Research Article - (2023) Volume 14, Issue 3
Role of Non-Coding Endogenous RNA in Osteoarthritis
Gunjan Kumar* and Amit SinghAbstract
The main objective of this project report is to study and brief about Osteoarthritis (OA), pathogenetic role of miRNAs and association or relation of Osteoarthritis with asthma and cardiovascular diseases. Osteoarthritis is a syndrome of joint pain caused by destruction or degeneration of joints and disability in an individual, the main causes of OA includes joint injuries, obesity, aging and genetic. The current review summarizes the critical role of miRNA in determining the complex gene expression patterns of OA and other diseases. Its role in the pathophysiology of OA, as well as the potential of miRNA as a biomarker and therapeutic target for OA, will be discussed and it also focuses in the relation and association of Osteoarthritis with Asthma and cardiovascular diseases.
Keywords
Osteoarthritis (OA), miRNAs, Pathogenesis, Asthma, Cardiovascular diseases
Introduction
The most well-known kind of joint pain and one of the main sources of incapacity is Osteoarthritis (OA). This degenerative and moderate joint illness influences around 250 million individuals world-wide. The populace with the most elevated gamble of creating OA is the old (roughly 35% of patients are 65 years of age), females, stout patients. Given the populace’s inclination to carry on with longer lives and our country’s ever-evolving expansion in corpulence, the quantity of impacted patients is relied upon to soar before very long. It is a concerning issue due to the practical impedance and inability related with this condition, as well as the adverse consequence on our general public’s social and monetary angles (Mora JC, et al., 2018).
Osteoarthritis can affect every joint, although the hands, knees, hips, and feet are the most affected. Pathologic alterations in cartilage, bone, synovium, ligament, muscle, and periarticular fat causes joint dysfunction, pain, stiffness, functional limitations, and the loss of treasured activities like walking and dancing. Risk factors include age (33% of those over 75 have symptomatic and radiographic knee OA), female sex, obesity, heredity, and significant joint damage. People with OA have more comorbidities and are less active than those without the disease. Physical inactivity is linked to a 20% increase in age-adjusted mortality. Two physical examination findings that can help diagnose the condition are bone enlargement in knee OA and discomfort caused by internal hip rotation in hip OA (Katz JN, et al., 2021) (Figure 1).
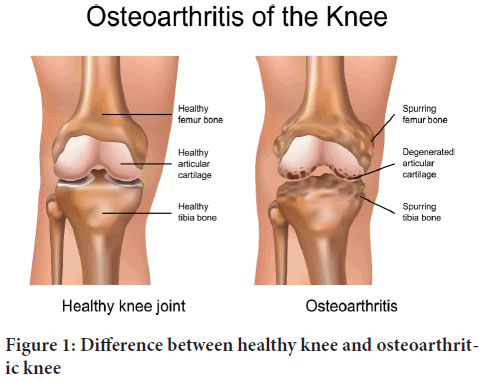
Figure 1: Difference between healthy knee and osteoarthritic knee
Symptoms are often accompanied by functional impairment. The condition is largely defined by cartilage degeneration although it also includes moderate to severe synovial membrane inflammation, subchondral bone re-modeling, and osteophytosis. The formation of an osteophyte is referred to as osteophytosis. Osteophytes are bony outgrowths at the joint margins that are covered in fibrocartilage. Osteophytes in OA often form on the exterior of the joints cortical bone and originate from the periosteum. Mechanical forces, growth factors, and cytokines that promote mesenchymal stem cell proliferation in the periosteum all contribute to osteophyte formation (Junker S, et al., 2016).
Literature Review
Types of osteoarthritis
Primary osteoarthritis: This is the most common type of Osteoarthritis, and it is thought to be primarily caused by “wear and tear” over time. As a result, it is associated with ageing; in fact, age is the most powerful risk factor for OA, and the longer a person uses their joints, the more likely it is that they will develop this type of OA. In theory, this indicates that if we live long enough, we will get primary OA.
This type of Osteoarthritis usually appears around the age of 55 or 60. Because it can be localized to specific joints, primary OA is usually classified by the site of involvement (hands and feet, knee, hip), though it can affect multiple joints (Yolanda S, 2022).
Secondary osteoarthritis: This form of Osteoarthritis consequences from situations that result in an alternate inside the microenvironment of the cartilage. Such situations include considerable trauma, congenital joint abnormalities, metabolic defects (Wilson ailment), infections, diseases (neuropathic), and problems that regulate the everyday shape and function of cartilage (Eg: Rheumatoid Arthritis, gout).
Secondary osteoarthritis tends to seem in tremendously younger people aged approximately 45 or 50, not unusual threat elements that may lead to secondary Osteoarthritis consist of:
Trauma: A fractured bone (which is common in sports) increases the likelihood of a person developing OA in the injured joint. Regrettably, this also implies that the character is much more likely to develop OA at a younger age than those who have primary OA.
Weight problems: Three to six times a person’s frame weight is transmitted across the knees in a single leg stance. As a result, it stands to reason that an increase in body weight could result in additional force across the knees while walking. This weight puts strain on the joints (particularly the knees and hips) and causes them to wear out faster.
Sedentary life-style: Not only does this promote weight loss, but it is also associated with weaker muscle tissues and tendons surrounding the joints. This increases the risk of developing OA because the muscle tissue is not strong enough to keep the joints effectively aligned, stable, and supported. Because of this, it is critical to participate in low-impact sports that emphasise stretching, strengthening, posture, and range of motion. These consist of aerobics, swimming and yoga.
Heredity: Epidemiological studies of own family history have currently shed proof of a genetic impact on OA (mainly within the hands, knees and hips). Twin studies have revealed that heritability varies depending on the affected joint, but on average, they suggest a heritability of OA of 50% or higher. Researches have also counseled the involvement of unique chromosomes (for example, 2q, 9q, 11q, and 16p) as well as genes like CRTM (Cartilage Matrix Protein), CRTL (Cartilage Link Protein), and collagen II, IX, and XI.
Joint overuse: Due to the occupations/hobbies, few people severally strain the joints by placing pressure on them for longer periods, which progressively causes the muscle groups become tired and not function as powerful joint protectors.
Other conditions: Those may additionally consist of peripheral neuropathies and neuromuscular problems that positioned strange stress at the joint. Sicknesses that purpose irritation, including rheumatoid arthritis, can increase your danger of having Osteoarthritis later in lifestyles (Yolanda S, 2022).
Symptoms and causes of osteoarthritis
Osteoarthritis symptoms are developed slowly and gets worsen over the period of time. Signs and symptoms include:
• Pain: Affected joints might hurt during or after work or movement.
• Stiffness: Joints Stiffness might be most seen upon awakening or after being at rest or inactive.
• Tenderness: Joints might feel tender when light pressure applied.
• Loss of flexibility: Movement of joints gets restricted or may not able to move through its full range of motion.
• Grating sensation: Grating sensation when the joints are used, popping or crackling can be heard.
• Bone spurs: Extra bone, which feels like hard lumps, can form around affected joints.
• Swelling: This could be due to soft tissue inflammation surrounding the joint.
Cartilage acts as cushion of the bone ends and when this cartilage or joints gets deteriorates then Osteoarthritis occurs. Cartilage acts as slippery, firm tissue for the frictionless joint motion. Osteoarthritis is additionally referred to as wear and tear tissues. Osteoarthritis may occur in whole joints due to the breakdown of the cartilage. Those connective tissues that hold the joint together and fasten muscle to the bone can causes the change in deteriorates of the bone. Within the joint lining it’s going to causes inflammation. Then the Osteoarthritis joint cartilage become stiffer and loses its elasticity and its make more vulnerable to damage. In some areas, Osteoarthritis cartilage may wear away with the age and its ability of shock absorption gets decreased. Cartilage deterioration can cause pain as a result of stretching the tendon and ligament. If condition worsen out then bones may rub against one another.
Osteoarthritis symptoms may develop gradually triggering aching within the joint and soreness, especially with movement. Overuse and long period of inactivity, it’s going to cause pain. After remainder of periods, it’s going to causes stiffness. Within the middle and end joints of finger, bone may get enlarged and it might not be painful but it causes swelling within the joints (Kellgren JH and Lawrence J, 1957).
Systems to measure or classify osteoarthritis
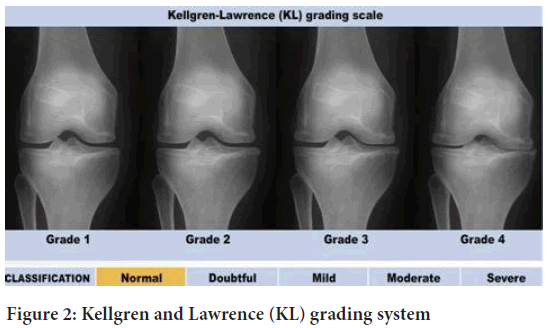
Kellgren and Lawrence system (KL grading system): The Kellgren and Lawrence system is a most common method used for classification of Osteoarthritis. It is a most used research tool in studies of Osteoarthritis and also used in development of radiographic features of Osteoarthritis.
Kellgren and Lawrence proposed this classification in 1957. It was later accepted as the radiological definition of Osteoarthritis by WHO (World Health Organization) in 1961 (Kellgren JH and Lawrence J, 1957). The KL grading system also assists healthcare providers with treatment, specifically defining which patients may benefit most surgical management.
Based on the data, the KL grading is typically applied specifically in knee Osteoarthritis.
Kellgren and Lawrence System of osteoarthritis classification:
•Osteoarthritis is not present in grade 0 (none).
•Grade 1 (doubtful or negligible): Possible osteophytes lipping and joint space narrowing (small osteophytes).
•Grade 2 (minimal):Definite osteophytes and possible joint space narrowing, possibly with Osteoarthritis.
•Grade 3 (moderate):Moderate multiple osteophytes, definite narrowing of joint space, some sclerosis, and bone end possibility.
•Large osteophytes, most narrowing of joint space, severe sclerosis, and definite deformity of bone ends in grade 4 (severe).
Osteophytes are the boney growth that develops on bone ends (Figure 2).
Figure 2: Kellgren and Lawrence (KL) grading system
Visual Analog Scale for pain (VAS pain): The Visual Analogue Scale (VAS) includes a straight line with the endpoints defining an intense limit which includes ‘no pain at all’ and ‘ache as horrific as it is able to be’. The affected person is requested to mark his pain stage on the road between the 2 endpoints. The gap between ‘no ache in any respect’ and the mark then defines the situation’s ache. This tool became first used in psychology by way of Freyd in 1923 (Haefeli M and Elfering A, 2006).
Hayes and Patterson used a Visual Analogue Scale (VAS) as one of the first pain rating scales in 1921. It is frequently used in epidemiologic and clinical research to assess the severity or frequency of different symptoms. A patient’s pain, for example, can range from none to extreme. From the patient’s point of view, this spectrum appears continuous; their pain does not appear to be discrete, as a categorization of none, mild, moderate, and severe would imply. The VAS was created to capture the idea of an underlying continuum (Physiopedia, 2022) (Figure 3).
Figure 3: Visual Analogue Scale (VAS)
The Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC): The Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) is a widely used, proprietary set of standardized questionnaires used by fitness specialists to assess the condition of patients with knee and hip Osteoarthritis, including pain, stiffness, and joint function. The WOMAC has also been used to assess rheumatoid arthritis, juvenile rheumatoid arthritis, systemic lupus erythematous, and fibromyalgia. It is self-administered and was developed in 1982 at Western Ontario and McMaster Universities.
Better scores suggest worse pain, stiffness, and useful boundaries. The WOMAC assesses pain (score range zero-20), stiffness (score range zero-8), and 17 practical issues (rating range zero-68). Bodily functioning questions cover everyday sports which includes stair use, status up from a sitting or mendacity position, standing, bending, strolling, moving into and out of a vehicle, buying, placing on or starting off socks, lying in mattress, entering into or out of a bathtub, sitting, and heavy and light household duties. The WOMAC questions are a subset of the Hip disability and Osteoarthritis outcome rating questions (HOOS). Hence, a HOOS survey can also be used to determine a WOMAC rating (Wikipedia, 2022) (Figure 4).
Figure 4: The Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) survey form
micro RNAs
microRNA is a single-stranded, non-coding RNA molecule found in plants, animals, and certain viruses that acts in RNA silencing and post-transcriptional gene control. miRNAs function by forming base pairs with complementary sequences in mRNA molecules. A team lead by Ambros and included Lee and Feinbaum found the first miRNA in 1993. Additional insight into its manner of operation, however, needed concurrently published work by Ruvkun’s team, which included Wightman and Ha. But the term was coined in 2001 (Wikipedia, 2022).
microRNAs (miRNAs) are a type of non-coding RNA molecules (21-25-NUCLEAOTIDES) found in Eukaryotes that regulate genetic expression at the post-transcriptional stage by interacting with the 3’ Untranslated Regions (UTRs).With the discovery of new miRNAs, researchers are paying more attention to their roles in biological processes.
There are more than 2000 types of micro RNAs are present.
microRNAs are known to share regulatory elements and to live in the introns of their pre-miRNA host genes. Its expression profile and primary transcript are similar. microRNA genes maintained their transcripts with the aid of their own promoter, and only a few primary transcripts were entirely identified. The microRNA was transcribed by RNA polymerase. The big RNA precursor, Pri-miRNA, is made up of 5’cap and poly-A tail.
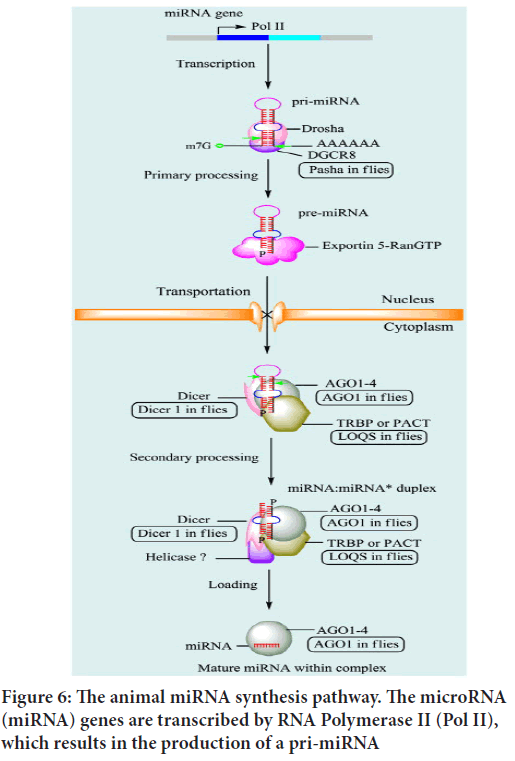
Synthesis of micro RNAs (in animals): miRNAs are single-stranded RNAs (ssRNAs) with 21-25 nucleotides that are synthesised from hairpin-shaped precursors (Wang XJ, et al., 2004). Following synthesis, miRNA transcripts are processed. In recent years, there has been a lot of research on how miRNAs are processed in animals and plants. miRNA genes are transcribed to a main miRNA in mammals (pri-miRNA). Within the nucleus, Drosha, a class 2 RNAase III enzyme, converts pri-miRNA to a precursor miRNA (pre-miRNA). Exportin-5 then facilitates the transfer of pre-miRNAs to the cytoplasm (EXP-5). They are then matured in the cytoplasm by Dicer, an RNAase III-like protein, and loaded onto the Argonaute (ago) protein to form the effector RNA-Induced Silencing Complex (RISC) (Wahid F, et al., 2010).
Genome, gene and transcriptions: Cloning and other molecular biology techniques have now identified hundreds of miRNAs and miRNA genes. Bioinformatics and computational technology methods have also been used to predict additional miRNAs and miRNA genes.
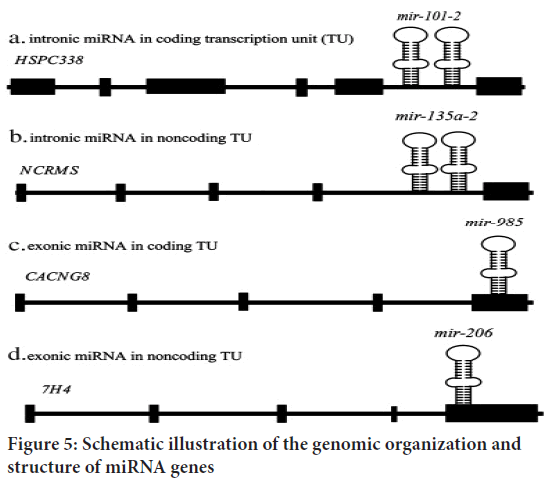
Early studies observed that most miRNAs are found in intergenic regions, with only a few found in intronic regions. Approximately half of all known miRNAs are clustered together. Polycistronic main transcripts are produced from these clustered miRNAs. In a few cases, some miRNAs can be transcribed as monocistronic primary transcripts from their own promoter. Based on their chromosomal positions, miRNA genes are classified as intronic miRNAs in coding Transcription Units (TUs), intronic miRNAs in noncoding TUs, exonic miRNAs in coding TUs, and exonic miRNAs in noncoding TUs. Although RNA polymerase II (Pol II) is primarily responsible for miRNA gene transcription, RNA polymerase III (Pol III) can transcribe a small group linked with Alu repeats (Le Graverand-Gastineau MP, 2009). Temporal control of Pol II-dependent miRNA gene expression allows for the synthesis of a specific collection of miRNAs in response to specific situations and cell types. The primordial miRNA (pri-miRNA) is the result of Pol II or Pol III-mediated production. It is several kilobases long and contains local stem loop structures (Wahid F, et al., 2010) (Figure 5).
Figure 5: Schematic illustration of the genomic organization and structure of miRNA genes
Nuclear processing: A variety of proteins are involved in miRNA processing. Initially, all animal miRNAs are processed in the nucleus. The Pol II-produced pri-miRNA is cleaved at the stem of the hairpin structure, releasing a 60-70 nt hairpin structure known as the precursor miRNA (premiRNA). Metazoan pri-miRNAs are typically made up of around 33 base pairs (bp) of stem loop, a terminal loop, and single-strand RNA (ssRNA) surrounding segments. DGCR8 interacts with and directs the ssRNA segment (Wahid F, et al., 2010) (Figure 6).
Figure 6: The animal miRNA synthesis pathway. The microRNA (miRNA) genes are transcribed by RNA Polymerase II (Pol II), which results in the production of a pri-miRNA
Transportation by Exportin-5 (EXP5): Pre-miRNAs are transported to the cytoplasm and processed further to become mature miRNAs. The nuclear pore complex, a large proteinaceous channel embedded in the nuclear envelope, transports pre-miRNA. Transport of pre-miRNA is mediated by the Ran-GTP-dependent nuclear transport receptor Exportin5 (EXP5). The proposed model of miRNA transport initiates pre-miRNA export when EXP5 recognizes a >14 bp double-stranded RNA (dsRNA) stem loop with a 3’overhang, and is then coordinated to both pre-miRNA and GTP. It is supposed to be combined with Ran, a binding cofactor in the cell nucleus. EXP5 bound to pre-miRNA is exported from the nucleus, and hydrolysis of GTP releases pre-miRNA.The proposed model of miRNA transport initiates pre-miRNA export when EXP5 recognizes a >14 bp double-stranded RNA (dsRNA) stem-loop with a 3’overhang, and is then coordinated to both pre-miRNA and GTP. EXP5 bound to pre-miRNA is exported from the nucleus, and hydrolysis of GTP releases pre-miRNA (Wahid F, et al., 2010).
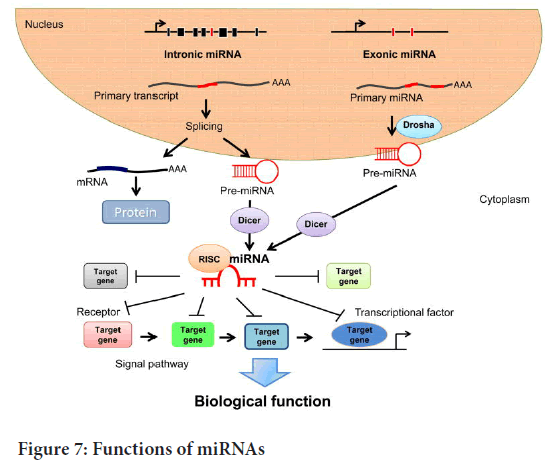
Mechanism of miRNAs: microRNAs guide miRNA-Induced Silencing Complexes (miRISCs) for specific messenger RNA recognition and down regulation of gene expression by one of two post-transcriptional mechanisms such as translational repression and mRNA cleavage. First, it was found that lin4RNA suppresses the translation of c. elegans lin14 mRNA (Wightman B, et al., 1993). According to recent studies, this allows miRISC to recognize and specifically suppress mRNA when it contains heterologous RNA recognition factors, even though there is no miRNA binding site (Telfer A and Poethig RS, 1998). According to recent research, this is the binding site for the majority of miRNAs in animal mRNAs in the 3’UTR (3’Untranslated Region) as multiple copies. Watson-Crick base pair-mediated animal mismatch explains about the binding of microRNAs to the bulge (Telfer A and Poethig RS, 1998). The miRNA binding site in plants is located near the centre of the complementary region. The majority of plant miRNAs have a high degree of sequence complementarity with the target mRNA sequence. The degree of complementarity of miRNA mRNA is very important in the process of regulatory mechanism (Pillai RS, et al., 2004). The high degree of complementarity allows precatalytic degradation of the target mRNA sequence through the mechanism process of mRNA cleavage. Therefore, there is a discrepancy between central degradation and omitted degradation, facilitating the process of translation mechanism (Carthew RW and Sontheimer EJ, 2009).
Functions of microRNAs: microRNA function manifests itself as gene regulation. microRNAs function in tandem with one or more messenger RNAs. 3`UTRs are complementary microRNA sites in animals, whereas in plants the mRNA coding region is complementary to miRNAs (Wang XJ, et al., 2004). Target RNA promotes RNA cleavage by complete or near-complete base pairing (Kawasaki H and Taira K, 2004). miRNAs provide important and powerful tools in gene regulation. Therefore, it offers a potential new class of therapeutic targets. miRNAs have a variety of physiological functions in animals and play an evolutionary conservative role in development. microRNAs, including target mRNAs, show limited complementarity in animals, but some physiological processes remain unregulated. They have been suggested to suppress the translation process, which is probably the starting step followed by mRNA degradation (Kato M and Slack FJ, 2008). During larval development, defects were found due to the loss of the mutational function of the two miRNAs first identified in c. elegans, lin4 (abnormal cellular language 4) and let7 (lethal7) (Reinhart BJ, et al., 2000). Early developmental stages were regulated by lin4, but late developmental let7 plays an important role in c. elegans and possibly some other animals (Boehm M and Slack F, 2005) (Figure 7).
Figure 7: Functions of miRNAs
Role of micro RNAs in pathogenesis of osteoarthritis
Several recent investigations have found that distinct miRNAs play different roles in OA pathogenesis. As a result, a comprehensive study of miRNA in OA is required, which will aid in the development of new treatment targets.
The miRNAs and signaling pathways implicated in OA pathogenesis, defining their functional processes, and showing how they interact with one another, with the goal of creating a basis for a better understanding of OA pathogenesis and potential treatment options (Xu B, et al., 2016).
OA has been found to have abnormal expression levels of many miRNAs. Only a few examples include miR-9, miR-27, miR-34a, miR-140, miR-146a, miR-558, and miR-602. Many of the dysregulated miRNAs have been shown to control the expression of inflammatory pathways in articular cartilage, such as interleukin-mediated or Matrix Metalloproteinase-13 (MMP-13)-mediated Extracellular Matrix breakdown (ECM). miRNAs may also play a role in pain pathways and, as a result, clinical symptom manifestation.
miRNA expression changes have been linked to a number of diseases, including obesity, cardiovascular disease, and cancer. The field of miRNAs has sparked a lot of interest, because of their stability and ease of biomarkers use for disease activity have become popular detection of toxins in the body’s fluids.
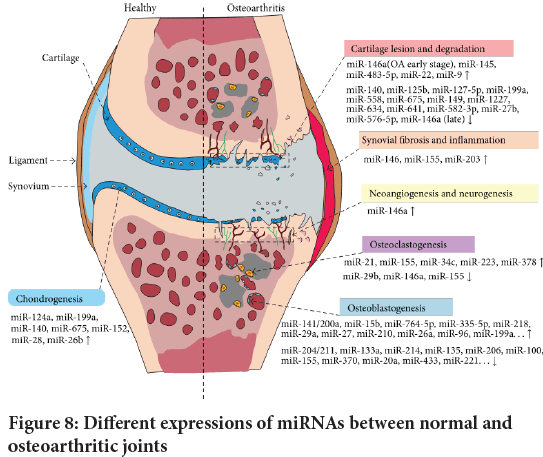
Changes in miRNA expression can lead to the destruction of joints homeostasis, involving alterations in chondrogenesis, cartilage degradation, synovial inflammation, neurogenesis, osteoblast genesis, and osteoclast genesis (Miyaki S and Asahara H, 2012) (Figure 8).
Figure 8: Different expressions of miRNAs between normal and osteoarthritic joints
More than 30 microRNAs, including miR-455s,miR-145,miR-27b,miR-146a,miR-199a,and miR-125b, are abnormally expressed in human OA samples, implying the potential implications of cartilage homeostasis and OA development.
The pathophysiology of OA and the potential involvement of miRNA OA have been linked to an age-related breakdown of the homeostatic balance between degradation and repair processes in joint tissues. miRNAs are required for the maintenance of cellular function by fine-tuning the expression of many target genes. Obesity and genetic alterations, for example, interact with epigenetic modifications and altered transcriptional regulation to impair OA signaling networks. miRNA overexpression and down regulation have both been linked to OA; addressing these alterations may be of future therapeutic value. Alternatively, miRNA levels might be employed as biomarkers (Miyaki S and Asahara H, 2012).
Regulation of gene expression in OA by miRNAs: miRNAs regulate gene expression in OA and the importance of epigenetic gene expression regulation in the development of OA has recently been reported. Recent epigenetic studies have identified a number of miRNAs as being involved in the pathogenesis of OA. miRNAs can directly bind to catabolic and anabolic mRNAs to regulate their expression at the post-transcriptional level in the cytoplasm by inducing cleavage and degradation or blocking translation. According to new research, the regulatory effect of miRNAs on the expression of catabolic and anabolic genes in OA may occur at upstream levels prior to transcription (Zhang M, et al., 2017). To begin, miRNAs direct their attention to upstream signalling pathways or transcription factors. Several signalling pathways, including the NF-kappaB pathway and the Wnt/beta-Catenin pathway, were found to be modulated by miRNAs in chondrocytes during the development of OA. Furthermore, miRNAs have been shown to regulate the SRY-box transcription factor 9 (SOX9) in the development of OA. Second, miRNAs specifically target upstream epigenetic factors. Histone deacetylase-2, -4, and the NAD-dependent deacetylase sirtuin-1 have been found to be regulated by miRNAs in OA cartilage, indicating that the interaction of different epigenetic mechanisms plays a role in OA pathogenesis (Zhang M, et al., 2017).
The pathogenesis of osteoarthritis is still unknown, which is a major impediment to the development of disease-modifying pharmacologic therapy for Osteoarthritis. The molecular finding in Osteoarthritis is well characterised for the aberrant expression of catabolic and anabolic genes; however, clinical trials focusing on a single inflammatory mediator or proteinase did not slow the progression of Osteoarthritis (Le Graver-and-Gastineau MP, 2009). Multiple factors are likely to be involved in the pathogenesis of Osteoarthritis. In this regards, the more favorable therapeutic target would be upstream molecular regulators. For the treatment of Osteoarthritis several gene may regulate by miRNA and could be potential upstream targets. In Osteoarthritis cartilage gene expression is regulate by miRNA at multiple level and in a specific manner sequence (Wang H, et al., 2017).
miRNA in a large number is recently identified in Osteoarthritis joint, tissue and several miRNA may also regulate one gene.
miRNAs as biomarkers in osteoarthritis: X-rays and MRI (Magnetic Resonance Imaging) are the accepted procedures for diagnosing OA in clinical practice. However, accurate blood testing to aid in the diagnosis and monitoring of OA progression is still being developed. Clinicians and scientists are looking for a new molecule or molecules that might be utilized as a biomarker for early OA identification and monitoring OA development (Zhang M, et al., 2017).
miRNAs may be appropriate blood-based diagnostics for OA due to the high frequency of miRNA expression in OA and the very stable form of miRNAs found in clinical plasma and serum samples (Zhang M, et al., 2017).
miRNA may be the best blood-based biomarkers for osteoarthritis. The expression of miRNA in Osteoarthritis is high, with a very stable form of miRNA detectable in clinical plasma and serum samples (Bernard NJ, 2014).
miRNA in osteoarthritis inflammation and matrix degradation: TNF alpha (TNF alpha), Interleukin (IL- and IL-6) and cytokine-inducible Cyclooxygenase-2 (COX-2) are all significantly elevated in Osteoarthritis, as are Matrix Metalloproteinase (MMPs), Nitric Oxide (NO), and Reactive Oxygen Species (ROS) (Rahmati M, et al., 2016). These pro-inflammatory mediators influence the damaged joints chondrocytes, synoviocytes, osteoblasts, osteoclasts, and macrophages, as well as a range of other cell types (Yu XM, et al., 2016).
TNF-, IL-, and IL-6 modulation by miRNAs in osteoarthritis: Several miRNAs control IL- and IL-6 either directly or indirectly via other protein targets. In the case of Osteoarthritis, Monocyte Chemoattractant Protein-Induced Protein 1 (MCPIP-1) functions as a negative regulator by down regulating the production of IL-6 (Makki MS, et al., 2015). The study found that miR-9 specifically targets the “seed-sequence” in the 3’UTR of MCPIP1 mRNA, resulting in IL-6 down regulation and increased production in human Osteoarthritis chondrocytes. miRNA-139 is abundant in osteoarthritic chondrocytes, which also target MCPIP1 (Makki MS and Haqqi TM, 2015).
miRNA and treatment of osteoarthritis: The development of disease-modifying pharmacologic therapy for OA currently faces significant challenges, owing to the fact that the pathogenesis of OA is unknown. Although the aberrant expression of catabolic and anabolic genes is a wellknown molecular finding in OA, clinical trials targeting a single inflammatory mediator or proteinase did not slow the progression of OA (Zhang M, et al., 2017).
This is most likely due to the involvement of multiple factors in OA pathogenesis. Upstream molecular regulators would be better therapeutic targets in this regard (Zhang M, et al., 2017).
The pathogenesis of Osteoarthritis remains unclear, because it currently faces the main obstacle for the event of disease-modifying pharmacologic therapy of Osteoarthritis. The molecular finding in Osteoarthritis is well characterized for the aberrant expression of catabolic and anabolic gene; however, the targeting of clinical trials for single inflammatory mediator or proteinase didn’t slow the Osteoarthritis progression (Le Graverand-Gastineau MP, 2009). The pathogenesis of Osteoarthritis is perhaps due to the involvement of multiple factors. During this regards, the more favourable therapeutic target would be upstream molecular regulators. For the treatment of Osteoarthritis several gene may regulate by miRNA and will be potential upstream targets. In Osteoarthritis cartilage organic phenomenon is regulate by miRNA at multiple level and during a specific manner sequence (Wang H, et al., 2017). miRNA during a sizable amount is recently identified in Osteoarthritis joint, tissue and a number of other miRNA can also regulate one gene (Wang H, et al., 2017).
miRNAs may be potential upstream targets for OA treatment because one miRNA can regulate multiple genes. Furthermore, miRNAs regulate gene expression at multiple levels and in a sequence-specific manner in OA cartilage. However, a large number of miRNAs have recently been discovered in OA joint tissues, and one gene could be regulated by multiple miRNAs (Li YH, et al., 2016).
Relation between osteoarthritis and other diseases
Role of micro RNA in pathogenesis of asthma: Asthma is a condition that affects the respiratory system and is marked by restricted airflow and persistent inflammation. It occurs as a result of prolonged mast cell and eosinophil invasion (Ebrahimi A and Sadroddiny E, 2015). Environmental and genetic variables are both crucial in the etiology of this illness. IL-4, IL-5, IL-9, and IL-13 are cytokines that are implicated in airway hypersensitivity, increased mucus output, eosinophil infiltration, and raised IgE levels (Ebrahimi A and Sadroddiny E, 2015).
Different asthma models have different levels of miRNA expression. A few investigations showed that the expression of miRNA in the exhaled breath condensate differed between asthma sufferers and healthy people (Sinha A, et al., 2013). When comparing mild asymptotic asthma patients to healthy individuals, a substantial differential in exosomal miRNA was discovered in bronchoalveolar lavage fluid (Levänen B, et al., 2013).
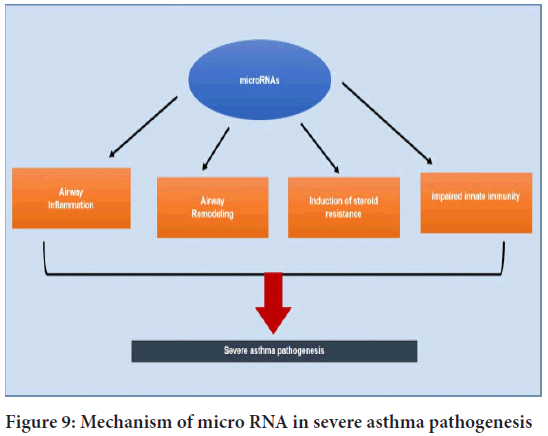
This stability is aberrantly dysregulated in bronchial allergies and airway epithelium turns into a relevant orchestrator of airway irritation. The manufacturing cytokines and chemokines that cause eosinophilia and neutrophilia in the airways suppression of the latter occurs outside the range of corticosteroid action, and neutrophil apoptosis is at least partially steroid-resistant (Koo HK, et al., 2012) (Figure 9).
Figure 9: Mechanism of micro RNA in severe asthma pathogenesis
Novel association between asthma and osteoarthritis: Asthma and Osteoarthritis (OA) are both diseases that limit physical activity and have a detrimental impact on quality of life. Despite the significant frequency, few researches have been conducted to investigate the comorbid condition and the relationship between asthma and Osteoarthritis (Koo HK, et al., 2012).
People over 40 years were diagnosed with OA and the prevalence of OA was higher with Asthma. The link between asthma and OA was significant independent of the existence of airflow limitation on spirometry, however the effects were less pronounced in individuals with an airflow limitation (Sonkoly E and Pivarcsi A, 2009).
Asthma and OA interact in a variety of ways. First, asthma has an immunological and inflammatory pathophysiology that may influence the development of OA. Endogenous and exogenous reactive oxygen and nitrogen species play important roles in airway inflammation, which influences the severity of asthma.
The previously mentioned oxidative stress is also known to play a role in the development of OA.
According to a recent paper, an SNP in the 3UTR of HLA-G, a known asthma susceptibility gene, affects the binding sites of three miRNAs (miR- 148a, miR-148b, and miR-152) targeting this gene. Although there is no direct proof, it is probable that previous observations on the HLA-G gene’s relationship with asthma susceptibility were correct (Sastre B, et al., 2017).
Different techniques have been developed to characterized miRNA expression and its involvement in asthma. As a result, the expression of miRNA in various tissues and cells implicated in the pathophysiology of asthma has been investigated to see if there is a difference in expression between healthy people and those with active asthmatic illness. miRNAs that were shown to be unregulated in asthmatic samples were subsequently investigated in asthma models, both in vitro and in vivo, to learn more about their roles in asthma (Ebrahimi A and Sadroddiny E, 2015).
Role of micro RNA in Cardiovascular Diseases (CVDs):
Formation of atherosclerotic plague: Atherosclerosis is regarded as an inflammatory and complicated condition caused by lipid buildup and subsequent oxidation, which includes various types of immune cells in the artery walls, including macrophages, T and B lymphocytes.
Platelets contain around 170 different miRNA types, as well as functional enzymes required for the processing of precursor miRNAs into mature miRNAs. Platelets are powerful regulators of several CVD molecular processes, including thrombus formation.
Following platelet activation, acute thrombus development occurs due to the changing of the original concentration of the circulating plateletderived exosome miRNAs. The main miRNAs that are responsible are miR- 143 and miR-145 (Aghabozorgi AS, et al., 2019).
miRNAs in heart failure: One of the repercussions of IR damage is heart failure, which comprises many metabolic and anatomical maladaptations against proper cardiac efficiency such as hypertrophy, fibrosis, and abnormal angiogenesis.
Recent data suggests that exosomes involved in intercellular communication between Cardiac Fibroblasts (CFs) and cardiomyocytes are involved in the etiology of hypertrophy and heart failure. Exosomal miR21 3p, which is shed from CFs and causes cellular hypertrophy by suppressing sarcoplasmic protein sorbin and SH3 domain containing protein 2 as well as PDZ and LIM domain 5 as its two putative targets in cardiomyocytes, can be treated by pharmacological suppression of miR21 3p. As a result, miR21 could be a promising therapeutic target for congestive heart failure (Aghabozorgi AS, et al., 2019).
miRNAs in Acute Myocardial Infarction (AMI): Acute Myocardial Infarction (AMI) is characterised by the death of cardiac myocytes (necrosis) as a result of persistent ischemia. Ischemia is caused by a perfusion dependent imbalance that reduces blood flow to the heart. Recent research has found that a changed exosomal miRNA profile in the bloodstream contributes to the sequence of changes in AMI onset, progression, and improvement of cardiac healing following myocardial injury. It has been shown repeatedly that one of the miR30 family members, miR30a, is dominantly expressed in the heart. However, altered miR30a expression has been seen in both heart failure and AMI. Exosomal miR30a is found in the serum of AMI patients (in vivo) and the culture media of hypoxia stimulated cardiomyocytes (in vitro). Exosomal miR30a has been found to protect cells against autophagy in a paracrine manner, resulting in the control of the autophagy process in cardiomyocytes following hypoxia. They also demonstrated that miR30a’s anti-autophagy activity is attributable to modulation of the autophagy core machinery, which includes Atg12 and LC3II/LC3I, as well as direct targeting of BECN1, which encodes Beclin1, and is recognised for its essential involvement in autophagy processes and these findings highlight the cardioprotective effects of exosomal miR181b released by CDCs upon reperfusion, the main miRNAs responsible for acute myocardial infarction are miR-30 for changing the sequence and miR-181b for releasing CDCs after reperfusion (Aghabozorgi AS, et al., 2019).
Association between osteoarthiritis and cardiovascular diseases: People with OA are roughly three times more likely than those without OA to develop Cardiovascular Disease (CVD) or heart failure (Arthritis Foundation, 2022).
The prevalence of cardiovascular illness in osteoarthritic patients is considerable. People with Osteoarthritis had a higher incidence of incident heart failure and ischemic heart disease when compared to matched controls. However, the link between Osteoarthritis and cardiovascular disease is complex, and further research is needed to better understand the potential shared pathways linking pathophysiological mechanisms (Hall AJ, et al., 2016).
Reason behind association between OA and CVDs:
Inflammation: Although OA has not historically been thought to be an inflammatory illness, evidence is beginning to reveal that it does. Longterm inflammation also plays a role in CVD.
Aging: Growing older increases chances of developing both illnesses. The arteries enlarge and harden as age, which can contribute to excessive blood pressure and heart problems. Years of usage and minor injuries cause joint degeneration.
Obesity: Obesity has been linked to OA as well as CVD. Excess body weight places strain on both the joints and the heart, which can have longterm consequences. Inflammatory chemicals are also produced by fat cells, which are detrimental to joints, the heart, and blood vessels.
Metabolic syndrome: This group of disorders, which includes high blood pressure, high blood sugar, abdominal fat, and abnormal cholesterol, raises the risk of CVD. Metabolic syndrome and OA frequently coexist. This cluster of disorders affects around 60% of patients with OA, compared to 23% of the general population. There is evidence that metabolic syndrome can induce or aggravate joint injury.
Osteoarthritis drugs can cause CVDs: A few of the medication you’re taking to manage joint pain can even shield your heart. The majority of Disease-Modifying Anti-Rheumatic Drugs (DMARDs), as well as methotrexate, sulfasalazine (Azulfidine), and anti-inflammatory drugs (Plaquenil), appear to be protective. Growth mortification issue inhibitors (TNF inhibitors) like adalimumab (Humira), Enbrel (Enbrel), and anti-TNF compound (Remicade) may also protect the guts by reducing inflammation within the body. However, for patients with RA who already have a heart condition or who develop heart failure while taking a tumour necrosis factor inhibitor, the American College of Rheumatology (ACR) “conditionally” recommends a non-TNF life DMARD or targeted artificial DMARD over a tumour necrosis factor inhibitor, according to the ACR’s 2021 Guideline for the Treatment of Arthritis. Concerns ought to be taken on an item-by-item basis (Wang H, et al., 2016).
On the flip side, alternative inflammatory disease medications truly damage the heart. Non-Steroidal Anti-Inflammatory Drugs (NSAIDs) became disreputable for increasing the chance for attack or stroke. In 2018, the federal agency issued a stern warning concerning these drugs, cautioning that they will cause heart events within a number of weeks of use. The chances of occurrence of CVD’s will increase with increase in the dose of the drug and time period of drug usage. Patients can use low doses of NSAIDs for shorter periods of time for managing the symptoms.
Conjointly risky is that the steroid drug, Deltasone. Despite the fact that it works by reducing inflammation, prednisone can raise blood pressure, increase sterol levels, and harden the arteries. So while administering steroids, the anti-inflammatory capacity of the drug should be observed to reduce the risks of heart diseases (Fernandes GS and Valdes AM, 2015).
Conclusion
The study is focused on the pathogenetic effect of microRNA in Osteoarthritis and cardiovascular diseases (heart failure, atherosclerotic plague and acute myocardial infarction) are related to OA.
Osteoarthritis still lacks a clear mechanism and a single most effective strategy to treat the symptoms and degeneration associated with it, despite being one of the most studied and more widespread disorders in our community. Exercises are a helpful therapy for these people in the early stages, and it is recommended by all medical organizations.
In Osteoarthritis, diagnosis plays an important role to identify the grade of the OA development, and pain level and problems patients faces to day-to-day lifestyle and gives a brief knowledge about the condition and grade the patient is suffering. There are three most commonly used methods KL grading, VAS pain scale and WOMAC.
Several recent investigations have found that distinct miRNAs play different roles in OA pathogenesis. As a result, a comprehensive study of miRNA in OA is required, which will aid in the development of new treatment targets.
microRNAs are the also responsible in a role of pathogenesis of OA, Changes in miRNA expression play a role in joint homeostasis or pathology. The expression of microRNAs (miRNAs) in joint tissue has been demonstrated. Normal cartilage is maintained by miR-140, miR-675, and miR-27b, which regulate cartilage homeostasis and differentiation. In synovium, miR-146, miR-155, and miR-203 have a role in the regulation of the inflammatory system. Change in miRNAs can cause destruction in synovial fluid which can lead to Osteoarthritis,
Changes in miRNA expression can lead to changes in chondrogenesis, cartilage degradation, synovial inflammation, neurogenesis, osteoblast genesis, and osteoclast genesis, all of which can disrupt joint homeostasis. More than 30 miRNAs are responsible for overexpressed in patient OA samples, indicating that cartilage homeostasis and OA development may be linked.
microRNAs are also play crucial role in gene regulation in Osteoarthritis, the miRNAs bind with catabolic and anabolic gene that changes the gene expressions the alteration in gene regulation can be reason of pathogenesis of OA.
microRNAs can be also used as biomarkers in diagnosis and development of OA. It’s also used to treat Osteoarthritis.
Osteoarthritis also showed association with other disease i.e., asthma and cardiovascular diseases.
Despite the excessive prevalence, few researches at the comorbid circumstance and the hyperlink among asthma and Osteoarthritis were undertaken. The courting among asthma and OA become sizable no matter whether or not or now no longer there has been an airflow limit on spirometry, but the results have been much less outstanding in people with an airflow limit.
The prevalence of cardiovascular illness in osteoarthritic patients is considerable. People with Osteoarthritis had a higher incidence of incident heart failure and ischemic heart disease when compared to matched controls.
The role of miRNAs is very complex for any disease. However, the link between Osteoarthritis and miRNAs, and further research is needed to better understand the potential shared pathways linking pathogenesis mechanisms.
References
- Mora JC, Przkora R, Cruz-Almeida Y. Knee osteoarthritis: Pathophysiology and current treatment modalities. J Pain Res. 2018; 11: 2189.
[Google Scholar] [Pubmed]
- Katz JN, Arant KR, Loeser RF. Diagnosis and treatment of hip and knee osteoarthritis: A review. JAMA. 2021; 325(6): 568-578.
[Crossref] [Google Scholar] [Pubmed]
- Junker S, Krumbholz G, Frommer KW, Rehart S, Steinmeyer J, Rickert M, et al. Differentiation of osteophyte types in osteoarthritis-proposal of a histological classification. Joint Bone Spine. 2016; 83(1): 63-67.
[Crossref] [Google Scholar] [Pubmed]
- Yolanda S. Osteoarthritis types. News Medical. 2022.
- Kellgren JH, Lawrence J. Radiological assessment of osteo-arthrosis. Ann Rheum Dis. 1957; 16(4): 494.
[Crossref] [Google Scholar] [Pubmed]
- Haefeli M, Elfering A. Pain assessment. Eur Spine J. 2006; 15(1): S17-24.
[Crossref] [Google Scholar] [Pubmed]
- Physiopedia. Visual Analogue Scale. Physiopedia. 2022.
- Wikipedia. WOMAC. Wikipedia. 2022.
- Wang XJ, Reyes JL, Chua NH, Gaasterland T. Prediction and identification of Arabidopsis thaliana microRNAs and their mRNA targets. Genome Biol. 2004; 5(9): 1-5.
[Crossref] [Google Scholar] [Pubmed]
- Wahid F, Shehzad A, Khan T, Kim YY. MicroRNAs: Synthesis, mechanism, function, and recent clinical trials. Biochim Biophys Acta. 2010; 1803(11): 1231-1243.
[Crossref] [Google Scholar] [Pubmed]
- Le Graverand-Gastineau MP. OA clinical trials: Current targets and trials for OA. Choosing molecular targets: what have we learned and where we are headed?. Osteoarthritis Cartilage. 2009; 17(11): 1393-1401.
[Crossref] [Google Scholar] [Pubmed]
- Wightman B, Ha I, Ruvkun G. Posttranscriptional regulation of the heterochronic gene lin-14 by lin-4 mediates temporal pattern formation in C. elegans. Cell. 1993; 75(5): 855-862.
[Crossref] [Google Scholar] [Pubmed]
- Telfer A, Poethig RS. HASTY: A gene that regulates the timing of shoot maturation in Arabidopsis thaliana. Development. 1998; 125(10): 1889-1898.
[Crossref] [Google Scholar] [Pubmed]
- Pillai RS, Artus CG, Filipowicz W. Tethering of human Ago proteins to mRNA mimics the miRNA-mediated repression of protein synthesis. RNA. 2004; 10(10): 1518-1525.
[Crossref] [Google Scholar] [Pubmed]
- Carthew RW, Sontheimer EJ. Origins and mechanisms of miRNAs and siRNAs. Cell. 2009; 136(4): 642-655.
[Crossref] [Google Scholar] [Pubmed]
- Kawasaki H, Taira K. MicroRNA-196 inhibits HOXB8 expression in myeloid differentiation of HL60 cells. Nucleic Acids Symp Ser. 2004; 48(1): 211-212.
[Crossref] [Google Scholar] [Pubmed]
- Kato M, Slack FJ. microRNAs: Small molecules with big roles-C. elegans to human cancer. Biol Cell. 2008; 100(2): 71-81.
[Crossref] [Google Scholar] [Pubmed]
- Reinhart BJ, Slack FJ, Basson M, Pasquinelli AE, Bettinger JC, Rougvie AE, et al. The 21-nucleotide let-7 RNA regulates developmental timing in Caenorhabditis elegans. Nature. 2000; 403(6772): 901-906.
[Crossref] [Google Scholar] [Pubmed]
- Boehm M, Slack F. A developmental timing microRNA and its target regulate life span in C. elegans. Science. 2005; 310(5756): 1954-1957.
[Crossref] [Google Scholar] [Pubmed]
- Xu B, Li YY, Ma J, Pei FX. Roles of microRNA and signaling pathway in osteoarthritis pathogenesis. J Zhejiang Univ Sci B. 2016; 17(3): 200-208.
[Crossref] [Google Scholar] [Pubmed]
- Miyaki S, Asahara H. Macro view of microRNA function in osteoarthritis. Nat Rev Rheumatol. 2012; 8(9): 543-552.
[Crossref] [Google Scholar] [Pubmed]
- Zhang M, Lygrisse K, Wang J. Role of MicroRNA in osteoarthritis. J Arthritis. 2017; 6(2): 239.
[Crossref] [Google Scholar] [Pubmed]
- Wang H, Zhang H, Sun Q, Wang Y, Yang J, Yang J, et al. Intra-articular delivery of antago-miR-483-5p inhibits osteoarthritis by modulating matrilin 3 and tissue inhibitor of metalloproteinase 2. Mol Ther. 2017; 25(3): 715-727.
[Crossref] [Google Scholar] [Pubmed]
- Bernard NJ. Circulating miRNAs-early osteoarthritis biomarkers?. Nat Rev Rheumatol. 2014; 10(4): 197.
[Crossref] [Google Scholar] [Pubmed]
- Rahmati M, Mobasheri A, Mozafari M. Inflammatory mediators in osteoarthritis: A critical review of the state-of-the-art, current prospects, and future challenges. Bone. 2016; 85: 81-90.
[Crossref] [Google Scholar] [Pubmed]
- Yu XM, Meng HY, Yuan XL, Wang Y, Guo QY, Peng J, et al. MicroRNAs’ involvement in osteoarthritis and the prospects for treatments. Evid Based Complement Alternat Med. 2016.
[Crossref] [Google Scholar] [Pubmed]
- Makki MS, Haseeb A, Haqqi TM. MicroRNA‐9 promotion of interleukin‐6 expression by inhibiting monocyte chemoattractant protein-induced protein 1 expression in interleukin‐1β-stimulated human chondrocytes. Arthritis Rheumatol. 2015; 67(8): 2117-2128.
[Crossref] [Google Scholar] [Pubmed]
- Makki MS, Haqqi TM. miR-139 modulates MCPIP1/IL-6 expression and induces apoptosis in human OA chondrocytes. Exp Mol Med. 2015; 47(10): e189.
[Crossref] [Google Scholar] [Pubmed]
- Li YH, Tavallaee G, Tokar T, Nakamura A, Sundararajan K, Weston A, et al. Identification of synovial fluid microRNA signature in knee osteoarthritis: Differentiating early-and late-stage knee osteoarthritis. Osteoarthritis Cartilage. 2016; 24(9): 1577-1586.
[Crossref] [Google Scholar] [Pubmed]
- Ebrahimi A, Sadroddiny E. MicroRNAs in lung diseases: Recent findings and their pathophysiological implications. Pulm Pharmacol Ther. 2015; 34: 55-63.
[Crossref] [Google Scholar] [Pubmed]
- Sinha A, Yadav AK, Chakraborty S, Kabra SK, Lodha R, Kumar M, et al. Exosome-enclosed microRNAs in exhaled breath hold potential for biomarker discovery in patients with pulmonary diseases. J Allergy Clin Immunol. 2013; 132(1): 219-222.
[Crossref] [Google Scholar] [Pubmed]
- Levänen B, Bhakta NR, Paredes PT, Barbeau R, Hiltbrunner S, Pollack JL, et al. Altered microRNA profiles in bronchoalveolar lavage fluid exosomes in asthmatic patients. J Allergy Clin Immunol. 2013; 131(3): 894-903.
[Crossref] [Google Scholar] [Pubmed]
- Koo HK, Song P, Lee JH. Novel association between asthma and osteoarthritis: A nationwide health and nutrition examination survey. BMC Pulm Med. 2021; 21(1): 1-8.
[Crossref] [Google Scholar] [Pubmed]
- Sonkoly E, Pivarcsi A. Advances in microRNAs: Implications for immunity and inflammatory diseases. J Cell Mol Med. 2009; 13(1): 24-38.
[Crossref] [Google Scholar] [Pubmed]
- Sastre B, Cañas JA, Rodrigo-Muñoz JM, del Pozo V. Novel modulators of asthma and allergy: Exosomes and microRNAs. Front Immunol. 2017; 8: 826.
[Crossref] [Google Scholar] [Pubmed]
- Aghabozorgi AS, Ahangari N, Eftekhaari TE, Torbati PN, Bahiraee A, Ebrahimi R, et al. Circulating exosomal miRNAs in cardiovascular disease pathogenesis: New emerging hopes. J Cell Physiol. 2019; 234(12): 21796-21809.
[Crossref] [Google Scholar] [Pubmed]
- Arthritis Foundation. Osteoarthiritis and your heart. Arthritis Foundation. 2022.
- Hall AJ, Stubbs B, Mamas MA, Myint PK, Smith TO. Association between osteoarthritis and cardiovascular disease: Systematic review and meta-analysis. Eur J Prev Cardiol. 2016; 23(9): 938-946.
[Crossref] [Google Scholar] [Pubmed]
- Wang H, Bai J, He B, Hu X, Liu D. Osteoarthritis and the risk of cardiovascular disease: A meta-analysis of observational studies. Sci Rep. 2016; 6(1): 1-7.
[Crossref] [Google Scholar] [Pubmed]
- Fernandes GS, Valdes AM. Cardiovascular disease and osteoarthritis: Common pathways and patient outcomes. Eur J Clin Invest. 2015; 45(4): 405-414.
[Crossref] [Google Scholar] [Pubmed]
Author Info
Gunjan Kumar* and Amit SinghCitation: Kumar G: Role of Non-Coding Endogenous RNA in Osteoarthritis
Received: 15-Feb-2023 Accepted: 10-Mar-2023 Published: 17-Mar-2023, DOI: 10.31858/0975-8453.14.3.146-154
Copyright: This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
ARTICLE TOOLS
- Dental Development between Assisted Reproductive Therapy (Art) and Natural Conceived Children: A Comparative Pilot Study Norzaiti Mohd Kenali, Naimah Hasanah Mohd Fathil, Norbasyirah Bohari, Ahmad Faisal Ismail, Roszaman Ramli SRP. 2020; 11(1): 01-06 » doi: 10.5530/srp.2020.1.01
- Psychometric properties of the World Health Organization Quality of life instrument, short form: Validity in the Vietnamese healthcare context Trung Quang Vo*, Bao Tran Thuy Tran, Ngan Thuy Nguyen, Tram ThiHuyen Nguyen, Thuy Phan Chung Tran SRP. 2020; 11(1): 14-22 » doi: 10.5530/srp.2019.1.3
- A Review of Pharmacoeconomics: the key to “Healthcare for All” Hasamnis AA, Patil SS, Shaik Imam, Narendiran K SRP. 2019; 10(1): s40-s42 » doi: 10.5530/srp.2019.1s.21
- Deuterium Depleted Water as an Adjuvant in Treatment of Cancer Anton Syroeshkin, Olga Levitskaya, Elena Uspenskaya, Tatiana Pleteneva, Daria Romaykina, Daria Ermakova SRP. 2019; 10(1): 112-117 » doi: 10.5530/srp.2019.1.19
- Dental Development between Assisted Reproductive Therapy (Art) and Natural Conceived Children: A Comparative Pilot Study Norzaiti Mohd Kenali, Naimah Hasanah Mohd Fathil, Norbasyirah Bohari, Ahmad Faisal Ismail, Roszaman Ramli SRP. 2020; 11(1): 01-06 » doi: 10.5530/srp.2020.1.01
- Manilkara zapota (L.) Royen Fruit Peel: A Phytochemical and Pharmacological Review Karle Pravin P, Dhawale Shashikant C SRP. 2019; 10(1): 11-14 » doi: 0.5530/srp.2019.1.2
- Pharmacognostic and Phytopharmacological Overview on Bombax ceiba Pankaj Haribhau Chaudhary, Mukund Ganeshrao Tawar SRP. 2019; 10(1): 20-25 » doi: 10.5530/srp.2019.1.4
- A Review of Pharmacoeconomics: the key to “Healthcare for All” Hasamnis AA, Patil SS, Shaik Imam, Narendiran K SRP. 2019; 10(1): s40-s42 » doi: 10.5530/srp.2019.1s.21
- A Prospective Review on Phyto-Pharmacological Aspects of Andrographis paniculata Govindraj Akilandeswari, Arumugam Vijaya Anand, Palanisamy Sampathkumar, Puthamohan Vinayaga Moorthi, Basavaraju Preethi SRP. 2019; 10(1): 15-19 » doi: 10.5530/srp.2019.1.3