Review Article - (2023) Volume 14, Issue 2
Abstract
The process of ageing is the consequence of both genetic and epigenetic alterations associated with metabolic disorders and characterized by mitochondrial dysfunctions generated due to reactive oxygen species. Current reports indicate that cellular ageing or senescence retrograde mitochondrial signaling disorders, telomere shortening, heterochromatin configuration, endoplasmic reticulum strain and unfolded protein responses. Supplemental vitamins, inhibition of cell cycle arrest and controlled expressions of tumor promoting genes p53, p21CIP1 and p16INK4a are robust telomere length longevity promoting interventions and prolong youthful cell functions. This review is aimed to provide an update on the molecular approach mediating the cellular ageing in developmental and programmed replicative ageing cascade with focus on DNA damage response in various cell types. Through rational ideas and critical investigation, we conclude that the combination of irretrievable alternations caused by metabolic reactions elicit the accelerated mechanisms of cellular ageing.
Keywords
DNA Damage Response (DDR), Mitochondrial signaling, Tumorigenesis, Telomerase
Introduction
The process of ageing or senescence is a degenerative process derived from a variety of distinct complex mechanisms which is implicated by different physiological, biochemical and molecular factors. The pathophysiology of aging is directly related to oxidative stress caused due to the overproduction of reactive oxygen species, thereby deteriorating DNA and is responsible for cellular transformation, carcinogenesis, mutations, vascular endothelial dysfunctions, atherosclerosis, neuro-oxidative stresses such as Parkinson’s disease, Alzheimer’s dementia and various age related syndromes. Until now different theories have defined the ageing process, each from different point of view (Adam R, et al., 2017). The most recent and strongest study supports the statistical progressive idea that oxidative stress is a substantial biomarker of ageing and probability of death increases with the age of the organism at any time.
The first proposed theory by Harman in 1950s was known as ‘free radical theory of ageing’ which was based on the fact of mitochondrial production of reactive oxygen species. It was stated as the age of the organisms can be determined by the degree of accumulation of free radical deterioration with the passage of time and increase in ROS generation accompany aging resulting in oxidative damage, functional alterations, pathological complications and eventually death. The biochemical and molecular mechanism of aging is associated with mitochondrial damage because it is the major site of ROS production and intracellular oxygen consumption from the electron transport chain and nitric oxide synthase reactions. Hydrogen peroxide and complex reactive oxygen species produced in human mitochondrial matrix are chemical signal molecules which regulate the whole cellular growth, aging and proliferation processes (Shekhidem AH, et al., 2019). The most commonly produced reactive oxygen species are superoxide and nitric oxide radicals in the presence of NADPH oxidases and nitric oxide synthase which highly regulate the whole metabolism of the human body including calcium ion concentration, activation of proteins such as glutathione (GSH) and modulation of signal transduction proteins such as protein kinase C, tyrosine kinase and Ca+2-ATPase responsible for aging and cell growth.
This redox regulation also causes many disorders associated with aging process (Figure 1). Ageing tissues experience a progressive decline in homeostatic and regenerative capacities which has been attributed to degenerative changes in the aged cells. This proposed mechanism involved in age dependent deterioration of cells is important to develop new therapies to slow down the ageing process and to wipe out the age related diseases that target the specific causes of age related functional decline.
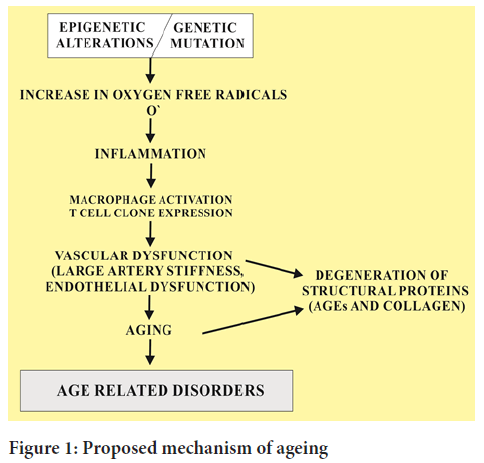
Figure 1: Proposed mechanism of ageing
The industrious exploration of geneticists has led to the discovery of many age-related genes which code for various transcriptional longevity proteins. These are categorized into four groups i.e. anti-stress genes (for example: Antioxidative stress proteins), metabolic regulator genes (for example: Insulin/IGF-1 signaling proteins, mitochondrial enzymes and cholesterol synthesis genes), mutation repairing genes (nuclear stability genes) and homeostasis proteins (hormone synthesis in a regular pattern). Some of the age-related genes been discovered are hsp-16, hsp-70, KLOTHO, HSF-1, isp-1, ras2p, lac-1, lag-1, PCMT, daf-2, DAF-16, daf-23, AGE-1, CLK-1,Pit-1, SOD-1, mth, α-MUPA, p66sh, PROP-1, SAG, spe-26, SIRT1 etc. (Aksenova AY and Mirkin SM, 2019). Other cellular proliferators such as IGF-1, MAPK, PI3K, P16 and CDK1 also regulate the cellular longevity. With respect to the physiology and pathology of a biological system, aging is related to the genetic networks that work in a synchronized manner to resist various environmental injuries. Thus, aging is a complex interaction of both intrinsic and acquired factors which focus on damage restoration and capability of defensive renovation (AFfAR AF, 2016).
Literature Review
Molecular mechanism of cellular aging
Multiple studies demonstrate that ageing of differentiated cells significantly demonstrates the age related damage to distinct cellular organelles and their associated functions. For example, mitochondrial DNA (mtDNA) mutations which are maternally transmitted underpin brain ageing related disorders. Similarly, endoplasmic reticulum and protein misfolding stress also participated in ageing of brain lesions and increased production of mitochondrial DNA denotes cardiac ageing process. A research study conducted on muscle cell ageing demonstrated that eukaryotic initiation factor 4E-Binding Protein (4E-BP) which is transcribed by the Fork Head Box O (FOXO) transcription factor through the autophagy lysosome system alleviates the muscle cell ageing due to the aggregation of protein. Specific oxidative agents which create oxidative stress in cellular organelles such as endoplasmic reticulum, mitochondria and lysosomes that accelerate the process of cellular ageing resulting in the loss of intrinsic cellular function (Arai Y, et al., 2015). The ageing spectrum of differentiated cells is broad as compared to the non-dividing cells because the cumulative damage to differentiated cells causes genetic and epigenetic alterations showing greater DNA Damage Response (DDR) in proliferating cells known as mitotic stress. However, the cell ageing is basically triggered by DNA damage response. The ageing of cell is identified by two types of factors i.e. replicative ageing and Oncogene (stress) Induced Premature Senescence (OIS). Multiple lines of evidence indicate that shortening of telomeres act as a biological clock which causes hematopoietic stem cells and cancerous cells to show replicative ageing.
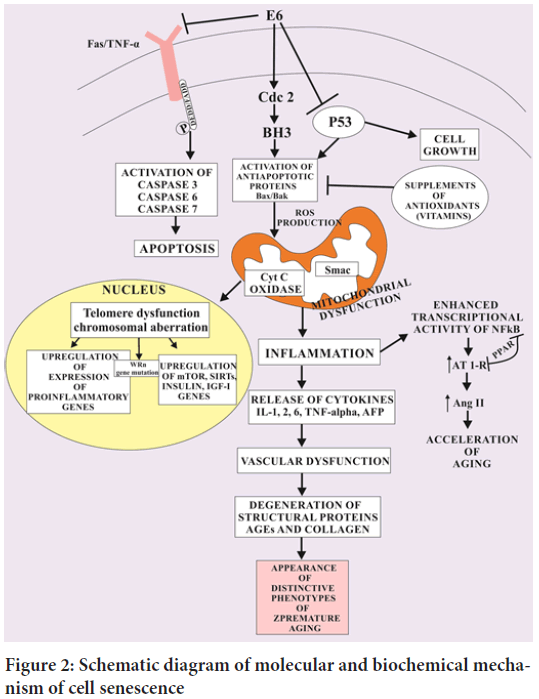
During the process of telomere shortening, the DNA Damage Response (DDR) kinases recruit Ataxia Telangiectasia Mutated (ATM), ataxia telangiectasia and Rad3 ATR to the altered foci of single and double stranded DNA resulting in DNA damage and activate p53 and p21CIP1. These activated genes trigger the cell cycle break due to which OIS separates the dividing the cells from the proliferative pool of cells. Thus, this crafted OIS creates a specific pathophysiological barrier to knock out tumor formation (Carvalho AC, et al., 2019) (Figure 2). Damage to mtDNA and organelles by free radicals leads to loss of mitochondrial function and loss of cellular energy. Mutations in mtDNA occur at 10-20 times the rate seen in nuclear DNA. A significant portion of ageing may be due to mtDNA deletions from singlet oxygen induced by ultraviolet light. Ageing mitochondria become enlarged. Ageing leads to the decline in coenzyme Q which is an important part of antioxadative defence system. In contrast to the normal human cells which undergo ageing (cell senescence) by the inhibition of DDR related OIS and activation of H-Ras V12 oncogene. The oncogene induced DNA replication activate both DDR and OIS which craft alternative changes in the succession of DNA replication fork with active replicons. When the progression of DNA replication fork and DNA double strand break is prematurely suppressed, it activates OIS resulting in accelerated carcinogenesis in albino mice. During the process of ageing, the senescent cells undergo increased activity of senescent associated β-galatosidase (SA-βGAl), Senescence Associated Heterochromatin Foci (SAHF) which ultimately aggravate histone H3 lysine 9 trimethylation (H3K9me3) and heterochromatin protein 1 ϒ (HP1ϒ) activity. It is very important in cellular senescence to amplify the levels of cyclin dependent protein kinase inhibitors p16INK4a and p21CIP1 in order to break cell cycle crafting a crucial halt in tumor formation (Arbeev KG, et al., 2020).
Figure 2: Schematic diagram of molecular and biochemical mechanism of cell senescence
Molecular biology of Developmental and Programmed Senescence (DPS)
The physiology of cellular ageing in developing embryonic tissues of nude mice is based on the recruitment of SA-βFal, H3K9me3, p21CIP1 and (HP1ϓ) activation and deactivation of cell proliferation biomarker Ki67 and Bromodeoxyuridine (BrdU) in embryonic tissues. The molecular mechanistic approach determined that there is specific difference between Development and Programmed Senescence (DPS) and OIS and replicative senescence such that DPS system is devoid of DDR-ATM-p53 genes and p38 and p16INK4a oncogenes instead is related to the cascade signalling Smad and p21CIP1. One of the most important feature of DPS is that it is associated with inflammatory cytokines and transforming growth factor β. However, many characteristics of DPS and OIS are common in them such as Upregulated gene expression of p15INK4b, SASP mediators, mitogen activated protein kinases and nuclear factor kappa-B signaling (Astuti Y, et al., 2017). SASP is indicated to control paracrine senescence mediating Vascular Endothelial Growth Factor (VEGF) and Tumor Growth Factor (TGF-β) which in turn regulates the genetic expression of p15INK4b and p21CIP1.
The mechanism of induction of DPS and its regulation by TGF-β is quite interesting. The cytokine family of TGF-β plays a crucial role in the maintenance of telomeres during the development of tumor. It helps in tissue remodeling, the hurdles of transplantations and inflammatory processes. However, Smad3 represses the Telomerase Reverse Transcriptase (TERT) gene being regulated by a number of cytokines. Instead, the checkpoints of telomere DNA double strand being p16INK4a and p15INK4b shows a putative mechanism of activation and cytokine induced telomerase inhibition. The eventual destiny of aged cells is apoptosis and clearance mediated by macrophages. Multiple researches indicate that developmental and programmed ageing is seen only in tissues with developmental birth abnormalities. A recent study also disclosed molecular biomarkers of cellular ageing in human placental syncytiotrophoblast during embryonic development. During the formation of embryo, the Human Endogenous Retrovirus-W-1 (ERVWE1) is involved in the formation of syncytiotrophoblast which acts as an interface between maternal and fetal tissues at the placenta mediating cellular fusion. However, in case of cellular senescence (ageing), the genetic expression of ERVWE1 causes hyperploid syncytia through the mechanism of p53 and p16-pRb signalling cascades exhibiting the process of aging (Aubert G, et al., 2012).
Mediation of cellular ageing by telomerase and telomere repeating units of RNA
Telomeres are the repeating DNA sequences of TTAGGG associated with the binding molecules at the end of each linear chromosome in all nucleated cells which do not fully replicate by DNA polymerases. Telomeres show variation in length from 4 to 14 kb which is maintained by a ribonucleic enzyme “telomerase” which add telomere repeating sequences to the ends of chromosomes. Thus, telomere shortening could be a biological prognostic biomarker that signals the replicative ageing, disease and premature morbidity in all cultured primary human cells. During the phase of mitosis, the cell chromosome undergoes replication (duplication) maintaining the length of telomeres.
Thus, every unit increase in the length of telomere reflects the number of replications happening in mitosis. Upon the achievement of specific length, the shortest telomere activates DNA Damage Response (DDR) element for cellular ageing (Aubert G, et al., 2012). In spite of this fact, many critical diseases such as heart diseases, immune-compromising and psychological disorders involve telomere shortening often diagnosed in peripheral blood leukocyte count. Thus, the degree of shortening of telomeres determines the degree of stress in the body. For example, in healthy women with premenopausal condition, increased mitochondrial oxidative stress is associated with telomere length determined in peripheral blood leukocytes. The women which depicted increased level of oxidative stress had shorter telomeres compared with females having lower levels of oxidative stress. Evidence indicated that sedentary lifestyles cause this telomere shortening and improved diet, stress management and comprehensive living improve the telomere length both qualitatively and quantitatively (Aunan JR, et al., 2016).
Discussion
Current research reported that a non-coding telomere unit of RNA (TERRA) code for telomere shortening causing the aggregation of TERRA molecules in nuclear centre. The human repeating units of UUAGGG of TERRA sequences form guanine quadruplexes (G- quadruplexes) leading to the formation of RNA: DNA hybrid loop. The RNase H enzyme in eukaryotes removes the RNA moiety from the telomeres containing RNA TERRA. Telomere shortening can also be induced by exonuclease-1 which resects the ends of chromosomes producing replication stress mediated telomere short phenotype. An important feature of TERRA is that it removes the heterogeneous nuclear ribonucleoprotein A1 (HNRNPA1) thus, prevents the binding of telomere 1 (POT1) to telomere of the single stranded DNA (ssDNA) leading to the telomeric stress. In spite of the TERRA regulation in telomere shortening, TERRA forms aggregates in extremely proliferating cancerous cells which indicate large diameter nuclear centers different from the phosphorylated histone H2AXϒ nuclear foci and normal progenitor neuronal cells of under developing cerebellum. This fact suggested that elevated TERRA in the chromosomes might act as prognostic biomarker of early telomere shortening during the early development of tumor and replication stress (Aviv A and Levy D, 2019). Various factors which alter the length of the telomere and consequently the activity of the telomerase are depicted in Table 1.
| Factors | Description | References |
|---|---|---|
| Oxidative stress | Oxidative stress is an important aspect of toxicology associated with telomere shortening | (Aviv A and Shay JW, 2018) |
| Immune response | High level of T-lymphocytes (CD8 and CD28+) in elder patients is associated with blunted immune risk. Telomerase induction is a solution to this immune response | (Banszerus VL, et al., 2019) |
| Chronic inflammation | Short telomere is associated with inflammatory disease e.g. rheumatoid arthritis | (Barnes RP, et al., 2019) |
| Insulin resistance | Inhibition of Insulin like growth factor-I signaling is a prompt cell longevity promoter | (Barnett AG, et al., 2005) |
| Menopause | There is a strong dependency of hormones on telomere length with milestones of aging | (Beijers R, et al., 2020) |
| Telomerase | Length of the telomere is shortened due the down regulation of telomerase expression | (Bell CG, et al., 2019) |
| Genetic transfer | The trait of telomere shortening is transferred from parents to the offspring (For example: Dolly sheep) | (Belsky DW, et al., 2018) |
| Metabolic syndromes | Type 2 diabetes and metabolic syndrome X are metabolic syndromes of premature aging | (Belsky J and Shalev I, 2016) |
| Gender | Telomere length tend to be longer in females than in males | (Benetos A, et al., 2013) |
| Age | Younger age individuals (children and adults) have longer telomeres as compared to the older individuals | (de Jesus BB and Blasco MA, 2011) |
Table 1: List of biochemical and physiological factors that alter the length of the telomere
Cell cycle arrest induced by cellular ageing
B-Raf is known to induce cell cycle arrest by a complex mechanism of inhibition of pyruvate dehydrogenase kinase 1 (PDK-1) and over expression of pyruvate dehydrogenase phosphatase 2 (PDP 2). As a result, the pyruvate is increased due to the elevated levels of Pyruvate Dehydrogenase (PDH) in citric acid cycle (Krebs’ cycle). The resultant electron transport chain is up regulated crafting redox stress which ultimately leads to the cellular ageing. The normal levels of pyruvate dehydrogenase phosphatase 2 and pyruvate dehydrogenase kinase 1 cause the downregulation of PDH and elevated OIS which ultimately leads to the synthesis of melanoma. Another important fact supported by many evidences is that the stimulation of PDH also causes the activation of p53 and p21CIP1 induced DNA damage response (Figure 3). Cancer and ageing are both accompanied by cellular damage. Cancer and cell longevity require a durable cell proliferation potential. Therefore, this mechanism limit the indefinite proliferation provide cancer protection that favor ageing. The overall balance between these convergent and divergent mechanisms ensures cancer free and healthy life until late adulthood for most individuals. The malate enzymes i.e. ME-1 and ME-2 also increases the expression of OIS in albino mice models. The specific reason of down regulated expression of malate enzymes ME-1 and ME-2 in OIS is supported by the relationship of tumor suppressor gene p53. However, it is a clear fact that p53 is inhibited by ME-1 and ME-2 in normal circumstances through the activation of AMP-activated protein kinase-A consequencing in the acceleration of ageing process. The convergence of the metabolic pathways of PDH, PDP2, ME-1 and ME-2 can elucidate the interconnection of replicative ageing and OIS (Bettin N, et al., 2019).
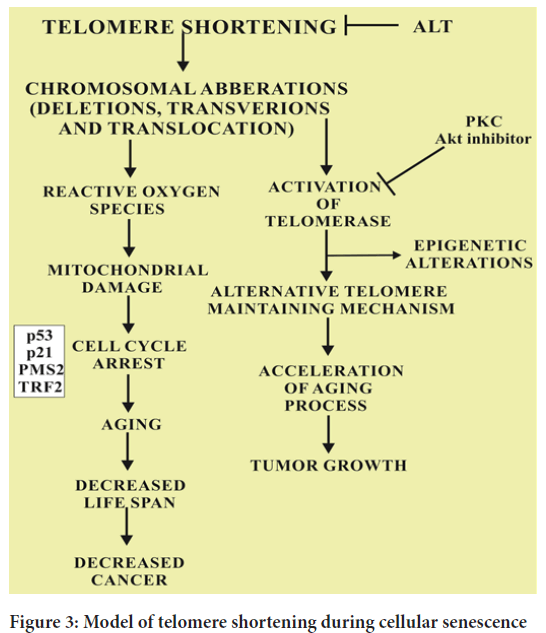
Figure 3: Model of telomere shortening during cellular senescence
Various antioxidants and nutraceutical which are responsible for active targeting of the telomere activity are depicted in Tables 2 and 3.
| Nutraceuticals | Modulation | References |
|---|---|---|
| Omega 3 fatty acids | Recent study of coronary heart disease patients reveal that there is an inverse relationship between levels of omega 3 fatty acids in blood and telomere shortening over a period of five years | (Boccardi M and Boccardi V, 2019) |
| Vitamin D | Vitamin D is an inducer of telomere length | (Bodelon C, et al., 2014) |
| Nicotinamide | Nicotinamide increases the life span of the fibroblasts in human beings due to the reduced production of ROS in mitochondria | (Calado RT and Dumitriu B, 2013) |
| Multivitamins | Use of multivitamins increases the telomere length, hence, improving the life span | (Canudas S, et al., 2020) |
| Folate B12 | Folate increases the length of the telomere in a non-linear manner by changing the DNA integrity and epigenetic alterations. However, decreased telomere length is associated with older males | (Celtikci B, et al., 2021) |
| Statins | Statins control the ROS balance of endothelial cells and increases the telomere length of lymphocytes | (Chen BH, et al., 2017) |
| Gingko biloba | Standardized extracts of Gingko biloba delay the onset of ageing by upregulating the signaling of P13kinase/Akt that increases the telomerase activity | (Chen R, et al., 2020) |
| Alpha-tocopherol | Also called vitamin E which is indicated to increase the telomerase activity of microvascular cells of the brain | (Chen W, et al., 2011) |
| N-acetylcystein | N-acetylcystein delays the onset of ageing by a complex mechanism of hTERT by blocking its entrance into the cell cytosol | (Cheng G, et al., 2013) |
| Antioxidants | Low levels of antioxidants is reported to increase the rate of breast cancer in females and prostate cancer in males | (Dagnall CL, et al., 2017) |
Table 2: Modulation of telomere structure and function by nutraceuticals
| Antioxidants | Proposed mechanism of slowing down of ageing | Interventions in preclinical trials | References |
|---|---|---|---|
| Vitamin E Idebenone clioquinol | The mitochondrial dysfunctioning disrupts cytochrome c oxidase activity, ultimately disturbs various homeostasis mechanisms | Caloric restricted diet administered in albino mice at 1 mg/kg body weight | (de Pedro N, et al., 2020) |
| Vitamin C and E | Neuronal dysfunction is generated in dopaminergic neurons producing endogenous and exogenous toxic secondary metabolites | Reduction of coenzyme Q and creatinine | (Delgado DA, et al., 2019) |
| Beta carotene | Several mitochondrial mutations occur due to SOD1 mutations resulting in loss of mitochondrial membrane potential | Creatinine and coenzyme Q | (Demanelis K, et al., 2020) |
| Deferoxamine | Iron metabolism is deteriorated due to the loss of iron sulfur enzymes | Histone deacetylase inhibitors CoQ | (Der G, et al., 2012) |
| Clioquinol | Impaired iron metabolism due to mtDNA mutations | Idebenone (Histone aceylase inhibitor) | (Dhabhar FS, et al., 2012) |
| Flavonoids | Reactive oxygen species generated in SOD1 mutations | Reduction in mitochondrial enzymes | (Dixit S, et al., 2019) |
| Selenium | Mitochondrial dysfunctions due to genetic mutations | Histone modification | (Dlouha D, et al., 2014) |
Table 3: Antioxidative therapy of ageing
Mediation of cellular senescence through p53, p21CIP1 and P16INK4a
Peroxisome proliferator-activated receptor (PPAR)-β/δ causes the inhibition of skin tumerogenesis by activating p53 dependent OIS. The increased expression of peroxisome proliferator-activated receptor (PPAR)-β/δ causes the phosphorylation of ERK thereby increasing the activity of kinase and down regulating p-AKT expression. This decreased p-AKT activity leads to the progression of cellular senescence by up regulating the expression of p53 and p27 (Daniali L, et al., 2013). The p53 protein 2 (ASPP2/53BP2L) is activated by tumor suppressor apoptosis gene which indirectly enhances the expression of p53 dependent apoptosis protein containing active C-terminal p53 binding domain. This p53 protein 2 has strong association with RAS by binding to its N-terminal domain resulting in the binding of Ras-GTP ultimately activating the RAS induced molecular senescence in non-transformed human cells (Eisenberg DT and Kuzawa CW, 2018).
Another inhibitor protein of cell senescence, Yes-Associated Protein (YAP) plays a crucial role in slowing down the cellular ageing process (Eisenberg DT, 2014). The deficiency of YAP activated cellular replicative ageing mechanism through the mediation of p53-p16-Rb protein and TEAD-Cdk6 dependent mechanisms. A recent study reported that the promoting of activity of SAHF protein causes the dissociation of oncogene induced tumor suppressor gene BRCA1 from the cellular chromatin which leads to its down regulation which drives the process of ageing further (El-Chemaly S, et al., 2018). The chromatin-remodeling factor associates with BRCA1 and carries out the phosphorylation of Retinoblastoma Protein (pRb) which activates the Brahma-related gene 1 (BRG1) causing the formation of SAHF protein and cellular senescence is induced by the degradation of oncogenic protein Ras and BRCA1 (Entringer S, et al., 2018). The up regulation of SAHF causes the induction of BRG1 and cellular ageing processes induced by chromatin dependent mechanisms. However, the stimulation of SAHF and BRG1 is inhibited by reducing pRb, p21CIPI and p16INK4a. Therefore, BRG1 is reported to down regulate BRCA1 and up regulate RAS activity and pRb, p21CIPI and p16INK4a to increase the process of cellular ageing or senescence (de Meyer T, et al., 2018).
Conclusion and Future Prospects
The activity of telomerase is a very important step towards the maintenance of life span of an organism. Telomere length as well as the production of telomerase plays the crucial role as the cellular targets of the viability and longevity of the cells. We have reviewed multiple factors which affect the telomerase activity and the length of the telomeres throughout the life span which include many genetic factors such as P13k/Akt signaling cascades, insulin resistance and tumor promoting genes etc. as well as the epigenetic factors such as the metabolic regulation through mitochondrial signaling, endoplasmic reticulum chronic stress and inflammatory diseases. The process of cellular senescence is amenable to molecular analysis and needs to determine the easily measured biological markers of ageing. Scientists believe that DNA chip arrays are very helpful to determine the gene expression of the entire genome which can provide the constructive biomarkers of cellular ageing. If the researchers are able to determine the characteristic pattern of changes in life span of the organism for example in nude mice, the DNA chip arrays will then be able to measure the rate of cellular senescence in genetically and physiologically transformed organisms. The genome analysis can also be an amiable indicators of relevant human polymorphic loci associated with cellular prolonged existence. Since, only limited key cascades are critical to control the cellular longevity, there must be specific molecular targets that work as therapeutic pharmacological interventions. Scientists have cloned the adult somatic cells which indicated that age related nuclear alterations may be reversible. These somatic cells that have been cloned may represent that they have fugitive age linked cellular events. In the past decade, the human life expectancy has been improved due to advances in therapeutic and medicinal approaches. The average life span is impacted probably to a small extent than that in the past two centuries. Slowing down the process of ageing increases the longevity and vitality of the organism over the whole life span. Of course, slowing down the ageing process faces many complex implications. Indeed, an over populated country, it is important to cut down the birth rates to achieve the significant effects on longevity and survival. Many industrialized countries have implicated this birth control strategy and have productive long lived individuals associated with improved health. The progress gradually depends on the increased knowledge and wisdom of the individuals to achieve premium benefits of organisms’ longevity.
Acknowledgement
Authors are highly thankful to Prof. Dr. Arif Malik (Dean of Sciences, Minhaj University, Lahore, Pakistan for refining and critically reviewing the manuscript.
Author Contributions
Sara Zahid-Writing and designing the original draft; Fatima Zahid-Reviewing and editing; Faisal Gulzar-Supervision
Data Availability
All relevant data is included in this manuscript
References
- Adam R, Diez-Gonzalez L, Ocana A, Šeruga B, Amir E, Templeton AJ. Prognostic role of telomere length in malignancies: A meta-analysis and meta-regression. Experimental and molecular pathology. 2017; 102(3): 455-74.
[Crossref] [Google scholar] [Pubmed]
- Shekhidem AH, Sharvit L, Leman E, Manov I, Roichman A, Holtze S, et al. Telomeres and longevity: A cause or an effect? Int J Mol Sci. 2019; 20(13): 3233.
[Crossref] [Google scholar] [Pubmed]
- Aksenova AY, Mirkin SM. At the beginning of the end and in the middle of the beginning: Structure and maintenance of telomeric DNA repeats and interstitial telomeric sequences. Genes. 2019; 10(2): 118.
[Crossref] [Google scholar] [Pubmed]
- AFfAR AF. Biomarkers of aging: An introduction to aging science brought to you by the American federation for aging research. American federation for aging research, New York, NY. 2016.
- Arai Y, Martin-Ruiz CM, Takayama M, Abe Y, Takebayashi T, Koyasu S, et al. Inflammation, but not telomere length, predicts successful ageing at extreme old age: A longitudinal study of semi-supercentenarians. EBioMedicine. 2015; 2(10): 1549-1558.
[Crossref] [Google scholar] [Pubmed]
- Carvalho AC, Mendes ML, da Silva Reis MC, Santos VS, Tanajura DM, Martins-Filho PR. Telomere length and frailty in older adults-A systematic review and meta-analysis. Ageing Res Rev. 2019; 54: 100914.
[Crossref] [Google scholar] [Pubmed]
- Arbeev KG, Verhulst S, Steenstrup T, Kark JD, Bagley O, Kooperberg C, et al. Association of leukocyte telomere length with mortality among adult participants in 3 longitudinal studies. JAMA netw open. 2020; 3(2): e200023.
[Crossref] [Google scholar] [Pubmed]
- Astuti Y, Wardhana A, Watkins J, Wulaningsih W. Cigarette smoking and telomere length: A systematic review of 84 studies and meta-analysis. Environ Res. 2017; 158: 480-489.
[Crossref] [Google scholar] [Pubmed]
- Aubert G, Baerlocher GM, Vulto I, Poon SS, Lansdorp PM. Collapse of telomere homeostasis in hematopoietic cells caused by heterozygous mutations in telomerase genes. PLoS genet. 2012; 8(5): e1002696.
[Crossref] [Google scholar] [Pubmed]
- Aubert G, Hills M, Lansdorp PM. Telomere length measurement-Caveats and a critical assessment of the available technologies and tools. Mutat Res. 2012; 730(1-2): 59-67.
[Crossref] [Google scholar] [Pubmed]
- Aunan JR, Watson MM, Hagland HR, Søreide K. Molecular and biological hallmarks of ageing. Br J Surg. 2016; 103(2): e29-46.
[Crossref] [Google scholar] [Pubmed]
- Aviv A, Levy D. Hemothelium, clonal hematopoiesis of indeterminate potential, and atherosclerosis: Role of telomere length dynamics. Circulation. 2019; 139(1): 7-9.
[Crossref] [Google scholar] [Pubmed]
- Aviv A, Shay JW. Reflections on telomere dynamics and ageing-related diseases in humans. Philos Trans R Soc Lond B Biol Sci. 2018; 373(1741): 20160436.
[Crossref] [Google scholar] [Pubmed]
- Banszerus VL, Vetter VM, Salewsky B, König M, Demuth I. Exploring the relationship of relative telomere length and the epigenetic clock in the LipidCardio cohort. Int J Mol Sci. 2019; 20(12): 3032.
[Crossref] [Google scholar] [Pubmed]
- Barnes RP, Fouquerel E, Opresko PL. The impact of oxidative DNA damage and stress on telomere homeostasis. Mech Ageing Dev. 2019; 177: 37-45.
[Crossref] [Google scholar] [Pubmed]
- Barnett AG, van Der Pols JC, Dobson AJ. Regression to the mean: What it is and how to deal with it. Int J Epidemiol. 2005; 34(1): 215-220.
[Crossref] [Google scholar] [Pubmed]
- Beijers R, Hartman S, Shalev I, Hastings W, Mattern BC, de Weerth C, et al. Testing three hypotheses about effects of sensitive-insensitive parenting on telomeres. Dev Psychol. 2020; 56(2): 237.
[Crossref] [Google scholar] [Pubmed]
- Bell CG, Lowe R, Adams PD, Baccarelli AA, Beck S, Bell JT, et al. DNA methylation aging clocks: Challenges and recommendations. Genome biology. 2019; 20: 1-24.
[Crossref] [Google scholar] [Pubmed]
- Belsky DW, Moffitt TE, Cohen AA, Corcoran DL, Levine ME, Prinz JA, et al. Eleven telomere, epigenetic clock, and biomarker-composite quantifications of biological aging: Do they measure the same thing? American journal of epidemiology. 2018; 187(6): 1220-1230.
[Crossref] [Google scholar] [Pubmed]
- Belsky J, Shalev I. Contextual adversity, telomere erosion, pubertal development, and health: Two models of accelerated aging, or one? Dev Psychopathol. 2016; 28(4pt2): 1367-1383.
[Crossref] [Google scholar] [Pubmed]
- Benetos A, Kark JD, Susser E, Kimura M, Sinnreich R, Chen W, et al. Tracking and fixed ranking of leukocyte telomere length across the adult life course. Aging cell. 2013; 12(4): 615-621.
[Crossref] [Google scholar] [Pubmed]
- de Jesus BB, Blasco MA. Aging by telomere loss can be reversed. Cell Stem Cell. 2011; 8(1): 3-4.
[Crossref] [Google scholar] [Pubmed]
- Bettin N, Oss Pegorar C, Cusanelli E. The emerging roles of TERRA in telomere maintenance and genome stability. Cells. 2019; 8(3): 246.
[Crossref] [Google scholar] [Pubmed]
- Boccardi M, Boccardi V. Psychological wellbeing and healthy aging: Focus on telomeres. Geriatrics. 2019; 4(1): 25.
[Crossref] [Google scholar] [Pubmed]
- Bodelon C, Savage SA, Gadalla SM. Telomeres in molecular epidemiology studies. Progress in molecular biology and translational science. 2014; 125: 113-131.
[Crossref] [Google scholar] [Pubmed]
- Calado RT, Dumitriu B. Telomere dynamics in mice and humans. Semin Hematol. 2013; 50(2): 165-174.
[Crossref] [Google scholar] [Pubmed]
- Canudas S, Becerra-Tomás N, Hernández-Alonso P, Galié S, Leung C, Crous-Bou M, et al. Mediterranean diet and telomere length: A systematic review and meta-analysis. Adv Nutr. 2020; 11(6): 1544-1554.
[Crossref] [Google scholar] [Pubmed]
- Celtikci B, Erkmen GK, Dikmen ZG. Regulation and effect of telomerase and telomeric length in stem cells. Curr Stem Cell Res Ther. 2021; 16(7): 809-823.
[Crossref] [Google scholar] [Pubmed]
- Chen BH, Carty CL, Kimura M, Kark JD, Chen W, Li S, et al. Leukocyte telomere length, T cell composition and DNA methylation age. Aging (Albany NY). 2017; 9(9): 1983.
[Crossref] [Google scholar] [Pubmed]
- Chen R, Zhan Y, Pedersen N, Fall K, Valdimarsdóttir UA, Hägg S, et al. Marital status, telomere length and cardiovascular disease risk in a Swedish prospective cohort. Heart. 2020; 106(4): 267-722.
[Crossref] [Google scholar] [Pubmed]
- Chen W, Kimura M, Kim S, Cao X, Srinivasan SR, Berenson GS, et al. Longitudinal versus cross-sectional evaluations of leukocyte telomere length dynamics: Age-dependent telomere shortening is the rule. J Gerontol A Biol Sci Med Sci. 2011; 66(3): 312-319.
[Crossref] [Google scholar] [Pubmed]
- Cheng G, Kong F, Luan Y, Sun C, Wang J, Zhang L, et al. Differential shortening rate of telomere length in the development of human fetus. Biochem Biophys Res Commun. 2013; 442(1-2): 112-115.
[Crossref] [Google scholar] [Pubmed]
- Dagnall CL, Hicks B, Teshome K, Hutchinson AA, Gadalla SM, Khincha PP, et al. Effect of pre-analytic variables on the reproducibility of qPCR relative telomere length measurement. PloS one. 2017; 12(9): e0184098.
[Crossref] [Google scholar] [Pubmed]
- de Pedro N, Díez M, García I, García J, Otero L, Fernández L, et al. Analytical validation of telomere analysis technology® for the high-throughput analysis of multiple telomere-associated variables. Biol Proced Online. 2020; 22(1): 1-3.
[Crossref] [Google scholar] [Pubmed]
- Delgado DA, Zhang C, Gleason K, Demanelis K, Chen LS, Gao J, et al. The contribution of parent-to-offspring transmission of telomeres to the heritability of telomere length in humans. Human genetics. 2019; 138: 49-60.
[Crossref] [Google scholar] [Pubmed]
- Demanelis K, Jasmine F, Chen LS, Chernoff M, Tong L, Delgado D, et al. Determinants of telomere length across human tissues. Science. 2020; 369(6509): eaaz6876.
[Crossref] [Google scholar] [Pubmed]
- Der G, Batty GD, Benzeval M, Deary IJ, Green MJ, McGlynn L, et al. Is telomere length a biomarker for aging: Cross-sectional evidence from the west of Scotland?. PLoS One. 2012; 7(9): e45166.
[Crossref] [Google scholar] [Pubmed]
- Dhabhar FS, Malarkey WB, Neri E, McEwen BS. Stress-induced redistribution of immune cells-From barracks to boulevards to battlefields: A tale of three hormones-Curt Richter award winner. Psychoneuroendocrinology. 2012; 37(9): 1345-1368.
[Crossref] [Google scholar] [Pubmed]
- Dixit S, Whooley MA, Vittinghoff E, Roberts JD, Heckbert SR, Fitzpatrick AL, et al. Alcohol consumption and leukocyte telomere length. Sci Rep. 2019; 9(1): 1404.
[Crossref] [Google scholar] [Pubmed]
- Dlouha D, Maluskova J, Lesna IK, Lanska V, Hubacek JA. Comparison of the relative telomere length measured in leukocytes and eleven different human tissues. Physiological Research. 2014; 63: S343.
[Crossref] [Google scholar] [Pubmed]
- Daniali L, Benetos A, Susser E, Kark JD, Labat C, Kimura M, et al. Telomeres shorten at equivalent rates in somatic tissues of adults. Nat commun. 2013; 4(1): 1597.
[Crossref] [Google scholar] [Pubmed]
- Eisenberg DT, Kuzawa CW. The paternal age at conception effect on offspring telomere length: Mechanistic, comparative and adaptive perspectives. Philos Trans R Soc Lond B Biol Sci. 2018; 373(1741): 20160442.
[Crossref] [Google scholar] [Pubmed]
- Eisenberg DT, Lee NR, Rej PH, Hayes MG, Kuzawa CW. Older paternal ages and grandpaternal ages at conception predict longer telomeres in human descendants. Proc Biol Sci. 2019; 286(1903): 20190800.
[Crossref] [Google scholar] [Pubmed]
- Eisenberg DT. Inconsistent inheritance of telomere length (TL): Is offspring TL more strongly correlated with maternal or paternal TL? Eur J Hum Genet. 2014; 22(1): 8-9.
[Crossref] [Google scholar] [Pubmed]
- El-Chemaly S, Cheung F, Kotliarov Y, O’Brien KJ, Gahl WA, Chen J, et al. The immunome in two inherited forms of pulmonary fibrosis. Front Immunol. 2018; 9: 76.
[Crossref] [Google scholar] [Pubmed]
- Entringer S, de Punder K, Buss C, Wadhwa PD. The fetal programming of telomere biology hypothesis: An update. Philos Trans R Soc Lond B Biol Sci. 2018; 373(1741): 20170151.
[Crossref] [Google scholar] [Pubmed]
- de Meyer T, Nawrot T, Bekaert S, de Buyzere ML, Rietzschel ER, Andrés V. Telomere length as cardiovascular aging biomarker: JACC review topic of the week. J Am Coll Cardiol. 2018; 72(7): 805-813.
[Crossref] [Google scholar] [Pubmed]
Author Info
Sara Zahid1, Fatima Zahid2, Faisal Gulzar2, Qurban Ali2, Ayesha Zahid1 and Arif Malik1*2Department of Pharmacy, The University of Lahore, Punjab, Pakistan
Citation: Zahid S: Telomere Shortening as Biological Hallmark of Cellular Senescence and Longevity-An Update
Received: 20-Jan-2023 Accepted: 03-Feb-2023 Published: 10-Feb-2023, DOI: 10.31858/0975-8453.14.2.125-130
Copyright: This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
ARTICLE TOOLS
- Dental Development between Assisted Reproductive Therapy (Art) and Natural Conceived Children: A Comparative Pilot Study Norzaiti Mohd Kenali, Naimah Hasanah Mohd Fathil, Norbasyirah Bohari, Ahmad Faisal Ismail, Roszaman Ramli SRP. 2020; 11(1): 01-06 » doi: 10.5530/srp.2020.1.01
- Psychometric properties of the World Health Organization Quality of life instrument, short form: Validity in the Vietnamese healthcare context Trung Quang Vo*, Bao Tran Thuy Tran, Ngan Thuy Nguyen, Tram ThiHuyen Nguyen, Thuy Phan Chung Tran SRP. 2020; 11(1): 14-22 » doi: 10.5530/srp.2019.1.3
- A Review of Pharmacoeconomics: the key to “Healthcare for All” Hasamnis AA, Patil SS, Shaik Imam, Narendiran K SRP. 2019; 10(1): s40-s42 » doi: 10.5530/srp.2019.1s.21
- Deuterium Depleted Water as an Adjuvant in Treatment of Cancer Anton Syroeshkin, Olga Levitskaya, Elena Uspenskaya, Tatiana Pleteneva, Daria Romaykina, Daria Ermakova SRP. 2019; 10(1): 112-117 » doi: 10.5530/srp.2019.1.19
- Dental Development between Assisted Reproductive Therapy (Art) and Natural Conceived Children: A Comparative Pilot Study Norzaiti Mohd Kenali, Naimah Hasanah Mohd Fathil, Norbasyirah Bohari, Ahmad Faisal Ismail, Roszaman Ramli SRP. 2020; 11(1): 01-06 » doi: 10.5530/srp.2020.1.01
- Manilkara zapota (L.) Royen Fruit Peel: A Phytochemical and Pharmacological Review Karle Pravin P, Dhawale Shashikant C SRP. 2019; 10(1): 11-14 » doi: 0.5530/srp.2019.1.2
- Pharmacognostic and Phytopharmacological Overview on Bombax ceiba Pankaj Haribhau Chaudhary, Mukund Ganeshrao Tawar SRP. 2019; 10(1): 20-25 » doi: 10.5530/srp.2019.1.4
- A Review of Pharmacoeconomics: the key to “Healthcare for All” Hasamnis AA, Patil SS, Shaik Imam, Narendiran K SRP. 2019; 10(1): s40-s42 » doi: 10.5530/srp.2019.1s.21
- A Prospective Review on Phyto-Pharmacological Aspects of Andrographis paniculata Govindraj Akilandeswari, Arumugam Vijaya Anand, Palanisamy Sampathkumar, Puthamohan Vinayaga Moorthi, Basavaraju Preethi SRP. 2019; 10(1): 15-19 » doi: 10.5530/srp.2019.1.3