Research Article - (2024) Volume 15, Issue 7
Abstract
A microbial protein termed as Single Cell Protein (SCP) is regarded as an alternative protein to complement the conventional proteins. As protein requirement is rising, high concentration of nucleic acid present in SCP serves as great limitation for its consumption as food. This study is aimed at producing SCP with reduced nucleic acids concentration from banana peels using Saccharomyces cerevisiae through submerged fermentation. The fermentation media was prepared as Non-supplemented Media (NM) (only processed peels hydrolysate with distilled water) and Supplemented Media (SM) (mineral salts with processed peels hydrolysate) after which, a high microbial growth and protein production (680 μg/μl) was observed on NM compared to 410 μg/μl that was obtained on SM after 120 hrs of fermentation. The products were further treated with 5%, 10% and 20% solution of each of Sodium Hydroxide (NaOH), Sodium Chloride (NaCl) and Glycine (Gly)-NaOH buffer at the pH of 10.3 to reduce the nucleic acids content. Treatment with NaCl showed high decrease in nucleic acid content (51.3 ng/μl-30.1 μg/μl) with corresponding greater decrease in protein content (410 μg/ μl-190 μg/μl) at 20% treatment. However, treatment with Gly-NaOH buffer has reduced the protein quantity from 680 to 330 μg/μl (SM) and 410 to 250 μg/ μl (SM) and decreased the nucleic acid content (47.9 to 23.3 μg/μl and 51.3 to 24.5 μg/μl on NM and SM respectively), while the protein and nucleic acid contents lost (680 to 110 μg/μl and 47.9 to 36.7 μg/μl) was observed on NaOH treatment. Amino Acids (AA) analysis indicated the presence of the 9 essential AA and 2 non-essential AA (cystine and tyrosine) in the product. The crystalized form of the product was also obtained through centrifugation at 4000 (revolutions per minute) rpm for 45 minutes. Therefore, NM banana peel hydrolysate proved to be good source of SCP with high protein content and more essential AA. The use of Gly-NaOH buffer in reducing the nucleic acid content of the SCP produced proved to be more effective, considering the less proteolytic effect they showed on the SCP produced.
Keywords
Supplemented media, Non-supplemented media, Single Cell Protein (SCP), Glycine
Introduction
The fast growing population of the world has led to an increased demand for protein rich food; this exerts great pressure on food and feed industries to produce sufficient quantities of protein to meet the high nutrient requirements of vulnerable populations (children and women). SCP has proven to be a sustainable approach since agricultural residues can be used for its production (Jones SW, et al., 2020).
Microbial proteins or SCP, represent a potential future nutrient source for human food and animal feed. The term SCP according to Israelis refers to dead, dry cells of microorganisms such as yeast, which grow on different carbon sources. These microbial proteins can be grown rapidly on substrates with minimum dependence on soil, water and climate conditions (Nangul A and Bhatia R, 2013). SCP is gaining popularity day by day because they require limited land area for growth and also help in recycling of waste (Suman G, et al., 2015). Application of agro-industrial residues in bioprocesses such as cultivation of SCP on one hand provides alternative substrates while on the other hand helps in solving pollution problems, which their disposal may otherwise cause some problems. Research on SCP has been stimulated by a concern over the eventual food crisis or food shortage that will occur if the world population is not controlled (Suman G, et al., 2015).
The major setback associated with SCP is the presence of nucleic acid. According to Milala MA, et al., 2018 a minimum of 107.7 ng/ μl of nucleic acid was obtained when SCP was produced at 37°C while at 55°C over 200 ng/μl was discovered. Khan MY and Dahot MU, 2010, also reported a presence of significant amount of nucleic acid obtained at 45°C. In human, excessive intake of nucleic acid is toxic due to the limited capacity of urinary excretion of the uric acid formed from nucleotide catabolism.
The microbial protein, SCP is regarded as an alternative protein source that can serve as a complementation to the conventional sources of protein as the requirement for protein is rising due to the global population growth; however, a research evident proved that SCP contains high concentration of nucleic acid that made it unhealthy for human consumption. Therefore, there is urgent need to devise a method for production of SCP with low nucleic acid content. The conversion of banana peel which is mostly regarded as waste to an edible protein (SCP) with reduced nucleic acid content serves as a cheap source of protein supplement in order to provide for the increasing protein requirement and mitigate the proliferating nucleic acid consumption related health problem (such as gout) that is resulting from high intake of nucleic acid from food source. This study is limited to the production of single protein with reduced nucleic acid content by Saccharomyces cerevisiae from a banana peel.
SCP as food
The production of SCP is not a recent development. Since 2500 BC a number of microorganisms have been used as a part of the diet in the form of fermented food (Saccharomyces spp.). Cultured dairy products contain 107-1010 lactic acid bacteria per gram of product. During the first century BC, edible mushrooms were extensively consumed in Rome. In the 16th century blue green algae (Spirulina) was consumed as a major source of protein. The mass production of micro-organisms as a direct source of microbial protein was realized during World War-I in Germany and consequently, modern baker’s yeast (Saccharomyces cerevisiae) was produced. In the 1960s, researchers at British petroleum developed proteins-from-oil process, a technology for producing microbial protein using yeast fed with waxy n-paraffin, a byproduct of oil refineries. This method had a capacity to produce 10000 tons of microbial protein/annum. The QuornTM brand (http://www.quorn.com/) was launched in 1985 by Marlow Foods (United Kingdom). QuornTM products contain myco-protein from the filamentous fungus Fusariu venenatum. The fungal biomass provides a texture that resembles meat products. QuornTM may be the only SCP product exclusively used for human nutrition and has been extensively branded, marketed and sold for that purpose. The company was recently (2015) acquired by the Philippine instant noodles maker Monde Nissin Food Corporation for 550 million pounds (http://www.reuters.com/article/quorn-maidUSL5N1204C720151001) spent brewer’s yeast (Saccharomyces cerevisiae) have been sold for more than a century in yeast extracts such as Marmite (Unilever and Sanitarium Health Food), Vegemite (Bega Cheese Ltd.), Cenovis (Gustav Gerig AG) and Vitam-RR (VITAM Hefe-Product GmbH). Yeast extracts provides a good source of 5 important group-B vitamins, but also protein. Another commercially available yeast, torula (Candida utilis, renamed as Pichia jadinii), a widely used flavoring agent, is also high in protein. Torula was used in Provesteen®T, produced by the Provesta Corporation in the 1980s, along with similar products using Pichia and Kluyveromyces yeast as studied by Ritala A, et al., 2009. Torula is rich in the amino acid glutamate and for this reason it has been used to replace the flavor enhancer Monosodium Glutamate (MSG).
Feed kind, like Uniprotein® is used in animal feed. Methane is an interesting substrate, since it is a major by-product of cattle and pig farming (Philippe FX and Nicks B, 2015), as well as being available from biogas production (landfills, waste). Excess methane is currently flared. VTT, Ltd., is investigating the reactor design and options for coupling farm methane generation with the production of microbial oil and feed protein (http://www.vttresearch.com/media/news/protein-feedand-bioplastic-from-farm-biogas) from the methanotrophic bacteria Methylococcus capsulatus, Methylosinus trichosporium, and Methylocystis parvus.
Discovery of SCP
Since the early fifties, intense efforts have been made to explore new, alternative and unconventional proteins. For this reason, in 1996, new sources mainly yeast, fungi, bacteria and algae named SCP was coined to describe the protein production from biomass, originating from different microbial source. Microbial biomass has been considered an alternative to conventional source of food or feed Milala MA, et al., 2018. SCP production technologies arose as a potential way to solve the problem of worldwide protein shortage. They evolved as bioconversion processes which turned low value by-products, often wastes, into products with added nutritional and market value. SCP is one of the most important steps for this goal and is an alternative and an innovative way to successfully solve the global food problems according to Kurbanoglu EB and Algur OF, 2002.
Production of SCP is achieved through actions of different microorganism on variety of substrates. The classes of microorganisms capable of carrying out the task include; yeast, fungi, bacteria and algae. Yeast was the 1st microorganism whose importance as animal feed supplement was recognized almost a century ago. Yeast is considered suitable for single cell production because of its superior nutritional quality. The use of SCP produced by yeast for supplementation of cereals, makes it as good as animal protein (Raja R, et al., 2008). Use of microbes as a food source may appear to be unacceptable to some people but the idea of consumption of microbes as food for man and animals is certainly innovative to solve the global food problem. SCP has many applications in food and feed industries The microorganisms which can be used as SCP include a variety of bacteria, marine microalgae, yeasts and molds production of SCP using cheap materials as substrate provides an economically feasible source of protein for use in animal feed or the processing of products for human consumption, as it often meets dietary requirements for protein. Many microorganisms have been used to convert various substrates into biomass. SCP production technologies arose as a promising way to solve the problem of worldwide protein shortage. They evolved as bioconversion processes which turned low-value by-products into products with added nutritional and market value and since SCP belongs to one of the cheapest protein products in the market, its production is profitable (Srividya AR, et al., 2013).
Materials and Methods
Sample collection and peels hydrolysate preparation
Banana peels were collected from fruit sellers within Maiduguri Metropolis, Borno state, Nigeria in a tightly closed container. The peels were oven- dried at 105°C for 24 hours (AOAC, 2016) and ground with mortar and pestle to smaller particles. An amount of 40 g of the ground peel was pretreated with 100 ml of 10% (v/v) HCl solution followed by addition of 200 ml of distilled water and incubated in water bath at 1000°C for one hour, and filtered with No. 1 Whatman filter paper, as adapted by Abarshi AM, et al., 2017.
Quantitative proximate analysis of banana peel
Protein content determination: The protein content was determined by Kjedal method. The principle behind the method is that, organic nitrogen is converted to ammonium in the presence of catalyst at approximately 370°C. In a distillation step the digested sample is made (oxidized) to alkaline with NaOH and the nitrogen is distilled off as Ammonia (NH3) and trapped in a boric acid solution. The amount of NH3 in the solution is quantified by titration with a standard HCl solution. The volume of HCl acid required for the neutralization through titration determine the percentage protein content of the given sample.
Fat content determination: It was determined by Soxhlet extraction method based on gravimetric estimation of fat from a dry powdered sample after a continuous extraction with light organic solvent such as petroleum ether.
Fiber content determination: Muslin cloth method was used in the estimation of fiber content. The sample was subjected to acid (0.3 N Sulfuric acid (H2SO4)) digestion followed by alkali (0.25 N NaOH) and the residue was washed, dried and weighed. The remaining weight of the sample gives the percentage fiber.
Moisture content determination: The percentage of the moisture was determined best on the loss of weight on drying the sample. The sample was weighed and dried at temperature of 105°C in an oven and was cooled with desiccator. The lost in weight is directly proportional to the moisture percentage of the sample.
Determination of carbohydrate content: The percentage content of carbohydrate in sample was obtained using the below given formula
Carbohydrate%=100-(moisture+fiber+ash+protein+fat)
Isolation of microorganism
The yeast Saccharomyces cerevisiae was obtained by resuscitating a baker yeast (an instant dry yeast (NAFDAC Registration No: 01-5909, produced by Chinese company named Eagle Industry). 0.5 g of dry yeast was suspended in 5 ml of distilled water and was shook to dissolve after which a few drops of the solution was poured on Potato Dextrose Agar (PDA) plates and incubated at 37°C for 48 hours using Stuart orbital Incubator (SI500). The yeast was identified by its morphological characteristics based on the method described by Al-Mohanna MT, 2016 on the growth obtained.
Inoculum preparation
An inoculum was prepared by washing the plate with 5 ml of sterile distilled water and stored in sterile container for use.
Media preparation
Media were prepared in 2 groups in a conical flask. The first group was prepared using 20% (v/v) of the fruit hydrolysate in 100 ml of distilled water and labeled as NM while the second group was prepared as the first group but enriched to the following mineral salt composition. 1% (w/v) of potassium dihydrogen phosphate (KH2PO4), 0.5% (w/v) magnesium sulfate heptahydrate (MgSO4.7H2O), 0.1% (w/v) calcium chloride (CaCl2), 0.1% (w/v) NaCl, 2% (w/v) ammonium sulfate ((NH4)2SO4) and 2% (w/v) glucose and labeled as SM. Both media were then sterilized at 121°C using autoclave as outlined by Abarshi AM, et al., 2017.
Fermentation
The media were inoculated with the yeast (Saccharomyces cerevisae) using the prepared inoculum and incubated at 37°C for the period of 124 hours. The fermentation was observed in triplet and the process was monitored at every 24 hours for biomass growth using spectrophotometer. The increase in turbidity translates to an increase in biomass concentration as outlined by Abarshi AM, et al., 2017.
Product optimization (nucleic acid reduction)
The products were treated with NaCl, NaOH and Gly-NaOH to optimally reduce the nucleic acid content as indicated below
Treatment with salt: The product from both NM and SM were each divided into 4 groups of which each group was further divided into 3 sub- groups. 5%, 10% and 20% of NaCl were prepared by dissolving 5, 10 and 20 g of NaCl in separate test tubes containing 100 ml of sterile distilled water.
The 1st, 2nd and 3rd sub-groups of the first group was picked and diluted to an equal volume of the following 5%, 10% and 20% NaCl based on the sub-grouping and labeled as NMS1, SMS1; NMS2, SMS2; and NMS3, SMS3 (S stands for NaCl). The solutions were allowed to stand at lower temperature and pressure for 30 min at room temperature before the spectrometry as outlined by Mosier N, et al., 2005.
Alkali treatment: The 2nd group was picked and subjected to alkaline hydrolysis with the procedure in 3.5.1 above and the samples were labelled as NMB1, SMB1; NMB2, SMB2; and NMB3, SMB3.
Buffer treatment: 5%, 10% and 20% of the Gly-NaOH buffer was prepared by adding 5, 10 and 20 ml of the buffer to separate test tubes containing 100 ml of sterile distilled water and the group three samples were treated using the same procedure as in 3.5.1 with and labeled as NMGB1, SMGB1; NMGB2, SMGB2; and, NMGB3, SMGB3 and incubated at 55°C for 3 hours as outlined by Yang HH, et al., 1979. Group four was diluted with sterile distilled water and labelled as NMC and SMC <3rd>and was used as a control group.
Bradford standard curve preparation
Standard curve was prepared by adding 250 μg/μl, 500 μg/μl, 750 μg/μl and 1500 μg/μl of egg albumin in sterile distilled water while blank was prepared as 0.15 M NaCl only. To 100 μl of each of the solution in a test tube, 5 ml of Bradford reagent (Coomassie blue G-250 dye) was added and the absorbance depicted red colour, at 595 nm wavelength. The principle behind the procedure is that, the Coomassie blue die is red in colour in cationic form as protonated. When it is in an anionic form (in protein solution) it propagates from highly protonated (reddish) to unprotonated (blue). The intensity of the blue colour is directly proportional to the amount of protein in the given solution.
Analysis of protein concentration: The protein quantity of the product (the produced SCP) was determined using Bradford method of whole protein determination using egg albumin Bovine Serum Albumin (BSA) as a standard. 5 ml of Coomassie blue G-250 (Bradford reagent) was added to 100 μl of the sample and absorbance was red at 595 nm wavelength with PEC medical (USA model) spectrophotometer after 5 minutes (Tables 1 and 2).
| S. no | Organism | Protein content (%) |
|---|---|---|
| 1 | Aphanizomenon flos-aquae | 60%-75% |
| 2 | Aphanothece microscopica | 42% |
| 3 | Arthrospira maxima (spirulina maxima) | 60-71% |
| 4 | Arthrospira platensis (spirulina platensis) | 46-63% |
| 5 | Chlorella pyrenoidosa | 45% |
| 6 | Chlorella sorokiniana | 46-65% |
| 7 | Chlorella spp. | 6%-68% |
| 8 | Chlorella vulgaris | 42%-55% |
| 9 | Euglena gracilis | 50%-70% |
| 10 | Scenesdesmus obliquus | 30%-50% |
Table 1: Recent reports of the protein content of some algae
| S. no | Organism | Substrate | Protein content (%) |
|---|---|---|---|
| 1 | Aspergillus flavus | Rice bran | 10% |
| 2 | Aspergillus niger | Apple pomace | 17%-20% |
| Banana wastes | 18% | ||
| 3 | Saccharomyces cerevisiae | Orange, molasses, brewer’s spent grain | 24% |
| 4 | Candida utilis | Poultry litter; waste capsicum powder | 29% |
| 5 | Fusarium semitectum | Rice bran | 10% |
| 6 | Fusarium venenatum | Glucose (Product:QuornTM) | 44% |
| 7 | Trichoderma harzianum | Cheese whey filtrate | 34% |
| 8 | Trichoderma | Virideae citrus pulp | 32% |
| 9 | Candida krusei | Cheese whey | 48% |
| 10 | Candida tropicalis | Molasses | 56% |
Table 2: Recent reports of fungal protein content produced from specific substrates for species investigated as potential sources of SCP
AA profile analysis: AA profile of the product was analyzed using AA analyzer (Agilent 1220 infinity Liquid chromatography (LC)) using the procedure outlined by the manufacturer. In the given sample, the proteins are hydrolyzed into its AA component, followed by subsequent separation into its AA constituent by high performance liquid chromatography and detection by spectrophotometer.
Determination of nucleic acid concentration
The nucleic acid concentration of the products was determined by spectrophotometric analysis of nucleic acids with Nanodrop 2000ºC spectrophotometer (Thermo Scientific product) based on the principle that a liquid sample containing nucleic acid absorbs Ultraviolet (UV) light at 260 nm wavelength. The amount of light absorbed is directly proportional to the quantity of nucleic acid present in the sample (Huss VA, et al., 1983).
Biomass precipitation
The product was subjected to centrifugation at 4000 rpm for 45 min as outlined by Zhou JL, 2012, using 80-2 electric centrifuge USA model to obtain the biomass cell in precipitated form.
Experimental design
The experimental design adopted was pretest-post-test method in which media was set up in 250 ml conical flask and the production process was observed in triplet; the mean value of the data was used.
Results
Proximate content of processed peel
The quantity in percentage by proximate analysis with the processed banana peel shows 50.99% of carbohydrate, 17.42% of moisture and 11.54% fiber respectively, while ash and protein are 10.28% and 1.58%. The fat content constitutes of 8.21% (Figure 1).
Figure 1: Pure culture of Saccharomyces cerevisae
Bradford standard curve
The Bradford standard curve as shown in Figure 2 was drawn from the readings of absorbance at 595 nm wavelength using spectrophotometer. The absorbance with 250 μg was at 0.23 and at 500 μg an absorbance of 0.55 was obtained while an absorbance of 0.69 was obtained at 750 μg. At 1500 μg an absorbance of 1.29 was recorded respectively as indicated in the graph (Figure 3).

Figure 2: Liquid form of the product (SCP)
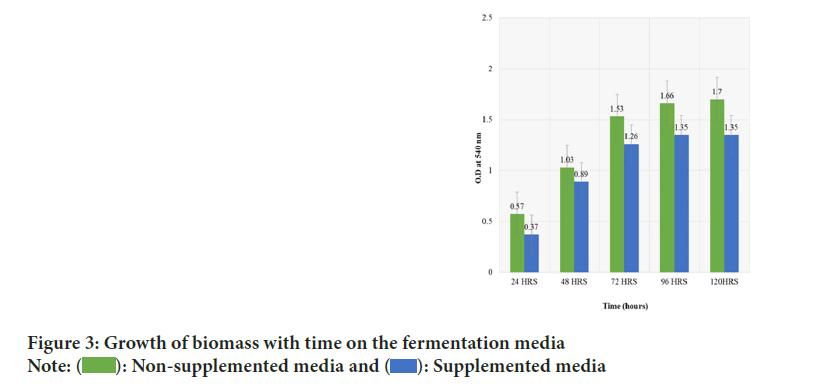
Figure 3: Growth of biomass with time on the fermentation media
Note: ( ): Non-supplemented media and (
): Non-supplemented media and ( ): Supplemented media
): Supplemented media
Biomass growth
It was estimated by turbidimetry method using spectrophotometer. The fermentation media were incubated for the period of 5 days in which the absorbance was red at 540 nm (turbidimetry) at 24, 72 and 124 hours respectively before the products were harvested for further treatment and analysis. Absorbance at 24 hours is 0.57 and 0.37 for NM and SM and 1.53 and 1.26 for NM and SM at 72 hours, while at 124 hours absorbance of 1.7 and 1.35 were observed for NM and SM respectively.
Protein concentration
680 μg/μl on NM and 410 μg/μl on SM media were observed respectively before the treatment of cells and followed by 530 μg/μl and 360 μg/μl of protein obtained at treatment of the product with 5% Gly-NaOH (Table 3). 360 μg/μl (NM) and 390 μg/μl (SM) protein were obtained at 5% NaOH treatment, at 10% NaOH treatment 320 μg/μl and 340 μg/μl protein were observed, while 110 μg/μl and 220 μg/μl of protein were obtained in both NM and SM media respectively at 20% NaOH treatment. On treatment with 5% NaCl 360 μg/μl of protein obtained both on NM and SM product while 330 μg/μl on NM and 310 μg/μl on SM protein were obtained at 10% NaCl treatment and also an amount of 290 μg/μl on NM and 190 μg/μl on SM were confirmed at 20% NaCl treatment. Gly-NaOH buffer treatment shows protein concentration 410 μg/μl on NM and 300 μg/μl on SM at 10% treatment, while 330 μg/μl and 250 μg/μl of protein was observed at 20% treatment on NM and SM respectively.
| Percentage treatment | Protein concentration (µg/µl) | |||||
|---|---|---|---|---|---|---|
| NM product | SM product | |||||
| 0% (control) | 680 | 410 | ||||
| Gly-NaOH B | NaCl | NaOH | Gly-NaOH B | NaCl | NaOH | |
| 5% | 530 | 360 | 360 | 360 | 360 | 390 |
| 10% | 410 | 330 | 320 | 300 | 340 | 330 |
| 20% | 330 | 290 | 110 | 250 | 220 | 190 |
Note: NM: Non-supplemented Media; SM: Supplemented Media; Gly: Glycine amino acid and B: Buffer
Table 3: Protein concentration obtained at different treatments level
Nucleic acid concentration
The highest concentration of 47.9 ng/μl on NM media and 51.3 ng/μl on SM media was observed prior to the treatment of the product. Treatment with NaOH shows 45.2 ng/μl and 46.2 ng/μl on NM and SM media respectively at 5% level. 43 ng/μl and 44 ng/μl of nucleic acid was obtained respectively on NM and SM when the products were treated with 10% NaOH. At 20% NaOH treatment level an amount of 36.7 ng/μl on NM media product and 27.2 ng/μl on SM media product were confirmed. Treatment with salt (NaCl) shows a drastic reduced in nucleic acid content in respect to the untreated product. 38.7 ng/μl for NM and 38.5 ng/μl for SM product of nucleic acid were obtained at 5% NaCl treatment level. At 10% NaCl treatment level, 28 ng/μl and 31.9 ng/μl for NM an SM product respectively of nucleic acid were observed. When the NaCl treatment level was increased to 20% the nucleic acid concentration was reduced to 23.3 ng/μl with NM product and 30.1 ng/μl with SM product respectively (Table 4).
| Percentage treatment | Nucleic acid concentration (ng/µl) at different treatment level | |||||
|---|---|---|---|---|---|---|
| NM product | SM product | |||||
| 0% (control) | 47.9 | 51.3 | ||||
| Gly-NaOH B | NaCl | NaOH | Gly-NaOH B | NaCl | NaOH | |
| 5% | 50.2 | 38.7 | 45.2 | 37.5 | 38.5 | 46.2 |
| 10% | 37.6 | 28 | 43 | 24.5 | 31.9 | 44 |
| 20% | 27.2 | 23.3 | 36.7 | 36.6 | 30.1 | 27.2 |
Note: NM: Non-supplemented Media; SM: Supplemented Media; Gly: Glycine amino acid and B: Buffer
Table 4: Nucleic acid concentration at different levels of Gly-NaOH buffer treatment
Treatment with buffer (Gly-NaOH) at pH 10.3 at different concentration levels denoted 50.2 ng/μl and 37.5 ng/μl nucleic acid concentration with NM and SM product respectively at 5%, while at 10% treatment level, nucleic acid concentration of 37.6 ng/μl and 24.5 ng/μl on NM and SM product were obtained respectively. The concentration of 27.2 ng/μl and 36.6 ng/μl of nucleic acid were obtained on NM and SM products were confirmed.
AA profile
The AA analysis observed indicated the present of 11 AA, which included both essential and non-essential AA. However, only 2 non-essential AA were confirmed but all of the essential AA were confirmed. Also there exist a variable in the concentrations of the AA (Table 5).
| S. no | AA | AA status | |
|---|---|---|---|
| SM product | NM product | ||
| 1 | Glycine | - | - |
| 2 | Alanine | - | - |
| 3 | Valine | + | + |
| 4 | Leucine | + | + |
| 5 | Isoleucine | ++ | + |
| 6 | Serine | - | - |
| 7 | Threonine | ++ | ++ |
| 8 | Cystine | ++ | + |
| 9 | Methionine | + | + |
| 10 | Phenylalanine | ++ | ++ |
| 11 | Tyrosine | ++ | ++ |
| 12 | Tryptophan | ++ | + |
| 13 | Lysine | ++ | + |
| 14 | Arginine | - | - |
| 15 | Histidine | + | + |
| 16 | Aspartate | - | - |
| 17 | Glutamate | - | - |
| 18 | Asparagine | - | - |
| 19 | Glutamine | - | - |
| 20 | Proline | - | - |
Note: -: Not confirmed; +: Confirmed at moderate concentration and ++: Confirmed at high concentration
Table 5: AA profile of the products
Observed biomass precipitation
After 45 minutes of centrifugation at 4000 rpm, the crystalized form of the product was obtained at 0.4 g/10 ml of product from it liquid form.
Discussion
Commercial baker’s yeast can be resuscitated to a viable cells of Saccharomyces cerevisiae with greater kinetic through an aseptic and effective procedure as outlined by (Al-Mohanna MT, 2016). The resuscitation of the baker’s yeast also makes it easier for other researchers that have interest on the yeast to obtain it with less effort and minimal error in the identification process.
The maximum reductive activity that was observed under NaCl treatment with reduction from 47.9 ng/μl at 0% treatment to 38.7 ng/μl at 5% treatment with NM product and from 51.3 ng/μl at 0% treatment to 38.5 ng/ μl at 5% treatment with SM product and the corresponding subsequent reduction on increasing the percentage of treatment actually uphold the findings by Mosier N, et al., 2005 that NaCl and NaOH have degradative effect on nucleic from a SCP. On treatment of the product with 10% NaCl, the nucleic acid was further decreased to 28 ng/μl and 31.9 ng/μl for NM and SM products respectively.
The lowest reduction in nucleic acid concentration was observed on treatment with the buffer (Gly-NaOH) which made it clear that it has less nucleic acid degradative capacity when compared to NaCl and NaOH. However, it’s (the buffer) minimal hydrolytic effect on the protein content makes it to be more valuable than both compounds especially when used on an average percentage level (10%).
The higher protein concentration that was observed in the product from NM actually contradicted the report by Abarshi AM, et al., 2017 that when a media (formulated with pineapple peel) for the production of SCP was supplemented with mineral salts, high protein content was produced, suggesting that the low biomass growth with the corresponding low protein content observed (on this work) might be an indication of antimicrobial activity (against yeast) from antimicrobial compound that might have formed as a result of the supplementation of the peel hydrolysate, or a sign of loss of valuable nutrient required for the yeast or the effect of heavy metals such as Iron (Fe) that were used in the supplementation of the media as many research works have indicated that heavy metals affect the folding of proteins, thus, making them susceptible for hydrolysis , proving the fact that heavy metals such as iron have proteolytic effect as presented by Tamás MJ, et al., 2014 and also the higher nucleic acid concentration that was obtained in the product from the SM supported the above statements.
AA profile analysis showed that the products contain both essential and non-essential AAs making it to be good source of essential AA as well as nutritionally valuable product, comparable to the findings by Ritala A, et al., 2017 that SCP can serve as a good source of AA, however, as all essential AA were confirmed but not all, except 2 of the non-essential AA (cystine and tyrosine) were confirmed. The confirmation of the essential amino acids lysing, methionine and threonine also showed that the result is comparable to the findings by Sharif M, et al., 2021 and also prove that SCP can replace the expensive sources of protein such as meat.
The crystalized form of the product was also obtained through subjecting the initial product (from it liquid form) to centrifugation at high rpm. Similar method was also reported by Dashmeet, 2018 on production of SCP through “bioprotein process” in which the biomass was continuously harvested by centrifugation and ultrafiltration prior to drying after the production of the SCP.
Conclusion
Conclusively, the use of Gly-NaOH buffer and NaCl in reducing the nucleic acid content of a SCP would be more valuable/considerable, considering their less proteolytic effect on the protein content and moderate nucleic acid degradative capacity compared to NaOH which proves to be highly protein hydrolyzing compound (though it reduced the nucleic acid content to a less concentration).
The product can serve as a good source of essential amino acids and can replace other expensive source of protein as it contains the entire 9 essential AA. It can be also concluded that a solidified or crystal form of the product can be obtained easily through centrifugation.
Based on the result presented above, we recommend that an additional research should be conducted to determine other micro nutrient content of the SCP produced. Further, other procedure and means of nucleic acid elimination process that can minimally affect or even increase the protein content with corresponding reduction/neutralization of the nucleic acid concentration should be devise.
References
- Jones SW, Karpol A, Friedman S, Maru BT, Tracy BP. Potential sources of single-cell protein products. Global Seafood Alliance. 2020.
- Nangul A, Bhatia R. Microorganisms: A marvelous source of single cell proteins. J Microbiol, Biotechnol Food Sci. 2013; 3(1): 15-158.
- Suman G, Nupur M, Anuradha S, Pradeep B. Single cell protein production: A review. Int J Curr Microbiol App Sci. 2015; 4(9): 251-262.
- Milala MA, Yakubu M, Burah B, Laminu HH, Bashir H. Production and optimization of single cell protein from orange peels by Saccharomyces cerevisiae. J Biosci Biotechnol Discov. 2018; 3: 99-104.
- Khan MY, Dahot MU. Effect of various agriculture wastes and pure sugars on the production of single cell protein by Penicillium expansum. World Appl Sci J. 2010; 8: 80-84.
- Ritala A, Häkkinen ST, Toivari M, Wiebe MG. Single cell protein-state-of-the-art, industrial landscape and patents 2001-2016. Front Microbiol. 2017; 8: 2009.
[Crossref] [Google Scholar] [PubMed]
- Philippe FX, Nicks B. Review on greenhouse gas emissions from pig houses: Production of carbon dioxide, methane and nitrous oxide by animals and manure. Agric Ecosyst Environ. 2015; 199: 10-25.
- Kurbanoglu EB, Algur OF. Single-cell protein production from ram horn hydrolysate by bacteria. Bioresour Technol. 2002; 85(2): 125-129.
[Crossref] [Google Scholar] [PubMed]
- Raja R, Hemaiswarya S, Kumar NA, Sridhar S, Rengasamy R. A perspective on the biotechnological potential of microalgae. Crit Rev Microbiol. 2008; 34(2): 77-88.
[Crossref] [Google Scholar] [PubMed]
- Srividya AR, Vishnuvarthan VJ, Murugappan M, Dahake PG. Single cell protein-A review. Int J Pharm Res Sch. 2013; 2: 472-485.
- AOAC. Official methods of analysis of AOAC International. 2016.
- Abarshi MM, Mada SB, Amin MI, Salihu A, Garba A, Mohammad HA. Effect of nutrient supplementation on single cell protein production from watermelon and pineapple peels. Nig J Basic Appl Sci. 2017; 25(1): 130-136.
- Al-Mohanna MT. Methods for fungal enumeration, isolation and identification. Origin of Ota. 2016: 155-241.
- Mosier N, Wyman C, Dale B, Elander R, Lee YY, Holtzapple M, et al. Features of promising technologies for pretreatment of lignocellulosic biomass. Bioresour Technol. 2005; 96(6): 673-686.
[Crossref] [Google Scholar] [PubMed]
- Yang HH, Thayer DW, Yang SP. Reduction of endogenous nucleic acid in a single-cell protein. Appl Environ Microbiol. 1979; 38(1): 143-147.
[Crossref] [Google Scholar] [PubMed]
- Huss VA, Festl H, Schleifer KH. Studies on the spectrophotometric determination of DNA hybridization from renaturation rates. Syst Appl Microbiol. 1983; 4(2): 184-192.
[Crossref] [Google Scholar] [PubMed]
- Zhou JL. Comprehensive sampling and sample preparation. Sampling Theory and Methodology. 2012.
- Tamás MJ, Sharma SK, Ibstedt S, Jacobson T, Christen P. Heavy metals and metalloids as a cause for protein misfolding and aggregation. Biomolecules. 2014; 4(1): 252-267.
[Crossref] [Google Scholar] [PubMed]
- Sharif M, Zafar MH, Aqib AI, Saeed M, Farag MR, Alagawany M. Single cell protein: Sources, mechanism of production, nutritional value and its uses in aquaculture nutrition. Aquaculture. 2021; 531: 735885.
- Dashmeet. Steps involved in SCP (Single Cell Protein) production from bacteria and yeast. Industrial Biotechnology. 2018.
Author Info
Mohammed Yakubu*, Hauwa Hajjagana Laminu and Mohammed Adamu MilalaCitation: Yakubu M: Production of Low Nucleic Acid Containing Single Cell Protein from Banana (Musa Spp.) Peels using the Yeast (Saccharomyces cerevisiae)
Received: 04-Jul-2024 Accepted: 20-Jul-2024 Published: 27-Jul-2024, DOI: 10.31858/0975-8453.15.7.244-250
Copyright: This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
ARTICLE TOOLS
- Dental Development between Assisted Reproductive Therapy (Art) and Natural Conceived Children: A Comparative Pilot Study Norzaiti Mohd Kenali, Naimah Hasanah Mohd Fathil, Norbasyirah Bohari, Ahmad Faisal Ismail, Roszaman Ramli SRP. 2020; 11(1): 01-06 » doi: 10.5530/srp.2020.1.01
- Psychometric properties of the World Health Organization Quality of life instrument, short form: Validity in the Vietnamese healthcare context Trung Quang Vo*, Bao Tran Thuy Tran, Ngan Thuy Nguyen, Tram ThiHuyen Nguyen, Thuy Phan Chung Tran SRP. 2020; 11(1): 14-22 » doi: 10.5530/srp.2019.1.3
- A Review of Pharmacoeconomics: the key to “Healthcare for All” Hasamnis AA, Patil SS, Shaik Imam, Narendiran K SRP. 2019; 10(1): s40-s42 » doi: 10.5530/srp.2019.1s.21
- Deuterium Depleted Water as an Adjuvant in Treatment of Cancer Anton Syroeshkin, Olga Levitskaya, Elena Uspenskaya, Tatiana Pleteneva, Daria Romaykina, Daria Ermakova SRP. 2019; 10(1): 112-117 » doi: 10.5530/srp.2019.1.19
- Dental Development between Assisted Reproductive Therapy (Art) and Natural Conceived Children: A Comparative Pilot Study Norzaiti Mohd Kenali, Naimah Hasanah Mohd Fathil, Norbasyirah Bohari, Ahmad Faisal Ismail, Roszaman Ramli SRP. 2020; 11(1): 01-06 » doi: 10.5530/srp.2020.1.01
- Manilkara zapota (L.) Royen Fruit Peel: A Phytochemical and Pharmacological Review Karle Pravin P, Dhawale Shashikant C SRP. 2019; 10(1): 11-14 » doi: 0.5530/srp.2019.1.2
- Pharmacognostic and Phytopharmacological Overview on Bombax ceiba Pankaj Haribhau Chaudhary, Mukund Ganeshrao Tawar SRP. 2019; 10(1): 20-25 » doi: 10.5530/srp.2019.1.4
- A Review of Pharmacoeconomics: the key to “Healthcare for All” Hasamnis AA, Patil SS, Shaik Imam, Narendiran K SRP. 2019; 10(1): s40-s42 » doi: 10.5530/srp.2019.1s.21
- A Prospective Review on Phyto-Pharmacological Aspects of Andrographis paniculata Govindraj Akilandeswari, Arumugam Vijaya Anand, Palanisamy Sampathkumar, Puthamohan Vinayaga Moorthi, Basavaraju Preethi SRP. 2019; 10(1): 15-19 » doi: 10.5530/srp.2019.1.3






