Research Article - (2022) Volume 13, Issue 3
Production and Escalation of L-Lysine via Bacterial Fermentation Utilizing Streptococcus sp
Shanzay Saleem*, Mehreen Sarfraaz, Zohaib Ahmad, Nuzhat Munawar, Zeeshan Rehman and Saira AhmadAbstract
Background and aim: The importance of L-lysine as an essential amino acid in the nutrition of human beings has made it desirable supplement of the diet in recent years. It can be produced in different ways among them fermentation is the most economical and practical means of producing lysine. In this method low temperature, low pressure and low-cost carbon sources are used and a biological form of lysine (L-lysine) is produced. Methods: In the present study, the production of L-lysine was achieved through fermentation developed from locally isolated bacterial strains. In total, twenty-nine (29) bacterial strains were isolated and tested using paper chromatographic technique. Six different parameters for optimization were scrutinized for improved bacterial growth and significant yield of lysine was obtained using selected strains. Results: For Streptococcus sp. molasses media with vitamins (w) formed 24.4 g/L, 40°C generated 24.4 g/L, addition of 1 mM solution of metal ion (mg) yielded 20.4 g/L, pH 6.5 delivered 6 g/L, fermentation period of 96 hours engendered 24.4 g/L, and 0.3 mL of inoculum results in 9.2 g/L of lysine. Conclusion: Laboratory scale production of L-lysine was carried out using 1 L Erlenmeyer flask. For Streptococcus sp. 23.4 g/L of lysine was produced after optimized conditions.
Keywords
Lysine, Fermentation, Bacteria, Streptococcus sp., Optimization, Media
Introduction
Amino acids are synthesized through microorganisms from the last 50 years. The most extensive formed amino acid (roughly 900,000 tons each year) occurs as L-glutamic acid, trailed by L-lysine (420,000 tons each year) as well as DL-methionine (350,000 tons each year) whiles the rest of the amino acids trail behind (Theodora T and Bustard MT, 2005). L-lysine is considered as the chief restraining amino acid that every single known cereal morsel has, therefore it takes a greater aptitude for increasing the protein content of cereal-based foods mostly around developing countries due to their huge reliance on cereal crops. It is also used for production of antibodies, enzymes and hormones inside the human body as well synthesis of antihypertensive agents and neutralizer for analgesic (Sadoul K, et al., 2008). Amino acid produced via fermentation has currently touched in a phase where it is frolicking an indispensable part for the cradle of usual amino acids at several manufacturing levels.
Micro-organisms that are already stated to produce L-lysine contain, ‘Bacillus megaterium (Ekwealor IA and Obeta JAN, 2005), Brevibacterium linens, Streptomyces albulus IFO (Shih IL and Shen MH 2006), Brevibacterium flavum (Irshad S, et al., 2015), M. methylophilis (Ishikawa, et al., 2008), B. lactofermentum (Goodfellow M and Schaal KP, 1979), B. subtilis and Bacillus laterosporus (Umerie SC, et al., 2006). As per nitrogen cradles, numerous inorganics plus organic salts as well as compounds like ammonium salts along with other parallel compounds, urea, pure proteolytic organic affluences like peptone plus casein hydrolysate plus yeast extract plus corn steep liquor plus soybean protein hydrolysate, plus numerous other extricates of vegetal as well as animal tissues may possibly be used (Nasab MS, et al., 2007). The final outcome is usually staged as a salt like lysine-HCl (lysine mono-chloridrate) (Junior LAL, et al., 2016) Nonetheless; it can similarly be staged as L-lysine liquid articulations or in granulated configuration. The present work is planned to maximize yields of free lysine obtained in a culture broth by using bacterial strain recover from various soil and water samples.
Literature Review
Isolation of bacteria
Techniques of microbial isolation undergo drastically changed throughout the past 50 years, as of a labor perspective through increasing mechanization, as well as in concern to the technology embroiled, and hence promptness and accuracy. The isolation of bacterial strains was done stepwise as:
• Preliminary screening test
• Final screening test
• Preliminary screening test
Preliminary screening was encompassed to restraint the presence of L-lysine by the amassed bacteria. The test consists of following parameters:
• Collection of samples
• Isolation medium
• Preparation of medium plates
• Serial dilution method
• Medium for sub-culturing of bacteria
• Streak plate method
• Screening medium for L-lysine production
• Cultivation
• Detection of L-lysine
• Collection of samples
Fifteen different samples were collected from the adjacent areas of Pakistan Council of Scientific and Industrial Research (PCSIR) laboratories complex Lahore during 2016-2017. The sample contains soil, soil with bird feces, soil with sanitary leakage, bird feces, sewage water, and waste water, soil with vegetation, tap water as well as drain water. All the samples were labeled and weighed as 5 g for soil samples and 5 mL for water samples in test tubes and petri plates respectively.
Isolation medium
A modified medium for the isolation of lysine producing bacteria (e.g., Corynebacterfium glutamicum) (Hussain A, et al., 2015; Xafenias N, et al., 2017; Rastegari H, et al., 2013) was employed having the composition as described by Nasab, et al. (Nasab MS, et al., 2007). Quantities in solution consumed were: Glucose (5 g), Peptone (2.5 g), Yeast extracts (2.5 g), NaCl (0.6 g), MgSO4.7H2O (0.7 g), MnSO4.H2O (0.02 g), K2HPO4 0.2 g, KH2PO4 (0.2 g), CaCO3 (2 g), (NH4)2SO4 (3 g), urea (1.2 g), agar (3.4 g). All these ingredients were accurately weighed and dissolved with the assistance of steady rate of stirring in 500 mL Pyrex beaker using distilled water. More distilled water was supplemented to the relevant mixture and the final volume was made to 250 mL. To facilitate the process of dis-solvation of agar, the beaker containing the medium was placed on the hot plate and was supplied with high temperature until the apparent light beige solution was formed. The pH was adjusted to 7.0 by using conc. HCl plus pure NaOH solution. Ultimately, the medium was dispensed in the 500 mL conical flask and then sterilized at 121°C temperature and 15 lb. pressure for 15 minutes.
Preparation of medium plates
The petri plates inside which medium had to be dispensed were subjected to autoclave for 15 minutes at 121°C temperature and 15 lb. pressure followed by thorough washing. However, petri plates could also be sterilized by heating them in oven overnight at 180°C. The 15 mL of medium was allocated to every petri plate and their lids were positioned on them. Subsequently the plates were preserved with the cling film and were stored in the uncontaminated location at room temperature until consumption. Still, it was recommended to employ the prepared petri plates within the time extent of 2-3 days to attain the correct affirmative consequences.
Serial dilution method
A serial dilution is basically a chain of sequential dilutions tapped to reduce an intense culture of cells into a more exploitable concentration. Each dilution can reduce the attentiveness of bacteria by an identifiable amount. So, by scheming the total dilution ended the entire chain, it is feasible to know how much bacteria you commenced with. 1 g or 1 mL of each sample was added to 9 mL sterile distilled water and a number of six-fold dilutions were prepared in the same diluent. Aliquots 0.1 mL of 10-6 diluted sample suspension was added to the agar plates prepared from the isolation medium and distributed evenly over the surface with the aid of sterile glass spreading rod. Following incubation at 37°C for 24 hours, those plates which contained the desired bacterial well isolated colonies were selected as the source of culture to be evaluated for the production of L-lysine.
Medium for sub-culturing of bacteria
Single colonies of the desired bacteria were then cultured on the nutrient agar medium. The medium was prepared with the American Public Health Association (APHA) standards and the ingredients were utilized according to Cruick-Shank, et al. The amount used (g/L) were: (Nutrient broth 8, Agar 15, Distilled water to raise volume up to 1 L). 8 g powdered nutrient broth was accurately weighed and dissolved with the assistance of steady rate of stirring in 1 L Pyrex beaker using 1 L distilled water. Eventually, 15 g agar was supplemented to the relevant mixture and the final volume was made to 1 L. To facilitate the process of dis-solvation of agar, the beaker containing the medium was placed on the hot plate and was supplied with high temperature until the apparent light beige solution was formed. Ultimately, the medium was dispensed in the 1 L conical flask and was autoclaved for 15 minutes at 121°C temperature and 15 lb. pressure. Plates were poured as described previously.
Streak plate method
The streaking is completed using a disinfected tool, like a cotton swab and normally an inoculation loop or needle. The inoculation loop or needle is first sterilized via passing it over a flame. Once the loop is chill, it is immersed into an inoculum like a broth or a petri plate containing the desired bacterial colony. The inoculation loop is subsequently dragged through the surface of culture plate containing agar back and forth wearing a zigzag motion till approximately 30% of culture plate has been obscured. The loop afterwards is re-sterilized as well as the plate is twisted 90 degrees. Beginning in the earlier streaked sector, the loop is hauled through these two to three stints continuing the zigzag configuration. The procedure is thenceforth repeated once more ensuing cautious to not tad the formerly streaked areas. Each spell the loop gathers rarer and rarer bacteria until it assembles just lone bacterial cells that have ability to grow into a cluster. The plate must show the heaviest progression in the first unit. The second unit will have less progression and a few sequestered colonies, while the ultimate unit will have the minimum amount of progression and many quarantined colonies.
Screening medium for L-lysine production
For the production of L-lysine, isolation medium with the exception of agar was prepared. All those ingredients were accurately weighed and dissolved to make the final volume up to 250 mL with distilled water in a 500 mL Pyrex beaker. The pH was adjusted to 7.1 by using conc. HCl plus pure NaOH solution. Ultimately, 10 mL of the medium was dispensed in the test tubes and then sterilized at 121°C temperature and 15 lb. pressure for 15 minutes.
Cultivation
Each test tube was inoculated with a loop-full of desired bacteria from each pure culture plate and incubated with shaking in a horizontal shaking water bath at 100 rpm maintained at 37°C for 48 and 72 hours respectively.
Detection of L-lysine
For the detection of L-lysine, paper chromatographic technique of Momose and Takagi (Momose H and Takagi T, 1978) was employed in the culture broth. This was run on a Whattman filter paper No. 1. Sample spots were applied using a sterile capillary tube, then heat-fixed on a chromatogram following a relevant sequence (Figure 1). It was cogitated as the stationary phase. The solvent systems applied included n-butanol: acetic acid: water (1: 2: 4, v/v). It was also contemplated as the mobile phase. Basically, amino acids have no color. So, all of these measures need to be conceded out "blind", plus the results were perceived when a revealing mediator (e.g., ninhydrin) was applied on the subsequent chromatogram. Hence, the spots were visualized by spraying with a solution of 0.5% ninhydrin in butanol. For each spot, calculated the Rf value (Rf means relative to front) by using the following formula: Rf value=distance moved by spot/distance moved by solvent front.
Figure 1:Chromatogram/TLC plate
Final screening test
Final screening test comprise of subsequent parameters-
1. Purification and maintenance of culture
2. Production medium
3. Cultivation
4. Identification of L-lysine
Purification and maintenance of culture: Organisms flaunting positive L-lysine production by fermentation were further purified by reiterated cultivation plus sub-culturing using streak plate method and maintained at 4°C on agar slants prepared by using nutrient agar medium.
Production medium: The composition and pH of the production medium was similar to that of the screening medium for lysine production. Aliquots 20 mL of this solution were decanted in 50 mL of Erlenmeyer flasks and sterilized by autoclaving for 15 min at 121°C temperature and 15 lb. pressure.
Cultivation: Each flask was inoculated with two loop-full of organism from each slant culture and incubated within a horizontal shaking water bath at 100 rpm maintained at 37°C for 24 hours. The cultivation was executed for those selected bacteria that revealed ameliorate results in the preliminary screening tests.
Identification of L-lysine: Ascending Thin Layer Chromatography (TLC) described by Nasab (Nasab, et al., 2007) was used for the detection of L-lysine in the culture broth. Spots on the TLC plate were concocted, following the same pattern of assembling chromatogram for paper chromatographic technique illustrated in the detection of L-lysine during preliminary screening. The solvent systems applied were also conceived as mentioned earlier. When the sample was put on the plate, the respective solvent or solvent amalgam (renowned as the mobile phase) strained up the plate by means of capillary action. Since dissimilar analytes ascend the respective TLC plate at altered rates, separation was achieved. L-Lysine didn't confer a strong positive response with ninhydrin and the color of spots emerged when heated at 60°C on a hot plate. For each spot appear, reckoned the Rf value by means of the formula.
Naming of bacteria
Naming of PCSIR-NL-42 was primarily based on the taxonomic comparison. The characteristic morphological, cultural, bio-chemical, physiological and chemical properties were taken into consideration (Bergey DH, et al., 1986; Bergey DH, et al., 1994; Goodfellow and Schaal, 1979).
Escalation of L-lysine production
In comparison, escalation means trying to accomplish the highest or supreme result or outcome deprived of the regard to cost and expenditure. Different parameters studied for escalation included different substrate ranges, different temperature ranges, different metal ions concentration (mM), different pH ranges, different incubation periods and different inoculum sizes.
Results
Production and escalation of L-lysine via bacterial fermentation utilizing Streptococcus sp. were conceded in Food and Biotechnology Research Centre (FBRC), Lahore. The rampant investigation was consummate and deliberated scrupulously.
Isolation of bacterial strains
For the isolation of bacteria fifteen different samples were collected from the adjacent areas of Pakistan Council of Scientific and Industrial Research (PCSIR) laboratories complex Lahore. A modified medium was employed having the composition as described by Nasab (Nasab, et al., 2007). In total, twenty-nine (29) bacterial strains were separated and tested for the accumulation of L-lysine (Table 1). The screening test for L-lysine producing bacterial strains was carried out with the media containing carbohydrate and nitrogen source as chief ingredients supplemented with inorganic salts.
| Serial no | Lab-Id (PCSIR-NL-NO) | Source | Sample no | Intensity of spots recorded |
|---|---|---|---|---|
| 1 | 37 | Soil with vegetation | 1 | +++ |
| 2 | 38 | Soil with bird faeces | 2 | + |
| 3 | 39 | Soil | 1 | + |
| 4 | 40 | Bird faeces | 3 | + |
| 5 | 41 | Drain water | 5 | + |
| 6 | 42 | Soil with bird faeces | 7 | +++ |
| 7 | 43 | Sewage water | 6 | ++ |
| 8 | 44 | Waste water | 6 | ++ |
| 9 | 45 | Soil with sanitary leakage | 5 | +++ |
| 10 | 46 | Waste water | 2 | + |
| 11 | 47 | Drain water | 4 | + |
| 12 | 48 | Sewage water | 8 | + |
| 13 | 49 | Soil | 11 | ++ |
| 14 | 50 | Soil with vegetation | 8 | + |
| 15 | 51 | Drain water | 3 | ++ |
| 16 | 52 | Waste water | 12 | + |
| 17 | 53 | Bird feces | 10 | + |
| 18 | 54 | Soil with sanitary leakage | 4 | ++ |
| 19 | 55 | Soil with vegetation | 11 | + |
| 20 | 56 | Sewage water | 7 | + |
| 21 | 57 | Soil | 9 | + |
| 22 | 58 | Bird faeces | 10 | ++ |
| 23 | 59 | Drain water | 14 | ++ |
| 24 | 60 | Waste water | 12 | + |
| 25 | 61 | Sewage water | 9 | ++ |
| 26 | 62 | Soil with vegetation | 13 | + + |
| 27 | 63 | Tap water | 15 | + |
| 28 | 64 | Soil with sanitary leakage | 14 | ++ |
| 29 | 65 | Sewage water | 13 | + |
Note: Where (PCSIR-NL-No.) is the Pakistan Council of Scientific and Industrial Research (PCSIR); (+++) denotes high L-lysine producing strains; (++) denotes medium L-lysine producing strains; (+) denotes low L-lysine producing strains
Table 1: Screening of bacterial strains as l-lysine producers
For the detection of L-lysine, paper chromatographic technique of Momose and Takagi (Momose H and Takagi T, 1978) was employed in the culture broth. The solvent systems applied included n-butanol: acetic acid: water (1: 2: 4, v/v). The spots were visualized by spraying with a solution of 0.5% ninhydrin in butanol. The intensity of spots designated as high (+++), medium (++), and low (+) which ultimately indicated the formation of L-lysine by each isolate.
The strains showing positive results were then subjected to purification into the same medium to evaluate their L-lysine producing capability and to identify the desired bacteria. Organisms were cultivated in Erlenmeyer flask containing screening medium at 37°C for 24 hours in a horizontal shaking water bath at 100 rpm.
The qualitative estimation of L-lysine and any other amino acid so produced was checked by ascending Thin Layer Chromatography (TLC) described by Nasab et al., (2007). Rf values were measured and compared with that of authentic amino acids. Table 2 shows the Rf values obtained from the authentic amino acids.
| Serial no | Amino acids | Abbreviations | Rf values |
|---|---|---|---|
| 1 | Lysine | Lys | 0.14 |
| 2 | Glutamic acid | Glu | 0.25 |
Table 2: Rf values of the standard amino acids
The quantitative estimation of L-lysine produces by selected strains was governed by a reliable method as mentioned by Shakoori (Shakoori FR, et al., 2012). Table 3 shows the results of the quantitative estimation of L-lysine by designated bacterial strains.
| Serial No | Strain (PCSIR-NL-No) | L-lysine produced (g/L) | Standard deviation |
|---|---|---|---|
| 1 | 37 | 13.3 ± 1.27 | 2.203 |
| 2 | 41 | 10.5 ± 2.27 | 3.946 |
| 3 | 42 | 20.1 ± 3.49 | 6.057 |
| 4 | 43 | 10.4 ± 5.06 | 8.772 |
| 5 | 44 | 8.1 ± 2.55 | 4.424 |
| 6 | 45 | 15.4 ± 0.87 | 1.514 |
| 7 | 46 | 12 ± 3.02 | 5.245 |
| 8 | 47 | 6.7 ± 2.41 | 4.174 |
| 9 | 48 | 7.8 ± 1.10 | 1.908 |
| 10 | 49 | 3.9 ± 0.43 | 0.75 |
| 11 | 50 | 3.5 ± 0.67 | 1.171 |
| 12 | 51 | 6.8 ± 1.04 | 1.814 |
| 13 | 52 | 5.8 ± 1.55 | 2.69 |
| 14 | 53 | 4.3 ± 0.45e | 0.793 |
| 15 | 54 | 9.3 ± 2.18 | 3.78 |
| 16 | 55 | 6.1 ± 0.35 | 0.611 |
Table 3: Quantitative estimation of L-Lysine
Naming of bacterial strains
The most significant task of bacteriology was to identify the pathogens commencing the clinical sample so that apposite treatment can be established. There are several procedures to identify the altered type of bacteria. Results of taxonomic comparison, i.e., characteristic morphological, cultural, biochemical and physical properties of the selected strains are given in Table 4.
| Strain (PCSIR-NL-NO) | Morphological Characteristics | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 42 | Gram stain | Cell form | Pleomorphism | Capsule stain | |||||||
| Positive (+) violet | Young cultures (12 to 24 hours old) |
Old cultures (72 to 120 hours old) |
+ | + | |||||||
| Rounded Cocci, mostly single, some occurs in pairs | Composed largely or entirely of coccoid cells | ||||||||||
| Biochemical characteristics | |||||||||||
| Indole test | Methyl red test |
Catalase test |
Starch hydrolysis test |
Casein hydrolysis test | |||||||
| - | + | - | - | - | |||||||
| Cultural characteristics | |||||||||||
| Small, smooth, entire, circular, convex, pale yellow in colour | |||||||||||
| Physiological characteristics | |||||||||||
| Optimum temperature |
Oxygen requirements |
Growth after heat (80°c) |
Growth in NaCl | ||||||||
| 5% | 10% | ||||||||||
| 27-45°C | FA | - | + | - | |||||||
Note: A=Aerobes; FA=Facultative anaerobes
Table 4: Identification of bacterial strain
Escalation of L-lysine production from Streptococcus sp.
Effect of different substrates on L-lysine production (Reza OA and Arenas TL, 2017) was revealed in Table 5 while their relationship in terms of lysine concentration to substrate ranges were prearranged in Figure 2. The ardent stimulatory effect on lysine production was reached using molasses media with vitamins (w) i.e., 24.4 g/L.
| Serial no | Substrate ranges | L-lysine produced (g/L) | Standard deviation |
|---|---|---|---|
| 1 | Glucose media with vitamins (g) | 22.8 ± 0.02 | 0.028 |
| 2 | Glucose media without vitamins (G) | 10 ± 0.04 | 0.056 |
| 3 | Molasses media with vitamins (w) | 24.4 ± 0.03 | 0.042 |
| 4 | Molasses media for bacillus without shaking (b) | 12 ± 0.02 | 0.028 |
| 5 | Molasses media for bacillus with shaking (B) | 21.2 ± 0 | 0 |
| 6 | Molasses media without vitamins (m) | 12.8 ± 0.01 | 0.014 |
| 7 | Simple glucose media (o) | 16.8 ± 0.01 | 0.014 |
Note: Significance level=0.05; Error mean square=0.001; Degree of freedom=7; LSD 0.05=0.07
Table 5: Effect of different substrates on L-lysine production by Streptococcus sp.
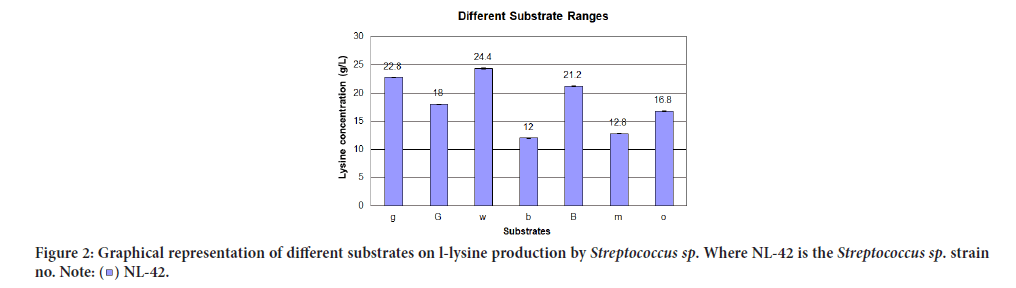
Figure 2:Graphical representation of different substrates on l-lysine production by Streptococcus sp. Where NL-42 is the Streptococcus sp. strain
no.
Influence of different temperatures on L-lysine production was mentioned in Table 6 while their relationship in terms of lysine concentration to temperature ranges were given in Figure 3. The potent stimulatory effect on lysine production was achieved at 40°C i.e., 24.4 g/L.
| Serial no | Temperature ranges | L-lysine produced (g/L) | Standard deviation |
|---|---|---|---|
| 1 | 25°C | 6.8 ± 0.01 | 0.041 |
| 2 | 27°C | 16.8 ± 0.01 | 0.041 |
| 3 | 30°C | 18 ± 0.02 | 0.028 |
| 4 | 35°C | 18 ± 0.03 | 0.042 |
| 5 | 37°C | 10.4 ± 0.01 | 0.014 |
| 6 | 40°C | 24.4 ± 0.02 | 0.028 |
| 7 | 45°C | 11.2 ± 0.01 | 0.014 |
Note: Significance level=0.05; Error mean square=6E-04; Degree of freedom=7; LSD 0.05=0.05
Table 6: Effect of different temperatures on l-lysine production by Streptococcus sp.
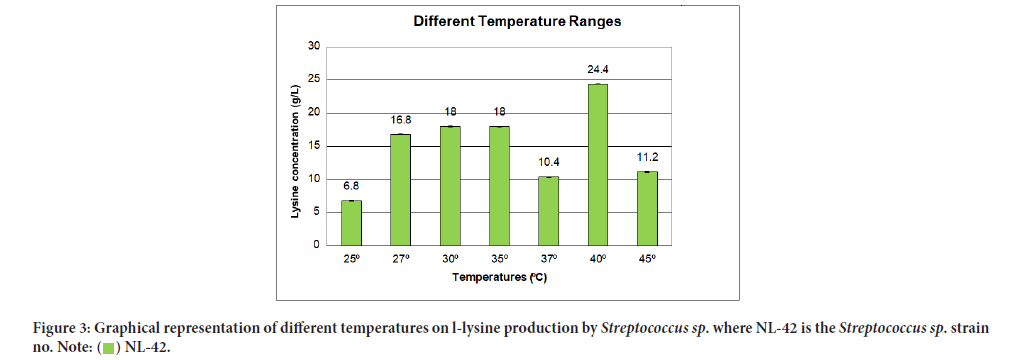
Figure 3:Graphical representation of different temperatures on l-lysine production by Streptococcus sp. where NL-42 is the Streptococcus sp. strain
no.
Upshot of different metal ions on L-lysine production was declared in Table 7 while their relationship in terms of lysine concentration to metal ions concentration was assumed in Figure 4. The powerful stimulatory effect on lysine production was succeeded by adding 1 mM solution of mg i.e., 20.4 g/L.
| Serial no | Metal ions (mm) | L-lysine produced (g/l) | Standard deviation |
|---|---|---|---|
| 1 | Ca | 12.4 ± 0.01 | 0.014 |
| 2 | Na | 11.6 ± 0.02 | 0.028 |
| 3 | K | 10.8 ± 0.02 | 0.028 |
| 4 | Mn | 9.6 ± 0.02 | 0.028 |
| 5 | Fe | 18 ± 0.01 | 0.014 |
| 6 | Mg | 20.4 ± 0.08 | 0.113 |
| 7 | Cu | 2.8 ± 0.01 | 0.014 |
Note: Significance level=0.05; Error mean square=0.002; Degree of freedom=7; LSD 0.05=0.112
Table 7: Effect of different metal ions on l-lysine production by Streptococcus sp.
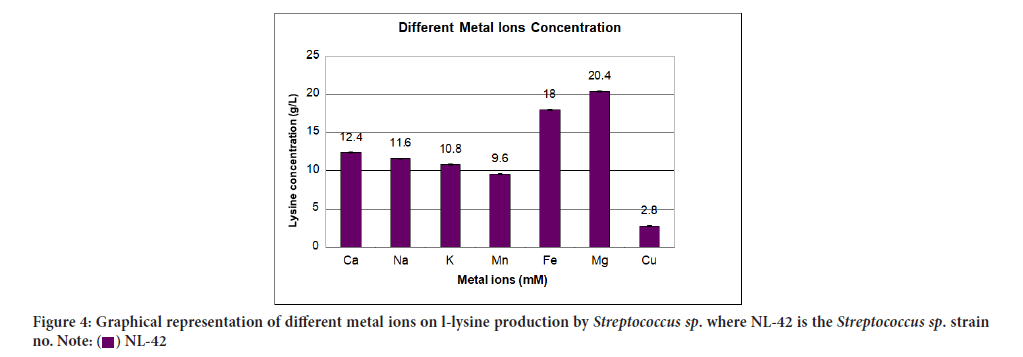
Figure 4:Graphical representation of different metal ions on l-lysine production by Streptococcus sp. where NL-42 is the Streptococcus sp. strain
no.
Consequence of different pH ranges on L-lysine production was avowed in Table 8 while their relationship in terms of lysine concentration to various pH ranges was snapped in Figure 5. The most ardent stimulatory effect on lysine production was comprehended at pH 6.5 i.e., 6 g/L.
| Serial no | PH ranges | L-lysine produced (g/L) | Standard deviation |
|---|---|---|---|
| 1 | 5.5 | 4 ± 0.02 | 0.028 |
| 2 | 6 | 2.4 ± 0.03 | 0.042 |
| 3 | 6.5 | 6 ± 0.05 | 0.07 |
| 4 | 7 | 2.8 ± 0.02 | 0.028 |
| 5 | 7.5 | 2.8 ± 0.01 | 0.014 |
| 6 | 8 | 2.4 ± 0.02 | 0.028 |
| 7 | 8.5 | 3.2 ± 0.02 | 0.028 |
Note: Significance level=0.05; Error mean square=0.001; Degree of freedom=7; LSD 0.05=0.09
Table 8: Effect of different pH ranges on l-lysine production by Streptococcus sp.
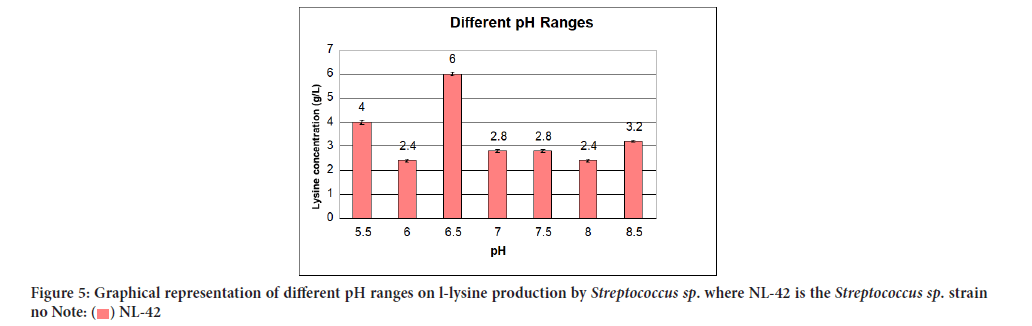
Figure 5:Graphical representation of different pH ranges on l-lysine production by Streptococcus sp. where NL-42 is the Streptococcus sp. strain
no
Outcome of different incubation periods on L-lysine production was said in Table 9 while their relationship in terms of lysine concentration to incubation periods was specified in Figure 6. The intense stimulatory effect on lysine production was succeeded after 96 hours i.e., 24.4 g/L.
| Serial no | Incubation period (hours) | L-lysine produced (g/l) | Standard deviation |
|---|---|---|---|
| 1 | 24 | 4 ± 0.02 | 0.028 |
| 2 | 48 | 2.8 ± 0.03 | 0.042 |
| 3 | 72 | 3.2 ± 0.01 | 0.014 |
| 4 | 96 | 24.4 ± 0.05 | 0.07 |
| 5 | 120 | 11.2 ± 0.03 | 0.042 |
| 6 | 144 | 1.6 ± 0.07 | 0.098 |
| 7 | 168 | 7.2 ± 0.03 | 0.042 |
| 8 | 192 | 9.2 ± 0.04 | 0.056 |
| 9 | 216 | 13.2 ± 0.06 | 0.084 |
| 10 | 240 | 10.8 ± 0.03 | 0.042 |
Note: Significance level=0.05; Error mean square=0.02; Degree of freedom=10; LSD 0.05=0.351
Table 9: Effect of different incubation periods on l-lysine production by Streptococcus sp.
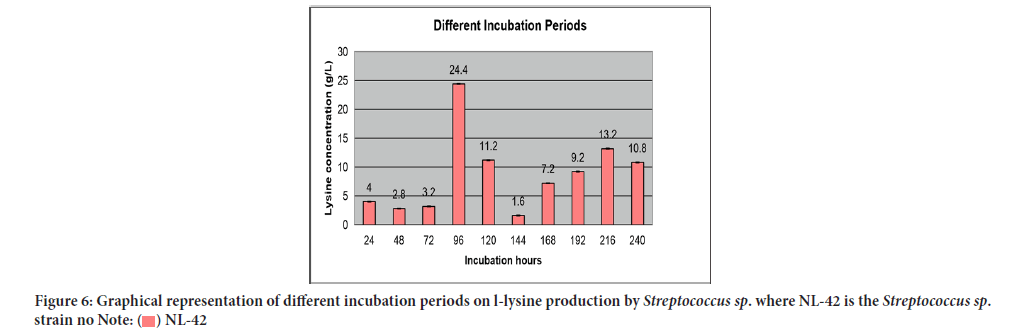
Figure 6:Graphical representation of different incubation periods on l-lysine production by Streptococcus sp. where NL-42 is the Streptococcus sp.
strain no
Result of different inoculum sizes on L-lysine production was revealed in Table 10 while their relationship in terms of lysine concentration to inoculum sizes was accorded in Figure 7. The fiercest stimulatory effect on lysine production was attained using 0.3 mL of inoculum i.e., 9.2 g/L.
| Serial no | Inoculum sizes (ml/l) | L-lysine produced (g/l) | Standard deviation |
|---|---|---|---|
| 1 | 0.1 | 6.8 ± 0.01 | 0.014 |
| 2 | 0.2 | 7.2 ± 0.03 | 0.042 |
| 3 | 0.3 | 9.2 ± 0.04 | 0.056 |
| 4 | 0.4 | 5.2 ± 0.05 | 0.07 |
| 5 | 0.5 | 6 ± 0.06 | 0.084 |
| 6 | 0.6 | 4.4 ± 0.03 | 0.042 |
| 7 | 0.7 | 4.8 ± 0.01 | 0.014 |
| 8 | 0.8 | 3.2 ± 0.03 | 0.042 |
| 9 | 0.9 | 2.8 ± 0.02 | 0.028 |
| 10 | 1 | 1.6 ± 0.03 | 0.042 |
Note: Significance level=0.05; Error mean square=0.002; Degree of freedom=10; LSD 0.05=0.108
Table 10: Effect of different inoculum sizes on l-lysine production by Streptococcus sp.
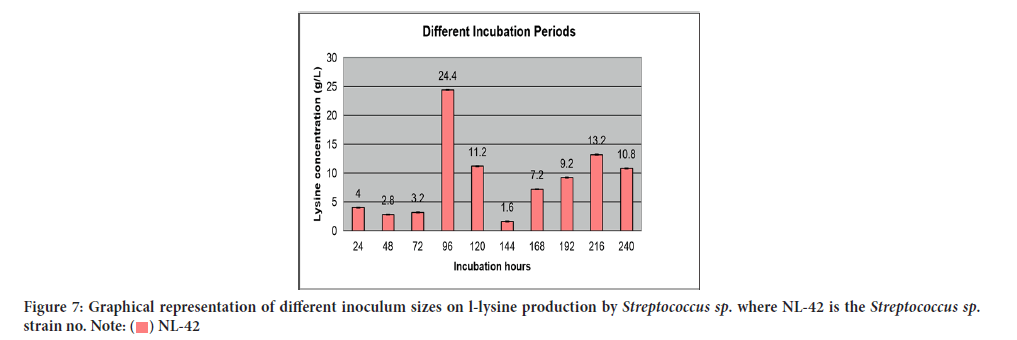
Figure 7:Graphical representation of different inoculum sizes on l-lysine production by Streptococcus sp. where NL-42 is the Streptococcus sp.
strain no.
Laboratory scale production of L-lysine was carried out using 1 L Erlenmeyer flask. Optimized conditions for each bacterium were maintained correspondingly. For Streptococcus sp. the parameters for optimization i.e., suitable substrate: (w) molasses media with vitamins-optimum temperature: 40°C-metal ion: Mg-optimum pH: 6.5-incubation period: 96 hours-inoculum size: 0.3 ml/L results in 23.4 g/L of lysine.
Discussion
The L-lysine production by fermentation was first reported by Casida and Beldwin (Casida LE and Baldwin NY, 1956), using a two-step method. In the first step, Di-Amino-Pimelic acid (DAP) was accumulated in the medium containing glycerol and corn steep liquor by a lysine requiring strain of Escherichia coli. Conversion of DAP to lysine was then accomplished with DAP-decarboxylase of Aerobacter aerogens. Subsequently, amino acid production by microorganisms continued to attract the attention of various workers throughout the world.
In the present study, the sewage and soil samples collected from different areas of Pakistan Council of Scientific and Industrial Research (PCSIR) laboratories complex Lahore indicated potential for the lysine producing bacteria. In total, twenty-nine (29) bacterial strains were separated and tested for the accumulation of L-lysine: 96.6% of total isolates. The soil samples were observed more potential than the sewage samples. The screening technique adapted in the present study was that described by Nasab, et al. with slight modification in the composition of screening medium. The cultivation and purification of all isolates on a maintenance or storage medium was carried out as it was certainly justified in the preliminary stage because their potential to synthesize the amino acid was determined.
For the detection of L-lysine, paper chromatographic technique of Momose and Takagi (Momose H and Takagi T, 1978) was employed in the culture broth. The solvent systems applied included n-butanol: acetic acid: water (1: 2: 4, v/v). The spots were visualized by spraying with a solution of 0.5% ninhydrin in butanol. Results given in Table 1 indicate the formation of L-lysine by each isolate which is according to the intensity of spots. Out of twenty-nine (29) strains, three (3) were found to produce high (+++), ten (10) as medium (++) and sixteen (16) as low (+) intensity producing L-lysine intensively.
The high intensity strains showing positive results were then subjected to purification into the same medium to evaluate their L-lysine producing capability and to identify the desired bacteria. Organisms were cultivated in Erlenmeyer flask containing screening medium at 37°C for 24 hours in a horizontal shaking water bath at 100 rpm. The qualitative estimation of L-lysine and any other amino acid so produced was checked by ascending Thin Layer Chromatography (TLC) described by Nasab, et al. The broth of each cultivated strain was applied on aluminium T.L.C. plates, 0.2 mm thick and up flow was made at 25°C. Since the free amino acids have been reported as marked hydrophilic compounds, the separation efficiency was noted with solvent systems, chloroform-methanol-17% ammonium hydroxide (2:2:1 v/v), n-butanol-acetic acid-water (4:1:1 v/v) and Phenol-water (3:1 v/v) (Brenner M, et al., 1969). However, in the present study, the solvent system n-butanol-acetic acid-water (1: 2: 4, v/v) provided best separation and was thus used throughout the whole study.
Apart from L-lysine, different amino acid like L-glutamic acid was also identified. Rf values were measured and compared with that of authentic amino acids. Table 2 shows the Rf values obtained from the authentic amino acids. The quantitative estimation of L-lysine produces by selected strains was governed by a reliable method as mentioned by Shakori, et al. Table 3 shows the results of the quantitative estimation of L-lysine by designated bacterial strains
| Serial No | Strain (PCSIR-NL-No) | L-lysine produced (g/L) | Standard deviation |
|---|---|---|---|
| 1 | 37 | 13.3 ± 1.27 | 2.203 |
| 2 | 41 | 10.5 ± 2.27 | 3.946 |
| 3 | 42 | 20.1 ± 3.49 | 6.057 |
| 4 | 43 | 10.4 ± 5.06 | 8.772 |
| 5 | 44 | 8.1 ± 2.55 | 4.424 |
| 6 | 45 | 15.4 ± 0.87 | 1.514 |
| 7 | 46 | 12 ± 3.02 | 5.245 |
| 8 | 47 | 6.7 ± 2.41 | 4.174 |
| 9 | 48 | 7.8 ± 1.10 | 1.908 |
| 10 | 49 | 3.9 ± 0.43 | 0.75 |
| 11 | 50 | 3.5 ± 0.67 | 1.171 |
| 12 | 51 | 6.8 ± 1.04 | 1.814 |
| 13 | 52 | 5.8 ± 1.55 | 2.69 |
| 14 | 53 | 4.3 ± 0.45e | 0.793 |
| 15 | 54 | 9.3 ± 2.18 | 3.78 |
| 16 | 55 | 6.1 ± 0.35 | 0.611 |
Table 3: Quantitative estimation of L-Lysine
A morphological screening test which was comprised of gram’s staining, pleomorphism, and capsule staining was first carried out. Strains identified as gram-positive were then subjected to detailed morphological, cultural, bio-chemical and physiological studies. Results of taxonomic comparison, i.e., characteristic morphological, cultural, biochemical and physical properties of the selected strains are given in Table 4.
| Strain (PCSIR-NL-NO) | Morphological Characteristics | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 42 | Gram stain | Cell form | Pleomorphism | Capsule stain | |||||||
| Positive (+) violet | Young cultures (12 to 24 hours old) |
Old cultures (72 to 120 hours old) |
+ | + | |||||||
| Rounded Cocci, mostly single, some occurs in pairs | Composed largely or entirely of coccoid cells | ||||||||||
| Biochemical characteristics | |||||||||||
| Indole test | Methyl red test |
Catalase test |
Starch hydrolysis test |
Casein hydrolysis test | |||||||
| - | + | - | - | - | |||||||
| Cultural characteristics | |||||||||||
| Small, smooth, entire, circular, convex, pale yellow in colour | |||||||||||
| Physiological characteristics | |||||||||||
| Optimum temperature |
Oxygen requirements |
Growth after heat (80°c) |
Growth in NaCl | ||||||||
| 5% | 10% | ||||||||||
| 27-45°C | FA | - | + | - | |||||||
Note: A=Aerobes; FA=Facultative anaerobes
Table 4: Identification of bacterial strain
The process intensification (especially, the media composition, addition of certain growth factors and cultural conditions) is indispensable for the scale up and commercial production of amino acids. Jyothi AN, et al., 2005 studied the characterization of glutamic-acid assembly commencing cassava arrowroot plant remains by Brevibacterium divaricatum. By using 0.7% ammonium-nitrate inside the mixture, maximum glutamate returns of 3.86%, grounded upon the mass of the remains stayed, attained at 38°C with a pH as 7.0. Also, maximum glutamate production remained chronicled using 5% inoculum mass with shakeup speed as 180 rpm. According to the present study, effect of different substrates (Table 5 and Figure 2) on lysine production is reached using molasses media with vitamins (w) i.e. 24.4 g/L. Influence of different temperatures (Table 6 and Figure 3) is achieved at 40°C i.e. 24.4 g/L. Upshot of different metal ions (Table 7 and Figure 4) is succeeded by adding 1 mM solution of Mg i.e. 20.4 g/L. Consequence of different pH ranges (Table 8 and Figure 5) is comprehended at pH 6.5 i.e. 6 g/L. Outcome of different incubation periods (Table 9 and Figure 6) is succeeded after 96 hours i.e. 24.4 g/L. Result of different inoculum sizes (Table 10 and Figure 7) is attained using 0.3 mL of inoculum i.e. 9.2 g/L. Similarly, Ezemba (Ezemba CC, et al., 2016) manifests the assembly of lysine by Microbacterium lacticum via immersed cultivation by many hydro-carbons, sweeten plus nitrogen cradles. Improving the fermented circumstances for M. lacticum under immersed media offered a met return as 2.99 mg/mL of L-lysine within the liquid fermentation following 96 hours.
| Serial no | Substrate ranges | L-lysine produced (g/L) | Standard deviation |
|---|---|---|---|
| 1 | Glucose media with vitamins (g) | 22.8 ± 0.02 | 0.028 |
| 2 | Glucose media without vitamins (G) | 10 ± 0.04 | 0.056 |
| 3 | Molasses media with vitamins (w) | 24.4 ± 0.03 | 0.042 |
| 4 | Molasses media for bacillus without shaking (b) | 12 ± 0.02 | 0.028 |
| 5 | Molasses media for bacillus with shaking (B) | 21.2 ± 0 | 0 |
| 6 | Molasses media without vitamins (m) | 12.8 ± 0.01 | 0.014 |
| 7 | Simple glucose media (o) | 16.8 ± 0.01 | 0.014 |
Note: Significance level=0.05; Error mean square=0.001; Degree of freedom=7; LSD 0.05=0.07
Table 5: Effect of different substrates on L-lysine production by Streptococcus sp.

Figure 2:Graphical representation of different substrates on l-lysine production by Streptococcus sp. Where NL-42 is the Streptococcus sp. strain
no.
| Serial no | Temperature ranges | L-lysine produced (g/L) | Standard deviation |
|---|---|---|---|
| 1 | 25°C | 6.8 ± 0.01 | 0.041 |
| 2 | 27°C | 16.8 ± 0.01 | 0.041 |
| 3 | 30°C | 18 ± 0.02 | 0.028 |
| 4 | 35°C | 18 ± 0.03 | 0.042 |
| 5 | 37°C | 10.4 ± 0.01 | 0.014 |
| 6 | 40°C | 24.4 ± 0.02 | 0.028 |
| 7 | 45°C | 11.2 ± 0.01 | 0.014 |
Note: Significance level=0.05; Error mean square=6E-04; Degree of freedom=7; LSD 0.05=0.05
Table 6: Effect of different temperatures on l-lysine production by Streptococcus sp.

Figure 3:Graphical representation of different temperatures on l-lysine production by Streptococcus sp. where NL-42 is the Streptococcus sp. strain
no.
| Serial no | Metal ions (mm) | L-lysine produced (g/l) | Standard deviation |
|---|---|---|---|
| 1 | Ca | 12.4 ± 0.01 | 0.014 |
| 2 | Na | 11.6 ± 0.02 | 0.028 |
| 3 | K | 10.8 ± 0.02 | 0.028 |
| 4 | Mn | 9.6 ± 0.02 | 0.028 |
| 5 | Fe | 18 ± 0.01 | 0.014 |
| 6 | Mg | 20.4 ± 0.08 | 0.113 |
| 7 | Cu | 2.8 ± 0.01 | 0.014 |
Note: Significance level=0.05; Error mean square=0.002; Degree of freedom=7; LSD 0.05=0.112
Table 7: Effect of different metal ions on l-lysine production by Streptococcus sp.

Figure 4:Graphical representation of different metal ions on l-lysine production by Streptococcus sp. where NL-42 is the Streptococcus sp. strain
no.
| Serial no | PH ranges | L-lysine produced (g/L) | Standard deviation |
|---|---|---|---|
| 1 | 5.5 | 4 ± 0.02 | 0.028 |
| 2 | 6 | 2.4 ± 0.03 | 0.042 |
| 3 | 6.5 | 6 ± 0.05 | 0.07 |
| 4 | 7 | 2.8 ± 0.02 | 0.028 |
| 5 | 7.5 | 2.8 ± 0.01 | 0.014 |
| 6 | 8 | 2.4 ± 0.02 | 0.028 |
| 7 | 8.5 | 3.2 ± 0.02 | 0.028 |
Note: Significance level=0.05; Error mean square=0.001; Degree of freedom=7; LSD 0.05=0.09
Table 8: Effect of different pH ranges on l-lysine production by Streptococcus sp.

Figure 5:Graphical representation of different pH ranges on l-lysine production by Streptococcus sp. where NL-42 is the Streptococcus sp. strain
no
| Serial no | Incubation period (hours) | L-lysine produced (g/l) | Standard deviation |
|---|---|---|---|
| 1 | 24 | 4 ± 0.02 | 0.028 |
| 2 | 48 | 2.8 ± 0.03 | 0.042 |
| 3 | 72 | 3.2 ± 0.01 | 0.014 |
| 4 | 96 | 24.4 ± 0.05 | 0.07 |
| 5 | 120 | 11.2 ± 0.03 | 0.042 |
| 6 | 144 | 1.6 ± 0.07 | 0.098 |
| 7 | 168 | 7.2 ± 0.03 | 0.042 |
| 8 | 192 | 9.2 ± 0.04 | 0.056 |
| 9 | 216 | 13.2 ± 0.06 | 0.084 |
| 10 | 240 | 10.8 ± 0.03 | 0.042 |
Note: Significance level=0.05; Error mean square=0.02; Degree of freedom=10; LSD 0.05=0.351
Table 9: Effect of different incubation periods on l-lysine production by Streptococcus sp.

Figure 6:Graphical representation of different incubation periods on l-lysine production by Streptococcus sp. where NL-42 is the Streptococcus sp.
strain no
| Serial no | Inoculum sizes (ml/l) | L-lysine produced (g/l) | Standard deviation |
|---|---|---|---|
| 1 | 0.1 | 6.8 ± 0.01 | 0.014 |
| 2 | 0.2 | 7.2 ± 0.03 | 0.042 |
| 3 | 0.3 | 9.2 ± 0.04 | 0.056 |
| 4 | 0.4 | 5.2 ± 0.05 | 0.07 |
| 5 | 0.5 | 6 ± 0.06 | 0.084 |
| 6 | 0.6 | 4.4 ± 0.03 | 0.042 |
| 7 | 0.7 | 4.8 ± 0.01 | 0.014 |
| 8 | 0.8 | 3.2 ± 0.03 | 0.042 |
| 9 | 0.9 | 2.8 ± 0.02 | 0.028 |
| 10 | 1 | 1.6 ± 0.03 | 0.042 |
Note: Significance level=0.05; Error mean square=0.002; Degree of freedom=10; LSD 0.05=0.108
Table 10: Effect of different inoculum sizes on l-lysine production by Streptococcus sp.

Figure 7:Graphical representation of different inoculum sizes on l-lysine production by Streptococcus sp. where NL-42 is the Streptococcus sp.
strain no.
From the results of the laboratory scale study, conclusion can be drawn that locally available cane molasses is a useful carbon source along with commercially available glucose. The quantity of L-lysine produced by such bacteria as well as the end products was quite comparable. Thus, keeping in view, the high cost of other carbon sources, commercially available glucose justified its use as a carbon source for L-lysine fermentation. Hence, a considerably less expensive L-lysine fermentation could be carried out using these raw materials.
Conclusion
From the results of the laboratory scale study, conclusion can be drawn that locally available cane molasses is a useful carbon source along with commercially available glucose. The quantity of L-lysine produced by such bacteria as well as the end products was quite comparable. Thus, keeping in view, the high cost of other carbon sources, commercially available glu- cose justified its use as a carbon source for L-lysine fermentation. Hence, a considerably less expensive L-lysine fermentation could be carried out using these raw materials.
Acknowledgement
I would like to thank Almighty ALLAH for giving me the hope, courage and opportunity to learn and work on this research. Indeed, without His help nothing could be accomplished. I am so grateful to Dr. Rana Abrar Hussain for providing me with excellent and valuable supervision, suggestions, guidance, critics and intellectual advice throughout my research work. I would like to thank from the core of my heart to the research officers and associates in Pakistan Council of Scientific and Industrial Research (PCSIR) Laboratories Complex, Lahore. I got to learn a lot in the kind assistance of them. With pleasure, I acknowledge the debt of affection and love to my parents and siblings for their kind and never-ending assistance in this piece of research work. I completely obliged to our parents for their great support. It was difficult to complete this research work without their financial support and moral assistance.
Authors' Contribution
SS did the main work for this research, MS helped in collecting of samples, ZH worked in applying stats to the results, NM helped in making graphs, ZR helped in compiling the information, SA helped in performing the identification.
References
- Theodora T, Bustard MT. Fermentative production of lysine by Corynebacterium glutamicum: Trans-membrane transport and metabolic flux analysis. Process Biochem. 2005; 40(2): 499-508.
- Sadoul K, Boyault C, Pabion M, Khochbin S. Regulation of protein turnover by acetyltransferases and deacetylases. Biochimie. 2008; 90(2): 306-312.
[Crossref] [google scholar] [Pubmed]
- Ekwealor IA, Obeta JAN. Studies on lysine production by Bacillus megaterium. African J Biotech. 2005; 7(4): 633-638.
- Shih IL, Shen MH. Optimization of cell growth and poly (L-lysine) production in batch and fed-batch cultures by Streptomyces albulus IFO 14147. Process Biochem. 2006; 41(7): 1644-1649.
- Irshad S, Faisal M, Hashmi AS, Javed MM, Baber ME, Awan AR, et al. Mass production and recovery of L-lysine by microbial fermentation by Brevibacterium flavum. J Anim Plant Sci. 2015; 25(1): 290-294.
- Ishikawa K, Murakoshi YT, Ohnishi F, Kondo K, Osumi T, Asano K. Medium composition suitable for L-lysine production by Methylophilus methylotrophusin fed-batch cultivation. J Biosci and Bioengin. 2008; 106(6): 574-579.
- Goodfellow M, Schaal KP. In identification methods for microbiologists: Actinomycetes in marine sediments. Academic Press London. 1979: 261.
- Umerie SC, Ekleawor IA, Nwagbo IO. Lysine production by Bacillus laterosporus from various carbohydrates and seed meals. Bioresource Technol. 2005; 75(3): 249-252.
- Hussain A, Mukhtar H, Ikram-ul-haq. Optimization of fermentation medium for L-lysine production by Corynebacterium glutamicum. Pak J Bot. 2015; 47(SI): 345-349.
- Xafenias N, Kmezik C, Mapelli V. Enhancement of anaerobic lysine production in Corynebacterium glutamicum electrofermentations. Bioelectrochem. 2017; 117: 40-47.
[Crossref] [google scholar] [Pubmed]
- Rastegari H, Mohsen C, Azim A, Sara C, Zahra S, Mohammad RM, et al. Improvement in the production of L-Lysine by over-expression of Aspartokinase (ASK) in C. glutamicum ATCC 21799. Trop J Pharm Res. 2013; 12(1): 51-56.
- Nasab MS, Ansari, Montazer Z. Fermentative production of lysine by Corynebacterium glutamicum from different carbon sources. Iran Agric Res. 2007; 25(1-2): 99-106.
- Junior LAL, Letti GVM, Soccol CR. Development of an L-Lysine enriched bran for animal nutrition via submerged fermentation by Corynebacterium glutamicum using agroindustrial substrates. Braz Arch Biol Technol. 2016; 59: 16150519.
- Momose H, Takagi T. Glutamic acid production in biotin-rich media by temperaturesensitive mutants of Brevibacterium lactofermentum, a novel fermentation process. Agric Biol Chem. 1978; 42 (10): 1911-1917.
- Bergey DH, Sneath PHA, Holt JG. Bergey’s manual of systematic bacteriology. Williams and Wilkins, Baltimore. 1986(2): 1261.
- Bergey DH, Holt JG. Bergey’s manual of determinative bacteriology. The Williams and Wilkins, Baltimore. 1994: 565.
- Shakoori FR, Butt AM, Ali NM, Zahid MT, Rehman A, Shakoori AR. Optimization of fermentation media for enhanced amino acids production by bacteria isolated from natural sources. Pakistan J Zool. 2012; 44(4): 1145-1157.
- Reza OA, Arenas TL. Comprehensive assessment of the L-lysine production process from fermentation of sugarcane molasses. Bioprocess Biosyst Eng. 2017.
[Crossref] [google scholar] [Pubmed]
- Casida LE, Baldwin NY. Preparation of diaminopimelic acid and lysine. US patent 2. 1956; 771: 369.
- Brenner M, Niedewieser A, Pataki G. Amino acids and derivatives, in thin layer chromatography. 1969: 730.
- Jyothi AN, Sasikiran K, Nambisan B, Balagopalan C. Optimisation of glutamic acid production from cassava starch factory residues using Brevibacterium divaricatum. Process Biochem. 2005; 40: 3576-3579.
- Ezemba CC, Ozokpo CA, Anakwenze VN, Anaukwu GC, Ogbukagu CM, Ekwealor CC, et al. Lysine production of Microbacterium lacticum by submerged fermentation using various hydrocarbon, sugar and nitrogen sources. J adv microbiol. 2016; 6: 797-810.
Author Info
Shanzay Saleem*, Mehreen Sarfraaz, Zohaib Ahmad, Nuzhat Munawar, Zeeshan Rehman and Saira AhmadCitation: Saleem S: Production and Escalation of L-Lysine via Bacterial Fermentation Utilizing Streptococcus sp
Received: 07-Feb-2022 Accepted: 22-Feb-2022 Published: 01-Mar-2022, DOI: 10.31858/0975-8453.13.3.148-157
Copyright: This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
ARTICLE TOOLS
- Dental Development between Assisted Reproductive Therapy (Art) and Natural Conceived Children: A Comparative Pilot Study Norzaiti Mohd Kenali, Naimah Hasanah Mohd Fathil, Norbasyirah Bohari, Ahmad Faisal Ismail, Roszaman Ramli SRP. 2020; 11(1): 01-06 » doi: 10.5530/srp.2020.1.01
- Psychometric properties of the World Health Organization Quality of life instrument, short form: Validity in the Vietnamese healthcare context Trung Quang Vo*, Bao Tran Thuy Tran, Ngan Thuy Nguyen, Tram ThiHuyen Nguyen, Thuy Phan Chung Tran SRP. 2020; 11(1): 14-22 » doi: 10.5530/srp.2019.1.3
- A Review of Pharmacoeconomics: the key to “Healthcare for All” Hasamnis AA, Patil SS, Shaik Imam, Narendiran K SRP. 2019; 10(1): s40-s42 » doi: 10.5530/srp.2019.1s.21
- Deuterium Depleted Water as an Adjuvant in Treatment of Cancer Anton Syroeshkin, Olga Levitskaya, Elena Uspenskaya, Tatiana Pleteneva, Daria Romaykina, Daria Ermakova SRP. 2019; 10(1): 112-117 » doi: 10.5530/srp.2019.1.19
- Dental Development between Assisted Reproductive Therapy (Art) and Natural Conceived Children: A Comparative Pilot Study Norzaiti Mohd Kenali, Naimah Hasanah Mohd Fathil, Norbasyirah Bohari, Ahmad Faisal Ismail, Roszaman Ramli SRP. 2020; 11(1): 01-06 » doi: 10.5530/srp.2020.1.01
- Manilkara zapota (L.) Royen Fruit Peel: A Phytochemical and Pharmacological Review Karle Pravin P, Dhawale Shashikant C SRP. 2019; 10(1): 11-14 » doi: 0.5530/srp.2019.1.2
- Pharmacognostic and Phytopharmacological Overview on Bombax ceiba Pankaj Haribhau Chaudhary, Mukund Ganeshrao Tawar SRP. 2019; 10(1): 20-25 » doi: 10.5530/srp.2019.1.4
- A Review of Pharmacoeconomics: the key to “Healthcare for All” Hasamnis AA, Patil SS, Shaik Imam, Narendiran K SRP. 2019; 10(1): s40-s42 » doi: 10.5530/srp.2019.1s.21
- A Prospective Review on Phyto-Pharmacological Aspects of Andrographis paniculata Govindraj Akilandeswari, Arumugam Vijaya Anand, Palanisamy Sampathkumar, Puthamohan Vinayaga Moorthi, Basavaraju Preethi SRP. 2019; 10(1): 15-19 » doi: 10.5530/srp.2019.1.3






