Research Article - (2023) Volume 14, Issue 3
Abstract
Background: Effective treatment of hypertension in neurocritical diseases is a current recommendation of guidelines. Clevidipine had showed effectiveness and safety for hypertension control in neurocritical patients.
Methods: Retrospective, observational and cross-sectional study in adult patients admitted to Intensive Care Unit for neurocritical disease requiring surgical or interventional treatment and presenting hypertension requiring urgent treatment with clevidipine as first line or rescue therapy after failure of different intravenous antihypertensive drugs. This study aimed to observe effectiveness and safety of clevidipine treatment in these patients. We conducted a subgroup analysis to observe possible confounder factors on effectiveness and safety of clevidipine treatment, major neurological complications and mortality.
Results: Thirty-three patients fulfilled inclusion criteria. Clevidipine was effective and safety for urgent control of hypertension in our patients. Effectiveness was higher in patients with larger brain hematomas and more severe subarachnoid hemorrhages treated with clevidipine as first line beginning within first 24 hours of admission. Subgroup of patients under effective treatment with clevidipine showed a slightly lower incidence of major neurological complications and mortality than global group. Early treatment within 24 hours of admission subgroup showed a lower incidence of major neurological complications than global group. No mortality was observed when clevidipine was first line treatment.
Conclusion: Clevidipine was effective and safe treatment of hypertension in our neurocritical patients. Severity of neurocritical disease is a key factor but effective treatment with clevidipine could contribute to reduce mortality and major neurological complications. Early treatment could prevent major neurological complications. First line treatment with clevidipine could reduce mortality. Nevertheless, our results based in a little size and retrospective study warranting further investigation in larger trials.
Keywords
Hypertension, Clevidipine, Intensive Care Unit (ICU), Neurocritical care
Introduction
Hypertension is a frequent problem of patients admitted to Intensive Care Unit (ICU) after Acute Ischemic Stroke (AIS), spontaneous Intracerebral Hemorrhage (ICH) and aneurysmal Subarachnoid Hemorrhage (aSAH) requiring surgical or interventional treatment and those under scheduled neurosurgical and neuroradiology procedures. Its effective management is challenging and must avoid significant decreases of blood pressure leading to lower Cerebral Perfusion Pressure (CPP) worsening ischemia and elevations probably associated with bleeding, rebleeding or hematoma expansion associated with poor prognosis (Whelton PK, et al., 2018; Williams B, et al., 2018).
Hypertension could be a risk factor as well a clinical finding of many neurocritical diseases (Hevesi M, et al., 2018) related with a higher risk of hemorrhagic, ischemic and recurrent stroke, hematoma expansion, death and poor neurological prognosis after ICH (Williams B, et al., 2018; Hevesi M, et al., 2018) and it is associated with higher rebleeding rates after aSAH (Tang C, et al., 2014; Larsen CC and Astrup J, 2013).
Target blood pressure in neurocritical patients in mainly unknown and controversial (Williams B, et al., 2018). Early control avoiding excessive reduction of hypertension leading to CPP decrease (Manoel AL, et al., 2016) and higher Systolic Blood Pressure (SBP) variability probably related with poor outcome according to evidence (Manning L, et al., 2014) should be the goal of treatment. Perioperative control of acute hypertension is especially especially important in patients presenting AIS, ICH and aSAH according to guidelines (Powers WJ, et al., 2019; Hemphill III JC, et al., 2015; Steiner T , et al., 2014; Graffagnino C, et al., 2013; Connolly Jr ES, et al., 2012) but the optimal levels are still controversial.
The ideal antihypertensive drug in this setting should be an arterial vasodilator of rapid onset and offset of action, low blood pressure variability and a few adverse effects (Manning L, et al., 2014; Der‐Nigoghossian C, et al., 2012). Labetalol, nicardipine and clevidipine are most often used intravenous drugs for hypertension control in neurocritical setting (Powers WJ, et al., 2019; Awad AS and Goldberg ME, 2010; Rosenfeldt Z, et al., 2018; Finger JR , et al., 2017; Bekker A, et al., 2010). Clevidipine is a short-acting, dihydropyridine L-type calcium channel antagonist approved in Europe for perioperative acute hypertension management, showing effectiveness and safety in patients with ICH (Graffagnino C, et al., 2013; Awad AS and Goldberg ME, 2010; Anderson CS, et al., 2013; Keating GM, 2014), AIS (Powers WJ, et al., 2019; Awad AS and Goldberg ME, 2010; Rosenfeldt Z, et al., 2018; Finger JR, et al., 2017; Keating GM, 2014), aSAH (Awad AS and Goldberg ME, 2010; Keating GM, 2014) and after neurosurgical procedures (Finger JR, et al., 2017; Bekker A, et al., 2010; Borrell-Vega J, et al., 2020), but little evidence exists regarding its effectiveness and safety in perioperative management of acute hypertension after mechanical thrombectomy, embolization of aneurysm causing aSAH, ICH requiring surgical treatment and interventional neuroradiology procedures. Clevidipine neither increases brain blood flow velocity or decreases brain reactivity to CO2 in healthy human volunteers according to evidence (Lemkuil BP, et al., 2016), but the real significance of this finding is unknown in damaged brain of neurocritical patients. This study aims to observe the effectiveness and safety of clevidipine for perioperative control of acute hypertension in neurocritical patients admitted to ICU.
Materials and Methods
Study design and population
Retrospective, single-group and cross-sectional study was conducted in neurocritical adult patients admitted to Cruces University Hospital Anesthesia ICU Department between January 1, 2017 and December 31, 2018. Adult patients older than 18 years presenting acute hypertension after urgent or scheduled neurosurgical or neuroradiology procedures requiring treatment using clevidipine as first line or after failure of different intravenous antihypertensive drugs based on ICU doctors decision were included. Patients presenting neurocritical condition not requiring surgical or interventional treatment or those not treated with clevidipine were excluded.
Retrospective and observational design were decided for investigators to describe urgent acute hypertension management using clevidipine as first line or rescue treatment in neurocritical patients without influencing ICU doctors decision regarding antihypertensive treatment. Ethical committee approved this study on May 28, 2019 (CEIC E19/17) and was registered on ClinicalTrials.gov NCT05168059 as NEURO-CLEV study. All methods were performed in accordance with Helsinki Declaration and Data Protection Law 3/2018. STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) guidelines were used for reporting results.
Data collection and variables
Patient data were obtained manually of hospital medical records platform (Osabide global, Osakidetza, Basque Country Healthcare Service) and critical care database ICCA (IntelliSpace Critical Care and Anesthesia information system, Phillips®) for 2 independent investigators (Blanca ER and Eunate AA) selecting patients according inclusion and exclusion criteria because retrospective design of this study.
Baseline variables were age, sex, chronic hypertension history, previous antihypertensive treatment, history of ischemic heart disease, stroke and cardiovascular risk factors, neurocritical disease, Blood Pressure (BP), Glasgow Coma Scale (GCS), ICH volume, National Institute of Health Stroke Scale (NIHSS), Fisher scale, neurosurgical and neuroradiology procedure. ICU variables were BP immediately before beginning clevidipine treatment, first line or rescue treatment, time of begin, effective doses achieving goal SBP, median doses, length and volume, infusion time frame with goal SBP, adverse effects, transition to oral antihypertensive treatment, Invasive Mechanical Ventilation (IMV) requirement, coagulopathy correction, deep venous thrombosis prevention, ICU and hospital stay. Evolution variables were major neurological and other complications during ICU stay, functional situation according to modified Rankin Scale (mRS) and mortality at 90 days. Rebleeding, hematoma expansion, brain swelling, intracranial hypertension (IH), vasospasm and neurological deterioration were considered major neurological complications.
Endpoints
Primary endpoint was observed effectiveness of clevidipine for hypertension control defined as percentage of patients achieving goal SBP within 1 hour of infusion beginning and maintaining target SBP between 48 to 72 hours without need of rescue treatment with different intravenous antihypertensive drugs. Goal SBP between 160 to 180 mm Hg in AIS, between 140 to 160 mm Hg in ICH, aSAH and scheduled neurosurgical or neuro-interventional procedures was based on current guidelines and ICU doctors experience. Effective treatment was described for ICU doctors according to medical records data using criteria defined earlier. BP was measured using a radial arterial catheter placed at ICU admission and registered for a nurse before begin of clevidipine infusion and then after 5,15,30,60,90 and 120 minutes for safety reasons because if treatment were ineffective ICU doctors could change to a different drug for achieve early hypertension control
Secondary endpoint was safety of clevidipine defined as percentage of significant adverse events related to infusion such tachycardia, atrial fibrillation, hypotension, fever and acute kidney failure.
Effectiveness and safety of clevidipine, major neurological complications, mortality and functional situation were analyzed in subgroups of patients to describe confounder factors probably influencing main outcomes.
Statistical analysis
Data of baseline, ICU and evolution variables were included for 2 independent investigators (Blanca ER and Eunate AA) and inconsistencies solved after discussion and agreement. Comparisons were made using Fisher exact test or chi-square for categorical variables and t or Mann Whitney U test for quantitative variables. Effectiveness was considered higher when clevidipine achieved primary endpoint in 70% to 100%, moderate in 51% to 69% and lower in 10% to 50% of patients. Adverse events were considered lower if presented in less than 20% of patients and severity was mild not requiring treatment stopped. Significance was set at 5% level. Analysis was performed by a biomedical statistician (Eunate AA) using IBM SPSS Statistics 23.0.
Results
100 patients were screened. 33 patients fulfilled all inclusion criteria and were included in this study. 67 patients were excluded (Figure 1).
Figure 1: Flow diagram of study design.
Baseline, ICU and evolution variables
Tables 1-3 showed baseline, ICU and evolution variables of patients. A mean age of 65 years old, 55% males, 45% females with history of chronic hypertension (67%; treated in 90% of patients), hypercholesterolemia (42%) and other cardiovascular risk factors (49%) were seen in these patients. Scheduled neurosurgical procedures (36%), AIS (27%), ICH (18%) and aSAH (18%) were most frequent diagnosis at ICU admission. Mean GCS was 11 points, half of presenting presented moderate to deep coma situation at admission and required IMV. Mean hematoma volumes of 25 ml and NIHHS of 9 points were seen at admission in patients with ICH and AIS, respectively. Fisher of 4 points were present in 67% of patients with aSAH. Major neurological complications were present in 51% of patients mainly between 24-48 hours after ICU admission because of intracranical hypertension (IH) secondary to hematoma, swelling, hematoma expansion and hydrocephalus. Mortality at 90 days was 30% and functional situation according to mRS at 90 days was moderate to severe disability in 79% of patients.
| Variable | Result |
|---|---|
| Age mean (SD) years | 65(13) |
| Gender % (n) | 55 males (18) |
| 45 females (15) | |
| Medical history | |
| Chronic hypertension % (n) | 67 (22) |
| Ischemic heart disease % (n) | 6 (2) |
| Stroke % (n) | 24 (8) |
| Diabetes mellitus % (n) | 21 (7) |
| Hypercholesterolemia % (n) | 42 (14) |
| Smoking % (n) | 12 (4) |
| Another cardiovascular risk factors % (n) | 49 (16) |
| Treated chronic hypertension % (n) | 90 (20) |
| 1 drug % (n) | 50 (10) |
| 2 drugs % (n) | 25 (5) |
| 3 or more drugs % (n) | 25 (5) |
| SBP, DBP and MBP baseline | |
| SBP mean (SD) mm Hg | 164 (29) |
| DBP mean (SD) mm Hg | 83 (19) |
| MBP mean (SD) mm Hg | 110 (19) |
| Glasgow coma scale | |
| Median (IQR) points | 11 (7) |
| 3-8 points % (n) | 30 (10) |
| 9-12 points % (n) | 21 (7) |
| 13-15 points % (n) | 49 (16) |
| Diagnosis and neurosurgical or neuro-interventional treatment | |
| Aneurysmal subarachnoid hemorrhage % (n) | |
| Embolization % (n) | 18 (6) |
| Embolization and intra cranial pressure monitoring sensor insertion % (n) | 50 (3) |
| Embolization, intra cranial pressure monitoring sensor insertion and intra-ventricular fibrinolysis % (n) | |
| Embolization, intra cranial pressure monitoring sensor insertion and craniotomy % (n) | 16.6 (1) |
| Intra cerebral hemorrhage % (n) | |
| Urgent craniotomy and intra cranial pressure monitoring sensor insertion % (n) | 16.6 (1) |
| Intracranial pressure monitoring sensor insertion % (n) | |
| Intraventricular fibrinolysis % (n) | 16.6 (1) |
| Acute Ischemic Stroke % (n) | 18 (6) |
| Mechanical thrombectomy % (n) | |
| Scheduled neurosurgical procedures % (n) | 33 (2) |
| Craniotomy % (n) | 50 (3) |
| Scheduled embolization of brain aneurysm % (n) | 16.7 (1) |
| Deep brain stimulation for parkinson’s disease % (n) | 27 (9) |
| 100 (9) | |
| 36 (12) | |
| 66.7 (8) | |
| 16.7 (2) | |
| 16.7 (2) | |
| NIHSS score | |
| Median (IQR) points | 9 (13) |
| Fisher score | |
| 3 points % (n) | 33 (2) |
| 4 points % (n) | 67 (4) |
| Hematoma volume at admission | |
| No intra-cerebral hemorrhage mean (SD) ml | 25 (16) |
| Intra-cerebral hemorrhage mean (SD) ml | 24 (18) |
| Note: IQR: Interquartile Range; SD: Standard Deviation; SBP: Systolic Blood Pressure; DBP: Diastolic Blood Pressure; MBP: Mean Blood Pressure | |
Table 1: Baseline variables.
| Variables | Result |
|---|---|
| SBP, DBP and MBP at begin of clevidipine | |
| SBP mean (SD) mm Hg | 180 (17) |
| DBP mean (SD) mm Hg | 80 (13) |
| MBP mean (SD) mm Hg | 112 (13) |
| Choice of treatment | |
| First line (%) | 18 |
| Rescue (%) | 82 |
| Time of begin of clevidipine (hours) | |
| Median (IQR) | 30 (46) |
| 0-24 hours % (n) | 45.5 (15) |
| 24-48 hours % (n) | 24 (8) |
| >48 hours % (n) | 30.3 (10) |
| First line median (IQR) hours | 33 (41) |
| Rescue median (IQR) hours | 34 (47) |
| Clevidipine effective doses Mean (SD) mg/h | 1.43 (1.40) |
| Clevidipine median doses Median (IQR) mg/h | 3 (2.5) |
| Length of clevidipine treatment median (IQR) hours | 39 (72.5) |
| 0-24 hours % (n) | 33.3 (11) |
| 24-48 hours % (n) | 18 (6) |
| >48 hours % (n) | 48.5 (16) |
| Clevidipine infusion volume median (IQR) ml | 210 (508) |
| Time within goal SBP median (IQR) hours | 61 (86.5) |
| 0-24 hours % (n) | 21 (7) |
| 24-48 hours % (n) | 18.2 (6) |
| >48 hours % (n) | 60.6 (20) |
| Adverse events % (n) | 3 (1) |
| Transition to oral treatment % (n) | 84.4 (28) |
| Intubation and mechanical ventilation between 24 hours to 7 days of ICU admission % (n) | 55 (18) |
| Normalization of coagulopathy at first 24 hours of ICU admission % (n) | 97 (32) |
| Mechanical thromboprophylaxis % (n) | 97 (32) |
| Pharmacology thromboprophylaxis % (n) | 76 (25) |
| ICU stay mean (SD) days | 12 (10) |
Table 2: ICU variables.
| Variables | Result |
|---|---|
| Major neurological complications % (n) | 51.5 (17) |
| Brain hematoma, hydrocephalus and intracranial hypertension % (n) | 35 (6) |
| Brain swelling and Intracranial hypertension % (n) | 17.6 (3) |
| Hematoma expansion and intracranial hypertension % (n) | 11.7 (2) |
| Hydrocephalus and Intracranial Hypertension % (n) | 11.7 (2) |
| Rebleeding, hydrocephalus and intracranial hypertension % (n) | 5.8 (1) |
| Vasospasm % (n) | 5.8 (1) |
| Hemorrhagic transformation and intracranial hypertension % (n) | 5.8 (1) |
| Neurological deterioration % (n) | 5.8 (1) |
| Time of complications onset | |
| <12 hours % (n) | 6 (1) |
| 12-24 hours % (n) | 6 (1) |
| 24-48 hours % (n) | 64.7 (11) |
| 48-72 hours % (n) | 17.6 (3) |
| >72 hours % (n) | 6 (1) |
| Other complications % (n) | 73 (24) |
| Neurological % (n) | 75 (18) |
| Thrombotic % (n) | 12.5 (3) |
| Hemorrhagic % (n) | 12.5 (3) |
| Cardiology % (n) | 4 (1) |
| Respiratory % (n) | 16.7 (4) |
| Infectious % (n) | 58 (14) |
| Psychiatry % (n) | 12.5 (3) |
| Functional situation (mRS) at 90 days | |
| 0-2 points % (n) | 21 (7) |
| 3-4 points % (n) | 30 (10) |
| 5-6 points % (n) | 49 (16) |
| Mortality at 90 days % (n) | 30 (10) |
| Hospital stay mean (SD) days | 12 (17) |
Table 3: Evolution variables.
Clevidipine treatment effectiveness
Clevidipine was effective for hypertension control in 73% of patients. Goal SBP was achieved in 97% of patients at 1 hour and target SBP was maintained for a median of 64 hours without requiring additional treatment with different intravenous antihypertensive drugs. Goal SBP was maintained also a median time of 42 hours after clevidipine infusion stopped. Effectiveness was 83% when clevidipine was first line treatment and 70% in patients with rescue treatment (Table 4).
| Effectiveness variable | Result |
|---|---|
| 1 hour to SBP goal % (n) | 97 (32) |
| Time within SBP goal median (IQR) hours | 64 (89) |
| Time within SBP goal after infusion stops median (IQR) hours | 42 (18) |
| Use of additional anti-hypertension drugs 24 to 72 hours of clevidipine treatment begin (%) | 0 |
| Effective treatment | |
| Global % (n) | 73 (24/33) |
| First line % (n) | 83 (5/6) |
| Rescue % (n) | 70 (19/27) |
| Diagnosis | |
| aSAH % (n) | 17 (4) |
| AIS % (n) | 25 (6) |
| Scheduled neurosurgical procedures % (n) | 46 (11) |
| ICH % (n) | 12 (3) |
Table 4: Clevidipine treatment effectiveness.
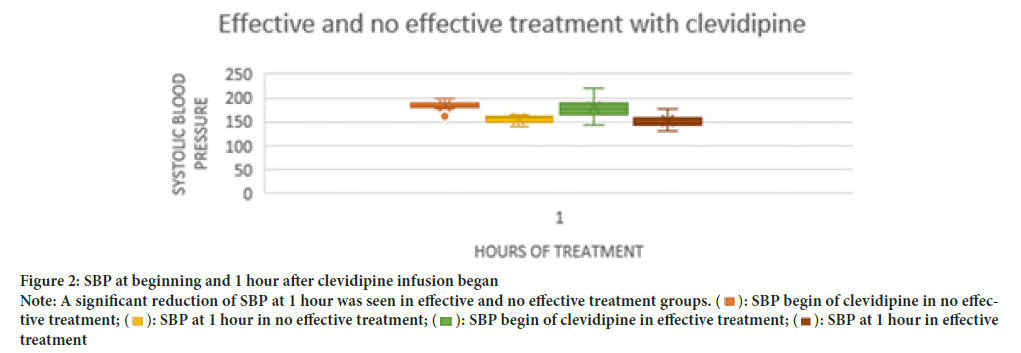
SBP at begin of clevidipine treatment was higher than 160 mm Hg without significant differences between patients with effective and no effective treatment (Table 5). SBP at 1 hour was significantly lower than SBP at begin of treatment in both effective and no effective treatment patients and without significant differences between groups (Figure 2 and Table 5). Time of clevidipine infusion begin, duration, volume, minimum and effective doses were not significantly different between effective and no effective treatment groups, but maximum and median doses were significantly higher in no effective treatment patients (Table 5). A significantly higher time with goal SBP during clevidipine infusion without additional intravenous antihypertensive drugs requirement were seen only in effective treatment group (Table 5). A significant reduction of SBP at 1 hour was seen in effective and no effective treatment groups (p<0.001) but without differences between these groups (p=0.709).
Note: A significant reduction of SBP at 1 hour was seen in effective and no effective treatment groups. (  ): SBP begin of clevidipine in no effective treatment; (
): SBP begin of clevidipine in no effective treatment; ( ): SBP at 1 hour in no effective treatment; (
): SBP at 1 hour in no effective treatment; ( ): SBP begin of clevidipine in effective treatment; (
): SBP begin of clevidipine in effective treatment; ( ): SBP at 1 hour in effective treatment.
): SBP at 1 hour in effective treatment.
Figure 2: SBP at beginning and 1 hour after clevidipine infusion began.
| Variable | Effective treatment | No effective treatment | Comparisons |
|---|---|---|---|
| SBP at begin of treatment (mm Hg) | No significant differences between effective and no effective treatment | ||
| Mean (SD) | 178 (19) | 183 (10) | p=0.401 |
| Median (IQR) | 178 (25) | 184 (10) | |
| SBP at 1 hour of treatment (mm Hg) | No significant differences between effective and no effective treatment | ||
| Mean (SD) | 151 (13) | 153 (12) | p=0.709 |
| Median (IQR) | 148 (18) | 157 (15) | |
| Time of treatment starts (hours) | No significant differences between effective and no effective treatment | ||
| Mean (SD) | 44 (56) | 60 (85) | p=0.839 |
| Median (IQR) | 28 (46) | 37 (81) | |
| Treatment duration (hours) | No significant differences between effective and no effective treatment | ||
| Mean (SD) | 56 (51) | 56 (39) | p=0.599 |
| Median (IQR) | 36 (70) | 50 (81) | |
| Time with goal SBP (hours) | Significant differences between effective and no effective treatment | ||
| Mean (SD) | 71 (48) | 55 (43) | p=0.01 |
| Median (IQR) | 64 (89) | 31 (62) | |
| (%) | 177 | 105 | |
| Doses (mg/h) | |||
| Minimum doses (mg/h) | No significant differences between minimum doses of effective and no effective treatment | ||
| Mean (SD) | 1 (1) | 2 (2) | p=0.340 |
| Median (IQR) | 1 (1) | 1 (2) | |
| Maximum doses (mg/h) | Significant differences between maximum doses of effective and no effective treatment | ||
| Mean (SD) | 5 (4) | 13 (11) | p=0.035 |
| Median (IQR) | 4 (5) | 8 (17) | |
| Median doses (mg/h) | Significant differences between median doses of effective and no effective treatment | ||
| Mean (SD) | 3.1 (2) | 7.3 (6) | p=0.035 |
| Median (IQR) | 2.4 (2) | 4.5 (10) | |
| Effective doses (mg/h) | Significant differences between median doses of effective and no effective treatment | ||
| Mean (SD) | 1.2 (0.8) | 2 (2.34) | p=0.039 |
| Median (IQR) | 1 (0.88) | 1 (1.5) | |
| Infusion volumes (ml) | No significant differences between effective and no effective treatment | ||
| Mean (SD) | 376 (472) | 474 (486) | p=0.466 |
| Median (IQR) | 162 (491) | 282 (792) | |
| Use of additional intravenous antihypertension drugs (%) | 0 | 78 | Significant differences between effective and no effective treatment, p<0.001 |
| Transition to oral Anti-hypertension drugs (%) | 71 | 89 | No significant differences between effective and no effective treatment, p<0.692 |
Table 5: Clevidipine treatment data.
Clevidipine safety: Secondary endpoint showed that clevidipine treatment had a lower number of adverse events (3%).
Subgroup analysis
Treatment effectiveness: Clevidipine treatment has effectiveness between 70% to 100% in both males and females with medical history of ischemic heart disease, cardiovascular risk factors, stroke and treated chronic High blood pressure (cHBP) presenting hypertension after urgent ruptured aneurysms embolization and mechanical thrombectomy or scheduled brain aneurysms embolization, deep brain stimulation and craniotomy. Subgroups of patients with poor prognosis factors like ICH with volumes higher than 30 ml and Fisher up to 3 points treated with clevidipine as first line, beginning within 24 hours of ICU admission with infusion length higher than 3 days and volumes higher than 500 ml also showed effectiveness between 70% to 100%.
Effectiveness of 50% to 69% of clevidipine was seen in patients younger than 65 years old, admitted for (IH) with deep coma situation as indicated for GCS lower than 8 points and requiring intracranial pressure monitoring sensor insertion. Clevidipine treatment had effectiveness between 10% to 49% in patients older than 65 years, admitted for ICH requiring urgent craniotomy and AIS with NIHSS higher than 10 points.
Treatment safety: No adverse events were observed in patients with begin of clevidipine treatment within 24 hours of ICU admission (0%). Adverse events were seen in a few patients of subgroups of effective treatment (4.2%) maintaining goal SBP higher than 48 hours (5%) and first line treatment (17%).
Major neurological complications: Major neurological complications were seen mainly in subgroups of patients treated with clevidipine as first line and maintaining goal SBP higher than 48 hours (Table 6). Mortality of patients presenting major neurological complications was 53.3%.
Mortality: Mortality was 0% in patients treated with clevidipine as first line; 16.7% when treatment was effective according to our criteria; 33% when clevidipine begins within 24 hours of ICU admission and 35% in patients with goal SBP maintained higher than 48 hours (Table 6).
| Subgroups | Major neurological complications | Mortality | Functional situation |
|---|---|---|---|
| Treatment begins within 24 hours of admission % (n) | 27 (5) | 33.3 (5) | mRS 0-2 points: 33 (5) |
| mRS 3-6 points: 67 (10) | |||
| Effective treatment % (n) | 38 (10) | 16.7 (4) | mRS 0-2 points: 25 (6) |
| mRS 3-6 points: 75 (18) | |||
| Goal SBP maintained >48 hours % (n) | 55 (12) | 35 (7) | mRS 0-2 points: 15 (3) |
| mRS 3-6 points: 85 (17) | |||
| First line treatment % (n) | 50 (4) | 0 (0) | mRS 0-2 points: 33 (2) |
| mRS 3-6 points: 67 (4) |
Table 6: Major neurological complications, mortality and functional situation in subgroups.
Functional situation: A functional situation of moderate to severe disability according with mRS were seen in all subgroups (Table 6).
Discussion
Hypertension is very frequent in patients admitted to ICU after AIS, ICH, aSAH and scheduled neurosurgical procedures (Graffagnino C, et al., 2013; Der‐Nigoghossian C, et al., 2019; Finger JR, et al., 2017; Anderson CS, et al., 2013) potentially causing complications like hemorrhagic transformation, hematoma expansion, brain swelling, rebleeding, longer hospital stay, worse functional outcomes and death (Tang C, et al., 2014; Graffagnino C, et al., 2013; Der‐Nigoghossian C, et al., 2019). Its exact pathophysiological mechanism is mostly unknown but increase of systemic vascular resistances because of damage of brain areas regulating arterial baroreceptor reflex and activation of renin-angiotensin-aldosterone, sympathetic autonomic and hypothalamic-pituitary-adrenal systems (Der‐Nigoghossian C, et al., 2019) probably plays a role. Cerebral autoregulation could be impaired in these patients causing vasoconstriction and ischemia or vasodilation and increase of cerebral blood flow volume and intracranial pressure (Der‐Nigoghossian C, et al., 2019), both associated with brain secondary injury. For this reason, its highly recommended a thoughtful management of hypertension in these patients.
In this study we aimed to observe effectiveness and safety of clevidipine for hypertension management in neurocritical patients admitted to ICU after AIS, ICH and aSAH requiring surgical management and scheduled neurosurgical procedures, because in Europe clevidipine is only approved for hypertension management in perioperative setting (Keating GM, 2014). Retrospective and observational study design was based in the unique profile of neurocritical patient requiring urgent and effective management of hypertension potentially avoiding serious life-threatening complications, death and poor functional outcomes according guidelines recommendations based on experience and preferences of ICU doctors regarding type and choice of antihypertensive drugs (Der‐Nigoghossian C, et al., 2019).
Our patients were median age, both gender with cHBP associated to other cardiovascular risk factors, as early published evidence regarding neurocritical population (Bekker A, et al., 2010). Treated cHBP seen in a higher number of patients, none with history of uncontrolled cHBP and probably unknown cHBP in a lower number of patients, could be paradoxical because severe neurocritical diseases of our patients should be expected if uncontrolled or unknown cHBP were present.
Our study included neurocritical patients admitted to ICU requiring safe and fast control of hypertension besides usual critical care management involving monitoring, airway control and thromboprophylaxis. Scheduled neurosurgical procedures, AIS, ICH and aSAH were most frequent diagnosis, requiring tailored approaches for hypertension management according to guidelines (Powers WJ, et al., 2019; Hemphill III JC, et al., 2015; Steiner T, et al., 2014; Connolly Jr ES, et al., 2012). Neurological situation at admission with half of patients presenting moderate to deep disturbance of consciousness level probably resulting of severe neurocritical diagnosis as indicated for high-risk neurosurgical procedures, hematoma volumes, NIHHS and Fisher scales. Hematoma volumes were higher (Anderson CS, et al., 2013; Qureshi AI, et al., 2016) but also similar (Graffagnino C, et al., 2013) than previously published, including patients in a higher risk of severe complications. Major neurological complications seen in half of patients were IH secondary to brain hematoma, swelling, hematoma expansion, hydrocephalus, rebleeding, hemorrhagic transformation and vasospasm, probably explaining IMV requirement and a moderate to severe disability according mRS in a higher number of patients. Mortality of 30% probably resulted of severe neurocritical disease and major neurologic complications.
Clevidipine showed a higher global effectiveness for hypertension treatment in this study. Several facts reflected its main advantages in neurocritical patients: 97% of patients achieved goal SBP within 1 hour of treatment begin, maintained it for 64 hours, and 42 hours after infusion stopped without need of additional different intravenous antihypertensive drugs. Clevidipine treatment was highly safe in this study presenting only 3% of transient mild adverse events not requiring infusion stopped. Fast and longer maintenance of goal SBP with one-drug presenting only a few transient mild adverse events could be good advantages of clevidipine for hypertension management in neurocritical patients. In Europe, Clevidipine is available in some centers only since a few years and different intravenous antihypertensive drugs like urapidil, nicardipine and labetalol are more used. This could explain that clevidipine was chosen after failure of different intravenous antihypertensive treatment in a higher number of our patients.
SBP thresholds requiring treatment are controversial also and vary according to neurocritical condition (Powers WJ, et al., 2019; Hemphill III JC, et al., 2015; Steiner T, et al., 2014; Graffagnino C, et al., 2013; Connolly Jr ES, et al., 2012). In this study, clevidipine treatment began with SBP higher than 160 mm Hg according to ICU doctors decision and significantly decrease to goal at first hour without differences between effective and no effective groups. Minimum and effective doses slightly lower than recommended for manufacturer (1 mg/h) and volumes than previously published (Finger JR, et al., 2017) with infusion length not significantly different between effective and no effective groups achieved goal SBP. Clevidipine infusion began not significantly different between first line and rescue patients or effective and no effective groups could be paradoxical, because in a higher number of patients with rescue treatment would be expected a later began of infusion than first line group, but probably the variable onset of hypertension requiring urgent treatment in each neurocritical disease explained this result. Transition to oral antihypertensive treatment required very frequently but not significantly different between effective and no effective treatment groups probably resulting of cHBP in a higher number of patients. A significantly longer time within goal SBP, requiring lower maximum (4 mg/h) and median doses (3 mg/h) without need of additional intravenous antihypertensive drugs seen only in effective group were also good advantages of clevidipine treatment in neurocritical patients.
Analysis of subgroups showed a higher effectiveness of clevidipine treatment in patients admitted to ICU after urgent embolization of aneurysm causing aSAH, mechanical thrombectomy for aIS, scheduled neurosurgical procedures and presenting poor prognosis factors like ICH volumes higher than 30 ml and Fisher scores up to 3 points. Clevidipine treatment as first line, beginning within 24 hours of ICU admission, maintained for higher than 3 days with volumes up to 500 ml showed a higher effectiveness also. These results could partly be explained for higher number of patients admitted for aSAH, AIS and scheduled neurosurgical procedures in this study, but factors related to treatment like first line, beginning within 24 hours and longer treatment for patients with severe neurocritical diseases as indicated for poor prognosis factors probably played a role and was a very interesting finding of this study not previously published as far as we know. Lower effectiveness of clevidipine treatment were probably explained for higher severity of neurocritical disease as indicated for coma, AIS with NIHHS score higher than 10 points, ICH and IH.
Clevidipine was safe in subgroups of patients with effective treatment beginning within 24 hours and maintaining goal SBP higher than 48 hours. Adverse events were more frequent in patients with first line treatment but as mentioned above it was lower, transient, mild and dose related not requiring treatment stopped.
Major neurological complications were lower in subgroups of patients treated with effective treatment with clevidipine beginning within 24 hours of admission. Mortality was lower in subgroups of patients with first line and effective treatment. Functional situation of moderate to severe disability was seen in a higher number of patients of all subgroups like than global group. These results seem to indicate the benefits of clevidipine treatment beginning within 24 hours of admission more than maintenance of goal SBP after 48 hours to reduce mortality and major neurological complications, but without changes in functional situation. First line treatment showed a higher effectiveness and lower mortality with a higher number of adverse events, probably because of a lower number of patients included in this subgroup, making this result difficult to interpretate. Nevertheless, severity of neurocritical disease probably is the key factor in these outcomes, probably making treatment facts of limited impact.
Our study has limitations related mainly to its little size and design. This retrospective and observational study made possible to describe hypertension management in neurocritical patients admitted to ICU without interfering with decision of ICU doctors regarding antihypertensive treatment. The little size of this investigation probably resulted of time of study of one year and based in one center because larger studies about neurocritical population should require inclusion of patients from different countries consuming more time. This study showed a little size and heterogeneous neurocritical population requiring urgent and effective treatment of hypertension using clevidipine mainly as a rescue therapy, but these patients probably take advantages of higher effectiveness and safety as well as lower mortality and major neurological complications of effective treatment.
Conclusion
In conclusion, we observed that clevidipine was effective and safe treatment in neurocritical patients with hypertension requiring urgent treatment with potential benefits related to mortality and major neurological complications. Clevidipine has pharmacological advantages in this population based on its shorter acting and smooth control of hypertension. Retrospective, observational and little size design of our study prevents us to make stronger recommendations. Nevertheless, this original study including larger population than previously published as far as we know underscore advantages of clevidipine for hypertension management in neurocritical patients.
Ethical Approval
Cruces University Hospital Ethical Committee of Clinical Investigation approved this study on May 28, 2019 (code CEIC E19/17). Neurocritical patients admitted to Anesthesia ICU of our hospital were affected for severe diseases with life-threatening complications. In some cases, patients did not survive and serious difficulties about obtaining relatives consent make us to propose anonymized methodology of medical records review protecting personal data of patients according Data Protection Law 3/2018. The need of informed consent was waived by Cruces University Hospital Ethical Committee due retrospective nature of the study.
Funding
This study was funding for Biocruces Health Research Institute regarding collection, statistical analysis of data and manuscript review.
Consent for Publication
Cruces University Hospital Ethical Committee and all authors of this manuscript gave consent for publish this study.
Data Availability Statement
Electronic medical records and anonymized dataset of patients included in present study could be found in Biocruces Health Research Institute archives and available for revision of Editorial Board Members and researchers wishing to use it for non-commercial purposes. Biocruces Health Research Institute archives are not publicly available in accordance with Spanish data protection law 3/2018 and autonomic Basque Country Healthcare Service regulations. Data however could be accessed under demand for peer-review or researchers from the corresponding author (Blanca ER) upon reasonable request with permission of Osakidetza (Basque Country Healthcare Service) and Biocruces Health Research Institute.
Author Contributions
Blanca ER and Eunate AA designed study, collected data and wrote method sections. Eunate AA and Blanca ER made statistical analysis of data. Blanca ER and Ismael AB wrote results and discussion sections. Alberto MR, Ismael AB, Maria JMB, Ane, GG wrote introduction and reviewed manuscript.
Competing Interests
None of authors received economic funding of clevidipine commercialization laboratory Ferrer® for this study. Dr. Blanca ER (corresponding and first author) presented a conference of acute high blood pressure management in neurocritical patients sponsored for Ferrer laboratories on April 25, 2019 in Spain. Dr. Alberto MR moderate a conference of acute high blood pressure management in perioperative setting sponsored for Ferrer laboratories on April 25, 2019 in Spain.
References
- Whelton PK, Carey RM, Aronow WS, Casey DE, Collins KJ, Himmelfarb CD, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: A report of the American college of cardiology/American heart association task force on clinical practice guidelines. Hypertension. 2018; 71(19): e127-248.
[Crossref] [Google scholar] [Pubmed]
- Williams B, Mancia G, Spiering W, Rosei EA, Azizi M, Burnier M, et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension: The task force for the management of arterial hypertension of the European Society of Cardiology (ESC) and the European Society of Hypertension (ESH). Eur Heart J. 2018; 39(33): 3021-3104.
[Crossref] [Google scholar] [Pubmed]
- Hevesi M, Bershad EM, Jafari M, Mayer SA, Selim M, Suarez JI, et al. Untreated hypertension as predictor of in-hospital mortality in intracerebral hemorrhage: A multi-center study. J Crit Care. 2018; 43: 235-239.
[Crossref] [Google scholar] [Pubmed]
- Tang C, Zhang TS, Zhou LF. Risk factors for rebleeding of aneurysmal subarachnoid hemorrhage: A meta-analysis. PloS one. 2014; 9(6): e99536.
[Crossref] [Google scholar] [Pubmed]
- Larsen CC, Astrup J. Rebleeding after aneurysmal subarachnoid hemorrhage: A literature review. World neurosurg. 2013; 79(2): 307-312.
[Crossref] [Google scholar] [Pubmed]
- Manoel AL, Goffi A, Zampieri FG, Turkel-Parrella D, Duggal A, Marotta TR, et al. The critical care management of spontaneous intracranial hemorrhage: A contemporary review. Crit Care. 2016; 20(1): 1-29.
[Crossref] [Google scholar] [Pubmed]
- Manning L, Hirakawa Y, Arima H, Wang X, Chalmers J, Wang J, et al. Blood pressure variability and outcome after acute intracerebral haemorrhage: A post-hoc analysis of INTERACT2, a randomised controlled trial. Lancet Neurol. 2014; 13(4): 364-373.
[Crossref] [Google scholar] [Pubmed]
- Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: A guideline for healthcare professionals from the American heart association/American stroke association. Stroke. 2019; 50(12): e344-418.
[Crossref] [Google scholar] [Pubmed]
- Hemphill III JC, Greenberg SM, Anderson CS, Becker K, Bendok BR, Cushman M, et al. Guidelines for the management of spontaneous intracerebral hemorrhage: A guideline for healthcare professionals from the American heart association/American stroke association. Stroke. 2015; 46(7): 2032-2060.
[Crossref] [Google scholar] [Pubmed]
- Steiner T, Salman RA, Beer R, Christensen H, Cordonnier C, Csiba L, et al. European Stroke Organisation (ESO) guidelines for the management of spontaneous intracerebral hemorrhage. Int J Stroke. 2014; 9(7): 840-855.
[Crossref] [Google scholar] [Pubmed]
- Graffagnino C, Bergese S, Love J, Schneider D, Lazaridis C, LaPointe M, et al. Clevidipine rapidly and safely reduces blood pressure in acute intracerebral hemorrhage: The ACCELERATE trial. Cerebrovasc Dis. 2013; 36(3): 173-180.
[Crossref] [Google scholar] [Pubmed]
- Connolly Jr ES, Rabinstein AA, Carhuapoma JR, Derdeyn CP, Dion J, Higashida RT, et al. Guidelines for the management of aneurysmal subarachnoid hemorrhage: A guideline for healthcare professionals from the American heart association/American stroke association. Stroke. 2012; 43(6): 1711-1737.
[Crossref] [Google scholar] [Pubmed]
- Der‐Nigoghossian C, Levasseur‐Franklin K, Makii J. Acute blood pressure management in neurocritically ill patients. Pharmacotherapy. 2019; 39(3): 335-345.
[Crossref] [Google scholar] [Pubmed]
- Awad AS, Goldberg ME. Role of clevidipine butyrate in the treatment of acute hypertension in the critical care setting: A review. Vasc Health Risk Manag. 2010; 6: 457.
[Crossref] [Google scholar] [Pubmed]
- Rosenfeldt Z, Conklen K, Jones B, Ferrill D, Deshpande M, Siddiqui FM. Comparison of nicardipine with clevidipine in the management of hypertension in acute cerebrovascular diseases. J Stroke Cerebrovasc Dis. 2018; 27(8): 2067-2073.
[Crossref] [Google scholar] [Pubmed]
- Finger JR, Kurczewski LM, Brophy GM. Clevidipine versus nicardipine for acute blood pressure reduction in a neuroscience intensive care population. Neurocrit Care. 2017; 26(2): 167-173.
[Crossref] [Google scholar] [Pubmed]
- Bekker A, Didehvar S, Kim S, Golfinos JG, Parker E, Sapson A, et al. Efficacy of clevidipine in controlling perioperative hypertension in neurosurgical patients: Initial single-center experience. J Neurosurg Anesthesiol. 2010; 22(4): 330-335.
[Crossref] [Google scholar] [Pubmed]
- Anderson CS, Heeley E, Huang Y, Wang J, Stapf C, Delcourt C, et al. Rapid blood-pressure lowering in patients with acute intracerebral hemorrhage. N Engl J Med. 2013; 368: 2355-2365.
[Crossref] [Google scholar] [Pubmed]
- Keating GM. Clevidipine: A review of its use for managing blood pressure in perioperative and intensive care settings. Drugs. 2014; 74(16): 1947-1960.
[Crossref] [Google scholar] [Pubmed]
- Borrell-Vega J, Uribe AA, Palettas M, Bergese SD. Clevidipine use after first-line treatment failure for perioperative hypertension in neurosurgical patients: A single-center experience. Medicine. 2020; 99(1).
[Crossref] [Google scholar] [Pubmed]
- Lemkuil BP, Gierl BT, Patel PM, Pearn ML, Nguyen LC, Minokadeh A, et al. The effect of clevidipine on cerebral blood flow velocity and carbon dioxide reactivity in human volunteers. J Neurosurg Anesthesiol. 2016; 28(4): 337-340.
[Crossref] [Google scholar] [Pubmed]
- Qureshi AI, Palesch YY, Barsan WG, Hanley DF, Hsu CY, Martin RL, et al. Intensive blood-pressure lowering in patients with acute cerebral hemorrhage. N Engl J Med. 2016; 375(11): 1033-1043.
[Crossref] [Google scholar] [Pubmed]
Author Info
Escontrela Rodriguez Blanca1*, Acevedo Bambaren Ismael2, Arana-Arri Eunate3, Maroño Boedo Maria Jesús1, Guereca Gala Ane1 and Martinez Ruiz Alberto1,32Department of Anesthesia and Intensive Care Unit, Hospital Universitario Ramón y Cajal, Madrid, Spain
3Biocruces Health Research Institute, Bizkaia, Spain 4Department of Surgery, Radiology and Physical Medicine, Universidad País Vasco, Leioa, Spain
Citation: Blanca ER: Perioperative Control of Acute High Blood Pressure in Neurosurgical Patients Admitted to Intensive Care Unit Using Clevidipine (NEURO-CLEV)
Received: 10-Feb-2023 Accepted: 24-Feb-2023 Published: 03-Mar-2023, DOI: 10.31858/0975-8453.14.3.209-217
Copyright: This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
ARTICLE TOOLS
- Dental Development between Assisted Reproductive Therapy (Art) and Natural Conceived Children: A Comparative Pilot Study Norzaiti Mohd Kenali, Naimah Hasanah Mohd Fathil, Norbasyirah Bohari, Ahmad Faisal Ismail, Roszaman Ramli SRP. 2020; 11(1): 01-06 » doi: 10.5530/srp.2020.1.01
- Psychometric properties of the World Health Organization Quality of life instrument, short form: Validity in the Vietnamese healthcare context Trung Quang Vo*, Bao Tran Thuy Tran, Ngan Thuy Nguyen, Tram ThiHuyen Nguyen, Thuy Phan Chung Tran SRP. 2020; 11(1): 14-22 » doi: 10.5530/srp.2019.1.3
- A Review of Pharmacoeconomics: the key to “Healthcare for All” Hasamnis AA, Patil SS, Shaik Imam, Narendiran K SRP. 2019; 10(1): s40-s42 » doi: 10.5530/srp.2019.1s.21
- Deuterium Depleted Water as an Adjuvant in Treatment of Cancer Anton Syroeshkin, Olga Levitskaya, Elena Uspenskaya, Tatiana Pleteneva, Daria Romaykina, Daria Ermakova SRP. 2019; 10(1): 112-117 » doi: 10.5530/srp.2019.1.19
- Dental Development between Assisted Reproductive Therapy (Art) and Natural Conceived Children: A Comparative Pilot Study Norzaiti Mohd Kenali, Naimah Hasanah Mohd Fathil, Norbasyirah Bohari, Ahmad Faisal Ismail, Roszaman Ramli SRP. 2020; 11(1): 01-06 » doi: 10.5530/srp.2020.1.01
- Manilkara zapota (L.) Royen Fruit Peel: A Phytochemical and Pharmacological Review Karle Pravin P, Dhawale Shashikant C SRP. 2019; 10(1): 11-14 » doi: 0.5530/srp.2019.1.2
- Pharmacognostic and Phytopharmacological Overview on Bombax ceiba Pankaj Haribhau Chaudhary, Mukund Ganeshrao Tawar SRP. 2019; 10(1): 20-25 » doi: 10.5530/srp.2019.1.4
- A Review of Pharmacoeconomics: the key to “Healthcare for All” Hasamnis AA, Patil SS, Shaik Imam, Narendiran K SRP. 2019; 10(1): s40-s42 » doi: 10.5530/srp.2019.1s.21
- A Prospective Review on Phyto-Pharmacological Aspects of Andrographis paniculata Govindraj Akilandeswari, Arumugam Vijaya Anand, Palanisamy Sampathkumar, Puthamohan Vinayaga Moorthi, Basavaraju Preethi SRP. 2019; 10(1): 15-19 » doi: 10.5530/srp.2019.1.3