimah Hasanah Mohd Fathil, Norbasyirah Bohari, Ahmad Faisal Ismail, Roszaman Ramli SRP. 2020; 11(1): 01-06 » doi: 10.5530/srp.2020.1.01
- Manilkara zapota (L.) Royen Fruit Peel: A Phytochemical and Pharmacological Review Karle Pravin P, Dhawale Shashikant C SRP. 2019; 10(1): 11-14 » doi: 0.5530/srp.2019.1.2
- Pharmacognostic and Phytopharmacological Overview on Bombax ceiba Pankaj Haribhau Chaudhary, Mukund Ganeshrao Tawar SRP. 2019; 10(1): 20-25 » doi: 10.5530/srp.2019.1.4
- A Review of Pharmacoeconomics: the key to “Healthcare for All” Hasamnis AA, Patil SS, Shaik Imam, Narendiran K SRP. 2019; 10(1): s40-s42 » doi: 10.5530/srp.2019.1s.21
- A Prospective Review on Phyto-Pharmacological Aspects of Andrographis paniculata Govindraj Akilandeswari, Arumugam Vijaya Anand, Palanisamy Sampathkumar, Puthamohan Vinayaga Moorthi, Basavaraju Preethi SRP. 2019; 10(1): 15-19 » doi: 10.5530/srp.2019.1.3






