Research Article - (2023) Volume 14, Issue 12
Preoperative Serum CA125 Levels for Predicting Peritoneal Dissemination of Colorectal Cancer
Nai-Lu Lin1,3, Shih-Ching Chang2,3, Jeng-Kae Jiang2,3 and Shung-Haur Yang1,2,3*Abstract
Objective: The present study aimed to analyze the association between tumor makers and Peritoneal Dissemination (PD) detection in patients with Colorectal Cancer (CRC).
Background: The eighth edition of the American Joint Committee on Cancer (AJCC) staging system expanded the classification of the M category with the addition of M1c for PD due to its worse prognosis compared to other visceral organ metastases. The lack of specific symptoms and varying sensitivities of imaging studies leads to difficulty in PD detection.
Methods: A total of 1,109 patients with CRC who underwent tumor resection between June 2000 and December 2017 were included in this retrospective study. Serum tumor markers, including Carcinoembryonic Antigen (CEA), Carbohydrate Antigen 19-9 (CA19-9), and CA125, were analyzed preoperatively. The grading of PD was performed based on the findings during surgical exploration and further confirmed by pathology.
Results: CA125 had the lowest sensitivity; however, it showed the highest specificity and diagnostic accuracy compared to CEA and CA19-9. Based on the analysis of tumor marker levels in advancing stages, the levels of CA125 did not rise until stage IVC. CEA and CA19-9 levels increased significantly at stage IV; however, it was not possible to differentiate between stage IVC and other organ metastases.
Conclusion: Among the three tumor markers, CA125 levels were closely correlated with PD compared to CEA and CA19-9 levels. The present analysis of tumor markers poses a better chance of diagnosis of stage IVC preoperatively.
Keywords
Colorectal Cancer (CRC), Cytoreductive surgery, CA125 level, Peritoneal dissemination
Introduction
Colorectal Cancer (CRC) is one of the most commonly diagnosed cancers worldwide. According to GLOBOCAN 2018, more than one million new CRC cases and 55,000 deaths were estimated worldwide (Bray F, et al., 2018). Approximately 5%-15% of patients with CRC were diagnosed with Peritoneal Dissemination (PD) at abdominal exploration (Jayne DG, et al., 2002; Stewart JH, et al., 2005; Klaver YL, et al., 2012). Poor Overall Survival (OS) of patients with CRC with PD has been reported in previous studies (Stewart JH, et al., 2005; Stillwell AP, et al., 2011).
Since the 1980s, Cytoreductive Surgery (CRS) combined with Hyperthermic Intraperitoneal Chemotherapy (HIPEC) has been proposed to treat PD (Spratt JS, et al., 1980; Sugarbaker PH, 2010). Currently, complete CRS and/or HIPEC are carried out in centers with experienced physicians for a selected patient with limited PD for whom R0 could be achieved (Mahmoud AM, et al., 2018; NCCN, 2021).
In 2004, we analyzed preoperative Carcinoembryonic Antigen (CEA), carbohydrate antigen 19-9 (CA19-9), and other parameters and found that abnormal CA19-9 was a risk factor for PD (Yang SH, et al., 2004). In 2016, we reported that preoperative carbohydrate antigen 125 (CA125) was a better tumor marker compared to CEA to predict PD in both genders (Huang CJ, et al., 2016).
In 2017, the eighth edition of the American Joint Committee on Cancer (AJCC) staging system made a new classification of stage IV. The M category classification for PD was expanded with the addition of M1c (M1a denotes metastases to one distant site or organ and M1b denotes metastases to more than one) (Byrd DR, et al., 2017). The rationale for AJCC’s M1c designation is PD that generally has a poor prognosis compared to other visceral organ metastases (Weiser MR, 2018). A better diagnosis of PD at this stage is required to enable timely treatment. The question arises whether we can specifically detect PD through serum marker analysis.
In the present study, we aimed to investigate which among the three markers-CEA, CA19-9, or CA125-is the most suitable marker to predict the incidence of PD of CRC in a larger patient population.
Materials and Methods
Patients
This retrospective analysis included a prospectively collected database with 1,253 patients who were diagnosed with CRC between June 2000 and December 2016. Elective resections were performed by two surgeons (S-HY and J-KJ) at the Taipei Veterans General Hospital. The Institutional Review Board of the Taipei Veterans General Hospital approved this study (2015-07-005AC). Written informed consent was obtained from all the patients. After excluding patients with synchronous cancers other than CRC (n=4), those with rectal cancer receiving neoadjuvant radiotherapy (n=134), those with pathology other than adenocarcinoma (n=4), or those with insufficient data (n=2), the data of 1,109 patients were included. The clinicopathological data were retrieved from the medical records, including age, sex, primary tumor site, histology, Lymphovascular Invasion (LVI), sites of metastases, grading of PD, and preoperative serum CEA, CA19-9, and CA125 levels. The presence and grading of PD were defined based on the findings of operative exploration and were confirmed by pathology. The TNM staging of the AJCC (8th edition) was used for staging (Byrd DR, et al., 2017). The grading system for PD was based on the Japanese Classification of Colorectal Cancer (JCCRC) criteria (Japanese Society for Cancer of the Colon and Rectum, 2019).
Tumor biomarkers
CEA, CA19-9, and CA125 levels were determined from peripheral blood samples within 1 month before surgery. The measurement methods were changed during the study period. In the early years of the study (June 2000-November 2012), radioimmunoassay kits were used: CEA, CEARIACT Kit (CIS bio international, Gif-sur-Yvette, France); CA19-9 and CA125, 125I IRMA Kit (DiaSorin, Stillwater Minnesota, USA). Further, between December 2012 and December 2016, ARCHITECT i2000SR (Abbott Diagnostics, Lake Forest, USA) was used to measure the levels of all three markers. The values of CEA <5.0 ng/ml, CA19-9 <37.0 U/ml, and CA125 <35.0 U/ml were defined as normal results.
Statistical analysis
Categorical variables were compared using Pearson’s Chi-squared test. The means between two groups were compared using the student’s t-test. Oneway analysis of variance with the Bonferroni post hoc test was used for multiple comparisons. Statistical analysis was performed using the Statistical Package for Social Sciences (SPSS) software version 20.0. (SPSS Inc., Chicago, IL, USA). The Receiver-Operating Characteristic (ROC) curve and the Area Under the Curve (AUC) were analyzed using the Stata Statistical Software (version 12.0, Stata Corporation, College Station, TX) where p<0.05 was considered as statistically significant.
Results
A total of 636 male (57.3%) and 473 female patients (42.7%) were included in this study, with a mean age of 65.1 years (range, 26-94 years). All 1,109 patients underwent resection of the primary lesion with curative or palliative intent and exploration for macroscopic PD. Patient characteristics are shown in Table 1. Tumor locations were as follows-
| Demographic characteristics | |
|---|---|
| Age (years), mean (range) | 65.1 (26.0-94.0) |
| Sex, n (%) | |
| Male | 636, 57.3% |
| Female | 473, 42.7% |
| Tumor site, n (%) | |
| Right colon | 374, 33.7% |
| Left colon | 470 (42.4%) |
| Rectum | 265 (23.9%) |
| Histology, n (%) | |
| Adenocarcinoma | 1051 (94.8%) |
| Mucinous adenocarcinoma, signet ring cell carcinoma | 58 (5.2%) |
| Differentiation, n (%) | |
| Well, moderate differentiation | 1020 (92%) |
| Poor differentiation, undifferentiation | 89 (8%) |
| Lymphovascular invasion, n (%) | |
| Negative | 854 (77%) |
| Positive | 255 (23%) |
| Peritoneal dissemination*, n | |
| P0 P1 P2 P3 |
1029 (92.8%) 28 (2.5%) 26 (2.3%) 26 (2.3%) |
| TNM stage, n (%) | |
| I II III IV |
239 (21.6%) 328 (29.6%) 307 (27.7%) 235 (21.2%) |
| CEA (ng/ml), median (range) | 3.5 (0.1-5663.5) |
| CA19-9 (U/ml), median (range) | 14.3 (0.1-53252.3) |
| CA125 (U/ml), median (range) | 12 (0.1-1503.0) |
Note: CEA=Carcinoembryonic Antigen; CA19-9, carbohydrate antigen 19-9; CA125: Carbohydrate Antigen 125; TNM: Tumor Node
Metastasis
*Japanese Classification of Colorectal Cancer criteria-P0: No Peritoneal Dissemination (PD); P1: Adjacent PD without distant PD; P2: A few
distant PD; P3: Numerous distant PD
Table 1: Demographic distribution
Right side (33.7%), left side (42.4%), and rectum (23.9%). Mucinous adenocarcinoma and signet ring cell carcinoma composed 5.2%, poor differentiation plus undifferentiation 8%, and LVI 23% of the whole population. Using the AJCC staging system, 239 (21.6%), 328 (29.6%), 307 (27.7%), and 235 (21.2%) patients had stage I, II, III, and IV disease, respectively. There were 80 (7.2%) cases of PD, with P1 (2.5%), P2 (2.3%), and P3 (2.3%). The median level of CEA was 3.5 ng/ml (range, 0.1-5633), and that of CA19-9 and CA125 were 14.3 U/ml (0.1-53252) and 12 U/ml (0.1-1503), respectively.
Table 2shows the correlation of PD and clinicopathological factors. In univariate analysis, presence of PD (PD+) correlated with younger age (<65 years), right side colon location, mucinous or signet ring cell carcinoma, poor differentiation, LVI+, high CEA, and high CA125 levels.
| Demographic characteristics | PD (-) n=1029 | Percentage (%) | PD (+) n=80 |
Percentage (%) | p-value |
|---|---|---|---|---|---|
| Sex, n (%) | |||||
| Male | 597 | 58% | 39 | 48.8% | 0.11 |
| Female | 432 | 42% | 41 | 51.2% | |
| Age, n (%) | |||||
| <65 years | 451 | 43.8% | 52 | 65% | <0.001 |
| >=65 years | 578 | 56.2% | 28 | 35% | |
| Tumor site, n (%) | |||||
| Right side colon | 339 | 32.9% | 35 | 43.8% | <0.01 |
| Left side colon | 435 | 42.3% | 35 | 43.8% | |
| Rectum | 255 | 24.8% | 10 | 12.5% | |
| Histology, n (%) | |||||
| Adenocarcinoma | 982 | 95% | 69 | 86.2% | <0.001 |
| Mucinous adenocarcinoma, signet ring cell carcinoma | 47 | 5% | 11 | 13.8% | |
| Differentiation, n (%) | |||||
| Well, moderate differentiation | 956 | 93% | 64 | 80% | <0.001 |
| Poorly differentiation, Undifferentiation | 73 | 7% | 16 | 20% | |
| Lymphovascular invasion, n (%) | |||||
| Negative | 822 | 79.9% | 32 | 40% | <0.001 |
| Positive | 207 | 20.1% | 48 | 60% | |
| CEA (ng/ml), median (range) | 3.3 | (0.1-5663) | 10.3 | (1.1-3267) | 0.03* |
| CA19-9 (U/ml), median (range) | 13.2 | (0.1-11240) | 54.4 | (0.7-53252) | 0.07* |
| CA125 (U/ml), median (range) | 11.5 | (0.1-1036) | 35.7 | (2.3-1503) | 0.001* |
Note: CEA=Carcinoembryonic Antigen; CA19-9: Carbohydrate Antigen 19-9; CA125: Carbohydrate Antigen 125
*p-values were analyzed from the comparison of the mean of the levels of each tumor maker
Table 2: Correlation of Peritoneal Dissemination (PD) and clinicopathological factors
Table 3lists the diagnostic parameters of the three markers. CEA had the highest sensitivity (0.69); however, it had the lowest specificity (0.62) and lowest diagnostic accuracy (0.63) for detecting PD. However, CA125 had the lowest sensitivity (0.51), the highest specificity (0.89), and the highest diagnostic accuracy (0.86). AUC analysis revealed a trend of difference among them (p=0.051). Post hoc analysis of AUC revealed that CA125 was a significantly better predictor compared to CEA (p=0.02), and there was no significant difference between the other two matching comparisons.
| Diagnostic parameters | CEA (5.0 ng/ml) | CA19-9 (37.0 U/ml) | CA125 (35.0 U/ml) | p-value |
|---|---|---|---|---|
| Sensitivity | 0.69 | 0.59 | 0.51 | 5.10% |
| Specificity | 0.62 | 0.78 | 0.89 | |
| Diagnostic accuracy* | 0.63 | 76.00% | 0.86 | |
| AUC | 0.7 | 74.00% | 0.79 |
Note: *Diagnostic accuracy=(PD(+) with positive test result+PD(-) with negative test result)/all patients tested
Table 3: Diagnosis parameters of CEA, CA19-9, and CA125 to detect PD
The levels of the three tumor markers according to the AJCC staging system are shown in Figure 1. For CEA, the levels increased significantly from stage I to IV with each advancing stage, however, with an exception between stages II and III. An abrupt increase in level was noted between stage III and IVA. Among the stage IV group, only the level of IVA was higher than IVB, and IVC was not significantly different from IVA or IVB.

Figure 1: The relationship between tumor marker levels and AJCC staging system (8th edition)
For CA19-9 also, the levels significantly increased from stage II to IVA at each advancing stage, and not so between stages I and II. An abrupt increase in level was noted between stages III and IVA. However, in the stage IV group, there was no significant difference in CA19-9 levels between the groups. Stage IVC showed a higher mean of CA19-9 levels, however, there was also a high variation of distribution. Stage IVC did not have any significant difference with any stage, even in stage I.
For CA125, there was no difference in levels between stage I and stage IVA. Stage IVB had a small increase in level of CA125, but with only a significant difference from stage I. An abrupt rise was noted between stages IVB and IVC. Stage IVC showed a significantly higher CA125 level than all stages, from stage I to stage IVB.
The levels of CA125 with respect to the grade of PD based on the JCCRS criteria are shown in Figure 2. As PD progressed, the level of CA125 increased; however, it showed a small drop at P2. CA125 levels of P1 were higher than those of P0, and the CA125 levels of P3 were significantly higher than those of the other grades. However, the levels of CA125 of P2 were not higher than those of P0.
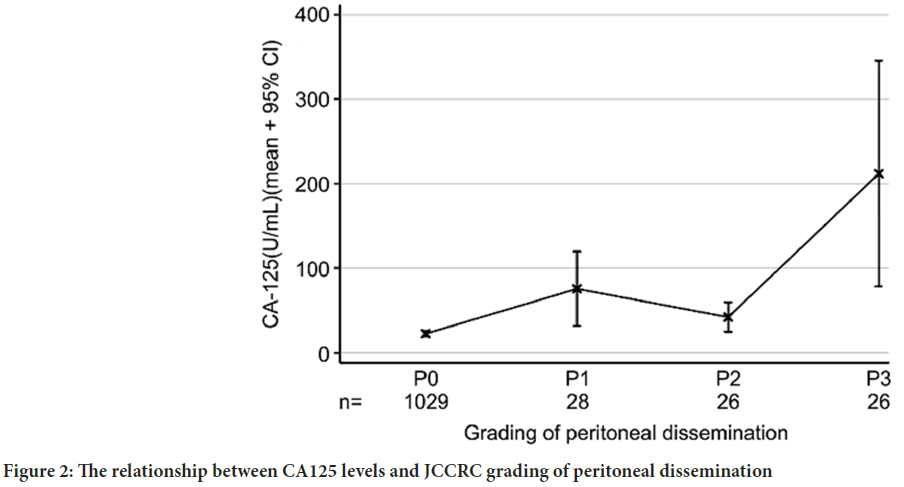
Figure 2: The relationship between CA125 levels and JCCRC grading of peritoneal dissemination
Discussion
With the aim of detecting PD using tumor markers in a large population, our study showed that CA125 has the highest diagnostic accuracy and highest specificity, but lowest sensitivity, compared to the other two markers. In contrast, CEA had the highest sensitivity, but the lowest specificity and diagnostic accuracy. CA19-9 is always ranked as the middle one among the three markers used for diagnostic analysis. This result is consistent with our previous suggestion that CA125 should be included in the evaluation of PD (Huang CJ, et al., 2016). In particular, our results demonstrate the potential of detecting the worst prognosis group, i.e., M1c, stage IVC.
Two studies reported better PD detection using CA19-9 than CEA (Takakura Y, et al., 2015; Kaneko M, et al., 2017). However, applying similar analysis methods in this study did not show that CA19-9 had better potential to detect PD compared to CEA. CA125 was the best tumor marker among the others analyzed. These studies also revealed that an elevated preoperative serum CA19-9 level in patients with CRC was found to be a predictor of cancer progression and was associated with peritoneal recurrence after curative tumor resection surgery (Takakura Y, et al., 2015; Kaneko M, et al., 2017; Yu H, et al., 2013). In this study, we focused on the relationship between tumor makers and the presence of PD, but not on tumor prognosis or peritoneal recurrence, which we may evaluate in a future study.
CEA is very sensitive in detecting distant metastases but cannot differentiate PD from others. Intriguingly, the performance of CA19-9 in the analysis of diagnostic parameters is always between these two markers. The curve pattern of CA19-9 levels is similar to that of CEA, and both have a similar abrupt rise between stages III and IV. This rise matches the severity of the disease and is very likely to indicate the total tumor burden. Unlike these two markers, CA125 levels did not rise significantly until stage IVC. This might be because CA125 is mainly released from the irritated peritoneum or gynecological organs involved (Kabawat SE, et al., 1983), instead of the colorectal tumor tissue, although CA125 has been reported to be detected in cancer tissues of the stomach and colon (Streppel MM, et al., 2012). Based on the curve pattern of the AJCC staging system, we postulate that CA125 released into the serum is not from the tumor tissue, because it does not match the total tumor burden. Based on the PD grading of JSC-CR, CA125 levels roughly correlated with progressing grades, except for P2. This implies that, although CA125 does not correlate with the total tumor burden, it does correlate with the severity of PD.
With the help of a larger population study, the diagnostic parameters showed some differences from our previous study on CEA and CA125 (Huang CJ, et al., 2016). The sensitivity of CEA to detect PD dropped from 0.75 to 0.69, and that of CA125 dropped from 0.61 to 0.51. However, the specificities of CEA (0.63 vs.0.62) and CA125 (0.90 vs.0.89) were similar. The high specificity of CA125 was confirmed in this study; however, its sensitivity was relatively low.
PD is associated with a poor prognosis and is a common process in the natural course of CRC (Jayne DG, et al., 2002; Chu DZ, et al., 1989). The incidence of PD was 7.2% in our study, which is comparable to that reported in other studies (Jayne DG, et al., 2002; Stewart JH, et al., 2005; Klaver YL, et al., 2012). Patients with colorectal PD had a lower survival rate than those without PD (Klaver YL, et al., 2012). A poor prognosis of PD results in a poor quality of life, including refractory malignant ascites, intractable abdominal pain, and irreversible intestinal obstruction (Kaneko M, et al., 2017). Eventually, the clinical course progressing gradually to mortality is irreversible. The existence and extent of PD lead to different treatment policies. Multimodality treatments with CRS and HIPEC were considered in selected patients and favored in the early stages of PD. However, a certain number of PD cases were first diagnosed during tumor resection surgery due to the lack of specific symptoms and varying sensitivities of imaging studies, especially for those who had early stage colorectal PD (Klaver YL, et al., 2012; Chu DZ, et al., 1989; Franiel T, et al., 2009; Marin D, et al., 2010).
The PRODIGE 7 trial (Quénet F, et al., 2021) questioned the efficacy of Hyperthermic Intraperitoneal Chemotherapy (HIPEC), as there was no significant difference in OS after the addition of HIPEC to CRS from CRS alone for patients with PD. However, for patients with a PCI (Peritoneal Cancer Index) (Huang CJ, et al., 2016; Byrd DR, et al., 2017; Weiser MR, 2018; Japanese Society for Cancer of the Colon and Rectum, 2019; Takakura Y, et al., 2015), the addition of HIPEC to CRS still showed some benefit in the median OS and relapse-free survival in the subgroup analysis. In addition, preclinical studies (Löffier MW, et al., 2019; Ubink I, et al., 2019), provided some evidence that current HIPEC regimens were insufficient to eradicate colorectal PD, and the selected drugs and methods of HIPEC could influence the study results. A more standardized regimen of intraperitoneal chemotherapy remains to be determined. The National Comprehensive Cancer Network (NCCN) guidelines (NCCN, 2021) suggest that complete CRS and/or intraperitoneal chemotherapy for selected patients with limited peritoneal metastases can be arranged in established centers with experienced physicians. Confirming the presence of PD remains a crucial part of preoperative planning. In this study, the level of CA125 had the best diagnostic ability to predict PD among the three tumor makers.
Conclusion
Our study has some limitations. First, this was a single-center study with a retrospective design. Second, the sensitivity and specificity of imaging were not analyzed in this study. Third, we used the JCCRC criteria to evaluate PD grading instead of the more specific PCI.
In conclusion, among the three markers analyzed to detect PD, CA125 had the highest diagnostic accuracy, highest specificity, and lowest sensitivity. Preoperative CA125 levels might pose a better chance of identifying stage IVC. Furthermore, a better preoperative treatment policy may be implemented.
References
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018; 68(6): 394-424.
[Crossref] [Google Scholar] [Pubmed]
- Jayne DG, Fook S, Loi C, Seow‐Choen F. Peritoneal carcinomatosis from colorectal cancer. Br J Surg. 2002; 89(12): 1545-1550.
[Crossref] [Google Scholar] [Pubmed]
- Stewart JH, Shen P, Levine EA. Intraperitoneal hyperthermic chemotherapy for peritoneal surface malignancy: Current status and future directions. Ann Surg Oncol. 2005; 12: 765-777.
[Crossref] [Google Scholar] [Pubmed]
- Klaver YL, Simkens LH, Lemmens VE, Koopman M, Teerenstra S, Bleichrodt RP, et al. Outcomes of colorectal cancer patients with peritoneal carcinomatosis treated with chemotherapy with and without targeted therapy. Eur J Surg Oncol. 2012; 38(7): 617-623.
[Crossref] [Google Scholar] [Pubmed]
- Stillwell AP, Ho YH, Veitch C. Systematic review of prognostic factors related to overall survival in patients with stage IV colorectal cancer and unresectable metastases. World J Surg. 2011; 35: 684-692.
[Crossref] [Google Scholar] [Pubmed]
- Spratt JS, Adcock RA, Sherrill W, Travathen S. Hyperthermic peritoneal perfusion system in canines. Cancer Res. 1980; 40(2): 253-255.
[Google Scholar] [Pubmed]
- Sugarbaker PH. Surgical responsibilities in the management of peritoneal carcinomatosis. J Surg Oncol. 2010; 101(8): 713-724.
[Crossref] [Google Scholar] [Pubmed]
- Mahmoud AM, Ismail YM, Hussien A, Debaky Y, Ahmed IS, Mikhael HS, et al. Peritoneal carcinomatosis in colorectal cancer: Defining predictive factors for successful cytoreductive surgery and hyperthermic intraperitoneal chemotherapy-A pilot study. J Egypt Natl Canc Inst. 2018; 30(4): 143-150.
[Crossref] [Google Scholar] [Pubmed]
- National Comprehensive Cancer Network (N.C.C.N). Colon Cancer Version 2. 2021.
- Yang SH, Lin JK, Lai CR, Chen CC, Li AF, Liang WY, et al. Risk factors for peritoneal dissemination of colorectal cancer. J Surg Oncol. 2004; 87(4): 167-173.
[Crossref] [Google Scholar] [Pubmed]
- Huang CJ, Jiang JK, Chang SC, Lin JK, Yang SH. Serum CA125 concentration as a predictor of peritoneal dissemination of colorectal cancer in men and women. Medicine. 2016; 95(47): e5177.
[Crossref] [Google Scholar] [Pubmed]
- Byrd DR, Brookland RK, Washington MK, Gershenwald JE, Compton CC, Hess KR, et al. AJCC cancer staging manual. New York: Springer. 2017.
- Weiser MR. AJCC 8th edition: Colorectal cancer. Ann Surg Oncol. 2018; 25: 1454-1455.
[Crossref] [Google Scholar] [Pubmed]
- Japanese Society for Cancer of the Colon and Rectum. Japanese classification of colorectal, appendiceal, and anal carcinoma: The 3rd English Edition [Secondary Publication]. J Anus Rectum Colon. 2019; 3(4): 175-195.
[Crossref] [Google Scholar] [Pubmed]
- Takakura Y, Ikeda S, Imaoka Y, Urushihara T, Itamoto T. An elevated preoperative serum carbohydrate antigen 19‐9 level is a significant predictor for peritoneal dissemination and poor survival in colorectal cancer. Colorectal Dis. 2015; 17(5): 417-425.
[Crossref] [Google Scholar] [Pubmed]
- Kaneko M, Ishihara S, Murono K, Sasaki K, Otani K, Yasuda K, et al. Carbohydrate antigen 19-9 predicts synchronous peritoneal carcinomatosis in patients with colorectal cancer. Anticancer Res. 2017; 37(2): 865-870.
[Crossref] [Google Scholar] [Pubmed]
- Yu H, Son GM, Joh YG. The clinical significance of preoperative serum levels of carbohydrate antigen 19-9 in colorectal cancer. J Korean Surg Soc. 2013; 84(4): 231-237.
[Crossref] [Google Scholar] [Pubmed]
- Kabawat SE, Bast Jr RC, Bhan AK, Welch WR, Knapp RC, Colvin RB. Tissue distribution of a coelomic-epithelium-related antigen recognized by the monoclonal antibody OC125. Int J Gynecol Pathol. 1983; 2(3): 275-285.
[Crossref] [Google Scholar] [Pubmed]
- Streppel MM, Vincent A, Mukherjee R, Campbell NR, Chen SH, Konstantopoulos K, et al. Mucin 16 (cancer antigen 125) expression in human tissues and cell lines and correlation with clinical outcome in adenocarcinomas of the pancreas, esophagus, stomach, and colon. Hum Pathol. 2012; 43(10): 1755-1763.
[Crossref] [Google Scholar] [Pubmed]
- Chu DZ, Lang NP, Thompson C, Osteen PK, Westbrook KC. Peritioneal carcinomatosis in nongynecologic malignancy. A prospective study of prognostic factors. Cancer. 1989; 63(2): 364-367.
[Crossref] [Google Scholar] [Pubmed]
- Franiel T, Diederichs G, Engelken F, Elgeti T, Rost J, Rogalla P. Multi-detector CT in peritoneal carcinomatosis: Diagnostic role of thin slices and multiplanar reconstructions. Abdom Imaging. 2009; 34: 49-54.
[Crossref] [Google Scholar] [Pubmed]
- Marin D, Catalano C, Baski M, Di Martino M, Geiger D, Di Giorgio A, et al. 64-Section multi-detector row CT in the preoperative diagnosis of peritoneal carcinomatosis: Correlation with histopathological findings. Abdom Imaging. 2010; 35: 694-700.
[Crossref] [Google Scholar] [Pubmed]
- Quénet F, Elias D, Roca L, Goéré D, Ghouti L, Pocard M, et al. Cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy versus cytoreductive surgery alone for colorectal peritoneal metastases (PRODIGE 7): A multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2021; 22(2): 256-266.
[Crossref] [Google Scholar] [Pubmed]
- Löffler MW, Seyfried N, Burkard M, Oswald B, Tolios A, Yurttas C, et al. Short-term oxaliplatin exposure according to established Hyperthermic Intraperitoneal Chemotherapy (HIPEC) protocols lacks effectiveness in vitro and ex vivo. bioRxiv. 2019: 709055. [Crossref]
- Ubink I, Bolhaqueiro AC, Elias SG, Raats DA, Constantinides A, Peters NA, et al. Organoids from colorectal peritoneal metastases as a platform for improving hyperthermic intraperitoneal chemotherapy. Br J Surg. 2019; 106(10): 1404-1414.
[Crossref] [Google Scholar] [Pubmed]
Author Info
Nai-Lu Lin1,3, Shih-Ching Chang2,3, Jeng-Kae Jiang2,3 and Shung-Haur Yang1,2,3*2Department of Surgery, Division of Colon and Rectal Surgery, Taipei Veterans General Hospital, Taipei, Taiwan
3Department of Medicine, National Yang Ming Chiao Tung University, Hsinchu, Taiwan
Citation: Systematic Review Pharmacy Lin NL: Preoperative Serum CA125 Levels for Predicting Peritoneal Dissemination of Colorectal Cancer
Received: 11-Dec-2023 Accepted: 25-Dec-2023 Published: 01-Jan-2024, DOI: 10.31858/0975-8453.14.12.762-766
Copyright: This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
ARTICLE TOOLS
- Dental Development between Assisted Reproductive Therapy (Art) and Natural Conceived Children: A Comparative Pilot Study Norzaiti Mohd Kenali, Naimah Hasanah Mohd Fathil, Norbasyirah Bohari, Ahmad Faisal Ismail, Roszaman Ramli SRP. 2020; 11(1): 01-06 » doi: 10.5530/srp.2020.1.01
- Psychometric properties of the World Health Organization Quality of life instrument, short form: Validity in the Vietnamese healthcare context Trung Quang Vo*, Bao Tran Thuy Tran, Ngan Thuy Nguyen, Tram ThiHuyen Nguyen, Thuy Phan Chung Tran SRP. 2020; 11(1): 14-22 » doi: 10.5530/srp.2019.1.3
- A Review of Pharmacoeconomics: the key to “Healthcare for All” Hasamnis AA, Patil SS, Shaik Imam, Narendiran K SRP. 2019; 10(1): s40-s42 » doi: 10.5530/srp.2019.1s.21
- Deuterium Depleted Water as an Adjuvant in Treatment of Cancer Anton Syroeshkin, Olga Levitskaya, Elena Uspenskaya, Tatiana Pleteneva, Daria Romaykina, Daria Ermakova SRP. 2019; 10(1): 112-117 » doi: 10.5530/srp.2019.1.19
- Dental Development between Assisted Reproductive Therapy (Art) and Natural Conceived Children: A Comparative Pilot Study Norzaiti Mohd Kenali, Naimah Hasanah Mohd Fathil, Norbasyirah Bohari, Ahmad Faisal Ismail, Roszaman Ramli SRP. 2020; 11(1): 01-06 » doi: 10.5530/srp.2020.1.01
- Manilkara zapota (L.) Royen Fruit Peel: A Phytochemical and Pharmacological Review Karle Pravin P, Dhawale Shashikant C SRP. 2019; 10(1): 11-14 » doi: 0.5530/srp.2019.1.2
- Pharmacognostic and Phytopharmacological Overview on Bombax ceiba Pankaj Haribhau Chaudhary, Mukund Ganeshrao Tawar SRP. 2019; 10(1): 20-25 » doi: 10.5530/srp.2019.1.4
- A Review of Pharmacoeconomics: the key to “Healthcare for All” Hasamnis AA, Patil SS, Shaik Imam, Narendiran K SRP. 2019; 10(1): s40-s42 » doi: 10.5530/srp.2019.1s.21
- A Prospective Review on Phyto-Pharmacological Aspects of Andrographis paniculata Govindraj Akilandeswari, Arumugam Vijaya Anand, Palanisamy Sampathkumar, Puthamohan Vinayaga Moorthi, Basavaraju Preethi SRP. 2019; 10(1): 15-19 » doi: 10.5530/srp.2019.1.3






