Research Article - (2022) Volume 13, Issue 12
Relative Antibody Mediated Protection against Influenza in Vaccinated Children
Charlotte Switzer1*, Chris P Verschoor1, Eleanor Pullenayegum1,2, Pardeep Singh1 and Mark Loeb3Abstract
Background: Adjuvanted influenza vaccine has been shown to elicit more robust vaccine immunogenicity, as measured by the Hemagglutination Inhibition (HAI) assay. Post-vaccination HAI titers are the accepted correlate of protection against influenza. However, the proportion of relative vaccine protection in adjuvanted vaccines that is mediated by increased HAI titers has not been investigated.
Methods: A cluster randomized controlled trial of influenza vaccines in Canadian Hutterite colonies was conducted from the 2016-2017 to the 2018-2019 influenza season. Children were vaccinated with either Quadrivalent Inactivated Influenza Vaccine (QIV), or the MF59 adjuvanted trivalent influenza vaccine (aTIV). Sera were collected prior to and four weeks after vaccination, and HAI titers against A/H3N2 vaccine antigens were measured. We tested for differences in the hazard rate of influenza A/H3N2 infection using Cox proportional hazards models, and the proportion of relative vaccine protection in adjuvanted vaccinees mediated by higher HAI titers was estimated using a causal mediation model.
Results: Antibody titers from 542 paired serum samples were available, with 32 laboratory-confirmed influenza infections. Most infections were due to A/ H3N2 and occurred during a season of antigenic mismatch in the vaccine. Of 22 A/H3N2 infections, two occurred in the aTIV group (0.8%), versus 20 in the QIV group (6.6%). We estimated the relative adjuvanted vaccine efficacy against A/H3N2 as 87.8% (95% CI: 43.6%, 97.4%). The rise in post-vaccination titers in adjuvanted vaccinees explained 9.0% of vaccine protection, relative to quadrivalent controls.
Conclusion: Relative vaccine protection in adjuvanted vaccinees is not conclusively mediated by the superior induction of HAI titers. Adjuvanted vaccine effectiveness may be attributable to other mechanisms, such as increased engagement of the innateor cell-mediated immune repertoire. As most cases occurred during an antigenically mismatched season, adjuvanted vaccine protection against heterologous strains of influenza may be driven by cellular immunity, and further work is required.
http://www.environmentjournals.com/
http://www.eventsupporting.org/
http://www.escientificreviews.com/
http://www.openaccesspublications.com/
http://www.imedpub.org/
http://www.jpeerreview.com/
http://www.escientificres.com/
http://www.scholarlyjournals.org/
http://www.eclinicaljournals.com/
http://www.scischolarsjournal.com/
http://www.intlscholarsjournal.com/
http://www.scholarsresjournal.com/
http://www.sysrevpharma.org/
http://www.environjournal.com/
http://www.jpeerres.com/
http://www.managjournal.org/
http://www.emedicalhub.org/
http://www.biomedresj.org/
http://www.aaccongress.com/
http://www.eclinicalres.org/
http://www.scholarlymed.com/
http://www.eclinicalres.com/
http://www.theresearchpub.com/
http://www.imedpubscholars.com/
http://www.scholarcentral.org/
http://www.journalpublications.org/
http://www.scholarlypub.com/
http://www.imedpublishing.org/
http://www.emedsci.com/
http://www.longdomjournals.org/
http://www.longdomjournal.org/
http://www.emedicalcentral.com/
http://www.lexisjournal.com/
http://www.geneticjournals.com/
http://www.scitecjournals.com/
http://www.microbialjournals.org/
http://www.engjournals.org/
http://www.eneurologyjournals.com/
http://www.pulsusjournal.org/
http://www.biochemjournal.org/
http://www.epharmacentral.com/
http://www.eclinicalsci.com/
http://www.eclinicalcentral.com/
http://www.eclinmed.com/
http://www.jopenaccess.org/
http://www.peerreviewedjournals.com/
http://www.immunologyjournals.com/
http://www.neurologyjournals.org/
http://www.clinicalmedicaljournals.com/
http://www.molecularbiologyjournals.com/
http://www.geneticsjournals.com/
http://www.biochemistryjournals.org/
http://www.psychiatryjournals.org/
http://www.pharmajournals.org/
http://www.alliedresearch.org/
http://www.medicalres.org/
http://www.medicalresjournals.com/
http://www.alliedsciences.org/
http://www.pediatricsjournals.org/
http://www.oncologyinsights.org/
Keywords
Influenza, Adjuvanted influenza vaccine, Vaccination, Hemagglutination inhibition assay titers
Introduction
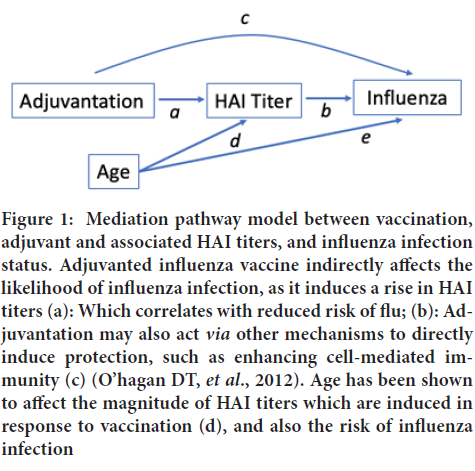
Seasonal influenza Vaccine Efficacy (VE) is measured by the robustness of hemagglutination antibody (HAI) titers, which have been shown to correlate with protection against infection (Hobson DR, et al., 1972). Vaccination influences numerous immune mechanisms, including antibody production, and the activation of dendritic cells and T-cells, which in turn mediate the outcome of influenza infection. Previous work has found that HAI titers account for the majority (57%) of vaccine induced protection against influenza B, when comparing seasonal Trivalent Inactivated Influenza Vaccine (TIV) with placebo (Cowling BJ, et al., 2019). The Adjuvanted seasonal influenza vaccine (aTIV) influences immune responses vianumerous pathways, leading to enhanced protection (Vesikari T, et al., 2011; Knuf M, et al., 2014; Vesikari T, et al., 2018). Studies have shown that the MF59 adjuvant directly effects the induction of cytokines, such as interferon-gamma and tumor necrosis factor, indicating that it is able to activate immune cells and enhance antigen uptake (O’hagan DT, et al., 2012; Cioncada R, et al., 2017). Other studies have demonstrated that it induces immunogenicity by the induction of chemokines, which increase the recruitment of immune cells such as neutrophils and monocytes; enhances differentiation of monocytes into dendritic cells; and facilitates dendritic cell migration into the lymph nodes, thus triggering an adaptive immune response (Zedda L, et al., 2015; Khurana S, et al., 2011). It is not surprising then that the MF59 adjuvant elicited significantly higher seroconversion rates and geometric mean titers against all vaccine antigens in children, as compared to TIV (Nolan T, et al., 2014). Moreover, this study demonstrated that the persistence of HAI titers was significantly improved in adjuvant vaccinated children after six months, suggesting that the adjuvant may increase magnitude and duration of protection. As discussed above, the MF59 adjuvant may mediate vaccine protection against influenza through its ability to induce higher post-vaccination HAI titers. However, to our knowledge no study has determined the proportion of adjuvant vaccine protection against influenza which is mediated by HAI titers. Our study used a causal mediation analysis to quantify the proportion of relative vaccine protection attributable to increased post-vaccination HAI titers in adjuvanted vaccinees, as compared to non-adjuvanted vaccines (Figure 1).
Figure 1: Mediation pathway model between vaccination, adjuvant and associated HAI titers, and influenza infection status. Adjuvanted influenza vaccine indirectly affects the likelihood of influenza infection, as it induces a rise in HAI titers (a): Which correlates with reduced risk of flu; (b): Adjuvantation may also act via other mechanisms to directly induce protection, such as enhancing cell-mediated immunity (c) (O’hagan DT, et al., 2012). Age has been shown to affect the magnitude of HAI titers which are induced in response to vaccination (d), and also the risk of influenza infection
Materials and Methods
Setting and study design
We conducted a cohort study, which made use of serum samples from the Adjuvanted Inactivated Vaccine versus Inactivated Influenza Vaccine in Hutterite Children Trial (NCT02871206). In the original cluster randomized controlled trial, 994 children aged 6 to 72 months were allocated to receive either quadrivalent inactivated influenza vaccine (QIV, Sanofi Pasteur: Fluzone®), or adjuvanted trivalent influenza vaccine (aTIV, Sequiris: Fluad PediatricTM). The parent study was conducted over three influenza seasons (2016-2019), and vaccines contained the recommended antigens for each season (Supplementary Table 1). Participants were then followed for the duration of the influenza season for any signs or symptoms of respiratory infection. Participants reporting two or more symptoms were sampled by nasopharyngeal swab for a multiplex respiratory pathogen panel and viral genotyping. Influenza infections were confirmed by reverse-transcriptase Polymerase Chain Reaction (PCR).
| Demographics | All children (n=542) | aTIV (n=239) | QIV (n=303) | |||
|---|---|---|---|---|---|---|
| n | % | n | % | n | % | |
| Age (months) (mean, SD) | 54.4 | 17.9 | 54.8 | 18 | 54.11 | 17.8 |
| Sex (Male=1) | 286 | 52.8 | 126 | 52.7 | 160 | 52.8 |
| PCR-confirmed influenza | 32 | 5.9 | 7 | 2.9 | 25 | 8.3 |
| A type | 23 | 4.2 | 3 | 1.3 | 20 | 6.6 |
| A/H1N1 | 1 | 0.2 | 0 | 0 | 1 | 0.4 |
| A/H3N2 | 22 | 4.1 | 2 | 0.8 | 20 | 6.6 |
| B type | 9 | 1.7 | 4 | 1.7 | 5 | 1.7 |
Note: Where n=542 observations from 330 unique children
Table 1: Characteristics of children with paired serum samples included in the study cohort
Laboratory testing
Our cohort included children in whom there were data available on post-vaccination HAI titers. In the parent study, sera were collected from study-vaccinated children at baseline and four weeks post-vaccination. Serum samples were tested by HAI assay against the four vaccine antigens recommended for each study year, per the standard protocol (13). In brief, samples were plated in serial twofold dilutions from an initial dilution of 1:10, and incubated with erythrocytes and a stock titer of each vaccine strain virus. HAI titers were determined to be the reciprocal of the last dilution at which hemagglutination was inhibited. Titers of <10 were imputed at 5 for the analysis.
Statistical analysis plan
The primary outcome of our analysis was time to influenza A infection, as confirmed by PCR. We hypothesized a mediation model (Figure 1), wherein adjuvanted influenza vaccination impacted HAI titers, as well as inducing other immune mechanisms against influenza infection. The adjuvant vaccine-induced protection against infection was mediated by the rise in HAI titers generated by a robust response to the vaccine (the mediating, “indirect” effect). Since age has been shown to affect the magnitude of HAI responses in children, as well as their likelihood of developing an influenza infection, we included this as a potential confounder in our models (Davenport FM, et al., 1953; Ranjeva S, et al., 2019).
Estimation of total effect
To estimate the total effect of vaccination on protection, we constructed a Cox proportional hazards model, where the independent variables were the vaccine allocation (QIV or aTIV) and age, and the dependent variable was time of PCR-confirmed influenza infection. We included a term for the colony (the clustered variable) to estimate cluster-robust variance. The relative vaccine efficacy was calculated as 1-the hazard ratio (HR) × 100%. We tested whether there was an association between the adjuvant-induced rise in post-vaccination HAI titers and the hazard of influenza infection by fitting a Cox proportional hazards model, with age and vaccine allocation as the predictors, and a cluster-robust estimation term for colony. We determined whether there was an interaction between vaccine formulation and the post-vaccination titers by adding an interaction term to the model.
Estimation of direct effect: Relative vaccine-induced protection
We estimated the direct effect of adjuvanted vaccination relative to quadrivalent vaccination on protection against influenza (the effect of the vaccine which does not act through the pathway of increased HAI titers) by first fitting a logistic regression model. We regressed the probability of vaccination with aTIV as the outcome onto post-vaccination HAI titers and age as predictors, adjusting for colony. The regression coefficients of this model were used to generate odds ratios for each child, from which we derived a weighted score for each vaccinated participant (1-OR) (WHO, 2011; Tchetgen TEJ, 2013). Weights for children receiving the QIV vaccine were pre-specified at 1. We then fit a proportional hazards model for the time to influenza infection, adjusting for age, vaccine group, and colony, weighted according to the scores generated by the odds ratios. The direct effect was estimated from the hazard ratio of this weighted model.
Estimation of indirect effect: HAI-mediated relative vaccine-induced protection
The indirect effect was calculated as the ratio between the total effect and the direct effect (steps described above). The proportion of effect which is attributed to the HAI titres following was estimated as (log (indirect effect HR))/(log(total effect HR)). We reported the estimates, 95% confidence intervals, and p-values from the models for total and direct effects. We conducted a sensitivity analysis in which we adjusted for pre-vaccination titers, to account for the possibility that vaccination would not have elicited a robust immune response due to a ceiling-effect for antibody titres in children with previously high titers. Models followed the same approach outlined above, with pre-vaccination titers for each participant included as a covariate. We included only samples with complete data on pre-and post-vaccination titers; due to study design, there were no observations with missing data. All analyses were done in the R environment, version 4.0.2, and an alpha of 0.05 was considered significant for all tests (GBIF, 2020).
Results
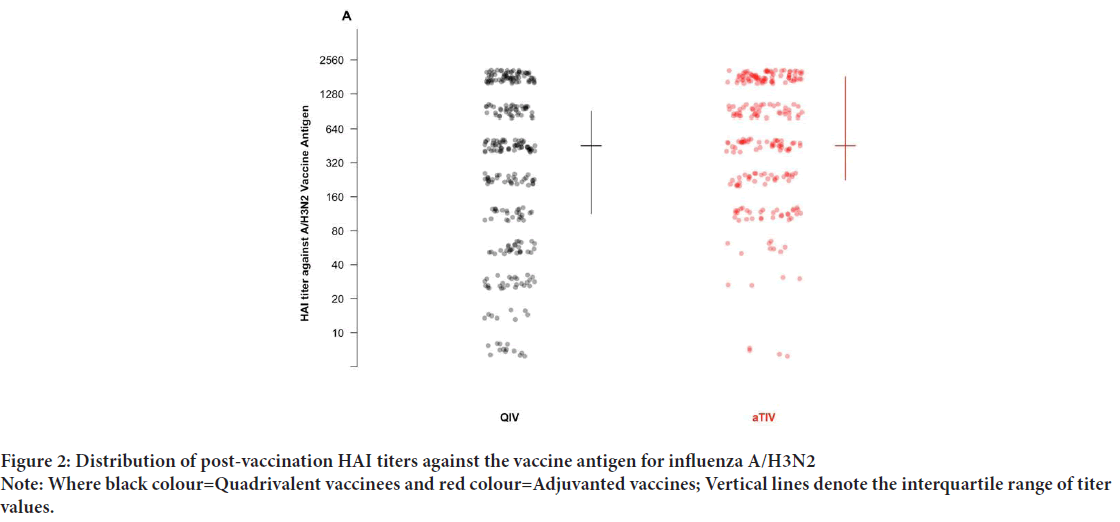
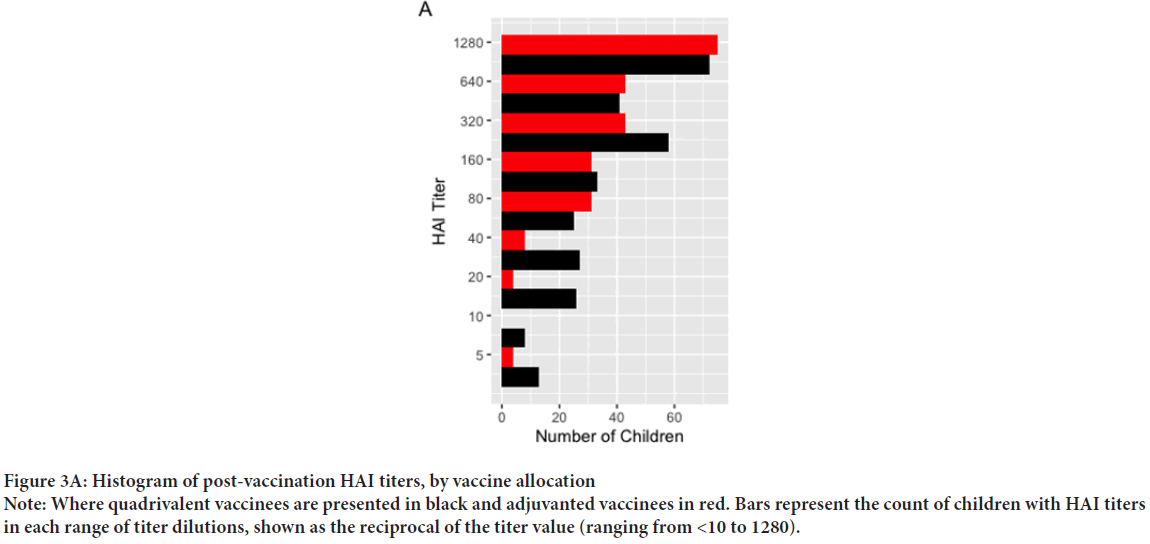
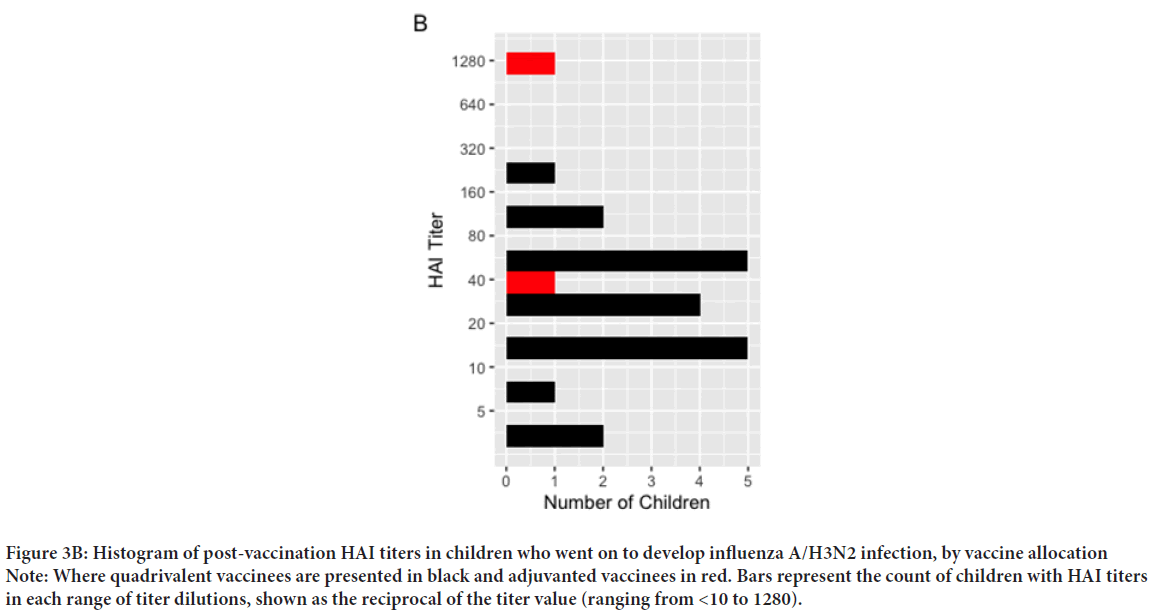
Our cohort included 542 post-vaccination serum samples from 330 unique children across the three influenza seasons of the original study (Table 1). The mean age was 54.4 months (SD: 17.9) and did not differ significantly across vaccine groups. Among these children, there were 32 PCR-confirmed influenza infections: One A/H1N1 case, 22 cases of A/H3N2, and 9 B infections (untyped). Given the small number of cases in the available samples, and the significant differences between vaccine groups in post-vaccination titers against A/H3N2, we focus on infections of this subtype. Of 22 A/H3N2 infections, two occurred in the aTIV group (0.8%), as compared to 20 in the QIV group (6.6%) (X-squared=9.97, 95% CI: -0.09, -0.02, p<0.001). Given the small number of events, and that 82% of cases occurred in season two, we pooled cases across all seasons. The post-vaccination HAI titers against A/H3N2 are shown in Figure 2, and Table 2. Of 542 observations, 239 were in aTIV recipients, and 303 in QIV recipients. The geometric mean HAI titers were 355 (SD: 3.49) in aTIV vaccinees, as compared to 202 (SD: 5.07) in QIV vaccines (p<0.001). Pre-vaccination titers did not differ significantly (68.80 ± 7.00 in aTIV vaccinees, vs. 54.22 ± 6.76 in QIV vaccinees, p=0.15). The distribution of titers in all children are shown in Figure 3A, and the distribution of post-vaccination titers in children who went on to develop flu infections in Figure 3B. We estimated the hazard of influenza A/H3N2 infection in children who received the adjuvanted vaccine, as compared to QIV. We estimated the total effect HR to be 0.122 (95% CI: 0.026, 0.565), corresponding to a relative vaccine efficacy of 87.8% (95% CI: 43.6%, 97.4%). Under our proposed causal framework, we estimated that the direct effect, or the amount of vaccine protection which was not mediated by the increase in post-vaccination HAI titers, was 0.148 (95% CI: 0.03, 0.706); the indirect effect was estimated at 0.827. Using the ratio between the estimated log HRs for indirect and total effects, we estimated the proportion of relative vaccine effect mediated by the rise in higher antibody titers in adjuvant vaccinated children as 9.02%. There was no interaction observed between the post-vaccination titers and the vaccine formulation (p=0.576).
| Antigen | Pre-vaccination titers | p | Post-vaccination titers | p | ||||
|---|---|---|---|---|---|---|---|---|
| All | aTIV | QIV | All | aTIV | QIV | |||
| A/ H1N1 | 56.21 | 80.93 | 42.16 | <0.001 | 211.72 | 478.88 | 111.21 | <0.001 |
| 7.27 | 6.71 | 7.39 | 5.53 | 3.21 | 6.03 | |||
| A/ H3N2 | 60.23 | 68.8 | 54.22 | 0.155 | 259.12 | 355.22 | 202.05 | <0.001 |
| 6.87 | 7 | 6.76 | 4.46 | 3.49 | 5.07 | |||
| B/ Victoria |
17.2 | 19.95 | 15.3 | 0.04 | 111.99 | 120.42 | 105.75 | 0.323 |
| 4.41 | 4.49 | 4.31 | 4.56 | 4.54 | 4.58 | |||
| B/ Yamagata |
25.85 | 26.73 | 25.17 | 0.667 | 148.37 | 122.89 | 172.15 | 0.014 |
| 5 | 4.97 | 5.04 | 4.84 | 5.07 | 4.59 | |||
Table 2: Geometric mean pre-and post-vaccination HAI titers (mean, SD) and significance of difference between vaccine groups (unadjusted)
Figure 2: Distribution of post-vaccination HAI titers against the vaccine antigen for influenza A/H3N2
Note: Where black colour=Quadrivalent vaccinees and red colour=Adjuvanted vaccines; Vertical lines denote the interquartile range of titer values.
Figure 3A: Histogram of post-vaccination HAI titers, by vaccine allocation
Note: Where quadrivalent vaccinees are presented in black and adjuvanted vaccinees in red. Bars represent the count of children with HAI titers in each range of titer dilutions, shown as the reciprocal of the titer value (ranging from <10 to 1280).
Figure 3B: Histogram of post-vaccination HAI titers in children who went on to develop influenza A/H3N2 infection, by vaccine allocation
Note: Where quadrivalent vaccinees are presented in black and adjuvanted vaccinees in red. Bars represent the count of children with HAI titers in each range of titer dilutions, shown as the reciprocal of the titer value (ranging from <10 to 1280).
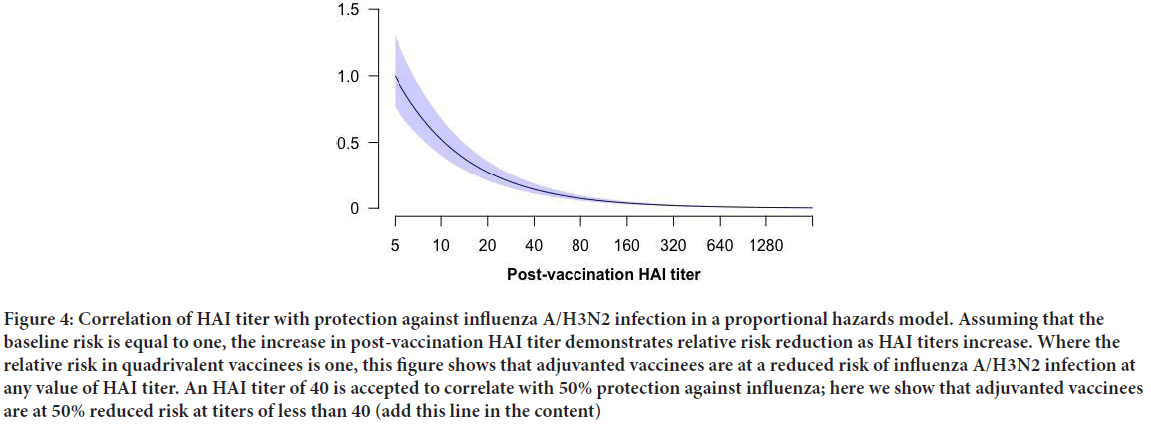
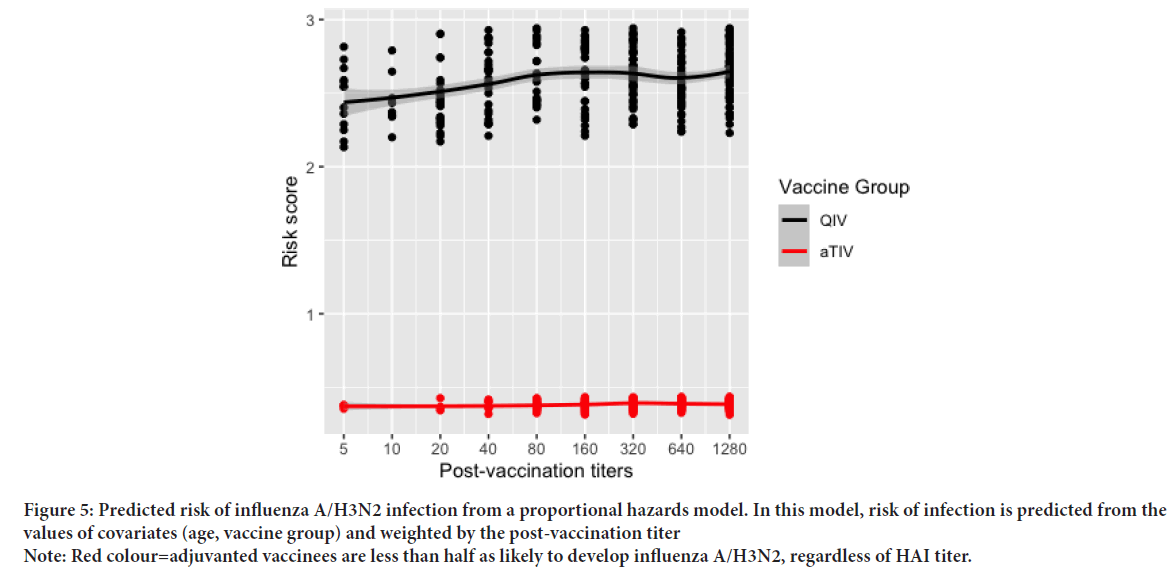
We conducted a sensitivity analysis to account for pre-vaccination HAI titers, and the potential antibody ceiling effect, which might impact the magnitude of post-vaccination titer increases or fold-change. In QIV vaccinated children, 21% of children had no change between pre and post-vaccination titers. Similarly, 18% of aTIV vaccinees had identical titers between time points. In our sensitivity model including pre-vaccination titers for each child, the total effect was estimated at 0.1305 (95% CI: 0.032, 0.532). The direct effect was 0.129 (95% CI: 0.032, 0.519). The indirect effect was estimated at 1.01%, suggesting no mediation as shown in Figure 4, the predicted hazard of infection in aTIV vaccinees relative to QIV vaccines declined well before the accepted correlate of influenza protection HAI titer of 1:40. To estimate a relative risk reduction of 50%, we conducted a post-hoc analysis of the predicted risk scores at each titer dilution. We tested for a difference in the mean risk scores at each titer value by vaccine group using Welch’s two-sample t-test, assuming unequal variance. Significant differences in the mean predicted risk scores were observed at every dilution (p<0.05 for all outcomes) (Table 3). Differences in risk scores by vaccine group where tested in each stratum of post-vaccination titers using Welch’s two sample t-test, assuming unequal variance. Of note, we observed that relative to QIV vaccinees, children in the aTIV group were predicted to have a risk reduction of 50% or greater, regardless of the post-vaccination titer (Figure 5).
| HAI Titer |
n (%) | Predicted risk score (mean, SD) | 95% CI | p | ||
|---|---|---|---|---|---|---|
| All children | aTIV | QIV | ||||
| 5 | 17 (3.1%) | 1.97 (0.94) | 0.37 (0.01) | 2.47 (0.22) | 1.96, 2.23 | <0.001 |
| 10 | 8 (1.5%) | 2.45 (0.19) | - | 2.45 (0.19) | - | - |
| 20 | 30 (5.5%) | 2.18 (0.74) | 0.37 (0.04) | 2.45 (0.19) | 2.00, 2.17 | <0.001 |
| 40 | 35 (6.5%) | 2.08 (0.96) | 0.38 (0.03) | 2.59 (0.21) | 2.13, 2.30 | <0.001 |
| 80 | 56 (10.3%) | 1.38 (1.13) | 0.38 (0.03) | 2.62 (0.20) | 2.16, 2.32 | <0.001 |
| 160 | 64 (11.8%) | 1.54 (1.15) | 0.38 (0.03) | 2.64 (0.22) | 2.18, 2.34 | <0.001 |
| 320 | 101 (18.6%) | 1.68 (1.12) | 0.39 (0.03) | 2.63 (0.18) | 2.19, 2.29 | <0.001 |
| 640 | 84 (15.5%) | 1.46 (1.11) | 0.39 (0.03) | 2.58 (0.18) | 2.13, 2.25 | <0.001 |
| 1280 | 147 (27.1%) | 1.50 (1.14) | 0.39 (0.03) | 2.65 (0.19) | 2.22, 2.31 | <0.001 |
Table 3: Mean predicted risk scores (SD) of post-vaccination titers, after adjustment for vaccine, age and colony
Figure 4: Correlation of HAI titer with protection against influenza A/H3N2 infection in a proportional hazards model. Assuming that the baseline risk is equal to one, the increase in post-vaccination HAI titer demonstrates relative risk reduction as HAI titers increase. Where the relative risk in quadrivalent vaccinees is one, this figure shows that adjuvanted vaccinees are at a reduced risk of influenza A/H3N2 infection at any value of HAI titer. An HAI titer of 40 is accepted to correlate with 50% protection against influenza; here we show that adjuvanted vaccinees are at 50% reduced risk at titers of less than 40 (add this line in the content)
Figure 5: Predicted risk of influenza A/H3N2 infection from a proportional hazards model. In this model, risk of infection is predicted from the values of covariates (age, vaccine group) and weighted by the post-vaccination titer
Note: Red colour=adjuvanted vaccinees are less than half as likely to develop influenza A/H3N2, regardless of HAI titer
Discussion
Our study evaluated the proportion of relative vaccine protection which is conferred by in rise in post-vaccination HAI titers induced by adjuvanted influenza vaccines. The MF59 adjuvant has been shown to increase flu vaccine immunogenicity and efficacy (Vesikari T, et al., 2009). Despite the observed changes in vaccine effectiveness, the mechanism by which MF59 acts to enhance protection remains unclear (O’hagan DT, et al., 2012). The HAI titer of <1:40 is the accepted correlate of protection against influenza, by which vaccine efficacy is assessed (FDA, 2017). In our study, we observed that the additional vaccine protection afforded by adjuvanted vaccinees was not largely driven by the greater HAI titers in this group. This finding offers interesting insight on both the utility of the HAI titer as a correlate of protection in children, and the adjuvant effect of MF59 on vaccine efficacy. Our finding suggests that post-vaccination HAI titers mediate little of the relative protection of adjuvanted influenza vaccine, an estimated 9.0%. Despite non-significant mediation attributable to HAI responses, adjuvanted vaccinees had significantly improved vaccine efficacy against A/H3N2. This suggests that the MF59 adjuvant, while it does induce stronger antibody responses to vaccination, does not improve protection viathis pathway. Studies in children have shown that seroprotection evoked by MF59-adjuvanted vaccines in children is superior to the immune responses following nonadjuvanted vaccines (Zedda L, et al., 2015; Nolan T, et al., 2014; Vesikari T, et al., 2015). However, studies evaluating the HAI threshold of <1:40 in children have found that it was not consistently correlated with protection against influenza, with new thresholds proposed of <1:110 (Black S, et al., 2011; Ng S, et al., 2019). Our study found that while immunogenicity was significantly enhanced in adjuvanted vaccinees, the relative protection of the adjuvanted vaccine was not conclusively mediated by the rise in HAI titers. This would suggest that MF59 adjuvant may confer improved relative vaccine efficacy via alternate pathways, such induction of innate-and cell-mediated immune responses. To our knowledge, our study is the first to use a causal mediation framework to assess relative vaccine protection. Cowling BJ, et al., 2019 used a similar framework to model the proportion of vaccine protection against influenza B that was mediated by HAI titers, as compared to unvaccinated children. This study found that post-vaccination HAI titers mediated 57% of an overall vaccine efficacy of 68% against influenza B. Strengths of this work included the use of an unvaccinated comparator group, and its randomized controlled study design. In contrast, our study assessed the relative protection of adjuvanted versus non-adjuvanted influenza vaccines against influenza A/H3N2. Given that all study participants were vaccinated and mounted HAI titers, we would expect to see a more modest effect than might have been observed if we had compared with an unvaccinated control group. Our study also differs in that it investigates influenza A/H3N2, rather than B. Both antibody and cell-mediated immune responses have been implicated in adaptive immunity against influenza A types (Sridhar S, et al., 2015; Eickhoff CS, et al., 2019; Ellebedy AH, et al., 2014). It is possible that the effect of HAI titers on vaccine-induced protection may differ according to influenza type. Further work to compare the relative proportion of protection mediated by antibody titers against influenza across types and subtypes would have valuable insight for novel vaccine development.
Studies have shown that early life exposures to influenza imprint the host immune system, influencing the immune responses and magnitude of protection against subsequent exposures (Davenport FM, et al., 1953; Gostic KM, et al., 2019; Dugan HL, et al., 2020; Fonville JM, et al., 2014; Nuñez IA, et al., 2017). Effects can include blunting of the immune response and epitope-biased responses, wherein antibody titers are increased to the novel exposure but absolute titers to primary exposure strains remain highest due to back boosting (Fonville JM, et al., 2014; Zarnitsyna VI, et al., 2016). In our cohort, most influenza A/H3N2 cases occurred in season two and the mean age of children enrolled during this season was 4.5 years. Hence, as suggested by the literature (Séblain GE, et al., 2019), children in this season would have already experienced an exposure to influenza, likely A/H3N2. This may mean that a significant proportion of HAI titers mounted in response to vaccination were memory responses to a previously encountered strain, rather than the vaccine antigen. Therefore, it is very likely that primary A/H3N2 exposures in study subjects were to antigenically drifted strains of A/H3N2 in previous seasons, which had acquired mutations in antigenic site B (Chambers BS, et al., 2015; Zost SJ, et al., 2017). These prior immune histories may have influenced the HAI responses to study vaccines, potentially limiting the quality and appropriateness of HAI titers and subsequent protection conferred.
There is a growing body of work on the effect of repeated vaccination on immunogenicity and efficacy of influenza vaccines (Belongia EA, et al., 2017; McLean HQ, et al., 2016; Thompson MG , et al., 2016; Vesikari T , et al., 2020; Ramsay LC , et al., 2019). A seminal modelling study by Smith DJ, et al., 1999 proposed the antigenic distance hypothesis, which predicts that vaccine efficacy is reduced when the vaccine antigen is closely similar to the prior season antigen, but dissimilar to the circulating strain. In season two of our study, the vaccine antigen was unchanged from the previous season recommendation; however, phylogenetic analyses of A/H3N2 evolution showed rapid mutation and clade diversification from 2013-2018 (Chambers BS, et al., 2015; Potter BI, et al., 2019; Skowronski DM, et al., 2022). Skowronski DM, et al., 2017 found that prior influenza vaccination had mixed effects across seasons, consistent with the antigenic distance hypothesis. Prior seasonal vaccination was significantly associated with negative impact on current VE during the 2017-2018 season, in which vaccine antigens were homologous with the previous season’s formulation but circulating strains had the greatest phylogenetic distance from the vaccine antigen (Skowronski DM, et al., 2017). This would suggest that both viral evolution and changes in host immunity due to serial vaccination may contribute to poor vaccine effectiveness during season two of our study.
As a result of the potential interference by repeat vaccinations and the acquisition of mutations in A/H3N2 which may have impeded efficient antibody binding during season two, HAI titers to the vaccine antigen may not have been sufficiently cross-protective. Therefore, it is challenging to ascertain the true proportion of relative vaccine protection which is mediated by the adjuvant-induced rise in HAI titers, had the vaccine been better matched to the seasonal circulating strain. Further study is warranted to investigate the causal mediation of relative adjuvanted vaccine protection by HAI titers during a matched influenza vaccine season. Our study has several other limitations, including the very small number of PCR-confirmed influenza cases, particularly in the adjuvanted group. It is possible that the low proportion of effect mediated by the rise in HAI titers in this group is underestimated due to the small sample size. Among confirmed influenza cases, HAI titers were not available for all children, further reducing our number of events and decreasing statistical power.
Conclusion
Our study provides unique insight on the potential mechanism of adjuvant-induced influenza vaccine protection. We found that while adjuvantation results in significant differences in post-vaccination titers, and confers significantly superior protection against influenza A/H3N2 infections, the increased HAI titers do not conclusively mediate this protection in adjuvanted vaccinees. We observed that relative to QIV vaccinees, the hazard of A/H3N2 in adjuvanted children was reduced by 50% at titers >1:20. Possible explanations for this include adjuvant-induced innate or cell-mediated immune responses. Moreover, influenza infections in our study were from a season of co-circulating H3N2 strains from divergent clades. Despite the differing epidemic strains, protection was significantly better in the adjuvanted group relative to non-adjuvanted vaccinees.
This suggests that adjuvanted influenza vaccine may confer enhanced cross-protection against novel influenza antigens, potentially in seasons of vaccine mismatch. This would provide strategic data for the development of novel vaccine candidates against influenza A types, particularly A/H3N2, which shows consistently weaker vaccine effectiveness. Further research on the potential cross-protection conferred by adjuvanted influenza vaccine would be powerful for the design of next generation vaccines and adjuvant formulations.
Acknowledgement
The authors would like to acknowledge the assistance of the McGill University Health Center, notably Dr. Brian Ward and his laboratory team.
References
- Hobson DR, Curry RL, Beare AS, Ward-Gardner A. The role of serum haemagglutination-inhibiting antibody in protection against challenge infection with influenza A2 and B viruses. J Hyg (Lond). 1972; 70(4): 767-777.
[Crossref] [Google scholar] [Pubmed]
- Cowling BJ, Lim WW, Perera RA, Fang VJ, Leung GM, Peiris JM, et al. Influenza hemagglutination-inhibition antibody titer as a mediator of vaccine-induced protection for influenza B. Clin Infect Dis. 2019; 68(10): 1713-1717.
[Crossref] [Google scholar] [Pubmed]
- Vesikari T, Knuf M, Wutzler P, Karvonen A, Kieninger-Baum D, Schmitt HJ, et al. Oil-in-water emulsion adjuvant with influenza vaccine in young children. N Engl J Med. 2011; 365(15): 1406-1416.
[Crossref] [Google scholar] [Pubmed]
- Knuf M, Leroux-Roels G, Rümke HC, Abarca K, Rivera L, Lattanzi M, et al. Immunogenicity and tolerability of an MF59-adjuvanted, egg-derived, A/H1N1 pandemic influenza vaccine in children 6-35 months of age. Pediatr Infect Dis J. 2014; 33: e320-e329.
[Crossref] [Google scholar] [Pubmed]
- Vesikari T, Kirstein J, Go GD, Leav B, Ruzycky ME, Isakov L, et al. Efficacy, immunogenicity, and safety evaluation of an MF59-adjuvanted quadrivalent influenza virus vaccine compared with non-adjuvanted influenza vaccine in children: A multicentre, randomised controlled, observer-blinded, phase 3 trial. Lancet Respir Med. 2018; 6(5): 345-356.
[Crossref] [Google scholar] [Pubmed]
- Oâ??hagan DT, Ott GS, de Gregorio E, Seubert A. The mechanism of action of MF59-an innately attractive adjuvant formulation. Vaccine. 2012; 30(29): 4341-4348.
[Crossref] [Google scholar] [Pubmed]
- Cioncada R, Maddaluno M, Vo HT, Woodruff M, Tavarini S, Sammicheli C, et al. Vaccine adjuvant MF59 promotes the intranodal differentiation of antigen-loaded and activated monocyte-derived dendritic cells. PloS one. 2017; 12(10): e0185843.
[Crossref] [Google scholar] [Pubmed]
- Zedda L, Forleo-Neto E, Vertruyen A, Raes M, Marchant A, Jansen W, et al. Dissecting the immune response to MF59-adjuvanted and nonadjuvanted seasonal influenza vaccines in children less than three years of age. Pediatr Infect Dis J. 2015; 34(1): 73-78.
[Crossref] [Google scholar] [Pubmed]
- Khurana S, Verma N, Yewdell JW, Hilbert AK, Castellino F, Lattanzi M, et al. MF59 adjuvant enhances diversity and affinity of antibody-mediated immune response to pandemic influenza vaccines. Sci Transl Med. 2011; 3(85): 85ra48.
[Crossref] [Google scholar] [Pubmed]
- Nolan T, Bravo L, Ceballos A, Mitha E, Gray G, Quiambao B, et al. Enhanced and persistent antibody response against homologous and heterologous strains elicited by a MF59 ®-adjuvanted influenza vaccine in infants and young children. Vaccine. 2014; 32(46): 6146-6156.
[Crossref] [Google scholar] [Pubmed]
- Davenport FM, Hennessy AV, Francis Jr T, With the Technical Assistance of Phyllis Fabisch. Epidemiologic and immunologic significance of age distribution of antibody to antigenic variants of influenza virus. J Exp Med. 1953; 98(6): 641-656.
[Crossref] [Google scholar] [Pubmed]
- Ranjeva S, Subramanian R, Fang VJ, Leung GM, Ip DK, Perera RA, et al. Age-specific differences in the dynamics of protective immunity to influenza. Nature Communications. 2019; 10(1): 1-1.
[Crossref] [Google scholar] [Pubmed]
- Manual for the laboratory diagnosis and virological surveillance of influenza. World Health Organization (WHO). 2011..
- Tchetgen TEJ. Inverse odds ratio-weighted estimation for causal mediation analysis. Stat Med. 2013; 32: 4567-4580.
[Crossref] [Google scholar] [Pubmed]
- Team RC. R: A language and environment for statistical computing. Global
Biodiversity Information Facility (GBIF). 2020. - Vesikari T, Pellegrini M, Karvonen A, Groth N, Borkowski A, Oâ??Hagan DT, et al. Enhanced immunogenicity of seasonal influenza vaccines in young children using MF59 adjuvant. Pediatr Infect Dis J. 2009; 28: 563-571.
[Crossref] [Google scholar] [Pubmed]
- Guidance for industry-clinical data needed to support the licensure of seasonal inactivated influenza vaccines. FDA. 2007.
- Vesikari T, Forstén A, Arora A, Tsai T, Clemens R. Influenza vaccination in children primed with MF59 ®-adjuvanted or non-adjuvanted seasonal influenza vaccine. Hum Vaccin Immunother. 2015; 11(8): 2102-2112.
[Crossref] [Google scholar] [Pubmed]
- Black S, Nicolay U, Vesikari T, Knuf M, Del Giudice G, Della Cioppa G, et al. Hemagglutination inhibition antibody titers as a correlate of protection for inactivated influenza vaccines in children. Pediatr Infect Dis J. 2011; 30(12): 1081-1085.
[Crossref] [Google scholar] [Pubmed]
- Ng S, Nachbagauer R, Balmaseda A, Stadlbauer D, Ojeda S, Patel M, et al. Novel correlates of protection against pandemic H1N1 influenza A virus infection. Nat med. 2019; 25(6): 962-967.
[Crossref] [Google scholar] [Pubmed]
- Sridhar S, Begom S, Hoschler K, Bermingham A, Adamson W, Carman W, et al. Longevity and determinants of protective humoral immunity after pandemic influenza infection. Am J Respir Crit Care Med. 2015; 191(3): 325-332.
[Crossref] [Google scholar] [Pubmed]
- Eickhoff CS, Terry FE, Peng L, Meza KA, Sakala IG, van Aartsen D, et al. Highly conserved influenza T cell epitopes induce broadly protective immunity. Vaccine. 2019; 37(36): 5371-5381.
[Crossref] [Google scholar] [Pubmed]
- Ellebedy AH, Krammer F, Li GM, Miller MS, Chiu C, Wrammert J, et al. Induction of broadly cross-reactive antibody responses to the influenza HA stem region following H5N1 vaccination in humans. Proc Natl Acad Sci USA. 2014; 111(36): 13133-13138.
[Crossref] [Google scholar] [Pubmed]
- Gostic KM, Bridge R, Brady S, Viboud C, Worobey M, Lloyd-Smith JO. Childhood immune imprinting to influenza A shapes birth year-specific risk during seasonal H1N1 and H3N2 epidemics. PLoS pathog. 2019; 15(12): e1008109.
[Crossref] [Google scholar] [Pubmed]
- Dugan HL, Guthmiller JJ, Arevalo P, Huang M, Chen YQ, Neu KE, et al. Preexisting immunity shapes distinct antibody landscapes after influenza virus infection and vaccination in humans. Sci Transl Med. 2020; 12(573): eabd3601.
[Crossref] [Google scholar] [Pubmed]
- Fonville JM, Wilks SH, James SL, Fox A, Ventresca M, Aban M, et al. Antibody landscapes after influenza virus infection or vaccination. Science. 2014; 346(6212): 996-1000.
[Crossref] [Google scholar] [Pubmed]
- Nuñez IA, Carlock MA, Allen JD, Owino SO, Moehling KK, Nowalk P, et al. Impact of age and pre-existing influenza immune responses in humans receiving split inactivated influenza vaccine on the induction of the breadth of antibodies to influenza A strains. PLoS One. 2017; 12(11): e0185666.
[Crossref] [Google scholar] [Pubmed]
- Zarnitsyna VI, Lavine J, Ellebedy A, Ahmed R, Antia R. Multi-epitope models explain how pre-existing antibodies affect the generation of broadly protective responses to influenza. PLoS pathog. 2016; 12(6): e1005692.
[Crossref] [Google scholar] [Pubmed]
- Séblain GE, Moureau A, Schiffler C, Dupuy M, Pepin S, Samson SI, et al. Epidemiology and burden of influenza in healthy children aged 6 to 35 months: Analysis of data from the placebo arm of a phase III efficacy trial. BMC infectious diseases. 2019; 19(1): 1-6.
[Crossref] [Google scholar] [Pubmed]
- Chambers BS, Parkhouse K, Ross TM, Alby K, Hensley SE. Identification of hemagglutinin residues responsible for H3N2 antigenic drift during the 2014-2015 influenza season. Cell rep. 2015; 12(1): 1-6.
[Crossref] [Google scholar] [Pubmed]
- Zost SJ, Parkhouse K, Gumina ME, Kim K, Perez DS, Wilson PC, et al. Contemporary H3N2 influenza viruses have a glycosylation site that alters binding of antibodies elicited by egg-adapted vaccine strains. Proc Natl Acad Sci USA. 2017; 114(47): 12578-12583.
[Crossref] [Google scholar] [Pubmed]
- Belongia EA, Skowronski DM, McLean HQ, Chambers C, Sundaram ME, de Serres G. Repeated annual influenza vaccination and vaccine effectiveness: review of evidence. Expert rev vaccines. 2017; 16(7): 723-736.
[Crossref] [Google scholar] [Pubmed]
- McLean HQ, Thompson MG, Sundaram ME, Meece JK, McClure DL, Friedrich TC, et al. Impact of repeated vaccination on vaccine effectiveness against influenza A (H3N2) and B during 8 seasons. Clin Infect Dis. 2014; 59(10): 1375-1385.
[Crossref] [Google scholar] [Pubmed]
- Thompson MG, Naleway A, Fry AM, Ball S, Spencer SM, Reynolds S, et al. Effects of repeated annual inactivated influenza vaccination among healthcare personnel on serum hemagglutinin inhibition antibody response to A/Perth/16/2009 (H3N2)-like virus during 2010-11. Vaccine. 2016; 34(7): 981-988.
[Crossref] [Google scholar] [Pubmed]
- Vesikari T, Ramsey K, Pitisuttithum P, Capeding R, Heijnen E, Sawlwin D, et al. Repeated exposure to an MF-59 adjuvanted quadrivalent subunit influenza vaccine (aQIV) in children: Results of two revaccination studies. Vaccine. 2020; 38(51): 8224-8231.
[Crossref] [Google scholar] [Pubmed]
- Ramsay LC, Buchan SA, Stirling RG, Cowling BJ, Feng S, Kwong JC, et al. The impact of repeated vaccination on influenza vaccine effectiveness: A systematic review and meta-analysis. BMC Med. 2019; 17(1): 1-6.
[Crossref] [Google scholar] [Pubmed]
- Smith DJ, Forrest S, Ackley DH, Perelson AS. Variable efficacy of repeated annual influenza vaccination. Proc Natl Acad Sci. 1999; 96: 14001-14006.
[Crossref] [Google scholar] [Pubmed]
- Potter BI, Kondor R, Hadfield J, Huddleston J, Barnes J, Rowe T, et al. Evolution and rapid spread of a reassortant A(H3N2) virus that predominated the 2017-2018 influenza season. Virus Evol. 2019; 5(2): vez046.
[Crossref] [Google scholar] [Pubmed]
- Skowronski DM, Leir S, Sabaiduc S, Chambers C, Zou M, Rose C, et al. Influenza vaccine effectiveness by A(H3N2) phylogenetic subcluster and prior vaccination history: 2016-2017 and 2017-2018 epidemics in Canada. J Infect Dis. 2022; 225(8): 1387-1398.
- Skowronski DM, Chambers C, de Serres G, Sabaiduc S, Winter A-L, Dickinson JA, et al. Serial vaccination and the antigenic distance hypothesis: Effects on influenza vaccine effectiveness during A(H3N2) epidemics in Canada, 2010-2011 to 2014-2015. J Infect Dis. 2017; 215: 1059-1099.
[Crossref] [Google scholar] [Pubmed]
Author Info
Charlotte Switzer1*, Chris P Verschoor1, Eleanor Pullenayegum1,2, Pardeep Singh1 and Mark Loeb32Department of Child Health Evaluative Sciences, The Hospital for Sick Children, Toronto, Ontario, Canada
3Department of Pathology and Molecular Medicine, McMaster University, Hamilton, Canada
Citation: Switzer C: Relative Antibody Mediated Protection against Influenza in Vaccinated Children
Received: 01-Nov-2022 Accepted: 25-Nov-2022 Published: 02-Dec-2022, DOI: 10.31858/0975-8453.13.10.847-854
Copyright: This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
ARTICLE TOOLS
- Dental Development between Assisted Reproductive Therapy (Art) and Natural Conceived Children: A Comparative Pilot Study Norzaiti Mohd Kenali, Naimah Hasanah Mohd Fathil, Norbasyirah Bohari, Ahmad Faisal Ismail, Roszaman Ramli SRP. 2020; 11(1): 01-06 » doi: 10.5530/srp.2020.1.01
- Psychometric properties of the World Health Organization Quality of life instrument, short form: Validity in the Vietnamese healthcare context Trung Quang Vo*, Bao Tran Thuy Tran, Ngan Thuy Nguyen, Tram ThiHuyen Nguyen, Thuy Phan Chung Tran SRP. 2020; 11(1): 14-22 » doi: 10.5530/srp.2019.1.3
- A Review of Pharmacoeconomics: the key to “Healthcare for All” Hasamnis AA, Patil SS, Shaik Imam, Narendiran K SRP. 2019; 10(1): s40-s42 » doi: 10.5530/srp.2019.1s.21
- Deuterium Depleted Water as an Adjuvant in Treatment of Cancer Anton Syroeshkin, Olga Levitskaya, Elena Uspenskaya, Tatiana Pleteneva, Daria Romaykina, Daria Ermakova SRP. 2019; 10(1): 112-117 » doi: 10.5530/srp.2019.1.19
- Dental Development between Assisted Reproductive Therapy (Art) and Natural Conceived Children: A Comparative Pilot Study Norzaiti Mohd Kenali, Naimah Hasanah Mohd Fathil, Norbasyirah Bohari, Ahmad Faisal Ismail, Roszaman Ramli SRP. 2020; 11(1): 01-06 » doi: 10.5530/srp.2020.1.01
- Manilkara zapota (L.) Royen Fruit Peel: A Phytochemical and Pharmacological Review Karle Pravin P, Dhawale Shashikant C SRP. 2019; 10(1): 11-14 » doi: 0.5530/srp.2019.1.2
- Pharmacognostic and Phytopharmacological Overview on Bombax ceiba Pankaj Haribhau Chaudhary, Mukund Ganeshrao Tawar SRP. 2019; 10(1): 20-25 » doi: 10.5530/srp.2019.1.4
- A Review of Pharmacoeconomics: the key to “Healthcare for All” Hasamnis AA, Patil SS, Shaik Imam, Narendiran K SRP. 2019; 10(1): s40-s42 » doi: 10.5530/srp.2019.1s.21
- A Prospective Review on Phyto-Pharmacological Aspects of Andrographis paniculata Govindraj Akilandeswari, Arumugam Vijaya Anand, Palanisamy Sampathkumar, Puthamohan Vinayaga Moorthi, Basavaraju Preethi SRP. 2019; 10(1): 15-19 » doi: 10.5530/srp.2019.1.3