Review Article - (2022) Volume 13, Issue 6
Solid Lipid Nanoparticles: Synthesis, Microstructural Elucidations and Future Prospects
Suraj B Kumbhar1, Vikram R Shinde1, Rajesh G Jadhao2,3, Poonam P Warade2, Parag R Patil2, Sharad Wakode3, Ankita R Wankhede2, Swati D Raysing4 and Dipak D Kumbhar2*Abstract
Solid Lipid Nanoparticles (SLNs) are useful carriers for delivering BCS II and IV drugs. SLNs microstructure is decisive parameter to attain significant drug payload. Its inability to retain hydrophilic molecules demands smart exploitation of SLNs that begins with careful selection of structural components. Besides, other challenges as lipid polymorphism and molecular interactions substantially influence SLNs properties asking for a detailed microstructural investigation. This work attempts to provide a comprehensive yet critical view on SLNs from microstructural perspective. These structural attributes are conclusive while entrusting their functionality. Besides with better understanding of their assembly mechanics it is practicable to devise SLNs of desired properties.
Keywords
Solid Lipid Nanoparticles (SLNs), Microstructure, Polymorphism, Neutraceuticals, Hydrophilic molecule
Abbreviations
SLNs: Solid Lipid Nanoparticles; HPH: High Pressure Homogenization; API: Active Pharmaceutical Ingredients; GRAS: Generally Regarded as Safe; LNP: Lipid Nano Particle; PS: Particle Size; PDI: Polydispersity Index; DLS: Dynamic Light Scattering; RESS: Rapid Expansion of Supercritical Solutions; GAS: Gas Anti-Solvent; SAILA: Supercritical Assisted Injection in a Liquid Anti-Solvent; PGSS: Particles from Gas-Saturated Solutions/Suspensions; SCF: Supercritical Fluid; SFEE: Supercritical Fluid Extraction of Emulsions; SFN: Supercritical Fluid Nucleation; PIT: Phase Inversion Temperature; WPN: Wide Pneumatic Nozzle; APN: Air Pressure Nozzle; MP: Melting Point; FA: Fatty Acid; SLM: Solid Lipid Microparticles; ST: Surface Tension; PCS: Photon Correlation Spectroscopy; LD: Laser Diffraction; SLS: Static Light Scattering; PIDS: Polarization Intensity Differential Scattering; ZP: Zeta Potential; SEM: Scanning Electron Microscopy; TEM: Transmission Electron Microscopy; RT: Room Temperature; DSC: Differential Scanning Calorimetry; WAXS: Wide Angel X-Ray Scattering; NMR: Nuclear Magnetic Resonance; LC: Loading Capacity; EE: Encapsulation Efficiency; NLC: Nanostructured Lipid Carriers; BBB: Blood Brain Barrier
Introduction
At nanoscale, molecules and atoms behave differently, allowing for several unexpected and intriguing applications. It increases the efficiency of new materials, including those for medical applications, where traditional techniques may be limited. The most challenging aspect in pharmaceutical sciences is delivering a drug to particular organ sites (Rosenblum D, et al., 2018; Singh N, et al., 2017). Figure 1 depicts vast array of nanocarriers useful for drug delivery purposes. This work comprehensively reviews the formulation and production aspects pertaining to SLNs and their specific role in bioavailability enhancement.
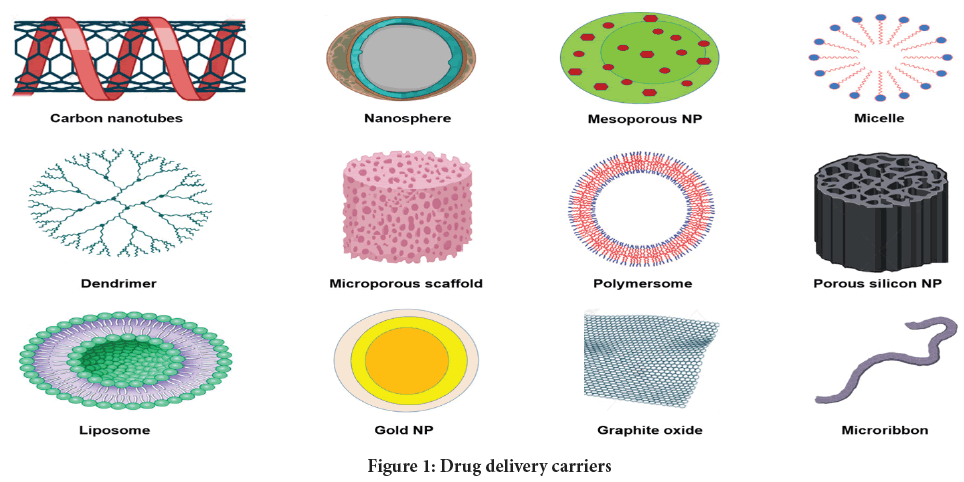
Figure 1: Drug delivery carriers
Literature Review
Solid Lipid Nanoparticles (SLNs)
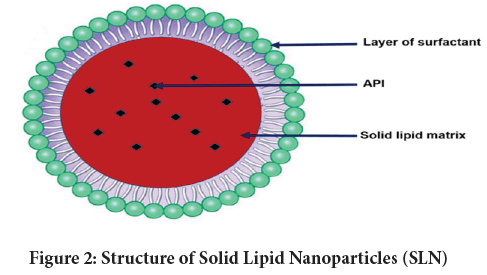
They are formed when solid lipid or its blend is disseminated in a water phase and if essential stabilized with surfactants (Khatak S and Dureja H, 2017; Naseri N, et al., 2015). SLNs size usually span within 50-1000 nm though it is challenging to produce them lower than 80 nm owing to least possibility of recrystallization during their production (Duong VA, et al., 2020; Salminen H, et al., 2020; Bahari LA and Hamishehkar H, 2016). The comprehensive list of diverse pharma actives that has been successfully formulated as SLNs (Shazly GA, 2017; Li S, et al., 2011). Moreover, to attain long term stability it is feasible to transform SLN dispersion into a dry form. For this techniques such as spray-drying or lyophilization (Abdelwahed W, et al., 2006) are usually employed. The schematic presentation of SLN structure is depicted in Figure 2, while the merits and demerits of SLN as drug carrier are summarized in Table 1.
| Merits | Demerits |
|---|---|
| Controlled and targeted drug release | Limited drug loading capacity |
| Improved stability of incorporated active | Drug expulsion during storage due to a polymorphic transition. |
| Carrier for both lipophilic and hydrophilic drugs | Particle growth and aggregation |
| Generally Regarded as Safe (GRAS) nature of lipid excipients render them | Gelation |
| Biocompatibility and ensure biosafety | |
| Can be formulated without use of organic solvents | |
| Easy to scale-up | |
| Sterilization feasibility | |
| Increased bioavailability of incorporated bioactive | |
| Allows encapsulation of nutraceuticals | |
| Known to attenuate nephrotoxicity and cardiotoxicity of an incorporated active | |
| Easy to gain regulatory approval |
Table 1: Solid Lipid Nanoparticles (SLN) merits and demerits in drug delivery
Figure 2: Structure of Solid Lipid Nanoparticles (SLN)
Drug incorporation models
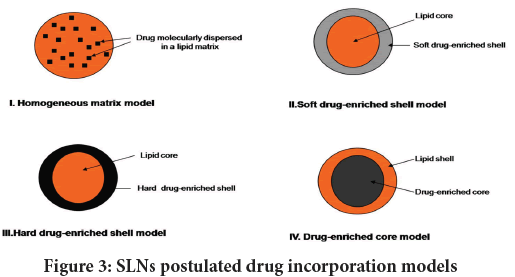
Among these API solubility in lipid, the physico-chemical features of SLN components, and the production method is of paramount importance (Üner M and Yener G, 2007; Mukherjee S, et al., 2009). Figure 3demonstrates various models for API incorporation into SLN.
Figure 3: SLNs postulated drug incorporation models
Homogeneous matrix model: This model (Model I) describes a situation in which an API is molecularly distributed or exist as an amorphous cluster within lipid matrix (Dolatabadi JE, et al., 2015). In the latter situation, the API is initially dissolved in bulk lipid materials and then subjected to the hot HPH processing to form SLN with a homogeneous API-containing structure. Likewise, this model can be created when oil droplets generated following the hot HPH are allowed to cool down and recrystallize to SLNs.
Soft and hard drug-enriched shell models: These models (Model II and III) describe SLNs with an inner core lipid, and enclosed by an external case supplemented with API. Here, an initial drug partitioning from a lipid phase into the water phase, and subsequent recrystallization of oil droplets brings about solidification of lipid core which is relatively drug-free. The subsequent recrystallization of an oily lipid shell results in drug-enriched lipid case around the core. The drug-enriched solid lipid shell may be soft or hard based upon the interactions amid API and lipid. The soft and the hard drug-enriched shell models are concomitant to burst release (Uprit S, et al., 2013) of the API integrated into the SLN.
Drug-enriched core model: This model (Model IV) describes the API incorporation into SLN that involves induction of drug-enriched core enclosed by a shell that is relatively free of drug (Aboud HM, et al., 2016). Further cooling lets oil droplets around the drug-enriched core to recrystallize forming a lipid shell that acts as a membrane to control API release from SLNs. This model exhibits a two phase drug release behavior (Hu FQ, et al., 2004) accentuated by an early burst then a sustained release.
SLN formulation aspects
The prime prerequisite to produce SLN includes a solid lipid or its blend, surfactants, water and a drug component. Since the lipids used to formulate SLN are of GRAS status they ensure biosafety (Souto EB, et al., 2020). Table 2 summarizes commonly used excipients in SLN formulation.
| Lipids | Emulsifiers/co-emulsifiers | |
|---|---|---|
| Triglycerides | Phospholipids | |
| Tricaprin | Soybean lecithin | |
| Trilaurin | (Lipoid® S 75, Lipoid® S 100) | |
| Trimyristin (Dynasan® 114) | Egg lecithin (Lipoid® E 80) | |
| Tripalmitin (Dynasan® 116) | Phosphatidylcholine | |
| Tristearin (Dynasan® 118) | (Epikuron® 170, Epikuron 200) | |
| Hydrogenated coco-glycerides (Softisan® 142) | ||
| Hard fat types | Ethylene oxide/propylene oxide | |
| Witepsol® W 35 | Copolymers | |
| Witepsol® H 35 | Poloxamer 188 | |
| Witepsol® H 42 | Poloxamer 182 | |
| Witepsol® E 85 | Poloxamer 407 | |
| Acyl glycerols | Sorbitan ethylene oxide/propylene oxide copolymers | |
| Glyceryl monostearate (Imwitor® 900) | Polysorbate 20 | |
| Glyceryl monooleate (Peceol) | Polysorbate 60 | |
| Glyceryl behenate (Compritol® 888 ATO) | Polysorbate 80 | |
| Glyceryl palmitostearate (Precirol® ATO 5) | Polysorbate 80 | |
| Miscellaneous | ||
| Stearic acid | Sodium glycocholate | |
| Cetyl palmitate | Dioctyl sodium sulfosuccinate | |
| Beeswax | Tyloxapol | |
| Decanoic acid | Butyric acid | |
Table 2: Formulation ingredients for SLN assembly
SLN production methods
Several methods have been testified to produce SLN (Trotta M, et al., 2003; Müller RH, et al., 2000). These include HPH, ultra-sonication, microemulsion, solvent emulsification-evaporation, solvent displacement, emulsification-diffusion, phase inversion, and solvent-injection techniques. Since this method is frequently used in SLNs manufacturing, it is described in more detail from a mechanical perspective while the other methods of production are overviewed.
High Pressure Homogenization (HPH): The method uses a vigorous movement of a microemulsion through a thin gap at a velocity (>1000 Km/hr), and pressure within 100-2000 bars (Jenning V, et al., 2002). These collisions render a shear stress through the turbulent flow that combines with the additional forces within the cavity, and brings about breakdown of microparticles into nanoparticles (Kluge J, et al., 2012). The microemulsion is typically positioned in a cylinder that has a comparatively large diameter than piston-gap located instantly next to cylinder.
The fluid arrives the thin piston-gap from a moderately large diameter cylinder preceding the gap, the dynamic pressure of the fluid inside the gap upsurges accompanying drop in its static pressure while the whole pressure in the gap remain constant. Consequently, the formed bubbles collapse forming cavitations and shockwaves inside the dispersion medium. Thus the induced cavitation’s and shockwaves alongside particles collision with piston-gap and the turbulent flow contribute to size reduction.
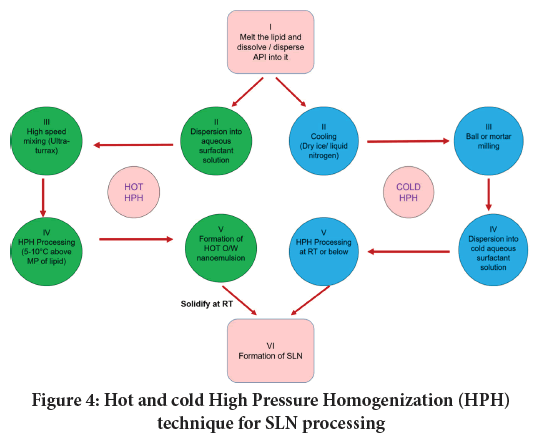
The power density is reliant on the pressure of homogenization and the width of the homogenization cavity gap (Gall V, et al., 2016). Thus, the HPH with a thin homogenization gap may attain power density within 1012-1013 W/m3. HPH processing is categorized into two as hot HPH and the cold HPH. Figure 4 gives schematic representation of hot and cold HPH technique for SLNs production. During the HPH processing lipid droplets size is crucial and determinant of final SLN quality. Though, such temperature might cause degradation of an API and/or that of lipid carrier. Moreover increasing the homogenization cycles in quest to achieve lesser sized particles is not always reasonable. Hence, it is recommended to use 3-5 homogenization cycles and the pressure ranging within 500-1500 bar to attain quality SLN (Loh ZH, et al., 2015). Hot HPH is preferred to yield SLNs of hydrophobic and thermostable API. Cold HPH does not avoid the contact of an API to relatively high temperatures since initially it is required to be dispersed in molten lipid before the rapid cooling stage. Thus, cold HPH can be preferred to produce SLN containing thermolabile substances. Moreover, using cold HPH it is feasible to incorporate hydrophilic agent into SLN owing to minimal drug partitioning effect.
Figure 4: Hot and cold High Pressure Homogenization (HPH) technique for SLN processing
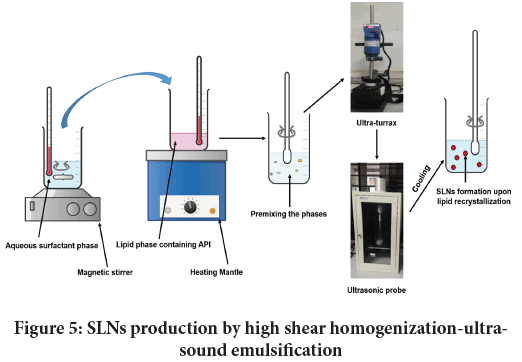
High shear homogenization-ultrasound emulsification: This is an affordable method to produce SLNs when compared to HPH. The process involves making of an oily (lipid) and an aqueous phase separately maintaining the equal temperature. Once these two phases are prepared, they are homogenized through high speed shearing machine like ultra-turrax to build a coarse emulsion. Thus formed emulsion is then processed under an ultrasonic horn with varied power and sonication time. The resultant dispersion upon cooling gives rise to SLN. The schematic representation of this method is portrayed in Figure 5. Here, the mechanism of nano sizing is based upon acoustic cavitation. Acoustic waves with frequency (>20 kHz) induces formation of micro bubbles (Sajjadi B, et al., 2015) which eventually colloid with one another, and implode resulting in cavitation.
Figure 5: SLNs production by high shear homogenization-ultrasound emulsification
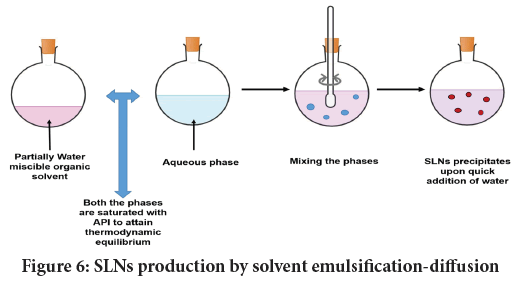
Solvent emulsification-diffusion: This process is summarized in Figure 6. The criterion for this technique is to use partially water-miscible solvent. In this regard, solvents as benzyl alcohol, isopropyl acetate, butyl lactate, and methyl acetate can be applied. Lipid is then dissolved/dispersed into water saturated solvent followed by emulsification with solvent-saturated surfactant. Upon dilution with excess water (1:5-1:10), SLN precipitates as solvent diffuse from emulsion droplets towards continuous phase. SLNs developed by this process ranges from 30-100 nm (Patravale VB and Mirani AG, 2019), and governed by lipid concentration, and the kind and amount of emulsifier used.
Figure 6: SLNs production by solvent emulsification-diffusion
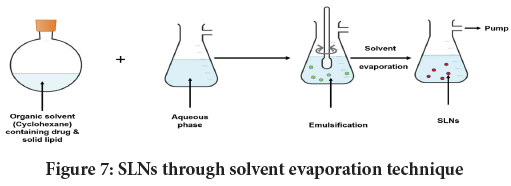
Solvent evaporation: Here, initially solid lipid is liquefied in cyclohexane, a water-insoluble organic solvent, and then subject to emulsification in aqueous phase. Aforesaid events are narrated in Figure 7. Using this technique it is feasible to obtain SLNs of about 25 nm. Pooja D, et al. used this technique to develop 5-fluorouracil-loaded SLNs (Pooja D, et al., 2016). Likewise, Siekmann and Western employed the same technique to yield cholesterol acetate SLNs of about 29 nm.
Figure 7: SLNs through solvent evaporation technique
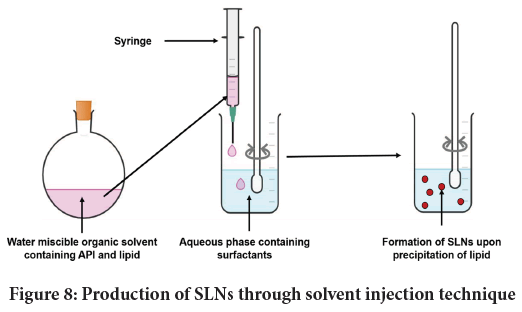
Solvent-injection: Here, the API and lipid is softened in water miscible organic solvent for instance isopropanol or ethanol. Figure 8 depicts the said method. Parameters like viscosity of continuous phase, nature of lipid and surfactants used can profoundly influence quality of final SLN. This process was used to design Ciprofloxacin Hydrochloride loaded SLNs (Pignatello R, et al., 2018). Likewise Mishra D, et al. employed the same method to design SLNs containing Hepatitis B surface antigen (Mishra D, et al., 2010).
Figure 8: Production of SLNs through solvent injection technique
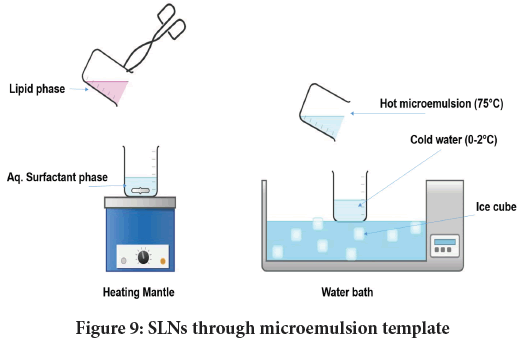
Micro emulsion based technique: Microemulsions are clear or faintly bluish solutions made of oily phase (e.g. lipid), surfactants, co-surfactant and water. They are thermodynamically stable and isotropic in nature (Tartaro G, et al., 2020). Figure 9shows basic steps allied with the microemulsion technique. The dispersal of hot microemulsion in cold water causes precipitation of lipid forming SLNs of about 300 nm or lower. Hot microemulsion (≈ 75°C) to cold water (2°C-3°C) ratio used is typically within 1:25-1:50. Unlike emulsion, microemulsions behaves dynamically and only be formed by significant lowering of tension at oil/water interface. Hence, generally co-surfactant is added while formulating microemulsion. This act not only brings about flexibility at oil/water interface but render significant stability to the system.
Figure 9: SLNs through microemulsion template
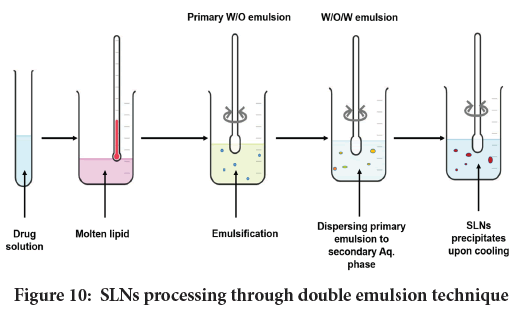
Double emulsion: Figure 10 summarize the assembly of SLNs using the said technique. Here, an API is dissolved in aqueous solution prior to emulsification in molten lipid. The said method is very useful to load hydrophilic agents. As here an API is encapsulated with a stabilizer that averts drug partitioning to external water phase. Li Z, et al. successfully obtained SLNs containing an isoflavonoid Puerarin through double emulsion (Li Z, et al., 2010).
Figure 10: SLNs processing through double emulsion technique
Spray drying: Here, an aqueous SLN dispersion is transformed into solid form. It is an alternative and relatively cheaper method to lyophilization. Lipids with higher Melting Point (MP) (75°C) are usually recommended for this technique. The method is liable for particle aggregation owing to high processing temperature, shearing and fractional melting of particles. Bag filter, electrostatic precipitator or cyclone is employed to separate dried particles from the gas. The prime benefit of a said technique is feasibility in manipulating various processing parameters. Though, thermal stress then high shear during the processing may induce sample degradation. Sebti T and Amighi K have successfully produced SLM through spray drying for the API delivery to lung (Sebti T and Amighi K, 2006).
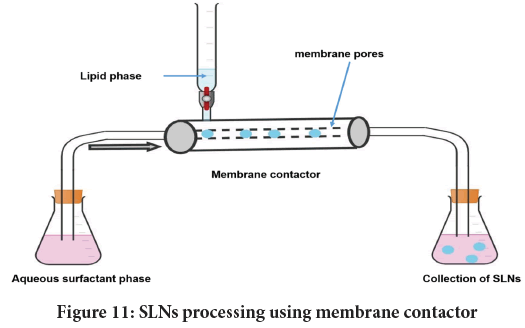
Membrane contactor: It is a distinctive method to formulate SLNs. The process involve squeezing of lipid (above its MP) over the membrane pores usually made of Kerasep ceramic with an active ZrO2 coating on an AlO2- TiO2 backing. This results in creation of tiny droplets. Subsequent cooling of sample yields SLNs. The whole process is briefed in Figure 11. During processing both the phases are maintained at same temperature while nitrogen assists generation of pressure for liquid phase.
Figure 11: SLNs processing using membrane contactor
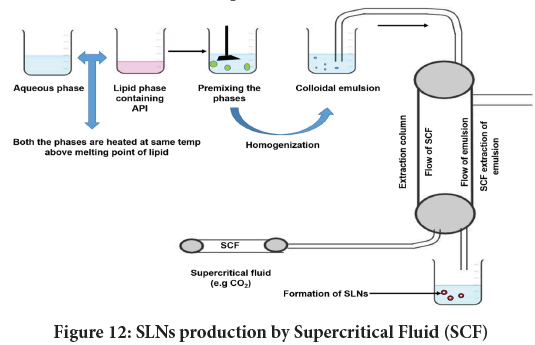
Supercritical Fluid (SCF) based methods: SCF is attained above its critical pressure and temperature wherein the substance solubility increases. CO2 is a choice of solvent in SCF owing to its small critical point (31°C and 74 bar), non-toxicity, and less cost (Khaw KY, et al., 2017). Figure 12 narrates SLN processing through the SCF technique. SCF processes are broadly categorized into four types.
Figure 12: SLNs production by Supercritical Fluid (SCF)
Rapid Expansion of Supercritical Solutions (RESS):The processing involves an initial dissolution of matrix in SCF following its expansion over a nozzle to form the particles. Albumin microcrystal has been accomplished through modified RESS (Dos Santos IR, et al., 2002).
Gas Anti-Solvent (GAS): This process is particularly important for nanosizing hydrophobic materials as they exhibit low SCF solubility. Park SJ and Yeo SD utilized GAS to recrystallize caffeine from its solution (Park SJ and Yeo SD, 2008). More recently Trucillo P and Campardelli R employed SAILA for making SLN (Trucillo P and Campardelli R, 2019).
Particles from Gas-Saturated Solutions/Suspensions (PGSS):The process comprises an initial solublization of CO2 in molten or liquid suspended substance(s). Prime benefit of said process is that a substance needn’t to have solubility in CO2.
Supercritical Fluid Extraction of Emulsions (SFEE): It is a convenient method of SLN production. SLNs are shaped by SFEE of organic solvent from an O/W emulsions. Subsequently the mixture is passed through HPH to attain fine emulsion with droplets within 30-100 nm. Supercritical CO2 boost the extraction efficiency and is far better when compared with solvent evaporation or diffusion. Using SFEE SLNs of about 20-90 nm could be attained (Chattopadhyay P, et al., 2006).
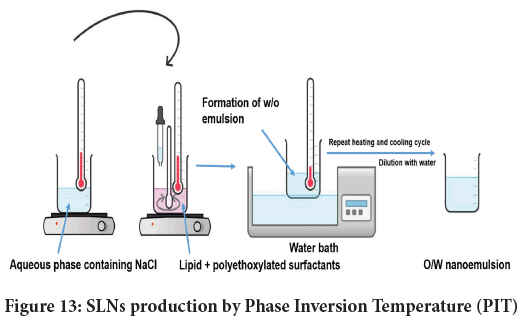
Phase Inversion Temperature (PIT): This technique is particularly useful in creation of nanoemulsions. These surfactants causes inversion of emulsion phase (O/W to W/O) above PIT, while below PIT it lowers the temperature to induce O/W nanoemulsion (Pavoni L, et al., 2020). The said technique is successfully used to design lipid nanocapsules (Anton N, et al., 2007; Kiani A, et al., 2017). Of late, the technique is amended for SLN formulations, and schematically represented in Figure 13. Here, two phases namely an oil phase holding lipid and surfactants (non-ionic) and an aqueous phase comprising NaCl are used. Both the phases are heated beyond PIT (≈ 90°C). Then the dropwise addition of an aqueous phase is done to oil phase using constant stirring.
Figure 13: SLNs production by Phase Inversion Temperature (PIT)
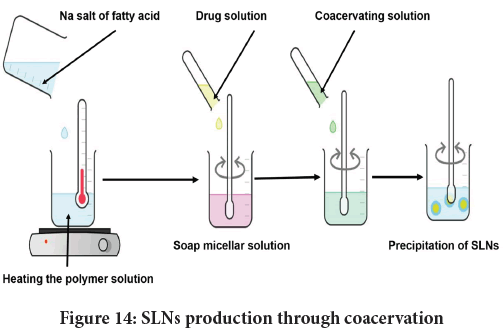
Coacervation: It is an inexpensive technique to yield SLNs as it evades complex equipment and toxic solvents while processing. Figure 14depicts SLN production by coacervation technique. The generation of FA nanoparticles ensues owing to proton exchange amid the soap and coacervating liquid (Battaglia L, et al., 2010). SLN precursor (soap micelle) is attained beyond its Krafft point temperature (Manojlović JŽ, 2012). To facilitate micellisation API is generally liquefied with micellar solution otherwise in a low quantity of ethanol. FA salts are preferably used within 1%-5% w/w (Vigani B, et al., 2021). The promising aspect of coacervation is the control over SLN size by altering reaction conditions.
Figure 14: SLNs production through coacervation
Cryogenic micronization: This involves melt dispersion/solvent stripping (API and lipid are liquefied in ethanol or benzyl alcohol) to acquire lipid matrices. Prior to micronization, API loaded matrices are cooled through liquid nitrogen approximately at -80°C. The resultant powder is then subjected to automatic sieving device. This technique produces SLM of about 1-500 μm (Battaglia L and Ugazio E, 2019).
Electrospray: This method enables the designing of monodispersed LNP and Lipid Micro Particles (LMP). Usually aliphatic alcohols are utilized to dissolve lipid. Nature of solvent, physico-chemical attribute of excipients and applied voltage influences SLN size together with shape. Increased electrical field brings about the transformation in hemispherical drops to conical shape that eventually disrupt into extremely charged droplets.
Spray congealing: Here, API is dissolved/dispersed in molten lipids. The hot drug lipid mixture is further subjected to atomization through pneumatic nozzle. Few variations in atomization device include WPN, APN and the ultrasound-atomizer (Passerini N, et al., 2010). Besides, rotating disc can be engaged for spray congealing (Mackaplow MB, et al., 2006), yielding SLMs within 50-2000 μm.
Microstructural characterization
SLNs characterization plays a significant role in establishing quality of a final product. Though, their colloidal dimension along with dynamic phenomena like hysterical kinetics and supercooling may complicate the analysis (Teeranachaideekul V, et al., 2017). So, care must be executed while characterizing SLNs. Following sections (4.1-4.11) narrates diverse sophisticated techniques useful to generate information relating to the inner and outer assembly, and the general behavior of SLN.
Particle Size (PS) and Polydispersity Index (PDI): SLNs are colloidal particles representing submicron range. A well fabricated SLN dispersion should display low PDI indicating narrow distribution of PS (Kunasekaran V and Krishnamoorthy K, 2016). Usually particles exceeding 1 µm size and their time dependent growth pinpoints physical instability of SLNs (Qian C, et al., 2013). Therefore, the measuring PS and the PDI is vital to ensure stability of designed SLNs.
Photon Correlation Spectroscopy (PCS)/Dynamic Light Scattering
(DLS): It is a convenient technique for the routine analysis of PS of SLN. Though the said method is unable to detect particles with sizes >3 µm. In general, PCS comprises of a laser beam source, a sample cell holder (temperature controlled), a light detector and a photomultiplier. Firstly, the SLN sample is irradiated through a laser beam, and then the scattered light by the particles Brownian motion is sensed by a detector.
Here, it is absolutely essential to understand that PCS works on the assumption that SLNs are spherical. However, several researchers have noted an anisometric shape of SLN (Jores K, et al., 2004; Noack A, et al., 2012; Schubert MA, et al., 2006). In such situation PS data gained using PCS could be deceptive. PCS is also valuable for determining the PDI of a colloidal system as a measure of width of PS distribution. Typically, monodispersed systems obey single exponential decay G (τ) (Stetefeld J, et al., 2016) while polydispersed systems exhibits polyexponential decay. PDI is a unitless parameter spanning within 0-1. A PDI value amid 0.03 and 0.06 reflects a monodispersed colloidal system. A narrow PSD is reflected by PDI value within 0.10-0.20, whereas a PDI amid 0.25-0.50 indicates wide PSD. Moreover, PDI values >0.50 are suggestive of broad PSD of SLN sample.
Laser Diffraction (LD)/Static Light Scattering (SLS): This technique is useful for measurement of the broader size of colloidal dispersions, spanning within 0.04-2000 µm. PS is derived from light diffracted from the particles surface at a stated angle. The detector then communicates the information to a computer and the PS is calculated using the Fraunhofer or the Mie theory (Blott SJ and Pye K, 2006). LD data is generally presented as volume distribution diameters of d50%, d90%, d95%, and d99%. The d99% specifies that 99% of dispersed particles are under a specified size or volume distribution. Nevertheless the real and imaginary refractive index of particles and the dispersion medium must be accounted (Nai-Ning W, et al., 1992). Besides, Mie theory assumes that particles must be spherical in morphology. Initial versions of LD equipment were intended to allow particles to scatter light up to an angle of 14° limiting its use to micrometric range. Though the new version of LD is more sensitive and grounded on PIDS technology, it detects light scattering at an angle between 60°-146° (Fu Q and Sun W, 2001).
Zeta Potential (ZP) and electrophoretic mobility: ZP is the most imperative parameter in SLNs characterization, and is determinant of long term colloidal stability. ZP represents an electric potential at hydrodynamic plane of shear separating a tinny liquid layer constituted of counter-ions, bound to a moving charged surface. The electric charges on the particles and a medium significantly influence ZP. Thus, the slight deviations in the pH or medium ionic strength affect ZP.
SLNs dispersion in distilled water or water with low conductivity facilitates better comprehension of ZP regarding aggregation processes inside colloidal systems. SLNs stability is assured if the ZP value is lower than -30 mV or more than a +30 mV (Skoglund S, et al., 2017), and often influenced by kind of ionic stabilizers present. Notably, this implies to the colloidal systems stabilized by an electrostatic interactions alone. It could be ascribed to covering of surface charges and decline in ZP due to a major shift in the particles shear plane.
Morphology analysis: Though PCS and LD are important tools to characterize SLNs PS, they fail to furnish the topographic information of particles. Here, microscopic techniques are crucial in that they not only provides details about PS of the disperse phase but also gives an insight to the shape, organization and morphology of SLNs. Various microscopic systems for example Light Microscopy (LM), SEM and TEM are valuable for the said purpose.
Light Microscopy (LM): This method is circumscribed by its low detection limit (≈ 200 nm). The technique utilizes polarized light to detect particles spanning 200-300 nm (Jacobs C and Müller RH, 2002). LM is more accurately and reliably utilized while estimating size of micro particles. Polarized light in LM can assist detection of drug nanocrystals which remained un-encapsulated during SLN processing.
Scanning Electron Microscopy (SEM): It is a very useful technique to visualize SLNs shape, lipid organization and morphology. The technique requires pretreatment of sample involving a drying process. Hence SLNs are visualized under dehydrated condition under a high vacuum (Sanderson MJ, et al., 2014). The procedure involves an initial deposition of aqueous SLN sample onto a graphite strip followed by drying at RT or in an oven (≈ 25°C-30°C) to evade the particles melting. This could possibly bring about a noteworthy change in SLN shape and morphology (Kyadarkunte AY, et al., 2015). Hence, now it is well recognized that SEM particle diameters are way smaller than PCS diameter, as the latter technique measures hydrodynamic particle diameter.
Transmission Electron Microscopy (TEM): This is a valued tool in the explication SLNs size, shape and morphology. However in contrary to SEM, this technique makes two dimensional (2D) images and usually has a better resolution. Contingent on the information to be pursued, different approaches as staining, freeze-fracturing and the usage of cryo-electron microscopy are exploited. Staining technique may be adequate to obtain data pertaining to SLNs shape and morphology. The dried sample is subsequently stained with dyes as Phosphotungstic Acid (PTA) or Uranyl Acetate (UA) (Franken LE, et al., 2017). Prior to TEM visualization, the stain is permitted to dry at RT for another 30 seconds.
Lipid crystallinity and polymorphism: Crystal structure then polymorphic form of lipid is essential parameters that should be extensively investigated when formulating SLNs. Now it is recognized that crystallinity of carrier lipid exerts substantial effect on API incorporation, its release or even expulsion (Kumbhar DD and Pokharkar VB, 2013). In this respect, technique such as DSC and WAXS are vital to elucidate the crystallinity and lipid modification. Besides, these techniques allow the formulator to analyze SLNs physical state, and the incidence of supercooled melt during its processing.
Differential Scanning Calorimetry (DSC): This technique elucidates modifications in crystallinity degree of a lipid and the API during SLN processing. Note that lipid modifications (α, β, and βi) (Pokharkar VB, et al., 2015) alters thermal events like MP significantly affecting systems enthalpy. The basic operating principle of DSC is to quantity the alterations in an amount of heat generated or lost between a sample and a reference pan with respect to temperature (Ribeiro AP, et al., 2015). Generally, DSC allows formulator to probe melting, vaporization, crystallization, condensation and/or glass transition state (Chiu MH and Prenner EJ, 2011) of that particular compound. The Power Compensated-DSC (PC-DSC) and Heat Flux-DSC (HF-DSC) can be effectively employed to assess SLNs thermal behavior (Leyva-Porras C, et al., 2019). The key adaptation amid the two systems is the supply of heat flow towards the investigational sample and a reference pan. In PC-DSC heat flow is derived from separate furnaces while a single furnace is utilized for HF-DSC. Melting enthalpy obtained from DSC investigation of SLNs assist assessment of Recrystallization Index (RI). It is a measure of percent lipid recrystallization during the storage period of SLNs.
Wide Angel X-Ray Scattering (WAXS): WAXS is substantially used in the investigation of the lamellar arrangement of lipid molecules, crystallinity degree of FA chains in triacylglycerides, and polymorphism (Shah M and Pathak K, 2010). The technique measures length of spacing (long and short) amid the alkyl side-chains of triacylglycerides and speculate about its polymorphic and crystalline nature. These appear as single or more reflections in wide angle region of a WAXS spectrum. WAXS allows making out crystalline and amorphous substances. It also shed a light on state of API after encapsulation in corresponding lipid matrix i.e. crystalline, amorphous or molecular.
Molecular analysis using vibrational spectroscopy: Crystal polymorphism is ubiquitous in small organic molecules. Grant defines it as propensity of a compound to exhibit different crystalline phases involving diverse arrangements or molecular conformations within a crystal lattice. In conformational polymorphism, substance exists in multiple molecular conformations in different solid-state forms (Bernstein J and Hagler AT, 1978). Vibrational spectroscopy methods are vital in identification and microstructural elucidations of pharmaceutical solids. Techniques as Fourier-Transform Infrared (FT-IR), Raman scattering, Terahertz spectroscopy (THz), and an Inelastic Neutron Scattering (INS) provides data pertaining to the molecular vibrational modes (Kalanoor BS, et al., 2017). Among them Raman scattering and Infrared spectroscopy (IR) are the mostly used as easy handling besides relatively low cost. THz radiation can stimulate low energy vibrations permitting probing of IR active low energy vibrational modes. In INS, molecules complete vibrational spectrum can be acquired by an inelastic scattering of thermal neutrons in solids. However, INS use is circumscribed owing to low resolution as against Raman and IR techniques. A crucial alteration amid the Raman and FT-IR is that Raman is a scattering measurement, while, FT-IR is absorption based technique. In contrast, in scattering technologies, the SLNs face a single wavelength of light usually in a form of laser, and a series of wavelengths are scrutinized for an emission of light.
Raman spectroscopy: Raman scattering is a commanding approach to examine polymorphism in API, and carrier lipid thanks to its remarkable sensitivity to transitions of molecular compounds. In the Raman analysis, low wavenumber (<200 cm-1) is vital to decipher materials polymorphic behavior. Given that at molecular level lattice deformations, liberations and translations usually occur below 200 cm-1 (Vega DR, et al., 2007). Note that the Raman spectra of lipid are profoundly contingent on the conformational packing and dynamic variations in hydrocarbon chains (Faried M, et al., 2019). Literature reports specific assignments to lipids Raman frequency including -CH3 rocking (800-900 cm-1), -C-C asymmetric stretch (1062 cm-1), -C-C symmetric stretch (1028 cm-1), -CH2 twist (1294 cm-1), -CH2 symmetric stretching (2845 cm-1), and -CH2 asymmetric stretch (2881 cm-1).
Fourier-Transform Infrared Spectroscopy (FT-IR): This is a routinely used vibrational method for material identification and authentication. Here, light absorption by the material of interest (API, lipid and SLN) is measured within 400-4,000 cm-1 consistent with essential vibrational modes.
Agrawal S, et al. employed FT-IR to characterize form I, II, and an amorphous rifampicin (Agrawal S, et al., 2004). Likewise, Bauer J, et al. identified conformational polymorphism in ritonavir (Bauer J, et al., 2001). This technique gives an insight to lipid organization and its possible molecular interactions with an incorporated API during processing SLNs and its storage. Like solids, Cary 630 is operable for liquid sample as such that is without transforming them to solid form. It allows the formulator to study intact SLNs microstructure under noninvasive conditions.
Microenvironment investigations: NMR is an expedient technique in studying the microenvironment of designed SLNs. For a better understanding of SLNs microenvironment, molecular, electronic and structural aspects are probed. NMR active nuclei of the interest are 1H, 13C, 19F, and 31P. A Simple 1H-NMR spectroscopy permits an easy and very rapid detection of a super cooled melt initiated by low line widths of lipid protons (Wissing SA, et al., 2004). The said technique utilizes proton relaxation time for diverse formulation states (liquid/semisolid/solid). Literature provides numerous reports on the usefulness of this method to distinguish the composition besides structure of LNP (Suzuki N and Kawasaki T, 2005; Jenning V, et al., 2000; Garcia-Fuentes M, et al., 2005; Zimmermann ES, et al., 2005). The 1H NMR spectra of NLC usually show slightly broaden proton peaks corresponding to oil molecules, which are rendered immobile by the matrix network. Conversely, SLNs on account of their restricted mobility and shortened relaxation time shows broad NMR signals by small amplitude (Jores K, et al., 2003). Thus NMR analysis is expected to reveal the mobility, arrangements, and the mode of liquid entrapment within solid matrices. Besides the technique also allows investigating the structural integrity and possible molecular exchanges amid the incorporated API and lipid. Though, these approaches need a substantial exploitation to get an understanding of structural dynamics of lipid dispersion.
Loading Capacity (LC) and Encapsulation Efficiency (EE): Drug LC must be evaluated to warrant the suitability of designed SLNs for a specific drug to be incorporated. In addition, such carrier systems should have a high EE besides long-term retention of an encapsulated API. The LC of SLN depends on API ability to get dissolved in molten lipid, drug-lipid miscibility, and lipid polymorphism (Rosenblatt KM and Bunjes H, 2017). The LC and EE of an API, is evaluated per unit weight of SLN dispersion by a validated analytical tool such as Ultraviolet (UV) spectrophotometry or High Performance Liquid Chromatography (HPLC). For the purpose API is separated from the SLNs by a high speed centrifuge as Allegra 64R (Beckman Coulter) usually above 15000 rpm for 30 min and at 4°C.
Drug release: Here, note that an incorporated API either present at core or exterior shell of SLN else forms a molecular dispersion with carrier matrix. This depends on various aspects as API solubility in used lipid, drug/ lipid ratio and the production technique. Thus, obtaining the desired drug release is still elusive for SLN and, usually, the release is accentuated by an early burst. Few were the first to report controlled prednisolone release from the SLNs shaped by HPH technique. They found that prednisolone release was dependent on extents of surfactant and lipid and the production parameters. They also established that the prednisolone release was independent of SLN size confirming that mass transfer was not diffusion limited. The rise in processing temperature on surfactant concentration usually tends to burst release. This might be inferred from drug partitioning into continuous phase while SLN processing adversely affecting sustained release (Shenoy VS, et al., 2013). Conversely, fall in processing temperature promotes lipid crystallization, and subsequent cooling diminishes API water solubility.
Dialysis tubing, reverse dialysis and Franz diffusion chamber are utilized to study SLN drug release. In the dialysis tubing method cellophane (mcw ≈ 12000-14000 Da) serves as a synthetic membrane. The SLN sample (≈ 5 ml) is positioned in dialysis tubing that has been prewashed and hermetically closed. The formed sac is then immersed in a suitable dissolution fluid, and dialyzed at RT. The specified quantity of sample is withdrawn at preset time interval, centrifuged, and then analyzed for drug content by UV/HPLC. This approach allows for dilution of SLNs, however does not quantify rapid drug release. Franz diffusion chambers (vertical/flow through) are typically used to scrutinize SLN drug release. The sample is located in a diffusion chamber with a synthetic membrane (cellophane, mcw ≈ 12000-14000 Da) in donor compartment. Following this dialysis is performed against an appropriate dissolution fluid. At pre-set time and preserving the sink state, withdrawn samples are examined for drug content by UV/HPLC.
Rheology: Rheology of LNP influences its potential for pharmaceutical application in a fundamental way. For dermal use SLNs should possess relatively high viscosity and a plastic or thixotropic flow (Lippacher A, et al., 2004). This would aid them to stick onto skin for an adequate time. Conversely, for parenteral use SLNs should possess low viscosity to ease withdrawal from the container and injectability. A very limited data is available on rheology of SLNs and that too pertaining to formulations with high lipid level (>20% m/m). Note that a lipid concentration and SLN size are the prime determinants of its rheology (Seetapan N, et al., 2010). On other side an oscillatory measurements (stress and frequency sweep) yield valued information about SLNs viscous and elastic nature and reveals the network structure formed by them (Lippacher A, et al., 2002; Lippacher A, et al., 2000). For the reason, parameters as Linear Viscoelastic Region (LVR), complex modulus (G*), storage modulus (G’) and the loss modulus (G”) are scrutinized. Thus, rheology offers a noninvasive mode to study SLNs viscoelastic behavior without disrupting its microstructure.
Stability: The disadvantages concomitant with SLNs particularly those regarding low drug LC, and API expulsion through extended storage periods directed the progress of NLC. The crystalline nature and polymorphic transition in lipids (Schoenitz M, et al., 2014) are the foremost reasons of drug eviction. Solid lipids exists in β-polymorphic form, though upon melting during SLN production, recrystallizes to lower ordered α- and/ or β'-polymorphic modifications. These polymorphic forms are relatively amorphous and permits API incorporation and their holding in the carrier matrix. This results in reduction in imperfections within matrix lipid and accounts for API expulsion from SLN.
Contrary to SLNs, which are prepared through extremely purified lipids, NLCs are manufactured by controlled mixing of solid lipids and oil. The company of oil increases the imperfections in crystal lattice (Khosa A, et al., 2018) enabling it to hold more drug quantity. Besides, formation of additional colloidal structures as liposomes, micelles, and even supercooled melt can adversely influence SLNs stability. Moreover, gelation of SLNs (Borges A, et al., 2020) ensues as PS rise unfavorably affecting its stability. Therefore in stability study of SLN, size, ZP and % drug encapsulation are vital parameters. Techniques like lyophilization, spray drying, or use of novel adsorbents are employed to transform SLN dispersion to dry powder. The subsequent section discusses the lyophilization pertaining to LNP.
Lyophilization: The utmost challenge in storing SLNs involves inhibition of hydrolytic reaction, and the protection of an initial PS (Hu FQ, et al., 2006). Ostwald ripening then temperature changes during shipping can adversely affect SLNs stability. It is now established that the judiciously produced SLNs provide stability over 12-36 months (del Pozo-Rodríguez A, et al., 2009). Though, their colloidal dimension and subsequent aggregation encourage rise in PS. The promising way to improve SLNs physicochemical stability is lyophilization. Besides, lyophilization of SLNs offer chances to incorporate LNP into various solid dosage forms as capsules, tablets and pellets (Attama AA and Umeyor CE, 2015). Moreover, lyophilization facilitates solid state analysis through X-ray diffraction, FT-IR, DSC besides Raman scattering. Though lyophilization of SLN results in better stability, two processing transformations augment stability concerns. The first transformation arises owing to freezing out effect (Soares S, et al., 2013) causing disparities in the osmolarity and pH. Also to note that lyophilization process can compromise surfactants protective effect. To ensure better redispersibility of dry product it is usually recommended to use cryoprotectant while processing SLN formulation. Usually for most SLNs cryoprotectants used are mannitol, trehalose, glucose and sorbitol (Zhang L, et al., 2008; Amis TM, et al., 2020). They work by lessening the osmotic activity of water besides crystallization while favoring the glassy frozen SLNs. Besides they form ‘pseudo hydration shell’ by networking with the surfactants polar head groups.
Applications of SLN’s
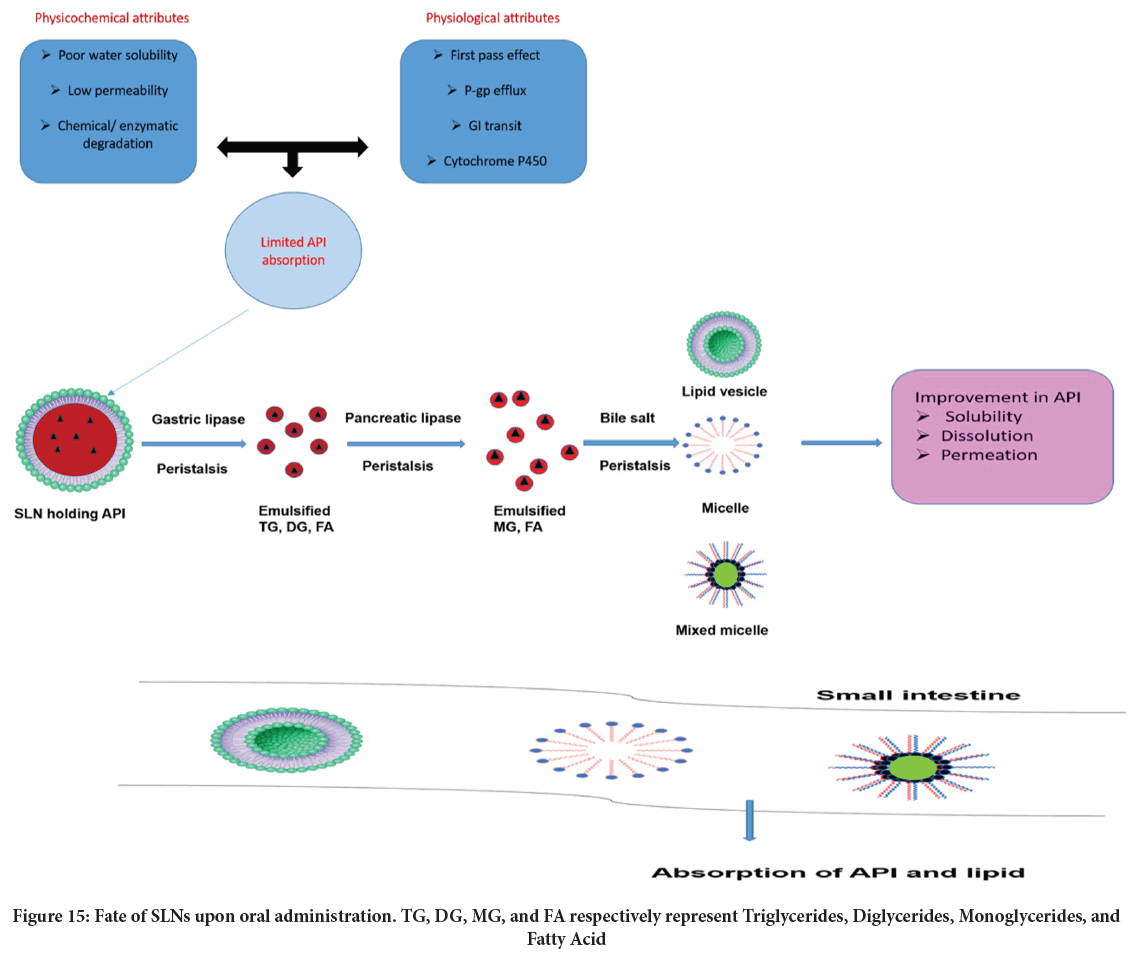
SLNs are capable to carry vast variety of pharmaceuticals, cosmaceuticals, neutraceuticals and pesticides. Biopharmaceutics Classification System (BCS) class II and IV API are amenable to SLNs and their absorption and subsequent bioavailability can be entrusted. Figure 15 depicts the mechanism by which SLNs brings about an enhancement in oral bioavailability of API belonging to said classes. Few demonstrated that SLN can improve drug holding in the eye for an adequate time. Thus with lipids biocompatibility then ease of metabolism, SLNs could effectively be formulated for ophthalmic delivery. SLNs are valuable in delivering drugs to lung. It is also likely to attain API controlled release upon localization of SLNs into bronchi and alveoli (de Menezes BR, et al., 2021; Gaspar DP, et al., 2017). Topical usage of SLNs is also well documented in literature (Küchler S, et al., 2010; Liu M, et al., 2020). Netto G and Jose J successfully developed silymarin containing SLN for better sun protection (Netto G and Jose J, 2018). The significant ionic interactions between DNA and cationic SLN accentuate gene delivery (Pedersen N, et al., 2006; Yu W, et al., 2009). Antitubercular drugs like isoniazid, pyrazinamide, and rifampicin (Pandey R, et al., 2005), have been productively encapsulated into SLNs. This improved the patient compliance by subsequent drop in drug dosing frequency. SLN also finds use in cancer therapeutics. Extended release tamoxifen SLNs are given intravenously to manage breast cancer (Fontana G, et al., 2005). Besides, methotrexate (Ruckmani K, et al., 2006) and camptothecin (Martins SM, et al., 2013) SLNs are conceivable for tumor targeting. Tupal A, et al. developed doxorubicin-loaded SLNs for skin cancer (Tupal A, et al., 2016). Lu B, et al. designed mitoxantrone containing SLNs for breast cancer and lymph node metastases (Lu B, et al., 2006). Resveratrol loaded SLNs exhibits a substantial activity against breast cancer (human) cell line (Wang W, et al., 2017). SLNs of an antimalarial agent have shown promise in attenuating drug toxicity besides improving its selectivity then bioavailability in animals. BBB is the prime culprit in attaining drug transport to brain and hence management of several Central Nervous System (CNS) disorders. Floxuridine (5-fluoro-2'-deoxyuridine) possess a poor BBB penetration (Wang JX, et al., 2002), and is associated with toxicities like myelosuppression and Gastrointestinal (GI) mucosal injury. Synthesis of 3’, 5’-Dioctanoyl-5- Fluoro-2'-deoxyuridine (DO-FUdR) and its subsequent incorporation into SLNs enables its entrance to brain while suppressing drug related toxicities. Several peculiarities of SLNs like occlusiveness, skin hydration, modified release, and skin permeability augers well for cosmaceuticals delivery. Of late, by dint of its GRAS nature, SLNs are gaining much consideration in delivering various bioactives (Aditya NP and Ko S, 2015), nutraceuticals (Nunes S, et al., 2017), and environmentally safe pesticides (Oliveira CR, et al., 2019; Nguyen HM, et al., 2012). Moreover, despite its excellent drug delivery properties, SLNs can attenuates the related toxicity of an integrated API. For instance nephrotoxicity associated with amphotericin B can be suppressed by formulating it as LNP (Ambisome®, Abelcet®) (Deray G, 2002). Likewise nano lipid formulation of doxorubicin (Doxil®, Myocet®) reduces cardiotoxicity pertaining to drug (Batist G, et al., 2001).
Figure 15: Fate of SLNs upon oral administration. TG, DG, MG, and FA respectively represent Triglycerides, Diglycerides, Monoglycerides, and Fatty Acid
Microstructural perspective
Though the lipids essentially hold its material properties while in colloidal dimension, several microstructural peculiarities need a probe while SLN processing. SLN dispersion displays a complex physicochemical behavior that differs considerably from bulk lipid. In particularly, at nanoscale the crystallization and polymorphism are significantly altered. These properties besides SLNs size, shape and microstructure get substantially affected by surfactants. Here, a careful appraisal of SLNs is mandatory since the structural attributes are decisive while defining their functionality. Not all expectations that were raised during the growth of SLNs are met yet. Still an open question, particularly regarding to drug-carrier interactions, microstructural understandings and encapsulation on a hydrophilic active in SLN pose a serious challenge. Therefore clinical use of SLNs is limited to BCS class II and IV drugs. The uncontrolled partitioning of hydrophilic molecule during SLN processing demands smart strategy to influence lipid matrix to accommodate the said molecule. Moreover, interactions among particles bring about a pivotal change in rheology significantly affecting SLNs microstructure. Note that colloidal size of SLNs encourages its aggregation, coagulation and even gelation affecting its stability. On the other side, the smart exploitation of SLNs usually instigates with a judicious choice of structural components. To attain maximum drug pay load modification in lipid matrix remains crucial. Incorporation of oil significantly modifies matrix architecture and allows for more drug loading followed by its retention for considerable time. Besides, as demonstrated in our earlier research to load hydrophilic active in SLNs use of C10-C30 alkyl acrylate was crucial. This approach based on polar lipid and hydrophobically modified polymer can assist encapsulation of diverse hydrophilic molecules. Though a detailed revelations are mandatory to confirm their formation, microstructure and stability. Additionally use of amphipathic polymer might serve to lower an interfacial tension at o/w interface while various block copolymers such as Poly (Dimethylsiloxane) (PDMS), Poly (Butadiene) (PBut), Poly (ε-Caprolactone) (PCL) and Poly (Isobutylene) (PIB) representing hydrophobic block and Poly (2-Methyloxazoline) (PMOXA) and Poly Ethylene Glycol (PEG) representing hydrophilic blocks can be explored to attain encapsulation of hydrophilic molecule in matrix lipid. To achieve significant encapsulation in the lipid matrix, cationic drug molecule can be ionically complexed in a correct molar ratio with an anionic polymer as dextran sulfate, sodium alginate and Gantrez AN-119. Though this appears to be a good strategy often results in a complex multicomponent system needing a molecular probe to confirm its functionality. Different techniques yield SLNs with an altered physico-chemical attributes and microstructure. Hence it is imperative to evaluate them thoroughly for thermal behavior, crystallization, rheology and morphology.
Discussion and Conclusion
This article provides the basic understanding of SLN production mechanics with respect to its microstructure and with this it is practicable to design SLNs of expected properties. In the upcoming time smartly designed SLNs with modified microstructure will open a new archetype to deliver diverse API, cosmeceuticals, and neutraceuticals. Besides with better understanding of their assembly mechanics it is practicable to devise SLNs of desired properties.
References
- Rosenblum D, Joshi N, Tao W, Karp JM, Peer D. Progress and challenges towards targeted delivery of cancer therapeutics. Nat Commun. 2018; 9(1): 1-2.
[Crossref] [Google Scholar] [Pubmed]
- Singh N, Joshi A, Toor AP, Verma G. Drug delivery: Advancements and challenges. Nanostructures for drug delivery. 2017; 865-886.
- Khatak S, Dureja H. Structural composition of solid lipid nanoparticles for invasive and non-invasive drug delivery. Curr Nanomater. 2017; 2(3): 129-153.
- Naseri N, Valizadeh H, Zakeri-Milani P. Solid lipid nanoparticles and nanostructured lipid carriers: Structure, preparation and application. Adv Pharm Bull. 2015; 5(3): 305.
[Crossref] [Google Scholar] [Pubmed]
- Duong VA, Nguyen TT, Maeng HJ. Preparation of solid lipid nanoparticles and nanostructured lipid carriers for drug delivery and the effects of preparation parameters of solvent injection method. Molecules. 2020; 25(20): 4781.
[Crossref] [Google Scholar] [Pubmed]
- Salminen H, Stübler AS, Weiss J. Preparation, characterization, and physical stability of cocoa butter and tristearin nanoparticles containing β-carotene. Eur Food Res Technol. 2020; 246(3): 599-608.
- Bahari LA, Hamishehkar H. The impact of variables on particle size of solid lipid nanoparticles and nanostructured lipid carriers: A comparative literature review. Adv Pharm Bull. 2016; 6(2): 143.
[Crossref] [Google Scholar] [Pubmed]
- Shazly GA. Ciprofloxacin controlled-solid lipid nanoparticles: Characterization, in vitro release, and antibacterial activity assessment. BioMed Res Int. 2017.
[Crossref] [Google Scholar] [Pubmed]
- Li S, Ji Z, Zou M, Nie X, Shi Y, Cheng G. Preparation, characterization, pharmacokinetics and tissue distribution of solid lipid nanoparticles loaded with tetrandrine. AAPS PharmSciTech. 2011; 12(3): 1011-1018.
[Crossref] [Google Scholar] [Pubmed]
- Abdelwahed W, Degobert G, Stainmesse S, Fessi H. Freeze-drying of nanoparticles: Formulation, process and storage considerations. Adv Drug Deliv Rev. 2006; 58(15): 1688-1713.
[Crossref] [Google Scholar] [Pubmed]
- Üner M, Yener G. Importance of solid lipid nanoparticles (SLN) in various administration routes and future perspectives. Int J Nanomedicine. 2007; 2(3): 289.
[Google Scholar] [Pubmed]
- Mukherjee S, Ray S, Thakur RS. Solid lipid nanoparticles: A modern formulation approach in drug delivery system. Indian J Pharm Sci. 2009; 71(4): 349.
[Crossref] [Google Scholar] [Pubmed]
- Dolatabadi JE, Valizadeh H, Hamishehkar H. Solid lipid nanoparticles as efficient drug and gene delivery systems: Recent breakthroughs. Adv Pharm Bull. 2015; 5(2): 151.
[Crossref] [Google Scholar] [Pubmed]
- Uprit S, Sahu RK, Roy A, Pare A. Preparation and characterization of minoxidil loaded nanostructured lipid carrier gel for effective treatment of alopecia. Saudi Pharm J. 2013; 21(4): 379-385.
[Crossref] [Google Scholar] [Pubmed]
- Aboud HM, Ali AA, el Menshawe SF, Abd Elbary A. Development, optimization, and evaluation of carvedilol-loaded solid lipid nanoparticles for intranasal drug delivery. AAPS PharmSciTech. 2016; 17(6): 1353-1365.
[Crossref] [Google Scholar] [Pubmed]
- Hu FQ, Hong Y, Yuan H. Preparation and characterization of solid lipid nanoparticles containing peptide. Int J Pharm. 2004; 273(1-2): 29-35.
[Crossref] [Google Scholar] [Pubmed]
- Souto EB, Baldim I, Oliveira WP, Rao R, Yadav N, Gama FM, et al. SLN and NLC for topical, dermal, and transdermal drug delivery. Expert Opin Drug Deliv. 2020; 17(3): 357-377.
[Crossref] [Google Scholar] [Pubmed]
- Trotta M, Debernardi F, Caputo O. Preparation of solid lipid nanoparticles by a solvent emulsification-diffusion technique. Int J Pharm. 2003; 257(1-2): 153-160.
[Crossref] [Google Scholar] [Pubmed]
- Müller RH, Mäder K, Gohla S. Solid Lipid Nanoparticles (SLN) for controlled drug delivery-a review of the state of the art. Eur J Pharm Biopharm. 2000; 50(1): 161-177.
[Crossref] [Google Scholar] [Pubmed]
- Jenning V, Lippacher A, Gohla SH. Medium scale production of Solid Lipid Nanoparticles (SLN) by high pressure homogenization. J Microencapsul. 2002; 19(1): 1-10.
[Crossref] [Google Scholar] [Pubmed]
- Kluge J, Muhrer G, Mazzotti M. High pressure homogenization of pharmaceutical solids. J Supercrit Fluid. 2012; 66: 380-388.
- Gall V, Runde M, Schuchmann HP. Extending applications of high-pressure homogenization by using Simultaneous Emulsification and Mixing (SEM): An overview. Processes. 2016; 4(4): 46.
- Loh ZH, Samanta AK, Heng PW. Overview of milling techniques for improving the solubility of poorly water-soluble drugs. Asian J Pharm Sci. 2015; 10(4): 255-2574.
- Sajjadi B, Raman AA, Ibrahim S. Influence of ultrasound power on acoustic streaming and micro-bubbles formations in a low frequency sono-reactor: Mathematical and 3D computational simulation. Ultrason Sonochem. 2015; 24: 193-203.
[Crossref] [Google Scholar] [Pubmed]
- Patravale VB, Mirani AG. Preparation and Characterization of solid lipid nanoparticles-based gel for topical delivery. Methods Mol Biol. 2019; 2000: 293-302.
[Crossref] [Google Scholar] [Pubmed]
- Pooja D, Tunki L, Kulhari H, Reddy BB, Sistla R. Optimization of solid lipid nanoparticles prepared by a single emulsification-solvent evaporation method. Data Brief. 2016; 6: 15-19.
[Crossref] [Google Scholar] [Pubmed]
- Pignatello R, Leonardi A, Fuochi V, Petronio G, Greco AS, Furneri PM. A method for efficient loading of ciprofloxacin hydrochloride in cationic solid lipid nanoparticles: Formulation and microbiological evaluation. Nanomaterials. 2018; 8(5): 304.
[Crossref] [Google Scholar] [Pubmed]
- Mishra D, Mishra H, Mishra PK, Nahar M, Dubey V, Jain NK. Evaluation of solid lipid nanoparticles as carriers for delivery of hepatitis B surface antigen for vaccination using subcutaneous route. J Pharm Pharmaceut Sci. 2010; 13(4): 495-509.
[Crossref] [Google Scholar] [Pubmed]
- Tartaro G, Mateos H, Schirone D, Angelico R, Palazzo G. Microemulsion microstructure (s): A tutorial review. Nanomaterials. 2020; 10(9): 1657.
[Crossref] [Google Scholar] [Pubmed]
- Li Z, Yu L, Zheng L, Geng F. Studies on crystallinity state of puerarin loaded solid lipid nanoparticles prepared by double emulsion method. J Therm Anal Calorim. 2010; 99(2): 689-693.
- Sebti T, Amighi K. Preparation and in vitro evaluation of lipidic carriers and fillers for inhalation. Eur J Pharm Biopharm. 2006; 63(1): 51-58.
[Crossref] [Google Scholar] [Pubmed]
- Khaw KY, Parat MO, Shaw PN, Falconer JR. Solvent supercritical fluid technologies to extract bioactive compounds from natural sources: A review. Molecules. 2017; 22(7): 1186.
[Crossref] [Google Scholar] [Pubmed]
- Dos Santos IR, Richard J, Pech B, Thies C, Benoit JP. Microencapsulation of protein particles within lipids using a novel supercritical fluid process. Int J Pharm. 2002; 242(1-2): 69-78.
[Crossref] [Google Scholar] [Pubmed]
- Park SJ, Yeo SD. Recrystallization of caffeine using gas antisolvent process. J Supercrit Fluid. 2008; 47(1): 85-92.
- Trucillo P, Campardelli R. Production of solid lipid nanoparticles with a supercritical fluid assisted process. J Supercrit Fluid. 2019; 143: 16-23.
- Chattopadhyay P, Huff R, Shekunov BY. Drug encapsulation using supercritical fluid extraction of emulsions. J Pharm Sci. 2006; 95(3): 667-679.
[Crossref] [Google Scholar] [Pubmed]
- Pavoni L, Perinelli DR, Bonacucina G, Cespi M, Palmieri GF. An overview of micro-and nanoemulsions as vehicles for essential oils: Formulation, preparation and stability. Nanomaterials. 2020; 10(1): 135.
[Crossref] [Google Scholar] [Pubmed]
- Anton N, Gayet P, Benoit JP, Saulnier P. Nano-emulsions and nanocapsules by the PIT method: An investigation on the role of the temperature cycling on the emulsion phase inversion. Int J Pharm. 2007; 344(1-2): 44-52.
[Crossref] [Google Scholar] [Pubmed]
- Kiani A, Fathi M, Ghasemi SM. Production of novel vitamin D3 loaded lipid nanocapsules for milk fortification. Int J Food Prop. 2017; 20(11): 2466-2476.
- Battaglia L, Gallarate M, Cavalli R, Trotta M. Solid lipid nanoparticles produced through a coacervation method. J Microencapsul. 2010; 27(1): 78-85.
[Crossref] [Google Scholar] [Pubmed]
- Manojlović JŽ. The Krafft temperature of surfactant solutions. Therm Sci. 2012; 16(2): 631-640.
- Vigani B, Valentino C, Sandri G, Listro R, Fagiani F, Collina S, et al. A composite nanosystem as a potential tool for the local treatment of glioblastoma: Chitosan-coated solid lipid nanoparticles embedded in electrospun nanofibers. Polymers. 2021; 13(9): 1371.
[Crossref] [Google Scholar] [Pubmed]
- Battaglia L, Ugazio E. Lipid nano-and microparticles: An overview of patent-related research. J Nanomaterials. 2019; 2019: 1-22.
- Passerini N, Qi S, Albertini B, Grassi M, Rodriguez L, Craig DQ. Solid lipid microparticles produced by spray congealing: Influence of the atomizer on microparticle characteristics and mathematical modeling of the drug release. J Pharm Sci. 2010; 99(2): 916-931.
[Crossref] [Google Scholar] [Pubmed]
- Mackaplow MB, Zarraga IE, Morris JF. Rotary spray congealing of a suspension: Effect of disk speed and dispersed particle properties. J Microencapsul. 2006; 23(7): 793-809.
[Crossref] [Google Scholar] [Pubmed]
- Teeranachaideekul V, Chantaburanan T, Junyaprasert VB. Influence of state and crystallinity of lipid matrix on physicochemical properties and permeation of capsaicin-loaded lipid nanoparticles for topical delivery. J Drug Deliv Sci Technol. 2017; 39: 300-307.
- Kunasekaran V, Krishnamoorthy K. Formulation and evaluation of nanoscale solid lipid particles containing a hydrophilic drug-rasagiline mesylate. J Appl Pharm Sci. 2016; 6: 44-50.
- Qian C, Decker EA, Xiao H, McClements DJ. Impact of lipid nanoparticle physical state on particle aggregation and β-carotene degradation: Potential limitations of solid lipid nanoparticles. Food Res Int. 2013; 52(1): 342-349.
- Jores K, Mehnert W, Drechsler M, Bunjes H, Johann C, Mäder K. Investigations on the structure of Solid Lipid Nanoparticles (SLN) and oil-loaded solid lipid nanoparticles by photon correlation spectroscopy, field-flow fractionation and transmission electron microscopy. J Control Release. 2004; 95(2): 217-227.
[Crossref] [Google Scholar] [Pubmed]
- Noack A, Hause G, Mäder K. Physicochemical characterization of curcuminoid-loaded solid lipid nanoparticles. Int J Pharm. 2012; 423(2): 440-451.
[Crossref] [Google Scholar] [Pubmed]
- Schubert MA, Harms M, Müller-Goymann CC. Structural investigations on lipid nanoparticles containing high amounts of lecithin. Eur J Pharm Sci. 2006; 27(2-3): 226-236.
[Crossref] [Google Scholar] [Pubmed]
- Stetefeld J, McKenna SA, Patel TR. Dynamic light scattering: A practical guide and applications in biomedical sciences. Biophys Rev. 2016; 8(4): 409-427.
[Crossref] [Google Scholar] [Pubmed]
- Blott SJ, Pye K. Particle size distribution analysis of sand‐sized particles by laser diffraction: An experimental investigation of instrument sensitivity and the effects of particle shape. Sedimentology. 2006; 53(3): 671-685.
- Nai-Ning W, Hong-Jian Z, Xian-Huang Y. A versatile Fraunhofer diffraction and Mie scattering based laser particle sizer. Adv Powder Technol. 1992; 3(1): 7-14.
- Fu Q, Sun W. Mie theory for light scattering by a spherical particle in an absorbing medium. Applied Optics. 2001; 40(9): 1354-1361.
[Crossref] [Google Scholar] [Pubmed]
- Skoglund S, Hedberg J, Yunda E, Godymchuk A, Blomberg E, Wallinder IO. Difficulties and flaws in performing accurate determinations of zeta potentials of metal nanoparticles in complex solutions-Four case studies. PLoS One. 2017; 12(7): 181735.
[Crossref] [Google Scholar] [Pubmed]
- Jacobs C, Müller RH. Production and characterization of a budesonide nanosuspension for pulmonary administration. Pharm Res. 2002; 19(2): 189-194.
[Crossref] [Google Scholar] [Pubmed]
- Sanderson MJ, Smith I, Parker I, Bootman MD. Fluorescence microscopy. Cold Spring Harb Protoc. 2014; 2014(10): 71795.
[Crossref] [Google Scholar] [Pubmed]
- Kyadarkunte AY, Patole MS, Pokharkar VB. Cellular interactions and photoprotective effects of idebenone-loaded nanostructured lipid carriers stabilized using PEG-free surfactant. Int J Pharm. 2015; 479(1): 77-87.
[Crossref] [Google Scholar] [Pubmed]
- Franken LE, Boekema EJ, Stuart MC. Transmission electron microscopy as a tool for the characterization of soft materials: Application and interpretation. Adv Sci. 2017; 4(5): 1600476.
[Crossref] [Google Scholar] [Pubmed]
- Kumbhar DD, Pokharkar VB. Physicochemical investigations on an engineered lipid-polymer hybrid nanoparticle containing a model hydrophilic active, zidovudine. Colloids and Surfaces A: Physicochem Eng Aspects. 2013; 436: 714-725.
- Pokharkar VB, Jolly MR, Kumbhar DD. Engineering of a hybrid polymer-lipid nanocarrier for the nasal delivery of tenofovir disoproxil fumarate: Physicochemical, molecular, microstructural, and stability evaluation. Eur J Pharm Sci. 2015; 71: 99-111.
[Crossref] [Google Scholar] [Pubmed]
- Ribeiro AP, Masuchi MH, Miyasaki EK, Domingues MA, Stroppa VL, de Oliveira GM, et al. Crystallization modifiers in lipid systems. J Food Sci Technol. 2015; 52(7): 3925-3946.
[Crossref] [Google Scholar] [Pubmed]
- Chiu MH, Prenner EJ. Differential scanning calorimetry: An invaluable tool for a detailed thermodynamic characterization of macromolecules and their interactions. J Pharm Bioallied Sci. 2011; 3(1): 39.
[Crossref] [Google Scholar] [Pubmed]
- Leyva-Porras C, Cruz-Alcantar P, Espinosa-Solís V, Martínez-Guerra E, Piñón-Balderrama CI, Compean Martínez I, et al. Application of Differential Scanning Calorimetry (DSC) and Modulated Differential Scanning Calorimetry (MDSC) in food and drug industries. Polymers. 2019; 12(1): 5.
[Crossref] [Google Scholar] [Pubmed]
- Shah M, Pathak K. Development and statistical optimization of solid lipid nanoparticles of simvastatin by using 23 full-factorial design. AAPS PharmSciTech. 2010; 11(2): 489-496.
[Crossref] [Google Scholar] [Pubmed]
- Bernstein J, Hagler AT. Conformational polymorphism: The influence of crystal structure on molecular conformation. J Am Chem Soc. 1978; 100(3): 673-681.
- Kalanoor BS, Ronen M, Oren Z, Gerber D, Tischler YR. New method to study the vibrational modes of biomolecules in the terahertz range based on a single-stage Raman spectrometer. ACS Omega. 2017; 2(3): 1232-1240.
[Crossref] [Google Scholar] [Pubmed]
- Vega DR, Polla G, Martinez A, Mendioroz E, Reinoso M. Conformational polymorphism in bicalutamide. Int J Pharm. 2007; 328(2): 112-118.
[Crossref] [Google Scholar] [Pubmed]
- Faried M, Suga K, Okamoto Y, Shameli K, Miyake M, Umakoshi H. Membrane surface-enhanced Raman spectroscopy for cholesterol-modified lipid systems: Effect of gold nanoparticle size. ACS Omega. 2019; 4(9): 13687-13695.
[Crossref] [Google Scholar] [Pubmed]
- Agrawal S, Ashokraj Y, Bharatam PV, Pillai O, Panchagnula R. Solid-state characterization of rifampicin samples and its biopharmaceutic relevance. Eur J Pharm Sci. 2004; 22(2-3): 127-144.
[Crossref] [Google Scholar] [Pubmed]
- Bauer J, Spanton S, Henry R, Quick J, Dziki W, Porter W, et al. Ritonavir: An extraordinary example of conformational polymorphism. Pharm Res. 2001; 18(6): 859-866.
[Crossref] [Google Scholar] [Pubmed]
- Wissing SA, Müller RH, Manthei L, Mayer C. Structural characterization of Q10-loaded solid lipid nanoparticles by NMR spectroscopy. Pharm Res. 2004; 21(3): 400-405.
[Crossref] [Google Scholar] [Pubmed]
- Suzuki N, Kawasaki T. Evaluation of solid state form of troglitazone by solid state NMR spectroscopy. J Pharm Biomed Anal. 2005; 37(1): 177-181.
[Crossref] [Google Scholar] [Pubmed]
- Jenning V, Mäder K, Gohla SH. solid Lipid Nanoparticles (SLN™) based on binary mixtures of liquid and solid lipids: A 1H-NMR study. Int J Pharm. 2000; 205(1-2): 15-21.
[Crossref] [Google Scholar] [Pubmed]
- Garcia-Fuentes M, Alonso MJ, Torres D. Design and characterization of a new drug nanocarrier made from solid-liquid lipid mixtures. J Colloid Interface Sci. 2005; 285(2): 590-598.
[Crossref] [Google Scholar] [Pubmed]
- Zimmermann ES, Souto EB, Müller RH. Physicochemical investigations on the structure of drug-free and drug-loaded Solid Lipid Nanoparticles (SLNTM) by means of DSC and 1H NMR. Die Pharmazie. 2005; 60(7): 508-513.
[Google Scholar] [Pubmed]
- Jores K, Mehnert W, Mäder K. Physicochemical investigations on solid lipid nanoparticles and on oil-loaded solid lipid nanoparticles: A nuclear magnetic resonance and electron spin resonance study. Pharm Res. 2003; 20(8): 1274-1283.
[Crossref] [Google Scholar] [Pubmed]
- Rosenblatt KM, Bunjes H. Evaluation of the drug loading capacity of different lipid nanoparticle dispersions by passive drug loading. Eur J Pharm Biopharm. 2017; 117: 49-59.
[Crossref] [Google Scholar] [Pubmed]
- Shenoy VS, Gude RP, Murthy RS. In vitro anticancer evaluation of 5-fluorouracil lipid nanoparticles using B16F10 melanoma cell lines. Int Nano Lett. 2013; 3(1): 1-9.
- Lippacher A, Müller RH, Mäder K. Liquid and semisolid SLN™ dispersions for topical application: Rheological characterization. Eur J Pharm Biopharm. 2004; 58(3): 561-567.
[Crossref] [Google Scholar] [Pubmed]
- Seetapan N, Bejrapha P, Srinuanchai W, Puttipipatkhachorn S, Ruktanonchai U. Nondestructive rheological measurement of aqueous dispersions of solid lipid nanoparticles: Effects of lipid types and concentrations on dispersion consistency. Drug Dev Ind Pharm. 2010; 36(9): 1005-1015.
[Crossref] [Google Scholar] [Pubmed]
- Lippacher A, Müller RH, Mäder K. Investigation on the viscoelastic properties of lipid based colloidal drug carriers. Int J Pharm. 2000; 196(2): 227-230.
[Crossref] [Google Scholar] [Pubmed]
- Lippacher A, Müller RH, Mäder K. Semisolid SLN™ dispersions for topical application: Influence of formulation and production parameters on viscoelastic properties. Eur J Pharm Biopharm. 2002; 53(2): 155-160.
[Crossref] [Google Scholar] [Pubmed]
- Schoenitz M, Joseph S, Nitz A, Bunjes H, Scholl S. Controlled polymorphic transformation of continuously crystallized solid lipid nanoparticles in a microstructured device: A feasibility study. Eur J Pharm Biopharm. 2014; 86(3): 324-331.
[Crossref] [Google Scholar] [Pubmed]
- Khosa A, Reddi S, Saha RN. Nanostructured lipid carriers for site-specific drug delivery. Biomed Pharmacother. 2018; 103: 598-613.
[Crossref] [Google Scholar] [Pubmed]
- Borges A, de Freitas V, Mateus N, Fernandes I, Oliveira J. Solid lipid nanoparticles as carriers of natural phenolic compounds. Antioxidants. 2020; 9(10): 998.
[Crossref] [Google Scholar] [Pubmed]
- Hu FQ, Jiang SP, Du YZ, Yuan H, Ye YQ, Zeng S. Preparation and characteristics of monostearin nanostructured lipid carriers. Int J Pharm. 2006; 314(1): 83-89.
[Crossref] [Google Scholar] [Pubmed]
- del Pozo-Rodríguez A, Solinís MA, Gascón AR, Pedraz JL. Short-and long-term stability study of lyophilized solid lipid nanoparticles for gene therapy. Eur J Pharm Biopharm. 2009; 71(2): 181-189.
[Crossref] [Google Scholar] [Pubmed]
- Attama AA, Umeyor CE. The use of solid lipid nanoparticles for sustained drug release. Ther Deliv. 2015; 6(6): 669-684.
[Crossref] [Google Scholar] [Pubmed]
- Soares S, Fonte P, Costa A, Andrade J, Seabra V, Ferreira D, et al. Effect of freeze-drying, cryoprotectants and storage conditions on the stability of secondary structure of insulin-loaded solid lipid nanoparticles. Int J Pharm. 2013; 456(2): 370-381.
[Crossref] [Google Scholar] [Pubmed]
- Zhang L, Liu L, Qian Y, Chen Y. The effects of cryoprotectants on the freeze-drying of ibuprofen-loaded solid lipid microparticles (SLM). Eur J Pharm Biopharm. 2008; 69(2): 750-759.
[Crossref] [Google Scholar] [Pubmed]
- Amis TM, Renukuntla J, Bolla PK, Clark BA. Selection of cryoprotectant in lyophilization of progesterone-loaded stearic acid solid lipid nanoparticles. Pharmaceutics. 2020; 12(9): 892.
[Crossref] [Google Scholar] [Pubmed]
- de Menezes BR, Rodrigues KF, Schatkoski VM, Pereira RM, Ribas RG, do Amaral MTL, et al. Current advances in drug delivery of nanoparticles for respiratory disease treatment. J Mater Chem B. 2021; 9(7): 1745-1761.
[Crossref] [Google Scholar] [Pubmed]
- Gaspar DP, Gaspar MM, Eleuterio CV, Grenha A, Blanco M, Gonçalves LM, et al. Microencapsulated solid lipid nanoparticles as a hybrid platform for pulmonary antibiotic delivery. Mol Pharm. 2017; 14(9): 2977-2990.
[Crossref] [Google Scholar] [Pubmed]
- Küchler S, Herrmann W, Panek-Minkin G, Blaschke T, Zoschke C, Kramer KD, et al. SLN for topical application in skin diseases: Characterization of drug-carrier and carrier-target interactions. Int J Pharm. 2010; 390(2): 225-233.
[Crossref] [Google Scholar] [Pubmed]
- Liu M, Wen J, Sharma M. Solid lipid nanoparticles for topical drug delivery: Mechanisms, dosage form perspectives, and translational status. Curr Pharm Des. 2020; 26(27): 3203-3217.
[Crossref] [Google Scholar] [Pubmed]
- Netto G, Jose J. Development, characterization, and evaluation of sunscreen cream containing solid lipid nanoparticles of silymarin. J Cosmet Dermatol. 2018; 17(6): 1073-1083.
[Crossref] [Google Scholar] [Pubmed]
- Pedersen N, Hansen S, Heydenreich AV, Kristensen HG, Poulsen HS. Solid lipid nanoparticles can effectively bind DNA, streptavidin and biotinylated ligands. Eur J Pharm Biopharm. 2006; 62(2): 155-162.
[Crossref] [Google Scholar] [Pubmed]
- Yu W, Liu C, Ye J, Zou W, Zhang N, Xu W. Novel cationic SLN containing a synthesized single-tailed lipid as a modifier for gene delivery. Nanotechnology. 2009; 20(21): 215102.
[Crossref] [Google Scholar] [Pubmed]
- Pandey R, Sharma S, Khuller GK. Oral solid lipid nanoparticle-based antitubercular chemotherapy. Tuberculosis. 2005; 85(5-6): 415-420.
[Crossref] [Google Scholar] [Pubmed]
- Fontana G, Maniscalco L, Schillaci D, Cavallaro G, Giammona G. Solid lipid nanoparticles containing tamoxifen characterization and in vitro antitumoral activity. Drug Deliv. 2005; 12(6): 385-392.
[Crossref] [Google Scholar] [Pubmed]
- Ruckmani K, Sivakumar M, Ganeshkumar PA. Methotrexate loaded Solid Lipid Nanoparticles (SLN) for effective treatment of carcinoma. J Nanosci Nanotechnol. 2006; 6(9-10): 2991-2995.
[Crossref] [Google Scholar] [Pubmed]
- Martins SM, Sarmento B, Nunes C, Lúcio M, Reis S, Ferreira DC. Brain targeting effect of camptothecin-loaded solid lipid nanoparticles in rat after intravenous administration. Eur J Pharm Biopharm. 2013; 85(3): 488-502.
[Crossref] [Google Scholar] [Pubmed]
- Tupal A, Sabzichi M, Ramezani F, Kouhsoltani M, Hamishehkar H. Dermal delivery of doxorubicin-loaded solid lipid nanoparticles for the treatment of skin cancer. J Microencapsul. 2016; 33(4): 372-380.
[Crossref] [Google Scholar] [Pubmed]
- Lu B, Xiong SB, Yang H, Yin XD, Chao RB. Solid lipid nanoparticles of mitoxantrone for local injection against breast cancer and its lymph node metastases. Eur J Pharm Sci. 2006; 28(1-2): 86-95.
[Crossref] [Google Scholar] [Pubmed]
- Wang W, Zhang L, Chen T, Guo W, Bao X, Wang D, et al. Anticancer effects of resveratrol-loaded solid lipid nanoparticles on human breast cancer cells. Molecules. 2017; 22(11): 1814.
[Crossref] [Google Scholar] [Pubmed]
- Wang JX, Sun X, Zhang ZR. Enhanced brain targeting by synthesis of 3′, 5′-dioctanoyl-5-fluoro-2′-deoxyuridine and incorporation into solid lipid nanoparticles. Eur J Pharm Biopharm. 2002; 54(3): 285-290.
[Crossref] [Google Scholar] [Pubmed]
- Aditya NP, Ko S. Solid lipid nanoparticles (SLNs): Delivery vehicles for food bioactives. RSC Adv. 2015; 5(39): 30902-30911.
- Nunes S, Madureira AR, Campos D, Sarmento B, Gomes AM, Pintado M, et al. Solid lipid nanoparticles as oral delivery systems of phenolic compounds: overcoming pharmacokinetic limitations for nutraceutical applications. Crit Rev Food Sci Nutr. 2017; 57(9): 1863-1873.
[Crossref] [Google Scholar] [Pubmed]
- Oliveira CR, Domingues CE, de Melo NF, Roat TC, Malaspina O, Jones-Costa M, et al. Nanopesticide based on botanical insecticide pyrethrum and its potential effects on honeybees. Chemosphere. 2019; 236: 124282.
[Crossref] [Google Scholar] [Pubmed]
- Nguyen HM, Hwang IC, Park JW, Park HJ. Enhanced payload and photo-protection for pesticides using nanostructured lipid carriers with corn oil as liquid lipid. J Microencapsul. 2012; 29(6): 596-604.
[Crossref] [Google Scholar] [Pubmed]
- Deray G. Amphotericin B nephrotoxicity. J Antimicrob Chemother. 2002; 49(1): 37-41.
[Crossref] [Google Scholar] [Pubmed]
- Batist G, Ramakrishnan G, Rao CS, Chandrasekharan A, Gutheil J, Guthrie T, et al. Reduced cardiotoxicity and preserved antitumor efficacy of liposome-encapsulated doxorubicin and cyclophosphamide compared with conventional doxorubicin and cyclophosphamide in a randomized, multicenter trial of metastatic breast cancer. J Clin Oncol. 2001; 19(5): 1444-1454.
[Crossref] [Google Scholar] [Pubmed]
Author Info
Suraj B Kumbhar1, Vikram R Shinde1, Rajesh G Jadhao2,3, Poonam P Warade2, Parag R Patil2, Sharad Wakode3, Ankita R Wankhede2, Swati D Raysing4 and Dipak D Kumbhar2*2Department of Pharmaceutics, KYDSCT’s College of Pharmacy, Maharashtra, India
3Department of Pharmacy, Delhi Institute of Pharmaceutical Sciences and Research (DIPSAR), New Delhi, India
4Department of Pharmacy, RC Patel Institute of Pharmaceutical Education and Research, Maharashtra, India
Citation: Kumbhar SB: Solid Lipid Nanoparticles: Synthesis, Microstructural Elucidations and Future Prospects
Received: 02-May-2022 Accepted: 27-May-2022 Published: 03-Jun-2022, DOI: 10.31858/0975-8453.13.6.417-429
Copyright: This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
ARTICLE TOOLS
- Dental Development between Assisted Reproductive Therapy (Art) and Natural Conceived Children: A Comparative Pilot Study Norzaiti Mohd Kenali, Naimah Hasanah Mohd Fathil, Norbasyirah Bohari, Ahmad Faisal Ismail, Roszaman Ramli SRP. 2020; 11(1): 01-06 » doi: 10.5530/srp.2020.1.01
- Psychometric properties of the World Health Organization Quality of life instrument, short form: Validity in the Vietnamese healthcare context Trung Quang Vo*, Bao Tran Thuy Tran, Ngan Thuy Nguyen, Tram ThiHuyen Nguyen, Thuy Phan Chung Tran SRP. 2020; 11(1): 14-22 » doi: 10.5530/srp.2019.1.3
- A Review of Pharmacoeconomics: the key to “Healthcare for All” Hasamnis AA, Patil SS, Shaik Imam, Narendiran K SRP. 2019; 10(1): s40-s42 » doi: 10.5530/srp.2019.1s.21
- Deuterium Depleted Water as an Adjuvant in Treatment of Cancer Anton Syroeshkin, Olga Levitskaya, Elena Uspenskaya, Tatiana Pleteneva, Daria Romaykina, Daria Ermakova SRP. 2019; 10(1): 112-117 » doi: 10.5530/srp.2019.1.19
- Dental Development between Assisted Reproductive Therapy (Art) and Natural Conceived Children: A Comparative Pilot Study Norzaiti Mohd Kenali, Naimah Hasanah Mohd Fathil, Norbasyirah Bohari, Ahmad Faisal Ismail, Roszaman Ramli SRP. 2020; 11(1): 01-06 » doi: 10.5530/srp.2020.1.01
- Manilkara zapota (L.) Royen Fruit Peel: A Phytochemical and Pharmacological Review Karle Pravin P, Dhawale Shashikant C SRP. 2019; 10(1): 11-14 » doi: 0.5530/srp.2019.1.2
- Pharmacognostic and Phytopharmacological Overview on Bombax ceiba Pankaj Haribhau Chaudhary, Mukund Ganeshrao Tawar SRP. 2019; 10(1): 20-25 » doi: 10.5530/srp.2019.1.4
- A Review of Pharmacoeconomics: the key to “Healthcare for All” Hasamnis AA, Patil SS, Shaik Imam, Narendiran K SRP. 2019; 10(1): s40-s42 » doi: 10.5530/srp.2019.1s.21
- A Prospective Review on Phyto-Pharmacological Aspects of Andrographis paniculata Govindraj Akilandeswari, Arumugam Vijaya Anand, Palanisamy Sampathkumar, Puthamohan Vinayaga Moorthi, Basavaraju Preethi SRP. 2019; 10(1): 15-19 » doi: 10.5530/srp.2019.1.3