Research Article - (2023) Volume 14, Issue 3
Percutaneous Intramyocardial Septal Radiofrequency Ablation in Patients with Hypertrophic Cardiomyopathy
Fatema Tashrifwala*, Manisha Purushotham, Sai Krishna Bhushan, Mohammad Omer Khan and Ishaque HameedAbstract
Aim: In this study, we aimed to Meta-analyze all available data to provide a holistic, well-powered assessment of the effect of PIMSRA on LVOT gradient, LVEF, and anterior and posterior IVS thickness.
Methods: PubMed, Cochrane Central, Embase, and Scopus were systematically searched from inception till October 2022 for published clinical trials assessing the efficacy of PIMSRA in patients with HCM using the keywords “PIMSRA” OR “Percutaneous Intramyocardial Septal Radiofrequency Ablation” OR “Liwen procedure.” Studies that reported change in either LVOT gradient, IVS thickness, or LVEF were selected. No time or language restrictions were applied. All case reports were excluded, and only clinical trials were included. The search and data extraction were carried out independently by two reviewers (MP, FT). A third reviewer (IH) was consulted to resolve discrepancies. All statistical analysis were conducted on Open Meta-analyst. Trials were pooled using a continuous random effects model (DL: DerSimonian-Laird), and results were presented with 95% Confidence Intervals (CIs). P value 75% regarded as substantial heterogeneity.
Results: All five studies reported a change in LVOT gradient from the baseline. Integrated analysis showed no significant change from baseline (MD:-69.506; 95% CI:-77.047, -61.966; P=0.089) (I2=50.4%). Subgroup analysis based on follow-up revealed that there was no significant difference noted at 6 months according to data from 2 studies (MD:-65.351; 95% CI: -85.253, -45.450; P=0.181) (I2=44.1%) however data from 3 studies at one year follow up did reveal a significant difference from baseline (MD:-71.010; 95% CI:-80.262, -61.758; P=0.047) (I2=67.2%). Only three studies reported changes in LVEF from the baseline. Integrated analysis Powered by Editorial Manager® and ProduXion Manager® from Aries Systems Corporation showed a significant change from baseline at one-year follow-up in patients who underwent PIMSRA (MD:-1.760; 95% CI:-3.239, -0.282; P=0.047) (I2=67.22%). The pooled analysis from four studies that reported anterior IVS thickness showed no significant change from baseline (MD:-9.707; 95% CI:- 10.819, -8.595; P=0.095) (I2=52.94%). Subgroup analysis based on follow-up revealed that there was no significant difference noted at 6 months (MD:-11.285; 95% CI:-12.825, -9.744; P=0.933) (I2=0%) or at oneyear follow-up (MD:-9.147; 95% CI:-9.837, -8.456; P=0.650) (I2=0%) The combined analysis of the four included studies reporting posterior IVS thickness showed no significant change from baseline (MD:- 8.859; 95% CI:-10.073, -7.644; P=0.119) (I2=48.73%). Additionally, there was no significant difference noted in the subgroup analysis at 6 months (MD:-10.278; 95% CI:-12.133, -8.423; P=0.934) (I2=0%) and at oneyear follow-up (MD:-8.335; 95% CI:-9.880, -6.789; P=0.072) (I2=69.14%)
Conclusion: This single-arm meta-analysis of 284 patients gathered from 5 clinical trials suggested that overall, after PIMSRA, LVOT gradient was reduced, and LVEF was slightly decreased. Additionally, no significant changes were observed in the anterior and posterior IVS thicknesses. Our results had substantial heterogeneity, which could be explained due to differences in the follow-up periods and studies with small sample sizes. It is important to note that even though no significant difference in follow-up was seen from the baseline at 6 months, there was indeed a significant difference noted at one year when assessing the outcomes of LVOT gradient. This could suggest that longer follow-up periods are imperative to truly observe the procedure’s efficacy.
Keywords
Hypertrophic Cardiomyopathy (HCM), Percutaneous Intramyocardial Septal Radiofrequency Ablation (PIMSRA), Left ventricular Outflow Tract (LVOT), Left Ventricular Ejection Fraction (LVEF), Anterior Interventricular septum (IVS) thickness, Posterior IVS Thickness, Efficacy
Abbrevations
HCM: Hypertrophic Cardiomyopathy; LVOT: Left ventricular Outflow Tract; US: United States; CMRI: Cardiovascular Magnetic Resonance Imaging; LVEF: Left Ventricular Ejection Fraction; ASA: Alcohol Septal Ablation; PIMSRA: Percutaneous Intramyocardial Septal Radiofrequency Ablation; IVS: Interventricular Septum; NYHA: New York Heart Association; CI: Confidence Interval; MD: Mean Deviation
Introduction
Hypertrophic Cardiomyopathy (HCM) is an autosomal dominant intrinsic disease of the myocardium characterized by asymmetric hypertrophy and stiffness of the left ventricle along with the systolic anterior motion of the mitral valve, leading to a Left Ventricular Outflow Tract (LVOT) obstruction. It affects one in five hundred people worldwide and is the most common genetic heart disease in the US (Zhou M, et al., 2022). The reduced volume of the heart chambers with a reduced capacity of the heart to pump blood results in a decreased stroke volume. LVOT obstruction is present in about one-third of the patients. The symptoms include exertional dyspnea, exercise intolerance, orthopnea, peripheral edema, and syncope (Marian AJ and Braunwald E, 2017). Diagnostic modalities include echocardiography, Electrocardiogram (ECG), and Cardiovascular Magnetic Resonance Imaging (CMRI). Identification of the HCM phenotype can be made by family screening and genetic testing in affected individuals (Qian D, et al., 2021; Tuohy CV, et al., 2020). The treatment goals are to reduce the severity of symptoms and prevent sudden cardiac death, particularly in young adults. Pharmacological treatment revolves around β-blockers or non-dihydropyridine calcium-channel blockers with the newer generation of drugs, including Mavacamten, Perhexiline, and Trimetazidine. Evaluation and follow-up are done by LVOT gradient, Left Ventricular Ejection Fraction (LVEF), and myocyte hypertrophy measured by Interventricular Septum (IVS) thickness (Waldman CB and Owens A, 2021). In patients who are refractory to maximum dosage (about 40%) or have intolerance to drugs, surgical options like septal myectomy and trans-catheter mitral valve repair may be considered. The downside to these is the need for a sternotomy. Minimally invasive procedures which can instead be done are high-intensity focused ultrasonography and radiofrequency ablation, or Alcohol Septal Ablation (ASA), which come with their own risk of complications with chances of occurrence ranging from 0.5%-5% (Liu L, et al., 2018). The suitability of ASA is limited by septal coronary anatomy and the risk of alcohol being injected into the wrong site. Advancements in techniques have now led to the use of Percutaneous Intramyocardial Septal Radiofrequency Ablation (PIMSRA) as a promising technique for such patients. This technique uses a radiofrequency electrode needle inserted under guidance into the hypertrophied IVS to achieve ablation (Tuohy CV, et al., 2020; Zuo L, et al., 2020). Trials have shown reductions in IVS thickness and LVOT gradients along with improvement in New York Heart Association (NYHA) functional classification following PIMSRA, but these studies have been limited by small sample sizes, thus being underpowered to reliably demonstrate any significant differences in outcomes. In this study, we aimed to meta-analyze all available data to provide a holistic, well-powered assessment of the effect of PIMSRA on LVOT gradient, LVEF, and anterior and posterior IVS thickness.
Materials and Methods
PubMed, Cochrane Central, Embase, and Scopus were systematically searched from inception till October 2022 for published clinical trials assessing the efficacy of PIMSRA in patients with HCM using the keywords “PIMSRA” OR “Percutaneous Intramyocardial Septal Radiofrequency Ablation” OR “Liwen procedure”. Studies that reported change in either LVOT gradient, IVS thickness, or LVEF were selected. No time or language restrictions were applied. All case reports were excluded, and only clinical trials were included. The search and data extraction were carried out independently by two reviewers (MP, FT). A third reviewer (IH) was consulted to resolve discrepancies. All statistical analysis were conducted on Open Meta-analyst. Trials were pooled using a continuous random effects model (DL: DerSimonian-Laird), and results were presented with 95% Confidence Intervals (CIs). P value <0.05 was considered significant. Subgroup analysis according to time at follow-up was done. Heterogeneity was evaluated using Higgins I2, with I2>75% regarded as substantial heterogeneity.
The initial search yielded 72 potential studies. After exclusions, 5 trials remained for analysis (Zhou M, et al., 2022; Qian D, et al., 2021; Tuohy CV, et al., 2020; Zuo L, et al., 2020; Liu LW, et al., 2019). The detailed literature search is highlighted in the PRISMA Flow Chart. The characteristics of the included studies have been summarized in Table 1.
| Included studies | Year | Characteristics of patients | Number of participants | Follow up | ||||
|---|---|---|---|---|---|---|---|---|
| Age in years | Gender | NYHA class | LVOT gradient | IVS thickness | ||||
| Zhou M, et al., 2022 | 2022 | 33-61 | M-125 F-75 |
>50 mmHg | 200 | 1 year | ||
| Qian D, et al., 2021 | 2021 | >50 mmHg | 15-25 mm | 30 | 1 year | |||
| Zuo L, et al., 2020 | 2020 | 15-79 | M-20 F-10 |
Chest pain, syncope, NYHA Class â?¢/IV | >50 mmHg | 30 | 1 year | |
| Liu L, et al., 2018 | 2018 | 24-57 | M-13 F-2 |
Chest pain, syncope, NYHA Class â?¢/IV | 15 | 6 months | ||
| Liu LW, et al., 2019 | 2019 | Two NYHA class â?¡ and Seven NYHA class â?¢ cases | 9 | 6 months | ||||
| Note: NYHA: New York Heart Association; LVOT: Left Ventricular Outflow Tract; IVS: Interventricular Septum | ||||||||
Table 1: Characteristics of included studies
Results and Discussion
All five studies reported a change in LVOT gradient from the baseline (Figure 1A). Integrated analysis showed no significant change from baseline (MD:-69.506; 95% CI:-77.047, -61.966; P=0.089) (I2=50.4%). Subgroup analysis based on follow-up revealed that there was no significant difference noted at 6 months according to data from 2 studies (MD: -65.351; 95% CI; -85.253, -45.450; P=0.181) (I2=44.1%) however data from 3 studies at one year follow up did reveal a significant difference from baseline (MD:-71.010; 95% CI:-80.262, -61.758; P=0.047) (I2=67.2%).
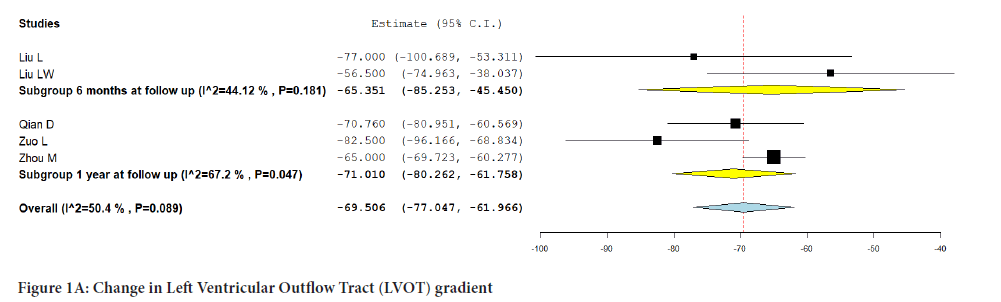
Figure 1A: Change in Left Ventricular Outflow Tract (LVOT) gradient.
Only three studies reported changes in LVEF from the baseline (Figure 1B). Integrated analysis showed a significant change from baseline at one- year follow-up in patients who underwent PIMSRA (MD:-1.760; 95% CI:- 3.239, -0.282; P=0.047) (I2=67.22%).
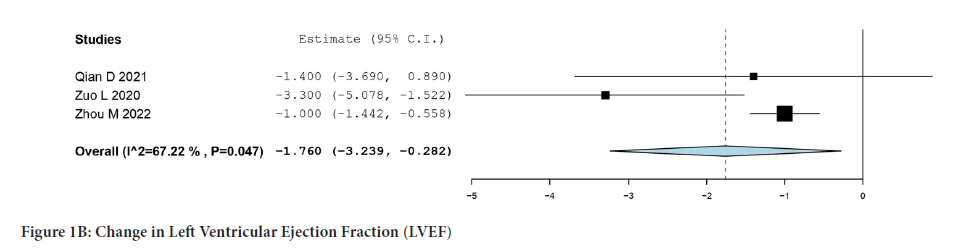
Figure 1B: Change in Left Ventricular Ejection Fraction (LVEF).
The pooled analysis from four studies that reported anterior IVS thickness showed no significant change from baseline (MD:-9.707; 95% CI:-10.819, -8.595; P=0.095) (I2=52.94%). Subgroup analysis based on follow-up revealed that there was no significant difference noted at 6 months (MD:- 11.285; 95% CI:-12.825, -9.744; P=0.933) (I2=0%) or at one-year follow-up (MD:-9.147; 95% CI:-9.837, -8.456; P=0.650) (I2=0%) (Figure 1C).
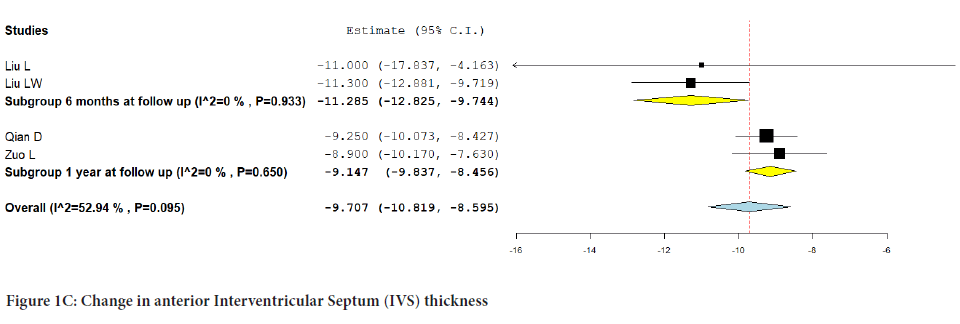
Figure 1C: Change in anterior Interventricular Septum (IVS) thickness.
The combined analysis of the four included studies reporting posterior IVS thickness showed no significant change from baseline (MD:-8.859; 95% CI:-10.073, -7.644; P=0.119) (I2=48.73%). Additionally, there was no significant difference noted in the subgroup analysis at 6 months (MD:- 10.278; 95% CI:-12.133, -8.423; P=0.934) (I2=0%) and at one-year follow-up (MD:-8.335; 95% CI:-9.880, -6.789; P=0.072) (I2=69.14%) (Figure 1D).
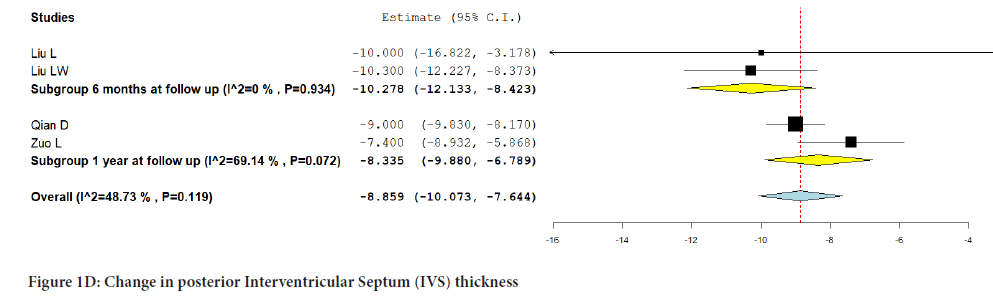
Figure 1D:Change in posterior Interventricular Septum (IVS) thickness.
Conclusion
This single-arm meta-analysis of 284 patients gathered from 5 clinical trials suggested that overall, after PIMSRA, LVOT gradient was reduced, and LVEF was slightly decreased. Additionally, no significant changes were observed in the anterior and posterior IVS thicknesses. Our results had substantial heterogeneity, which could be explained due to differences in the follow-up periods and studies with small sample sizes. It is important to note that even though no significant difference in follow-up was seen from the baseline at 6 months, there was indeed a significant difference noted at one year when assessing the outcomes of LVOT gradient. This could suggest that longer follow-up periods are imperative to truly observe the procedure’s efficacy.
Our meta-analysis had some limitations. The number of studies and the sample size available for analysis was limited, and most patients were of Chinese origin. Furthermore, no studies have evaluated the long-term effects on cardiac conduction due to the scar tissue formed following ablation. Therefore, further studies with long-term follow-up having large sample sizes from different patient populations and ethnicities should be done to demonstrate the effectiveness of PIMSRA with more statistical power. Outcomes measuring procedure safety, such as intraoperative and postoperative complications, should also be recorded and compared with a control group.
Author Declarations
Author contribution
All authors had access to the data and a role in writing the manuscript. All authors have reviewed the manuscript and approved it in its current form.
Code availability
Followed PRISMA guidelines and Software used was Open Meta analyst.
Availability of data and material
Transparent.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
References
- Zhou M, Ta S, Hahn RT, Hsi DH, Leon MB, Hu R, et al. Percutaneous intramyocardial septal radiofrequency ablation in patients with drug-refractory hypertrophic obstructive cardiomyopathy. JAMA Cardiol. 2022; 7(5): 529-538.
[Crossref] [Google Scholar] [Pubmed]
- Marian AJ, Braunwald E. Hypertrophic cardiomyopathy: Genetics, pathogenesis, clinical manifestations, diagnosis, and therapy. Circ Res. 2017; 121(7): 749-770.
[Crossref] [Google Scholar] [Pubmed]
- Qian D, Zhou X, Liu H, Cao L. Clinical value of 2D speckle tracking imaging in evaluating the effect of percutaneous intramyocardial septal radiofrequency ablation in patients with hypertrophic obstructive cardiomyopathy. J Clin Ultrasound. 2021; 49(6): 554-562.
[Crossref] [Google Scholar] [Pubmed]
- Tuohy CV, Kaul S, Song HK, Nazer B, Heitner SB. Hypertrophic cardiomyopathy: The future of treatment. Eur J Heart Fail. 2020; 22(2): 228-240.
[Crossref] [Google Scholar] [Pubmed]
- Waldman CB, Owens A. A plain language summary of the EXPLORER-HCM study: Mavacamten for obstructive hypertrophic cardiomyopathy. Future Cardiol. 2021; 17(7): 1269-1275.
[Crossref] [Google Scholar] [Pubmed]
- Liu L, Li J, Zuo L, Zhang J, Zhou M, Xu B, et al. Percutaneous intramyocardial septal radiofrequency ablation for hypertrophic obstructive cardiomyopathy. J Am Coll Cardiol. 2018; 72(16): 1898-1909.
[Crossref] [Google Scholar] [Pubmed]
- Zuo L, Hsi DH, Zhang L, Zhang Q, Shao H, Liu B, et al. Electrocardiographic QRS voltage amplitude improvement by intramyocardial radiofrequency ablation in patients with hypertrophic obstructive cardiomyopathy and one year follow up. J Electrocardiol. 2020; 61: 164-169.
[Crossref] [Google Scholar] [Pubmed]
- Liu LW, Zuo L, Zhou MY, Li J, Zhou XD, He GB, et al. Efficacy and safety of transthoracic echocardiography-guided percutaneous intramyocardial septal radiofrequency ablation for the treatment of patients with obstructive hypertrophic cardiomyopathy. Zhonghua Xin Xue Guan Bing Za Zhi. 2019; 47(4): 284-290.
[Crossref] [Google Scholar] [Pubmed]
Author Info
Fatema Tashrifwala*, Manisha Purushotham, Sai Krishna Bhushan, Mohammad Omer Khan and Ishaque HameedCitation: Tashrifwala F: Percutaneous Intramyocardial Septal Radiofrequency Ablation in Patients with Hypertrophic Cardiomyopathy
Received: 15-Feb-2023 Accepted: 10-Mar-2023 Published: 17-Mar-2023, DOI: 10.31858/0975-8453.14.3.195-198
Copyright: This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
ARTICLE TOOLS
- Dental Development between Assisted Reproductive Therapy (Art) and Natural Conceived Children: A Comparative Pilot Study Norzaiti Mohd Kenali, Naimah Hasanah Mohd Fathil, Norbasyirah Bohari, Ahmad Faisal Ismail, Roszaman Ramli SRP. 2020; 11(1): 01-06 » doi: 10.5530/srp.2020.1.01
- Psychometric properties of the World Health Organization Quality of life instrument, short form: Validity in the Vietnamese healthcare context Trung Quang Vo*, Bao Tran Thuy Tran, Ngan Thuy Nguyen, Tram ThiHuyen Nguyen, Thuy Phan Chung Tran SRP. 2020; 11(1): 14-22 » doi: 10.5530/srp.2019.1.3
- A Review of Pharmacoeconomics: the key to “Healthcare for All” Hasamnis AA, Patil SS, Shaik Imam, Narendiran K SRP. 2019; 10(1): s40-s42 » doi: 10.5530/srp.2019.1s.21
- Deuterium Depleted Water as an Adjuvant in Treatment of Cancer Anton Syroeshkin, Olga Levitskaya, Elena Uspenskaya, Tatiana Pleteneva, Daria Romaykina, Daria Ermakova SRP. 2019; 10(1): 112-117 » doi: 10.5530/srp.2019.1.19
- Dental Development between Assisted Reproductive Therapy (Art) and Natural Conceived Children: A Comparative Pilot Study Norzaiti Mohd Kenali, Naimah Hasanah Mohd Fathil, Norbasyirah Bohari, Ahmad Faisal Ismail, Roszaman Ramli SRP. 2020; 11(1): 01-06 » doi: 10.5530/srp.2020.1.01
- Manilkara zapota (L.) Royen Fruit Peel: A Phytochemical and Pharmacological Review Karle Pravin P, Dhawale Shashikant C SRP. 2019; 10(1): 11-14 » doi: 0.5530/srp.2019.1.2
- Pharmacognostic and Phytopharmacological Overview on Bombax ceiba Pankaj Haribhau Chaudhary, Mukund Ganeshrao Tawar SRP. 2019; 10(1): 20-25 » doi: 10.5530/srp.2019.1.4
- A Review of Pharmacoeconomics: the key to “Healthcare for All” Hasamnis AA, Patil SS, Shaik Imam, Narendiran K SRP. 2019; 10(1): s40-s42 » doi: 10.5530/srp.2019.1s.21
- A Prospective Review on Phyto-Pharmacological Aspects of Andrographis paniculata Govindraj Akilandeswari, Arumugam Vijaya Anand, Palanisamy Sampathkumar, Puthamohan Vinayaga Moorthi, Basavaraju Preethi SRP. 2019; 10(1): 15-19 » doi: 10.5530/srp.2019.1.3






