Research Article - (2022) Volume 13, Issue 8
Sub-acute Effects of Vaccination With a Messenger RNA-based Vaccine against Coronavirus Disease 2019 on Elderly Japanese Patients with Cardiac Disorder
Ryuichi Tamimoto1,2, Toshiharu Fujii2,3, Hirofumi Nagamatsu2,3, Tsutomu Murakami2,3, Koji Miyazaki2,4, Shinya Goto3, Yasunori Cho1 and Hidezo Mori1,2,5*Abstract
Background: The incidence of acute myocarditis as a sub-acute side effect after vaccination with an mRNA- based vaccine against Coronavirus Disease 2019 (COVID-19) in elderly patients with cardiac dysfunction is unknown. This study assessed adverse cardiac events following vaccination.
Methods: This observational study evaluated all- cause mortality and deterioration of heart failure in 100 Japanese patients who underwent vaccination with an mRNA-based vaccine against COVID-19. Heart failure status was assessed using the Brain Natriuretic Peptide (BNP) ratio, for which the BNP or N Terminal (NT)-pro BNP values were divided by their Upper Normal Limits (UNL). Deterioration was assessed based on changes in the BNP ratio; values (i.e., post-BNP levels minus pre-BNP levels) ≥ 10-fold higher than the UNL denoted significant increment.
Results: The BNP ratio increased from 4.5 (Interquartile range: 1.5-13.6) to 5.9 (Interquartile range: 1.8-19.5) after vaccination (P<0.01). After the first vaccination (Follow-up: 20-222 days), six patients expired and 15 patients exhibited significant increment of the BNP ratio; these patients had a pre-BNP ratio ≥ 4-fold higher than the UNL. Forty-five patients with pre-BNP ratio <4-fold higher than the UNL did not expire or show significant increase in the BNP ratio. The pre-BNP ratio was reliable in predicting cardiac deterioration (Crude Hazard Ratio: 1.02; 95% Confidence Interval (CI): 1.01-1.03; P<0.01).
Conclusion: A BNP ratio ≥ 4-fold higher than the UNL was associated with a high risk of death in elderly patients after vaccination against COVID-19. The BNP ratio may be useful for assessing the cardiac status of elderly patients in this setting.
Keywords
Coronavirus Disease 2019 (COVID-19), Vaccine, Death, Brain Natriuretic Peptide (BNP), Heart failure
Abbreviations
ACE2: Angiotensin-Converting Enzyme 2; BNP: Brain Natriuretic Peptide; CI: Confidence Interval; COVID-19: Coronavirus Disease 2019; CRP: C-Reactive Protein; HR: Hazard Ratio; UNL: Upper Normal Limits
Introduction
It is established that vaccination using an mRNA-based vaccine against Coronavirus Disease 2019 (COVID-19) is effective in preventing the spread of infection. Moreover, acute myocarditis in young individuals has been reported as a side effect of this type of vaccination (Witberg G, et al., 2021; Mevorach D, et al., 2021; Truong DT, et al., 2021; Ministry of Health, Labor and Welfare, 2021; Mevorach D, et al., 2022; Ministry of Health, Labor and Welfare, 2021).
However, it is currently unknown whether vaccination with an mRNA-based vaccine against COVID-19 has serious side effects on elderly patients with cardiac diseases in the sub-acute phase. Therefore, the objective of this study was to investigate the occurrence of adverse cardiac events 1-8 months following vaccination with an mRNA-based vaccine against COVID-19 (COMIRNATY® from BIONTECH and Pfizer Pharmaceutical Co. Ltd., Tokyo Japan).
Materials and Methods
Patient selection
The study included 100 Japanese patients (Median age: 84 years; Interquartile range: 78–89.5 years) with cardiovascular disorders, as described in Table 1.They received the first vaccination with an mRNA-based vaccine against COVID-19 (COMIRNATY® 0.3 mL IM; BIONTECH and Pfizer Pharmaceutical, Co. Ltd.) between April 23 and July 30, 2021; all patients underwent blood examination before and after the vaccination. Ninety-nine patients received the second vaccination 21-30 days after the first dose, whereas one patient denied the second vaccination due to dyspnoea on effort after the first dose. All patients included in this study were treated by the corresponding author at home/care facilities (80 patients) or at the outpatient clinics (20 patients) of two hospitals belonging to the Tokai University School of Medicine (Isehara, Japan) during the observation period (i.e., from the day of first vaccination to that of death, last blood sampling, or November 31, 2021).
| Characteristic | Baseline | Post vaccination | Pvalue |
|---|---|---|---|
| Age, years | 84 (78-89.5) | NA | NA |
| Males, n | 45 (45%) | NA | NA |
| Height, cm | 155.5 (145.5-163.5) | NA | NA |
| Weight, kg | 51 (42.5-60) | NA | NA |
| BMI | 21.4 (19.0-24.3) | NA | NA |
| Hypertension, n | 64 (64%) | NA | NA |
| Dyslipidemia, n | 54 (54%) | NA | NA |
| Diabetes mellitus, n | 21 (21%) | NA | NA |
| Prior myocardial infarction, n | 16 (16%) | NA | NA |
| Anticoagulation, n | 35 (35%) | NA | NA |
| Warfarin | 17 (17%) | NA | NA |
| DOAC | 18 (18%) | NA | NA |
| Serum creatinine, mg/dL | 0.9 (0.7-1.1) | 0.9 (0.7-1.1) | 0.26 |
| Urea nitrogen, mg/mL | 20.6 (15.9-26.0) | 21.6 (16.6-26.1) | 0.33 |
| Missing, n | 4 | 3 | |
| AST, IU/L | 21 (18-25.5) | 22 (18-25) | 0.22 |
| Missing, n | 4 | 3 | |
| ALT, IU/L | 15 (12-21) | 16 (11-22) | 0.92 |
| Missing, n | 4 | 3 | |
| HbA1c, % | 5.8 (5.4-6.2) | 5.8 (5.4-6.1) | 0.02 |
| Missing, n | 16 | 19 | |
| Troponin T, ng/mL | 0.03 (0.01-0.07) | 0.03 (0.02-0.07) | 0.55 |
| Missing, n | 32 | 24 | |
| CRP, mg/dL | 0.09 (0.04-0.26) | 0.09 (0.05-0.28) | 0.02 |
| Missing, n | 28 | 15 | |
| Abnormal Q wave, n | 16 (16%) | 12 (22%) | |
| Missing, n | 2 | 45 | |
| Observation period, days§ | 152.5 (20-222) | NA | |
| BNP ratio | 4.5 (1.5-13.6)† | 5.9 (1.8-19.5)‡ | <0.01 |
| <4-fold higher than the UNL, n | 45 (45%) | 41 (40%) | <0.01 |
| ≥ 4, <10-fold higher than the UNL, n | 27 (27%) | 22 (22%) | |
| ≥ 10-fold higher than the UNL, n | 28 (28%) | 37 (37%) | |
| Death, n | NA | 6 (6%) | NA |
Note: ALT: Alanine aminotransferase; AST: Aspartate aminotransferase; BMI: Body Mass Index; BNP: Brain Natriuretic Peptide; CRP: C-Reactive Protein; DOAC: Direct Oral Anticoagulant; HbA1c: Hemoglobin A1c; UNL: Upper Normal Limit. †Average value prior to first vaccine/upper limit within normal range. ‡Peak value after second vaccine/upper limit within normal range. §Duration between the day of first vaccination and that of death or blood examination.
Table 1: Baseline characteristics (n=100)
Study protocol
To achieve the objective of this study, we compared changes in clinical data before and 1-8 months after vaccination against COVID-19. Thereafter, multivariate analysis was performed to determine the risks linked to the vaccination of patients with cardiac disorders.
Clinical data analysis included NT-pro Brain Natriuretic Peptide (BNP) or BNP (in 80 and 20 patients, respectively), serum creatinine, blood urea nitrogen, aspartate aminotransferase, alanine aminotransferase, haemoglobin A1C, complete blood count, and electrocardiograms. In most patients, troponin T and C-Reactive Protein (CRP) were also measured, and echocardiography was performed. Missing parameters are summarized in Table 1.
Definitions
To obtain the BNP ratio, we divided the BNP or NT-pro BNP values (pg/ mL) by their Upper Normal Limits (UNL; 18.4 pg/mL and 125 pg/mL, respectively) to equalize both BNP and NT-pro BNP data. The BNP ratio prior to vaccination, termed pre-BNP ratio, was calculated as the mean values of the BNP ratio measured in the previous 12 months (the BNP ratio was measured 1-12 times in the 100 patients; Mean: 3.98 ± 2.73 times). For the ratio after vaccination, termed post-BNP, we used the maximum BNP or NT-pro BNP value recorded during the observation period. To assess the worsening of heart failure, the degree of change in the BNP ratio before and after the vaccination was determined as follows: Post-BNP ratio minus pre-BNP ratio, a value ≥ 10-fold higher than the UNL denoted significant increment.
Statistical analysis
Numerical factors are shown as the median (Interquartile range). The Wilcoxon rank-sum test was performed to compare unpaired numerical parameters. For the comparison of paired numerical parameters, the Wilcoxon signed-rank test was used. Pearson’s chi-squared test was applied to determine differences in categorical variables. Time to event was analysed with a Cox proportional-hazards model. The proportional-hazards assumption was tested using Schoenfeld residuals after fitting a model with the Cox proportional-hazards model. Hazard Ratio (HR) with 95% Confidence Interval (CI) was described as crude and adjusted HR for possible confounders, namely pre-BNP ratio, age, sex, body mass index, serum creatinine, and anticoagulation therapy.
Results
Baseline characteristics are summarized in Table 1. Six patients expired during a median follow-up of 152.5 days (Interquartile range: 20-222 days) after the first vaccination. The pre- and post-BNP ratios of patients who expired and survived are demonstrated in Figure 1. Both pre- and post- BNP ratios were higher in patients who expired (23.9 (12.6-43.6) and 144 (33.8-280), respectively) than in those who survived (4.2 (1.4-9.5) and 5.2 (1.8-16.1), respectively). The degree of increase after vaccination (i.e., post- BNP ratio minus pre-BNP ratio) was significantly high (≥ 10-fold higher than their UNL) in five patients who expired and 15 patients who survived. Death and/or remarkable increase in the BNP ratio (i.e., ≥ 10-fold higher than the UNL) were observed only in patients with a pre-BNP ratio ≥ 4-fold higher than the UNL (21/55, 38.2%) (Table 2). In contrast, 45 patients with a pre-BNP ratio <4-fold higher than the UNL in the pre-vaccination period did not expire or show remarkable increase in the BNP ratio (0/45, 0%) (Table 2). Hence, the pre-BNP ratio was a reliable parameter for predicting cardiac deterioration 1-8 months after vaccination (Crude HR: 1.02 (95% CI: 1.01-1.03; P<0.01)); Adjusted HR: 1.02 (95% CI: 1.00-1.03; P=0.03)) (Table 3).
| Combined outcome, n | Pre-BNP ratio | |
|---|---|---|
| <4 | ≥ 4 | |
| None | 45 (100%) | 34 (61.8%) |
| Presence | 0 | 21 (38.2%) |
Table 2: Proportions of combined outcome according to the pre-Brain Natriuretic Peptide (pre-BNP) ratio
| Characteristics | Crude HR | P value |
|---|---|---|
| Pre-BNP ratio | 1.02 (1.01-1.03) | 0.01 |
| Adjusted HR | ||
| Pre-BNP ratio | 1.02 (1.00-1.03) | 0.03 |
| Age | 1.07 (0.99-1.16) | 0.1 |
| Males | 1.63 (0.55-4.88) | 0.38 |
| BMI | 0.98 (0.83-1.15) | 0.8 |
| Serum creatinine | 0.26 (0.05-1.28) | 0.1 |
| Anticoagulation therapy | 5.05 (1.54-16.6) | 0.01 |
Note: BMI: Body Mass Index; BNP: Brain Natriuretic Peptide
Table 3: Hazard Ratio (HR) for the combined outcome
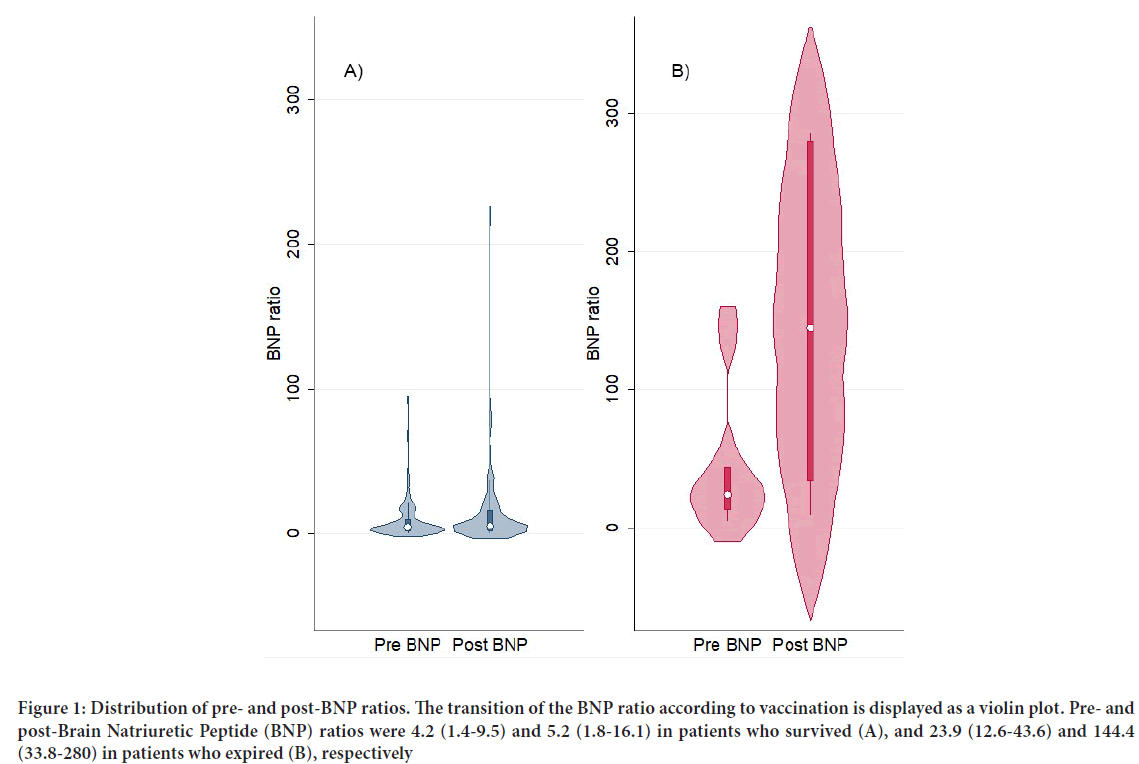
Figure 1: Distribution of pre- and post-BNP ratios. The transition of the BNP ratio according to vaccination is displayed as a violin plot. Pre- and post-Brain Natriuretic Peptide (BNP) ratios were 4.2 (1.4-9.5) and 5.2 (1.8-16.1) in patients who survived (A), and 23.9 (12.6-43.6) and 144.4 (33.8-280) in patients who expired (B), respectively
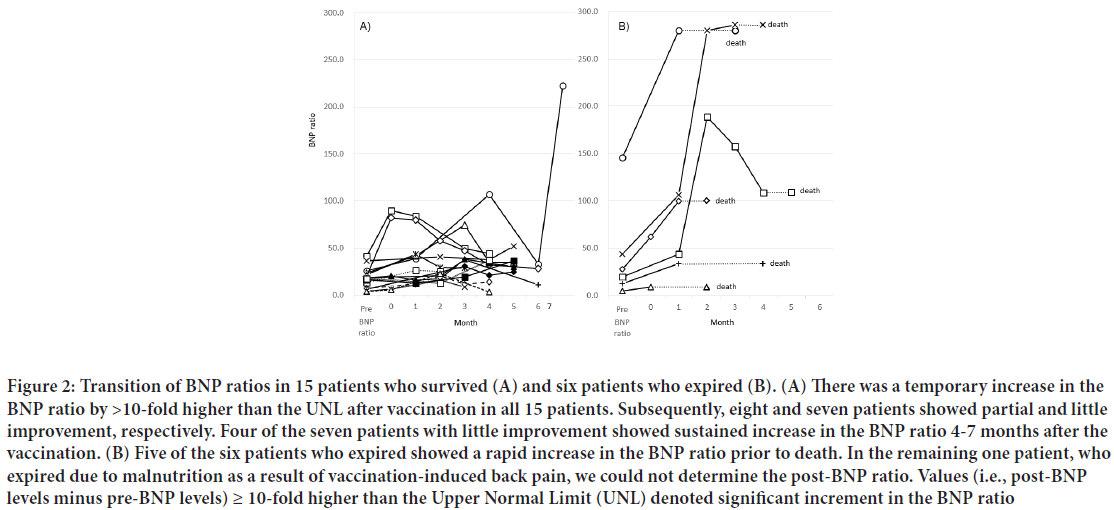
Six patients expired 47-156 days after the first vaccination. Their pre-BNP ratios ranged 4.8-145.8-fold higher than the UNL (605-18,225 pg/mL in NT-pro BNP) (Figure 2A). Five of the six patients with a pre-BNP ratio ranging 12.6-145.8-fold higher than the UNL (1,575-18,225 pg/mL in NT-pro BNP) expired due to deterioration of congestive heart failure; a remarkable increase in the BNP ratio (21.2-242.4-fold higher than the UNL) was observed in these patients. The remaining patient (Age: 89 years), with the lowest pre-BNP ratio (4.8-fold higher than the UNL) expired due to malnutrition caused by severe back pain which developed after the vaccination. In this patient, we could not determine the BNP ratio after the vaccination. Echocardiographic examination, performed during both the pre- and post-vaccination periods, revealed markedly deteriorated left ventricular wall motion after vaccination in four of the six patients who expired. The six patients who expired did not have newly developed abnormal Q waves. A distinct increase in CRP levels (i.e., >2.0 mg/dL) was observed in one of those patients.
Figure 2: Transition of BNP ratios in 15 patients who survived (A) and six patients who expired (B). (A) There was a temporary increase in the BNP ratio by >10-fold higher than the UNL after vaccination in all 15 patients. Subsequently, eight and seven patients showed partial and little improvement, respectively. Four of the seven patients with little improvement showed sustained increase in the BNP ratio 4-7 months after the vaccination. (B) Five of the six patients who expired showed a rapid increase in the BNP ratio prior to death. In the remaining one patient, who expired due to malnutrition as a result of vaccination-induced back pain, we could not determine the post-BNP ratio. Values (i.e., post-BNP levels minus pre-BNP levels) ≥ 10-fold higher than the Upper Normal Limit (UNL) denoted significant increment in the BNP ratio
The 15 patients who survived despite a significant increase in the BNP ratio had a relatively low pre-BNP ratio (4.2-41.3-fold higher than the UNL) and increase in BNP ratio (11.5-60.2-fold higher than the UNL) compared with the six patients who expired. In addition, they did not show changes in the electrocardiogram or the troponin T levels, suggesting newly developed myocardial infarction as described above. Seven of the 15 patients who survived exhibited an increase in CRP (i.e., >2.0 mg/dL) in the post-vaccination period. Their post-BNP ratio gradually decreased 1-4 months after the vaccination; however, four patients sustained this increase in the BNP ratio 4-7 months after vaccination (Figure 2A). In contrast, all patients who expired showed extreme increment of the BNP ratio, excluding one patient who expired due to malnutrition (Figure 2B).
Acute side effects, such as anaphylactic hemodynamic deterioration or sustained high fever in the acute phase (i.e., within 1 week) were not observed in this study. Most patients did not show significant changes in other blood parameters (i.e., serum creatinine, blood urea nitrogen, aspartate aminotransferase alanine, and aminotransferase) after vaccination.
Discussion
This study showed that vaccination against COVID-19 was associated with a high risk of death or decompensated heart failure for Japanese elderly patients with severe cardiac dysfunction and a high pre-BNP ratio ≥ 4-fold higher than the UNL (500 ng/mL in NT-pro BNP). However, the risk was negligible in patients with a pre-BNP ratio <4-fold higher than the UNL (Table 2). The incidence of death and/or remarkable increase in the BNP ratio (i.e., ≥ 10-fold higher than the UNL) in the post-vaccination period was strongly dependent on a pre-BNP ratio ≥ 4-fold higher that the UNL (500 ng/mL in NT-pro BNP in Table 2). Cardiovascular death after the vaccination developed only in patients with a pre-BNP ratio >12.6-fold higher than the UNL (1,575 in NT-pro BNP). Patients with a pre-BNP ratio <4-fold higher than the UNL in the pre-vaccination period did not expire or show remarkable increase in the BNP ratio after vaccination. This cardiac deterioration was not accompanied by side effects, such as anaphylactic hemodynamic deterioration or sustained high fever in the acute phase, or myocardial infarction, liver dysfunction, or renal dysfunction (Table 1) both in the acute and sub-acute phases. An increase in CRP (>2 mg/ dL) was noted in one patient who expired and seven of the surviving 15 patients with a remarkable increase in the BNP ratio (≥ 10-fold higher than the UNL). The most likely explanation for this sustained myocardial involvement in the sub-acute phase would be autoimmune myocarditis; in other words, anti-idiotype antibodies-dependent myocarditis recently proposed by Murphy and Longo (Murphy WJ and Longo DL, 2022; Paque RE and Miller RU, 1991; Talotta R and Robertson ES, 2021; Plotz P, 1983). The primary antibody induced by vaccination against COVID-19 has antigen-binding domains with a mirror image structure of the spike protein of COVID-19. Hence, it may induce a secondary antibody with a mirror image structure of the antigen-binding domains of the primary anti body. Consequently, the secondary antibodies may be able to bind to the Angiotensin-Converting Enzyme 2 (ACE2) receptor as the spike protein of COVID-19, thereby inducing the release of inflammatory cytokines. This hypothesis may explain our observation that myocardial dysfunction was sustained for several months after vaccination and led to the death of patients with severe damage. Nevertheless, massive myocardial necrosis was not noted. The transition of the BNP ratio suggests that myocardial damage appears to be somewhat reversible (Figure 2) in certain cases, but sustained in others.
The BNP and/or NT-pro BNP levels in patients with severe cardiac dysfunction may increase during the natural course of the diseases. Therefore, one may argue that the increase in the BNP ratio after vaccination may be caused by the natural course of the diseases. However, in the previous 4 years, the cardiac death rate every 6 months among patients treated by the corresponding author at home or care facilities was 5.0%-7.1% (seven terms). This was accompanied by a maximum BNP ratio ranging 12.7-280-fold higher than the UNL (Median: 49.4; Interquartile range: 39.7-103.2) and 1,590-35,000 pg/mL in NT-pro BNP (Median: 6,180, Interquartile range: 3,710-12,900). Nonetheless, the cardiac death rate in the previous 6 months (May 1 to October 31, 2021) was 12 cases (13.5%), including the six patients who expired after vaccination with an mRNA-based vaccine against COVID-19. The cardiac death rate among patients treated at home or care facilities who did not receive the vaccination during the same period was six cases (6.7%).
Conclusion
A BNP ratio ≥ 4-fold higher than the UNL was associated with a high risk of death in elderly patients after vaccination against COVID-19. The present study revealed that assessment of the cardiac status, including the BNP ratio, in elderly patients with severe cardiac dysfunction may be useful for determining the risk associated with the booster or initial vaccin ation against COVID-19. In patients at high risk, lower-dose vaccination should be considered as an alternative option.
Declarations
Ethical approval
Tokai University school of medicine ethical committee approved this research project (May 10, 2022, Approval Number: 21R216)
Author contributions
The roles of the authors were as follows: conception and design (RT, KM, HM); data analysis (TF, TM, YC); data analysis and interpretation (TF, HN, SG); and drafting of the manuscript (RT, TF).
References
- Witberg G, Barda N, Hoss S, Richter I, Wiessman M, Aviv Y, et al. Myocarditis after COVID-19 vaccination in a large health care organization. N Engl J Med. 2021; 385: 2132-2139.
[Crossref] [Google Scholar] [Pubmed]
- Mevorach D, Anis E, Cedar N, Bromberg M, Haas EJ, Nadir E, et al. Myocarditis after BNT162b2 mRNA vaccine against COVID-19 in Israel. N Engl J Med. 2021; 385(23): 2140-2149.
[Crossref] [Google Scholar] [Pubmed]
- Truong DT, Dionne A, Muniz JC, McHugh KE, Portman MA, Lambert LM, et al. Clinically suspected myocarditis temporally related to COVID-19 vaccination in adolescents and young adults. Circulation. 2021; 145(5): 345-356.
[Crossref] [Google Scholar] [Pubmed]
- Ministry of Health, Labor and Welfare. 74th health science council vaccination vaccine subcommittee vaccine reaction study subcommittee, 25th pharmaceutical affairs food sanitation council pharmaceutical affairs subcommittee safety measures subcommittee safety measures investigation committee (jointly held). Ministry of Health, Labor and Welfare. 2021.
- Mevorach D, Anis E, Cedar N, Hasin T, Bromberg M, Goldberg L, et al. Myocarditis after BNT162b2 Vaccination in Israeli Adolescents. N Engl J Med. 2022; 386: 998-999.
[Crossref] [Google Scholar] [Pubmed]
- Ministry of Health, Labor and Welfare. The 77th health science council vaccination/vaccine subcommittee vaccine reaction study subcommittee, the 30th pharmaceutical affairs/food hygiene council pharmaceutical affairs subcommittee safety measures subcommittee meeting (jointly held). Ministry of Health, Labor and Welfare. 2021.
- Murphy WJ, Longo DL. A possible role for anti-idiotype antibodies in SARS-CoV-2 infection and vaccination. N Engl J Med. 2022; 386(4): 394-396.
[Crossref] [Google Scholar] [Pubmed]
- Paque RE, Miller RU. Autoanti-idiotypes exhibit mimicry of myocyte antigens in virus-induced myocarditis. J Virol. 1991; 65(1): 16-22.
[Crossref] [Google Scholar] [Pubmed]
- Talotta R, Robertson ES. Antiphospholipid antibodies and risk of post-COVID-19 vaccination thrombophilia: The straw that breaks the camel's back? Cytokine Growth Factor Rev. 2021; 60: 52-60.
[Crossref] [Google Scholar] [Pubmed]
- Plotz P. Autoantibodies are anti-idiotype antibodies to antiviral antibodies. Lancet. 1983; 322(8354): 824-826.
[Crossref] [Google Scholar] [Pubmed]
Author Info
Ryuichi Tamimoto1,2, Toshiharu Fujii2,3, Hirofumi Nagamatsu2,3, Tsutomu Murakami2,3, Koji Miyazaki2,4, Shinya Goto3, Yasunori Cho1 and Hidezo Mori1,2,5*2Department of Cardiovascular Surgery, Shoju Sagamihara Clinic, Sagamihara, Japan
3Department of Cardiovascular Medicine, Tokai University School of Medicine, Isehara, Japan
4Department of Cardiovascular Surgery, Tokai University Hachiouji Hospital, Hachiouji, Japan
5Department of Cardiovascular Surgery, Mori Clinic, Tokyo, Japan
Citation: Tamimoto R: Sub-acute Effects of Vaccination With a Messenger RNA-based Vaccine against Coronavirus Disease 2019 on Elderly Japanese Patients with Cardiac Disorder
Received: 01-Jul-2022 Accepted: 25-Jul-2022 Published: 01-Aug-2022, DOI: 10.31858/0975-8453.13.8.552-556
Copyright: This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
ARTICLE TOOLS
- Dental Development between Assisted Reproductive Therapy (Art) and Natural Conceived Children: A Comparative Pilot Study Norzaiti Mohd Kenali, Naimah Hasanah Mohd Fathil, Norbasyirah Bohari, Ahmad Faisal Ismail, Roszaman Ramli SRP. 2020; 11(1): 01-06 » doi: 10.5530/srp.2020.1.01
- Psychometric properties of the World Health Organization Quality of life instrument, short form: Validity in the Vietnamese healthcare context Trung Quang Vo*, Bao Tran Thuy Tran, Ngan Thuy Nguyen, Tram ThiHuyen Nguyen, Thuy Phan Chung Tran SRP. 2020; 11(1): 14-22 » doi: 10.5530/srp.2019.1.3
- A Review of Pharmacoeconomics: the key to “Healthcare for All” Hasamnis AA, Patil SS, Shaik Imam, Narendiran K SRP. 2019; 10(1): s40-s42 » doi: 10.5530/srp.2019.1s.21
- Deuterium Depleted Water as an Adjuvant in Treatment of Cancer Anton Syroeshkin, Olga Levitskaya, Elena Uspenskaya, Tatiana Pleteneva, Daria Romaykina, Daria Ermakova SRP. 2019; 10(1): 112-117 » doi: 10.5530/srp.2019.1.19
- Dental Development between Assisted Reproductive Therapy (Art) and Natural Conceived Children: A Comparative Pilot Study Norzaiti Mohd Kenali, Naimah Hasanah Mohd Fathil, Norbasyirah Bohari, Ahmad Faisal Ismail, Roszaman Ramli SRP. 2020; 11(1): 01-06 » doi: 10.5530/srp.2020.1.01
- Manilkara zapota (L.) Royen Fruit Peel: A Phytochemical and Pharmacological Review Karle Pravin P, Dhawale Shashikant C SRP. 2019; 10(1): 11-14 » doi: 0.5530/srp.2019.1.2
- Pharmacognostic and Phytopharmacological Overview on Bombax ceiba Pankaj Haribhau Chaudhary, Mukund Ganeshrao Tawar SRP. 2019; 10(1): 20-25 » doi: 10.5530/srp.2019.1.4
- A Review of Pharmacoeconomics: the key to “Healthcare for All” Hasamnis AA, Patil SS, Shaik Imam, Narendiran K SRP. 2019; 10(1): s40-s42 » doi: 10.5530/srp.2019.1s.21
- A Prospective Review on Phyto-Pharmacological Aspects of Andrographis paniculata Govindraj Akilandeswari, Arumugam Vijaya Anand, Palanisamy Sampathkumar, Puthamohan Vinayaga Moorthi, Basavaraju Preethi SRP. 2019; 10(1): 15-19 » doi: 10.5530/srp.2019.1.3