Research Article - (2022) Volume 13, Issue 11
The Effectiveness and Safety of Trastuzumab Biosimilar ABP 980 plus Pertuzumab and Docetaxel in the Therapy of HER-2 Positive Metastatic Breast Cancer: Real World Experiences from the National Research Institute of Oncology in Warsaw
Karolina Miacz, Agnieszka Jagiello-Gruszfeld*, Michal Kunkiel, Izabela Lemanska, Anna Gorniak, Zbigniew Nowecki and Anna NiwinskaAbstract
Human Epidermal growth factor Receptor-2 (HER-2) overexpression can be found in 15%-20% of Breast Cancers (BC), and it strongly correlates with aggressive clinical behavior and adverse prognosis. The first- line treatment for HER-2 positive Metastatic Breast Cancers is the combination of trastuzumab, pertuzumab, and taxane (PTH). ABP 980 is a biosimilar of the innovator trastuzumab and is characterized by highly comparable effectiveness.
The group of 61 patients with HER-2 positive Metastatic Breast Cancer MBC received biosimilar ABP 980 plus pertuzumab and docetaxel from November, 18, 2018 to December, 24, 2019. The response to therapy, Overall Survival (OS), Progression-Free Survival (PFS), metastases, and adverse effects among patients were determined and analyzed. Initially, 42 women responded partially to the treatment and their median PFS was 27 months. Median PFS for the whole group was 18 months. Cardiotoxicity of treatment was noticed in all patients in the form of the reduction in Left Ventricular Ejection Fraction (LVEF) but only in 2 cases, it was the reason for withdrawing from therapy.
Biosimilar ABP 980 is registered in the same indications as the innovator trastuzumab and their effectiveness, as well as side effects, is comparable. The costs of biosimilar make the therapy more accessible and thus more patients with MBC around the world can receive relevant treatment.
Keywords
Biosimilar, Trastuzumab, Metastatic Breast Cancer (MBC), Human Epidermal growth factor Eeceptor-2 (HER-2) positive, Trastuzumab, pertuzumab, and taxane (PTH) therapy
Introduction
Breast Cancer (BC) is the most common type of cancer in the world, and despite the undoubted advances in methods used for cancer diagnoses and treatment; it has a high mortality rate (Sung H, et al., 2021; Cao W, et al., 2021). The Human Epidermal growth factor Receptor-2 (HER-2) is overexpressed in 15%- 20% of Breast Cancers (Cao W, et al., 2021; Wolff AC, et al., 2013; Nader-Marta G, et al., 2022; Valabrega G, et al., 2007), which defines BC as strongly associated with aggressive clinical behavior, high recurrence rates or metastases and worse prognosis (Cao W, et al., 2021; Wolff AC, et al., 2013; Nader-Marta G, et al., 2022). HER-2 levels correlate strongly with carcinogenesis and are associated with increased resistance to some chemotherapeutic drugs (Valabrega G, et al., 2007; Mukohara T, 2011). A huge break- through in the treatment of this biological subtype of BC has been the development of anti-HER2 targeted therapies. The first drug of this type is the humanized monoclonal antibody Trastuzumab (Slamon DJ, et al., 1989). It was approved for use in patients with HER2-positive Metastatic Breast Cancer (MBC) by the U.S. Food and Drug Administration (FDA) in 1998 and by the European Medicines Agency (EMA) in 2000 (FDA, 2017; EMA, 2017). In 2006, it was approved by the EMA and FDA for adjuvant therapy, and in 2011 for neoadjuvant therapy (FDA, 2017; EMA, 2017). The past two decades have seen rapid development of anti-HER2 therapies, which has significantly improved the prognosis in this group of patients (Simmons C, et al., 2022; Wynn CS and Tang SC, 2022). Still, trastuzumab and pertuzumab in combination with taxane (PTH) remain the standard first-line therapy for most MBC patients (Nader-Marta G, et al., 2022; Gennari A, et al., 2021). The current guidelines are based on the results of the randomized phase 3 CLEOPATRA study, in which the use of two anti-HER2 drugs, pertuzumab, and trastuzumab in combination with chemotherapy, was proven to be more effective than the use of trastuzumab plus the chemotherapy, both in terms of response rates, increasing the median PFS and median OS (Swain SM, et al., 2013; Swain SM, et al., 2020).
For more than 5 years now, biosimilars have been on the market in addition to the original Herceptin®. Biosimilars are biological products that are highly like a referenced anti-HER2 antibody in terms of their structure and functionality and have demonstrated similarity to trastuzumab in terms of safety, clinical efficacy, and tolerability (Cuellar S, 2020; Blackwell K, et al., 2018). While the cost of therapy with the original product can be a problem in some low-income countries, the use of biosimilars involves lower financial expenses, providing easier access to BC therapies worldwide (Blackwell K, et al., 2018; Cherny N, et al., 2016; Lammers P, et al., 2014). The guidelines for registration of biosimilars assume their registration in all indications that the original product has, provided the biosimilar product is like a registered originator product (i.e., reference product) in terms of safety, efficacy, and immunogenicity in at least one indication (EMA, 2017; EDA, 2017). One of the biosimilars is the monoclonal antibody ABP 980 (Kanjinti), which is approved for the treatment of HER2-positive early and Metastatic Breast Cancer and metastatic gastric cancer (Dhillon S, 2018; Hutterer KM, et al., 2019). APB 980 has been compared with Herceptin ® for the neoadjuvant therapy of Early Breast Cancer (EBC). However, the efficacy and safety of this drug in combination with pertuzumab in MBC have not been assessed.
The present study was conducted to evaluate the toxicity and efficacy of ABP 980 in combination with pertuzumab and docetaxel for first-line therapy of HER2-positive MBC at the National Research Institute of Oncology in Warsaw, the Breast Cancer, and Reconstructive Surgery Department.
Materials and Methods
We analyzed medical records of Breast Cancer patients who were treated with a PTH regimen with APB 980 (Kanjinti ®) as first-line therapy of MBC from Nov/28/2018 to Dec/24/2019.
All patients met the following criteria: Electrocorticogram (ECOG) 0-1 performance status, histopathological diagnosis of HER2-positive MBC, and baseline Left Ventricular Ejection Fraction (LVEF) of ≥ 50%. The key exclusion criteria included relapse within 1 year of the last dose of previous adjuvant (including neoadjuvant) any-HER2 treatment.
All patients received the PTH regimen (docetaxel, trastuzumab, and pertuzumab) at the following doses: Docetaxel 75 mg/m2, once every 3 weeks (6-8 cycles), biosimilars trastuzumab: Loading dose 8 mg/kg followed by 6 mg/kg intravenously, pertuzumab: Loading dose 840 mg followed by 420 mg intravenously, once every 3 weeks to disease progression or unacceptable toxicity. All patients with positive hormone receptors also received hormone therapy after the end of docetaxel.
All patients were evaluated for response every 12 weeks using a Computed Tomography (CT) scan according to RECIST 1.1 criteria.
Adverse events were assessed according to the Common Terminology Criteria for Adverse Events, version 4.0 (NCI, 2017). Echocardiography was used to monitor LVEF; it was performed before the start of treatment and then every 12 weeks during the therapy.
Statistical analysis
The normality of the distribution of the individual parameters evaluated in the study was verified using the Shapiro-Wilk test. In the case of normal distribution, Student’s t-distribution test was used to compare the mean values of independent variables. For the other parameters without normal distributions, appropriate methods of statistical analysis were selected based on non-parametric tests. The Mann-Whitney U test was used to compare numerical variables between the two groups observed. A Kruskal-Wallis test was used for the three groups. A non-parametric Wilcoxon signed-rank test was used to check for the differences between the results of the various parameters analyzed in the study group at different periods (dependent variables). A paired t-test was used to analyze two dependent variables with normal distributions. A linear mixed-effects model was used to examine the relationship between the variables. All calculations and graphs were performed using the R statistical package version 4.0.2.
Ethics statement
The study protocol was approved by the Ethics Committee of Maria Sklodowska-Curie National Research Institute of Oncology (No 22/13/2021). The study was performed per Good Clinical Practice standards and the ethical principles that have their origin in the Declaration of Helsinki. All patients provided informed consent for use of their data for research purposes.
Results
The inclusion criteria for the study were met by 61 patients. The median age of the entire group was 57 years. Most patients (75.4%) were below the age of 65. Most patients (78.3%) were diagnosed with luminal B HER2-positive cancer. More than half of the patients (55.7%) had previously received systemic therapy for EBC, of which 40% were treated with trastuzumab. Most cases (65.6%) were diagnosed with multiple metastases. Only four patients (6.6%) had Central Nervous System (CNS) metastases. The characteristics of the patients are shown in Table 1.
| Variable | Parameter | Total | <65 years old | ≥ 65 years old |
|---|---|---|---|---|
| N | 61 | 46 (75.4%) | 15 (24.6%) | |
| Age (years) | Mean (SD) | 56.39 (10.76) | 48.2 (8.2) | 68 (6) |
| Median | 57 | 49 | 68 | |
| Range | 28-76 | 28-64 | 65-76 | |
| Estrogen Receptor (ER) | Positive | 78.3% (N=47) | 75.60% | 86.70% |
| Negative | 21.7% (N=13) | 24.40% | 13.30% | |
| Progesterone Receptor (PGR) | Positive | 60% (N=36) | 53.30% | 80% |
| Negative | 40% (N=24) | 46.70% | 20% | |
| Single metastasis | Yes | 34.4% (N=21) | 37% | 26.70% |
| No | 65.6% (N=40) | 63% | 73.30% | |
| Bone metastases | Yes | 49.2% (N=30) | 47.80% | 53.30% |
| No | 50.8% (N=31) | 52.20% | 46.70% | |
| Liver metastases | Yes | 42.6% (N=26) | 39.10% | 53.30% |
| No | 57.4% (N=35) | 60.90% | 46.70% | |
| Lung metastases | Yes | 52.5% (N=32) | 45.70% | 73.30% |
| No | 47.5% (N=29) | 54.30% | 26.70% | |
| Central Nervous System (CNS) metastases | Yes | 6.6% (N=4) | 8.70% | 0% |
| No | 93.4% (N=57) | 91.30% | 100% | |
| Metastasis to soft tissues/lymph nodes | Yes | 60.7% (N=37) | 63% | 53.30% |
| No | 39.3% (N=24) | 37% | 46.70% | |
| Prior treatment due to Early Breast Cancer (EBC) | Yes | 55.7% (N=34) | 39.10% | 40% |
| No | 44.3% (N=27) | 60.90% | 60% | |
| Prior treatment with trastuzumab | Yes | 39.3% (N=24) | 47.80% | 40% |
| No | 60.7% (N=37) | 52.20% | 60% | |
| Prior treatment with anthracyclines | Yes | 41.0% (N=25) | 53.50% | 13.30% |
| No | 59.0% (N=36) | 46.50% | 86.70% | |
| Adjuvant radiotherapy | Yes | 32.7% (N=20) | 36.40% | 26.70% |
| No | 67.3% (N=41) | 63.60% | 73.30% | |
| Diabetes | Yes | 14.8% (N=9) | 15.20% | 13.30% |
| No | 85.2% (N=52) | 84.80% | 86.70% | |
| Body Mass Index (BMI)-breakdown | Normal value | 34.4% (N=21) | 34.80% | 33.30% |
| Overweight | 29.5% (N=18) | 30.40% | 26.70% | |
| Obesity | 36.1% (N=22) | 34.80% | 40% |
Table 1: Patient characteristics
Treatment toxicity
No grade 4 toxicity was found. No deaths due to toxicity were observed. There were also no unexpected toxicities that had not been described concerning treatment with the PTH regimen in the CLEOPATRA study. The most common side effects were weakness (52.4%), diarrhea (39%), neutropenia (36%), and anemia (32.8%). It should be noted that diarrhea was more frequently observed in patients aged 65 and older (53.3% vs. 34.7%), of which 2 patients (13.3%) had grade 3 diarrhea. The toxicity data are shown in Table 2.
| Toxicity | Incidence (any grade) | Grade 3 or 4 | ||||
|---|---|---|---|---|---|---|
| Any | Patients <65 | Patients ≥65 | Any | Patients <65 | Patients ≥65 | |
| Fatigue | 32 (52.4%) | 19 (41.3%) | 13 (86.7%) | 2 (3.3%) | 1 (2.2%) | 1 (6.7%) |
| Diarrhea | 24 (39%) | 16 (34.7%) | 8 (53.3%) | 2 (3.3%) | 0 | 2 (13.3%) |
| Neutropenia | 22 (36%) | 17 (37%) | 5 (33.3%) | 4 (6.5%) | 2 (4.3%) | 2 (13.3%) |
| Anemia | 20 (32.8%) | 16 (34.7%) | 4 (26.7%) | 2 (3.3%) | 1 (2.2%) | 1 (6.7%) |
| Thrombocytopenia | 13 (21.3%) | 10 (21.7%) | 3 (20%) | 0 | 0 | 0 |
| Neuropathy | 12 (19.7%) | 7 (15.2%) | 5 (33.3%) | 0 | 0 | 0 |
| Mucositis | 3 (4.9%) | 2 (4.3%) | 1 (6.7%) | 0 | 0 | 0 |
| Cardiac dysfunction | 0 | 0 | 0 | 0 | 0 | 0 |
Table 2: Treatment toxicity
LVEF analysis at selected time points
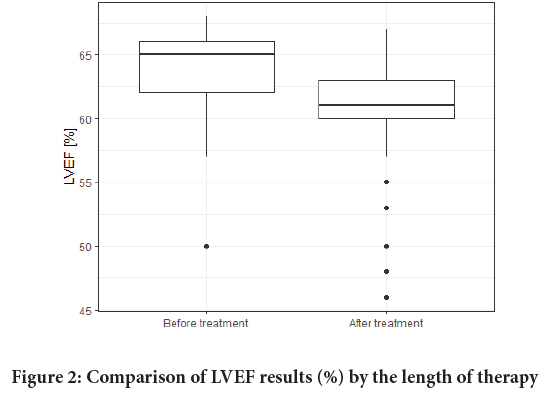
LVEF values were monitored in the study. There were no significant differences in baseline LVEF values which would depend on the age of the patients (Figure 1). LVEF before the therapy averaged 63.67% whereas the value of the last LVEF measure was 60.77%. The LVEF results are shown in Table 3 and Figure 2. No correlations between patients’ LVEFs and Body Mass Index (BMIs) were observed. Also, type II diabetes had no effect on LVEF values during the therapy.
| Variable | Parameter | Before treatment | Last measurement during therapy | ||||
|---|---|---|---|---|---|---|---|
| Total | <65 years old | ≥ 65 years old | Total | <65 years old | ≥ 65 years old | ||
| LVEF (%) | Number of patients | 61 | 46 | 15 | 61 | 46 | 15 |
| Mean (SD) | 63.67 (3.43) | 63.24 (4.08) | 65 (2.67) | 60.77 (4.1) | 60.57 (4.42) | 61.4 (2.97) | |
| Median (IQR) | 65 (62-66) | 64 (62-65) | 65 (65-67) | 61 (60-63) | 61 (60-63) | 62 (60-63) | |
| Range | 50-68 | 50-68 | 57-68 | 46-67 | 46-67 | 55-65 | |
| 10%-15% LVEF decrease | Yes | - | - | - | 11.5% (N=7) | 6.5% (N=3) | 26.7% (N=4) |
| No | - | - | - | 88.5% (N=54) | 93.5% (N=43) | 73.3% (N=11) | |
| <50% LVEF decrease | Yes | - | - | - | 3.3% (N=2) | 4.3% (N=2) | 0% (N=0) |
| No | - | - | - | 96.7% (N=59) | 95.7% (N=44) | 100% (N=15) | |
| Other cardiac issues during the treatment | Yes | - | - | - | 6.5% (N=4) | 6.5% (N=3) | 6.7% (N=1) |
| No | - | - | - | 93.5% (N=57) | 93.5% (N=43) | 93.3% (N=14) | |
Table 3: Comparison of Left Ventricular Ejection Fraction (LVEF) results (%)
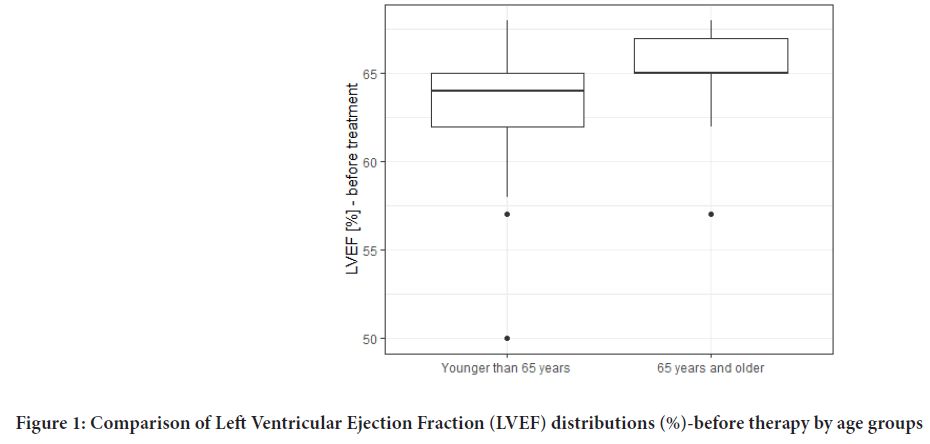
Figure 1: Comparison of Left Ventricular Ejection Fraction (LVEF) distributions (%)-before therapy by age groups
Figure 2: Comparison of LVEF results (%) by the length of therapy
An LVEF reduction of 10%-15% was observed in 7 patients (11.5%), and a <50% reduction affected only 2 patients (3.3%). Other cardiac problems, supraventricular arrhythmias, were observed in 4 patients (6.5%).
Central Nervous System (CNS) metastases
A statistical analysis of risk factors for the development of Central Nervous System (CNS) metastases was performed. The only correlation observed was the one with the absence of Estrogen Receptor (ER) expression. The CNS metastases patients had a median PFS of 7 months, while the rest of the group had a median PFS of 12 months (Table 4).
| Variable | Parameter | CNS metastases (N=4) | No metastasis to the CNS (N=57) | Test | p-value |
|---|---|---|---|---|---|
| Estrogen Receptor (ER) | Positive | 25% (N=1) | 82.1% (N=46) | Fisher | 0.029 |
| Negative | 75% (N=3) | 17.9% (N=10) | |||
| Time to progression (months) | N | 4 | 38 | Student’s t distribution | <0.001 |
| Mean (SD) | 7 (0.82) | 19.89 (9.19) | |||
| Median (IQR) | 7 (6.75-7.25) | 19 (7-20.75) | |||
| Range | 6-8 | 1-28 |
Table 4: Comparison of Central Nervous System (CNS) metastases variables
Response to treatment
68.9% (N=42) of patients had a Partial Response (PR), 23% (N=14) achieved Stabilization of the Disease (SD), and there were no cases with Complete Regression (CR). 8.2% (N=5) of patients had no response whatsoever (Progressive Disease (PD) in the first response assessment). The most common reason for therapy discontinuation was disease progression-39 patients (63.9%), two patients discontinued therapy due to cardiotoxicity (3.3%), and one patient discontinued therapy for other reasons (1.7%). At the time when the data were summarized (April/21/2022), 19 patients (31.1%) were continuing the therapy with trastuzumab plus pertuzumab.
Survival analysis
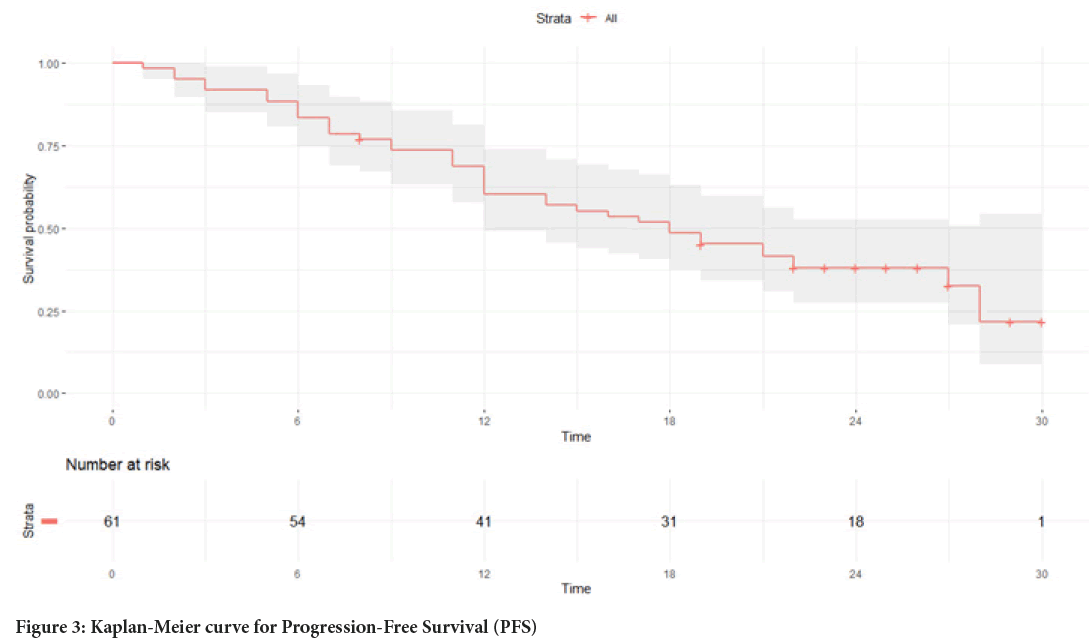
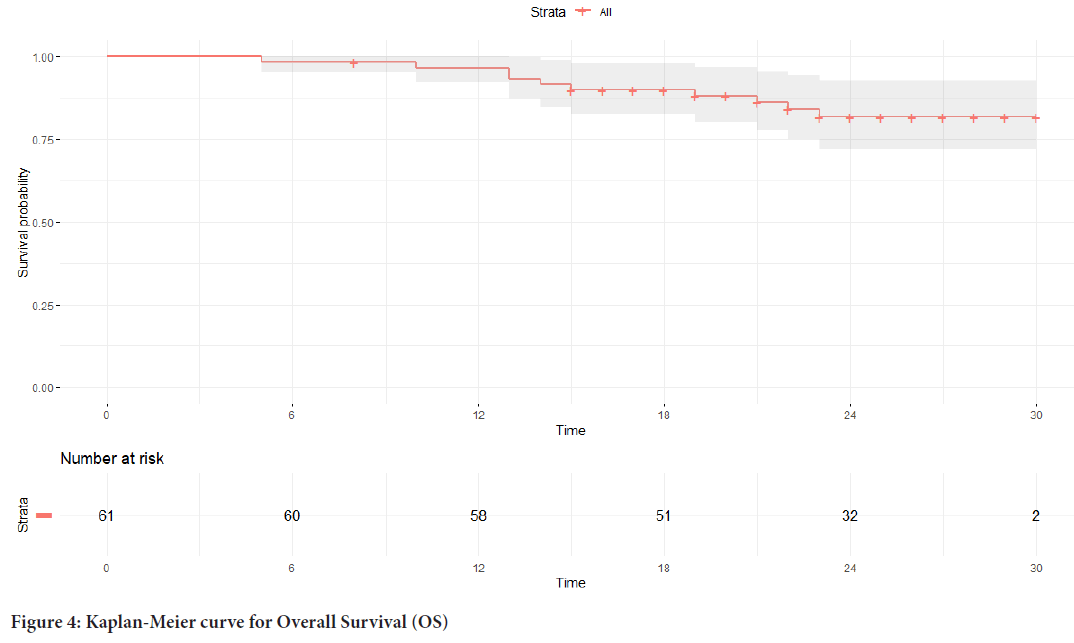
The median PFS in the study group was 18 months (Figure 3), and the median OS was not reached (Figure 4).
Figure 3: Kaplan-Meier curve for Progression-Free Survival (PFS)
Figure 4: Kaplan-Meier curve for Overall Survival (OS)
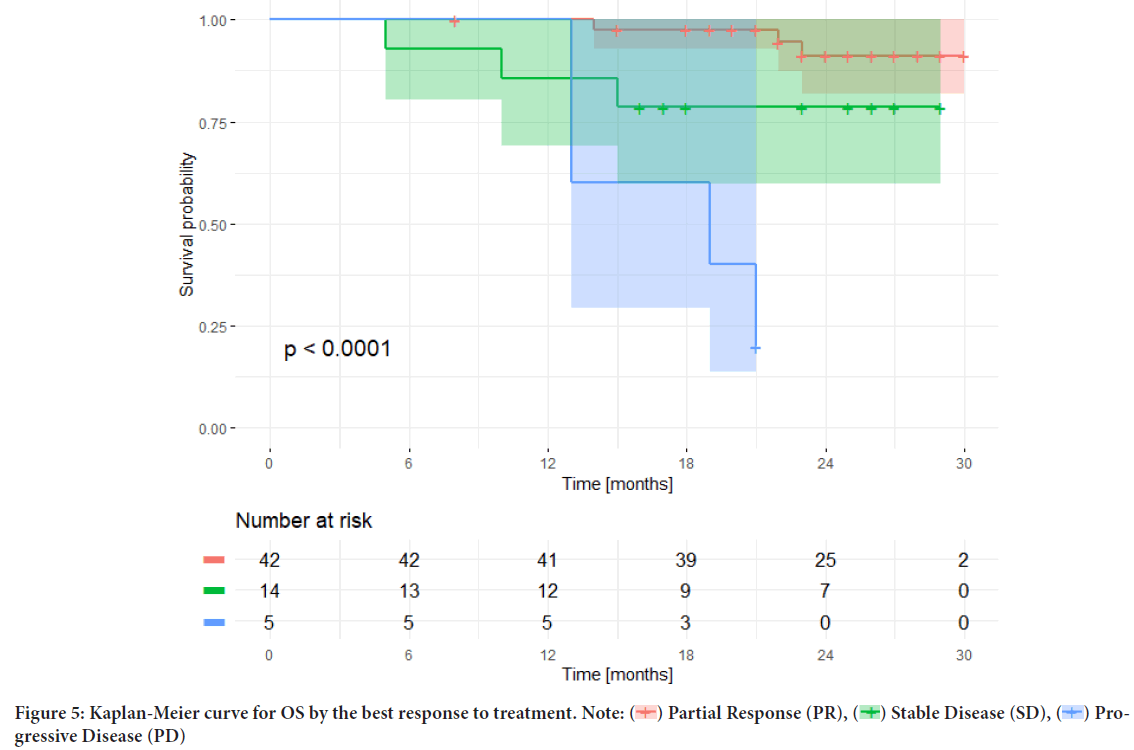
The median OS was analyzed depending on the response achieved; the median OS for the subgroup of patients who did not respond to first-line therapy was achieved and totaled only 19 months, despite using subsequent lines of anti-HER2 therapy (trastuzumab-emtansine, lapatinib plus capecitabine, trastuzumab deruxtecan), while the median OS was not achieved in the subgroups with PR or SD (Figure 5).
Figure 5: Kaplan-Meier curve for OS by the best response to treatment. Note:  Partial Response (PR),
Partial Response (PR),  Stable Disease (SD),
Stable Disease (SD),  Progressive Disease (PD)
Progressive Disease (PD)
Discussion
Breast Cancer with high HER2 expression levels is associated with worse prognosis and the disease progressing more dynamically. Significant improvements in therapy outcomes for this biologic subtype were achieved when HER2 blockers were introduced into routine management. The dual antibody blockade of HER2 with pertuzumab, trastuzumab plus taxanes has been shown to significantly increase the median OS in first-line therapy of MBC patients (Swain SM, et al., 2013; Swain SM, et al., 2020). In the CLEOPATRA study, the median OS was 57.1 months and the median PFS was 18.7 months (Swain SM, et al., 2020). In our study, the mOS had not been reached yet, while the mPFS was 18 months.
The therapy described in the CLEOPATRA study has been duplicated in several other studies, including those involving Asian populations (the Japanese COMACHI study and the Chinese PUFFIN study); the results thereof were consistent with the CLEOPATRA study results. The median PFS achieved in these studies ranged between 14.5 and 27.8 months (de Placido S, et al., 2018; Robert NJ, et al., 2017; Bachelot T, et al., 2019; Takahashi M, et al., 2021; Xu B, et al., 2020). Equivalent results were also presented in a study evaluating a trastuzumab biosimilar (SB3); the mPFS obtained in this study was 12.7 months (Celik A, et al., 2022). Table 5 shows a comparison of the results of the most frequently quoted studies evaluating the efficacy and safety of PTH therapy.
| Author, year of publication | Number of patients treated with pertuzumab+ trastuzumab | Trastuzumab used | Age (Average) | Hormone receptor status, n (%) | Prior adjuvant or neoadjuvant therapy | Type of study | ORR | M follow-up | mPFS | mOS |
|---|---|---|---|---|---|---|---|---|---|---|
| Baselga J, et al., 2012 | 402 | Herceptin | 54 | Positive-47%, Negative-52.7% | Yes-45.8% | Randomized, double-blind, placebo-controlled, phase 3 trial, | 80.20% | 99.9 | 18.7 | 57.1 |
| Swain SM, et al., 2020 | Unknown-0.2% | No-54.2% | ||||||||
| Dai WF, et al., 2022 | 912 | Herceptin (Roche) | 58.2 | Positive-51.1%, Negative-48.9% | Yes-41%, No-59% | Population-based retrospective comparative | N/A | N/A | N/A | 40.2 |
| Lee YP, et al., 2022 | 228 | N/A | 60 | Positive-54.3%, negative-41.2% unknown-4.3% | Yes-29.3%, No-70.6% | Retrospective | 86.80% | 28.7 | 19.1 | 58.3 |
| Bachelot T, et al., 2019; Miles D, et al., 2021 | 1436 | Herceptin (Roche) | 54 | Positive-64%, Negative-35.6% unknown-0.4% | Yes-27.8%, No-72.2% | Open-label, single-arm phase IIIb | 79% | 68.7 | 20.7 | 65.3 |
| Suleman K, et al., 2021 | 75 | Herceptin (Roche) | 45 | Positive-54.7% negative-53% | Yes-46%, No-54% | Retrospective, observational | 74.70% | 36 | 36 | N/A |
| Ramagopalan SV, et al., 2021 | 546 | Herceptin (Roche) | 59 | Positive-61%, Negative-31% unknown-8.1% | N/A | Population-based retrospective | N/A | 45.3 | N/A | 48.6 |
| Takahashi M, et al., 2021 | 132 | Herceptin (Roche) | 56.5 | Positive-54.5%, Negative-45.5% | Yes-28.8%, No-71.2% | Prospective, phase IV | 83.90% | 46.9 | 22.8 | N/A |
| Xu B, et al., 2020 | 122 | Herceptin (Roche) | 51 | Positive-56.6%, Negative-43.4% | Yes-62.3%, No-37.7% | Prospective, randomized, phase III, double-blind | 79% | 13.7 | 14.5 | N/A |
| Robert NJ, et al., 2017 | 266 | Herceptin (Roche) | 57.3 | Positive-58.3%, Negative-41.7% | N/A | Retrospective, observational | N/A | 16.4 | 16.9 | N/A |
| de Placido S, et al., 2018 | 155 | Herceptin (Roche) | 52 | Positive-70%, Negative-30% | Yes-48%, No-48%, Missing-4% | Retrospective, observational | N/A | 80 | 27.8 | N/A |
| Celik A, et al., 2022 | 117 | Biosimilar trastuzumab, ontruzant (SB3) | 60 | Positive-63%, Negative-37% | Yes-55%, No-45% | Retrospective, observational | N/A | 11.1 | 15.4 | N/A |
| Our study | 61 | Biosimilar trastuzumab ABP 980, | 54 | Positive-78.3%, Negative-27.1% | Yes-55.7%, No-44.3% | Retrospective, observational | 68.90% | 36 | 18 | N/A |
Note: ORR: Objective Response Rate; mPFS: median Progression Free Survival; mOS: median Overall Survival
Table 5: Comparison of the results of studies evaluating trastuzumab, pertuzumab, and taxane (PTH) therapy
In our study, a significant percentage of patients (78.3%) were found to have the ER receptor in cancer cells. Patients with HER2-positive, HR-positive Breast Cancer also prevailed in other studies, but the percentage of those patients was lower (47%-70%), which could translate into a higher Objective Response Rate (ORR). A significant percentage of patients in our study had previously received systemic therapy for Early Breast Cancer (55.7%), while in other studies, except for the Chinese study (Xu B, et al., 2020) and the SB3 evaluation study (Celik A, et al., 2022), this group of patients usually did not exceed 50% (27.8%-48%). These differences in the structure of the patient group also could potentially translate into slightly poorer therapy outcomes, as well as potentially higher rates of cardiotoxic complications (Dai WF, et al., 2022; Lee YP, et al., 2022; Miles D, et al., 2021).
Our follow-ups included only four patients with CNS metastases. These patients had significantly shorter mPFS compared to the rest of the population (Suleman K, et al., 2021; Ramagopalan SV, et al., 2021; Baselga J, et al., 2012).
The median age of the patients evaluated in our study was 54 years. It is close to the median age of most similar studies (CLEOPATRA study-54). Only 24.6% of the patients in our study were 65 or older. Their response to the therapy they received was like that of younger patients, but older patients were more likely to experience more intensive diarrhea and weakness, which is like the observations of other authors (Yankulina O, et al., 2020; Proctor T and Schumacher M, 2016).
Undoubtedly, there were no significant cardiotoxic complications in the group we evaluated. The LVEF reduction below 50% was observed during the therapy in only 2 patients. This occurred in patients below the age of 65 who had previously received anthracyclines as part of their perioperative treatment. Similar cardiotoxicity results have also been reported by other authors (de Placido S, et al., 2018; Bachelot T, et al., 2019; Dai WF, et al., 2022). When it comes to cardiotoxicity, it should be noted that no differences were found between patients’ age groups. Other authors have also observed this complication (Aladwani A, et al., 2021).
Based on our observations, it can be concluded that the use of ABP 980 biosimilar in combination with pertuzumab and docetaxel is an effective and safe therapy for patients with generalized HER2-positive Breast Cancer. Responses to therapy are like the results of therapy with the original trastuzumab in this indication. The safety of the therapy is also extremely high, with toxicities identical to the original regimen. This is a particularly important observation, given the pharmacoeconomic evaluations being conducted around the world and the search for opportunities to reduce the already exceedingly inflated cost of oncology treatment (Trapani D and Curigliano G, 2021; Cheng LJ, et al., 2021; Moriwaki K, et al., 2021). Our study results confirm that biosimilars used in oncology are not only safe and effective but also a less costly option of therapy (Trapani D and Curigliano G, 2021; Liu T, et al., 2022).
This study has clearly had several restrictions, namely the group size was small, there was no control arm of the study, and the follow-up time was short. Nevertheless, it reflects the therapy conditions in daily clinical practice.
Conclusion
Our study is another report confirming the safety and efficacy of ABP 980 biosimilar in combination with pertuzumab and docetaxel in the first-line therapy of HER2-positive MBC patients. What should be emphasized is a low rate of serious complications observed among patients, especially a low rate of cardiotoxicity. The good tolerability of biosimilar therapy in the population of patients over the age of 65 has also been confirmed, which is important given the increasing incidence of Breast Cancer among this age group and the rising cost of oncological therapies.
Acknowledgments
We thank BIOSTAT ® (especially Magdalena Mrozinska) for any help with the statistical calculations.
References
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021; 71(3): 209-249.
[Crossref] [Google Scholar] [Pubmed]
- Cao W, Chen HD, Yu YW, Li N, Chen WQ. Changing profiles of cancer burden worldwide and in China: A secondary analysis of the global cancer statistics 2020. Chin Med J. 2021; 134(07): 783-791.
[Crossref] [Google Scholar] [Pubmed]
- Wolff AC, Hammond ME, Hicks DG, Dowsett M, McShane LM, Allison KH, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol. 2013; 31(31): 3997-4013.
[Crossref] [Google Scholar] [Pubmed]
- Nader-Marta G, Martins-Branco D, de Azambuja E. How we treat patients with metastatic HER2-positive breast cancer. ESMO Open. 2022; 7(1): 100343.
[Crossref] [Google Scholar] [Pubmed]
- Valabrega G, Montemurro F, Aglietta M. Trastuzumab: Mechanism of action, resistance and future perspectives in HER2-overexpressing breast cancer. Ann Oncol. 2007; 18(6): 977-984.
[Crossref] [Google Scholar] [Pubmed]
- Mukohara T. Mechanisms of resistance to anti‐human epidermal growth factor receptor 2 agents in breast cancer. Cancer Sci. 2011; 102(1): 1-8.
[Crossref] [Google Scholar] [Pubmed]
- Slamon DJ, Godolphin W, Jones LA, Holt JA, Wong SG, Keith DE, et al. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science. 1989; 244(4905): 707-712.
[Crossref] [Google Scholar] [Pubmed]
- FDA. Herceptin (trastuzumab) US prescribing information. Genentech. 2017.
- EMA. Herceptin (trastuzumab) summary of product characteristics. European Medicines Agency (EMA). 2017.
- Simmons C, Rayson D, Joy AA, Henning JW, Lemieux J, McArthur H, et al. Current and future landscape of targeted therapy in HER2-positive advanced breast cancer: Redrawing the lines. Ther Adv Med Oncol. 2022; 14: 17588359211066677.
[Crossref] [Google Scholar] [Pubmed]
- Wynn CS, Tang SC. Anti-HER2 therapy in metastatic breast cancer: Many choices and future directions. Cancer Metastasis Rev. 2022: 1-7.
[Crossref] [Google Scholar] [Pubmed]
- Gennari A, André F, Barrios CH, Cortés J, de Azambuja E, de Michele A, et al. ESMO Clinical Practice Guideline for the diagnosis, staging and treatment of patients with metastatic breast cancer. Ann Oncol. 2021; 32(12): 1475-1495.
[Crossref] [Google Scholar] [Pubmed]
- Swain SM, Kim SB, Cortés J, Ro J, Semiglazov V, Campone M, et al. Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA): Overall survival results from a randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. 2013; 14(6): 461-471.
[Crossref] [Google Scholar] [Pubmed]
- Swain SM, Miles D, Kim SB, Im YH, Im SA, Semiglazov V, et al. Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA): End-of-study results from a double-blind, randomised, placebo-controlled, phase 3 study. Lancet Oncol. 2020; 21(4): 519-530.
[Crossref] [Google Scholar] [Pubmed]
- Cuellar S. Integrating trastuzumab biosimilars and HER2-directed therapies into HER2-positive breast cancer management. Am J Manag Care. 2020; 26(2): 32-40.
[Crossref] [Google Scholar] [Pubmed]
- Blackwell K, Gligorov J, Jacobs I, Twelves C. The global need for a trastuzumab biosimilar for patients with HER2-positive breast cancer. Clin Breast Cancer. 2018; 18(2): 95-113.
[Crossref] [Google Scholar] [Pubmed]
- Cherny N, Sullivan R, Torode J, Saar M, Eniu A. ESMO European Consortium Study on the availability, out-of-pocket costs and accessibility of antineoplastic medicines in Europe. Ann Oncol. 2016; 27(8): 1423-1443.
[Crossref] [Google Scholar] [Pubmed]
- Lammers P, Criscitiello C, Curigliano G, Jacobs I. Barriers to the use of trastuzumab for HER2+ breast cancer and the potential impact of biosimilars: A physician survey in the United States and emerging markets. Pharmaceuticals. 2014; 7(9): 943-953.
[Crossref] [Google Scholar] [Pubmed]
- EMA. Committee for Medicinal Products for Human Use (CHMP): Guideline on similar biological medicinal products. European Medicines Agency (EMA). 2017.
- FDA. Scientific considerations in demonstrating biosimilarity to a reference product. US Food and Drug Administration. 2017.
- Dhillon S. ABP 980: A trastuzumab biosimilar. BioDrugs. 2018; 32(5): 511-514.
[Crossref] [Google Scholar] [Pubmed]
- Hutterer KM, Polozova A, Kuhns S, McBride HJ, Cao X, Liu J. Assessing analytical and functional similarity of proposed Amgen biosimilar ABP 980 to trastuzumab. BioDrugs. 2019; 33(3): 321-333.
[Crossref] [Google Scholar] [Pubmed]
- NCI. Common Terminology Criteria for Adverse Events (CTCAE). National Cancer Institute (NCI). 2017.
- de Placido S, Giuliano M, Schettini F, von Arx C, Buono G, Riccardi F, et al. Human epidermal growth factor receptor 2 dual blockade with trastuzumab and pertuzumab in real life: Italian clinical practice versus the CLEOPATRA trial results. Breast. 2018; 38: 86-91.
[Crossref] [Google Scholar] [Pubmed]
- Robert NJ, Goertz HP, Chopra P, Jiao X, Yoo B, Patt D, et al. HER2-positive metastatic breast cancer patients receiving pertuzumab in a community oncology practice setting: Treatment patterns and outcomes. Drugs Real World Outcomes. 2017; 4(1): 1-7.
[Crossref] [Google Scholar] [Pubmed]
- Bachelot T, Ciruelos E, Schneeweiss A, Puglisi F, Peretz-Yablonski T, Bondarenko I, et al. Preliminary safety and efficacy of first-line pertuzumab combined with trastuzumab and taxane therapy for HER2-positive locally recurrent or metastatic breast cancer (PERUSE). Ann Oncol. 2019; 30(5): 766-773.
[Crossref] [Google Scholar] [Pubmed]
- Takahashi M, Ohtani S, Nagai SE, Takashima S, Yamaguchi M, Tsuneizumi M, et al. The efficacy and safety of pertuzumab plus trastuzumab and docetaxel as a first-line therapy in Japanese patients with inoperable or recurrent HER2-positive breast cancer: The COMACHI study. Breast Cancer Res Treat. 2021; 185(1): 125-134.
[Crossref] [Google Scholar] [Pubmed]
- Xu B, Li W, Zhang Q, Shao Z, Li Q, Wang X, et al. Pertuzumab, trastuzumab, and docetaxel for Chinese patients with previously untreated HER2-positive locally recurrent or metastatic breast cancer (PUFFIN): A phase III, randomized, double-blind, placebo-controlled study. Breast Cancer Res Treat. 2020; 182(3): 689-697.
[Crossref] [Google Scholar] [Pubmed]
- Celik A, Berg T, Nielsen LB, Jensen MB, Ejlertsen B, Knoop A, et al. First-line Treatment of HER2-positive metastatic breast cancer with dual blockade including biosimilar trastuzumab (SB3): Population-based real-world data from the DBCG. Breast Cancer. 2022; 16: 11782234221086992.
[Crossref] [Google Scholar] [Pubmed]
- Dai WF, Beca JM, Nagamuthu C, Liu N, de Oliveira C, Earle CC, et al. Comparative effectiveness and safety of pertuzumab and trastuzumab plus chemotherapy vs. trastuzumab plus chemotherapy for treatment of metastatic breast cancer. JAMA Netw Open. 2022; 5(2): 2145460.
[Crossref] [Google Scholar] [Pubmed]
- Lee YP, Lee MS, Kim H, Kim JY, Ahn JS, Im YH, et al. Real-world evidence of trastuzumab, pertuzumab, and docetaxel combination as a first-line treatment for Korean patients with HER2-positive metastatic breast cancer. Cancer Res Treat. 2022.
[Crossref] [Google Scholar] [Pubmed]
- Miles D, Ciruelos E, Schneeweiss A, Puglisi F, Peretz-Yablonski T, Campone M, et al. Final results from the PERUSE study of first-line pertuzumab plus trastuzumab plus a taxane for HER2-positive locally recurrent or metastatic breast cancer, with a multivariable approach to guide prognostication. Ann Oncol. 2021; 32(10): 1245-1255.
[Crossref] [Google Scholar] [Pubmed]
- Suleman K, Mushtaq AH, Haque E, Badran AA, Ajarim D, Elashwah AM, et al. Trastuzumab, pertuzumab, and docetaxel as the first line for HER-2-Positive metastatic breast cancer among Arabs. Breast Care. 2021; 16(1): 59-65.
[Crossref] [Google Scholar] [Pubmed]
- Ramagopalan SV, Pisoni R, Rathore LS, Ray J, Sammon C. Association of pertuzumab, trastuzumab, and docetaxel combination therapy with overall survival in patients with metastatic breast cancer. JAMA Netw Open. 2021; 4(1): 2027764.
[Crossref] [Google Scholar] [Pubmed]
- Baselga J, Cortés J, Kim SB, Im SA, Hegg R, Im YH, et al. Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N Engl J Med. 2012; 366(2): 109-119.
[Crossref] [Google Scholar] [Pubmed]
- Yankulina O, Zullo AR, Cabral SE, Huynh JP, Hutchinson JA, Lopresti ML, et al. Pertuzumab-associated diarrhea in HER2/neu-positive breast cancer patients: A comparison of trials to actual practice. J Oncol Pharm Pract. 2020; 26(4): 912-917.
[Crossref] [Google Scholar] [Pubmed]
- Proctor T, Schumacher M. Analysing adverse events by time‐to‐event models: The CLEOPATRA study. Pharm Stat. 2016; 15(4): 306-314.
[Crossref] [Google Scholar] [Pubmed]
- Aladwani A, Mullen A, Alrashidi M, Alfarisi O, Alterkait F, Kumar A, et al . Comparing trastuzumab-related cardiotoxicity between elderly and younger patients with breast cancer: A prospective cohort study. Eur Rev Med Pharmacol Sci. 2021; 25(24): 7643-7653.
[Crossref] [Google Scholar] [Pubmed]
- Trapani D, Curigliano G. The global landscape of drug development of trastuzumab biosimilars. J Cancer Policy. 2021; 28: 100273.
[Crossref] [Google Scholar] [Pubmed]
- Cheng LJ, Loke L, Lim EH, Pearce F, Aziz MI, Ng K. Cost-effectiveness of pertuzumab and trastuzumab biosimilar combination therapy as initial treatment for HER2-positive metastatic breast cancer in Singapore. Expert Rev Pharmacoecon Outcomes Res. 2021; 21(3): 449-456.
[Crossref] [Google Scholar] [Pubmed]
- Moriwaki K, Uechi S, Fujiwara T, Hagino Y, Shimozuma K. Economic evaluation of first-line pertuzumab therapy in patients with HER2-positive metastatic breast cancer in Japan. Pharmacoecon Open. 2021; 5(3): 437-447.
[Crossref] [Google Scholar] [Pubmed]
- Liu T, Liu D, Jin Y, Dong M. Trastuzumab biosimilars vs. trastuzumab originator in the treatment of HER2-positive breast cancer: A systematic review and network meta-analysis. Immunopharmacol Immunotoxicol. 2022: 1-7.
[Crossref] [Google Scholar] [Pubmed]
Author Info
Karolina Miacz, Agnieszka Jagiello-Gruszfeld*, Michal Kunkiel, Izabela Lemanska, Anna Gorniak, Zbigniew Nowecki and Anna NiwinskaCitation: Miacz K: The Effectiveness and Safety of Trastuzumab Biosimilar ABP 980 plus Pertuzumab and Docetaxel in the Therapy of HER-2 Positive Metastatic Breast Cancer: Real World Experiences from the National Research Institute of Oncology in Warsaw
Received: 20-Oct-2022 Accepted: 03-Nov-2022 Published: 10-Nov-2022, DOI: 10.31858/0975-8453.13.11.785-793
Copyright: This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
ARTICLE TOOLS
- Dental Development between Assisted Reproductive Therapy (Art) and Natural Conceived Children: A Comparative Pilot Study Norzaiti Mohd Kenali, Naimah Hasanah Mohd Fathil, Norbasyirah Bohari, Ahmad Faisal Ismail, Roszaman Ramli SRP. 2020; 11(1): 01-06 » doi: 10.5530/srp.2020.1.01
- Psychometric properties of the World Health Organization Quality of life instrument, short form: Validity in the Vietnamese healthcare context Trung Quang Vo*, Bao Tran Thuy Tran, Ngan Thuy Nguyen, Tram ThiHuyen Nguyen, Thuy Phan Chung Tran SRP. 2020; 11(1): 14-22 » doi: 10.5530/srp.2019.1.3
- A Review of Pharmacoeconomics: the key to “Healthcare for All” Hasamnis AA, Patil SS, Shaik Imam, Narendiran K SRP. 2019; 10(1): s40-s42 » doi: 10.5530/srp.2019.1s.21
- Deuterium Depleted Water as an Adjuvant in Treatment of Cancer Anton Syroeshkin, Olga Levitskaya, Elena Uspenskaya, Tatiana Pleteneva, Daria Romaykina, Daria Ermakova SRP. 2019; 10(1): 112-117 » doi: 10.5530/srp.2019.1.19
- Dental Development between Assisted Reproductive Therapy (Art) and Natural Conceived Children: A Comparative Pilot Study Norzaiti Mohd Kenali, Naimah Hasanah Mohd Fathil, Norbasyirah Bohari, Ahmad Faisal Ismail, Roszaman Ramli SRP. 2020; 11(1): 01-06 » doi: 10.5530/srp.2020.1.01
- Manilkara zapota (L.) Royen Fruit Peel: A Phytochemical and Pharmacological Review Karle Pravin P, Dhawale Shashikant C SRP. 2019; 10(1): 11-14 » doi: 0.5530/srp.2019.1.2
- Pharmacognostic and Phytopharmacological Overview on Bombax ceiba Pankaj Haribhau Chaudhary, Mukund Ganeshrao Tawar SRP. 2019; 10(1): 20-25 » doi: 10.5530/srp.2019.1.4
- A Review of Pharmacoeconomics: the key to “Healthcare for All” Hasamnis AA, Patil SS, Shaik Imam, Narendiran K SRP. 2019; 10(1): s40-s42 » doi: 10.5530/srp.2019.1s.21
- A Prospective Review on Phyto-Pharmacological Aspects of Andrographis paniculata Govindraj Akilandeswari, Arumugam Vijaya Anand, Palanisamy Sampathkumar, Puthamohan Vinayaga Moorthi, Basavaraju Preethi SRP. 2019; 10(1): 15-19 » doi: 10.5530/srp.2019.1.3