Research Article - (2022) Volume 13, Issue 2
Abstract
The immunotherapy’s, as a modern therapeutic approach, get attention because of their potential to treat a large number of different medical disorders. Immunomodulation effects of low titres (10 ha/ml) of NDV (Newcastle Disease Virus) ZG1999HDS or La Sota were tested on TLT (Human macrophage cell line) bound to PBMC (Peripheral Blood Mononuclear Cells). During the immunomodulation, the amount of NO, H2O2, lysozyme and induced antibacterial activity against gram-positive bacteria (Staphylococcus aureus, Methicillin-Resistant Staphylococcus aureus (MRSA), Streptococcus pyogenes, Streptococcus agalactiae and Streptococcus mutants) and against gram-negative bacteria (Escherichia coli, Pseudomonas aeruginosa, Proteus mirabilis and Acinetobacter baumanii) were analysed. In addition, the secretion of cytokines Interleukin 1 alpha (IL-1 α), IL-2, IL-4, Granulocyte Macrophage Colony-Stimulating Factor (GM-CSF), Tumor necrosis factor (TNF-α), Interferons (IFN-α) and IFN-γ was evaluated. First, the TLT cells are activated through the NDV ZG1999HDS or La Sota binding, followed by the NO “burst” and H2O2 and lysozyme level increase. Then, after the binding to the TLT cells and interaction with the PBMCs, the decrease of GM-CSF and an increase of TNF-α and IFN-γ were found. Simultaneously, the decrease of pro-inflammatory cytokine IFN-α, and the differential increase of IL-1 α, IL-2 and IL-4 were observed. During the induction of the antibacterial response, against gram-positive bacteria (Staphylococcus aureus, MRSA, Streptococcus pyogenes, Streptococcus agalactiae and Streptococcus mutants) the effect was one third higher with NDV ZG1999HDS compared to La Sota. Antibacterial response against gram-negative bacteria (Escherichia coli, Pseudomonas aeruginosa, Proteus mirabilis and Acinetobacter baumanii) was not so clear. In general, NDV ZG1999HDS or La Sota activated TLT cells, further bound to PBMC; the ZG1999HDS is stronger immunomodulator than La Sota.
Keywords
Newcastle Disease Virus ZG1999HDS, Newcastle Disease Virus La Sota, Immunomodulation, TLT (Human macrophage cell line) with PBMCs (Peripheral Blood Mononuclear Cells), Cytokines, Lysozyme, Antibacterial activity, Gram-positive bacteria, Gram-negative bacteria
Introduction
The substances interacting with the immune system aiming to increase or decrease the host response are named immunomodulators (Ortuño-Sahagún D, et al., 2017; Stanilova SA, et al., 2005; Utoh-Nedosa AU, et al., 2009). They are also known as biologic response modifiers or immunoregulators, and function as a drug leading mostly to a non-specific stimulation of immunological defence mechanisms of the body (Tzianabos AO, 2000; Ooi VE and Liu F, 2000). To enhance immune system, agents such as chemicals, proteins and/or viruses are used (Kobayashi S, et al., 2008). Among viruses, the Newcastle Disease Virus (NDV) was found to have pleiotropic immunomodulatory properties in addition to good cell-binding and selective proliferation in replicating cells. This virus also has the ability to introduce T-cell co-stimulatory activity and induce cytokines such as IFN-α, IFN- β and TNF-α which can affect T-cell recruitment and activation (Schirrmacher V, et al., 1998; Schirrmacher V, et al., 1999; Schirrmacher V, et al., 2000). The cellular cytotoxicity of PBMC was enhanced significantly after the co-incubation of NDV with effector cells (Zorn U, et al., 1994; Burke S, et al., 2020). The study found the NK (Natural Killer) cells to be the main mediator of the cell lysis. The increased cytotoxicity also correlates with the induction of TNF-α and with the reduced synthesis of IFN-α in PBMC by NDV. The ZG1999HDS NDV strain that was recently isolated was patented and genetically characterized (Mazija H, et al., 2011; Mazija H, 2011; Nedeljković G, 2011). Additionally, the ZG1999HDS NDV strain was deposited in “Collection National de Cultures de Microorganisms (CNCM)” in 2013 and in the “Gene Bank” in 2014 (Collection National de Cultures de Microorganism, 2013). The ZG1999HDS NDV strain was only isolated from lung tissue of broiler chickens suffering from a respiratory disease and was not present in the brain tissue, as it was described by Biđin M and Mazija H, 2009. Due to its lentogenic properties, it caused death of 74.6% of chickens because of the virus tropism for the respiratory system. It belongs to genotype II of class II NDV that is closely related to the La Sota and Hitcher NDV strains. In the same group, there are the following strains: La Sota, Ulster and Queensland. Cytolytic characteristics of the ZG1999HDS NDV strain were investigated in vitro on tumour cell cultures and in vivo on mice and compared to the impact of the La Sota NDV strain. The ZG1999HDS NDV strain is a relatively strong inducer of human type I IFN, more precisely Human Interferon (HuIFN)-α N3, in the PBMCs from human buffy coats. 100 HA of the ZG1999HDS NDV strain can induce 483 ± 45 pg/ml of the HuIFN-α N3. The Reversed-Phase High-Performance Liquid chromatography (RP- HPLC) profile of the HuIFN-α N3 show: subtypes α α 2, α 1A and α 2b (Ginting TE, et al., 2019). For the biological activity of the NDV induced HuIFN-α N3, the relative ratio between α 1 and α 2 subtypes is important (Antonelli G, 2008).
The presented experiments were aimed to analyse the immunomodulatory activity in vitro of the ZG1999HDS NDV strain in comparison to the La Sota NDV strain.
Material and Methods
Viruses used in the experiments
The ZG1999HDS NDV strain virus was obtained from The Croatian Institute for Experimental and Translation Oncology (CIETO), Koledinečka 03, 10040 Zagreb, Croatia. The La Sota NDV strain was obtained from different commercial sources. Both were multiplied in SPF (Specific Pathogen Free) chicken embryos, and concentrated by lyophilisation. The Median Embryo Infectious Dose (EID50) that was determined in the SPF chicken embryos was 2.0 × 107 for NDV ZG1999HDS and 2.5 × 107 for NDV La Sota.
Tissue culture medium and cells
Eagle's medium with high content of glucose, L-glutamine, 25 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) and antibiotics (Penicillin, Streptomycin and Gentamycin) and Trypsin solution were prepared in the CIETO laboratory. FBS (Foetal bovine serum) was obtained from Euro clone (Pero, Italy). Human TLT (macrophage) cell line was obtained from Lidija Gradišnik from the Institute of Biomedical Sciences, Medical Faculty University of Maribor, Slovenia. The TLT cells were cultivated in Eagle's medium with high content of glucose, L-glutamine, 25mM HEPES and antibiotics with addition of 10% of FBS.
Isolation of PBMC (Peripheral Blood Mononuclear Cells) from human buffy coats
The buffy coats were combined and centrifuged at 1700 RPM for 20 min at 4°C. The supernatant containing plasma and part of the leukocytes were resuspended in the PBS containing 1% glucose. Nine parts of 0.83% NH4Cl (ammonium chloride) were added to the sediment containing erythrocytes, lymphocytes, macrophages and granulocytes. The erythrocyte lysis was performed at 4°C for 15-20 min. Next, black chocolate coloured cell suspension was centrifuged at 2,500 RPM for 20 minutes at 4°C. The supernatant was removed and the white cell sediments were resuspended in the PBS (Phosphate Buffer Saline) containing 1% glucose. Both supernatants were combined and white cells were sediment by centrifugation at 2500 RPM for 20 min at 4°C. The percentage of viable cells was determined by Trypan blue staining.
Bacterial species used in the experiments
Bacterial species selected for the study were obtained from the “Microbecollection” at the Institute for Microbiology and Immunology, Medical Faculty, University of Ljubljana, Slovenia. Gram-positive bacteria were: Staphylococcus aureus, MRSA (Metycilline-Resistant Staphylococcus aureus), Micrococcus luteus,Streptococcus pyogenes, Streptococcus agalactiae and Streptococcus mutants. Gram-negative bacteria were: Escherichia coli, Pseudomonas aeruginosa, Proteus mirabilis and Acinetobacter baumanii.
Cell treatment
In the 10.0 ml flat bottom glass vials with rubber stoppers, the Human macrophage (TLT) cell line was cultivated in the Eagle's medium with 10% FBS. After reaching confluence, the supernatant was discarded and 2.0 ml of PBMC suspension (106 cells/ml) was added. After two hours, 2.5 ml of Eagle’s medium with 2.0% FBS was added. To exert the immunomodulation and the antimicrobial activity, 0.5 ml of 10.0 Haemagglutinin units (HAU)/ml of the ZG1999HDS or La Sota NDV strains were added and vials were stored at 37°C for 24 and 48 hours (Figure 1). Each treatment was performed in triplicate and repeated two to three times. After the indicated time (24 or 48 hours at 37°C) the vials were centrifuged at 1700 RPM/20 minutes, and clear supernatants were collected, filtered through 0.2 μm filters and stored before the analyses at -20°C.
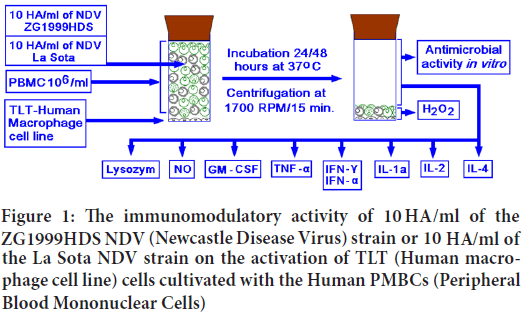
Figure 1: The immunomodulatory activity of 10 HA/ml of the ZG1999HDS NDV (Newcastle Disease Virus) strain or 10 HA/ml of the La Sota NDV strain on the activation of TLT (Human macrophage cell line) cells cultivated with the Human PMBCs (Peripheral Blood Mononuclear Cells)
The H2O2 (hydrogen peroxide) determination
Quantity (μM/ml) of H2O2 produced by the cultivated cells (TLT with PBMC) after the treatment or in untreated control was determined according to the method developed by Orsi RO, et al., 2000. In brief: 500.0 all of the Phenol red solution (50 ml) containing 140.0 mM NaCl, 10.0 mM K2HPO4, 5.5 mM dextrose and 8.5 U/ml of Horseradish peroxidase was added to a fresh sediment of cells (TLT with PBMC). After four hours of incubation at 37°C, 100.0 μl of 1.0 M NaOH was added, and solution was diluted 10 times with 4.5 ml of saline. The optical density of the solution was measured at 620.0 nm. As the control, 10.0 mM of H2O2 was used.
Lysozyme determination
The amount of lysozyme in the cells’ (TLT with PMBC) supernatant was determined by the method developed by Nash JA, et al., 2006. In brief: one thousand CFU (Colony Forming Units) of Streptococcus pyogenes in 200.0 μl of 10.0 mM PBS (Potassium phosphate buffer) (pH 7.4) and 200.0 μl of sample or 1.0, 10.0 and 100.0 μg/ml of lysozyme were added to the 1.6 ml of MH (Mueller Hinton) broth (pH 6.5) and incubated overnight at 37°C. On the next day, the Optical Density (OD) was measured at 595 nm. The amount of lysozyme (μM/ml) was calculated in relation to the bacterial OD after 24 hours.
The NO (Nitrite) assay
The concentration of stable NO (Nitrite), that is the end product of the nitric oxide present in the supernatant of treated or untreated human macrophages (TLT with PMBC) was measured by the method based on the Griess reaction, which was described by More P and Pai K, 2011. In brief, 50 μl of the cell supernatant was incubated with the equal volume of Griess reagent composed of 1% sulphanilamide in 2,5% H3PO4 and 0,1% NEDD (Naphytyl-ethylene-diamine-dihydrochloride) in distilled water. Both solutions were mixed in the 1:1 ratio at room temperature for 30 minutes. The absorbance at 550 nm was measured in a microtitre plate reader. The standard curve for nitrite was prepared using 10-100 μM sodium nitrites in distilled water.
HuIFN-α N3 monoclonal ELISA (Enzyme-Linked Immunosorbent Assay)
The quantity of induced HuIFN-α N3 (pg/ml) was determined by Human IFN ELISA kit "Platinum ELISA" by eBioscience (Vienna, Austria). In the assay, the international HuIFN-α N3 standard was used (Human IFN-α Platinum ELISA (BMS 216/BMS 216 TEN), Affymetrix, eBioscience, USA). The assay was performed according to the manufacturer’s instructions, with the final reading by the ELISA reader at 620 nm and calculating the pg-s of HuIFN-α N3/ml.
HuIFN-α N3 RP-HPLC (Reverse Phase High Performance Liquid Chromatography) analysis
The HuIFN-α N3 subtype composition was analysed by RP-HPLC (Reverse Phase High Performance Liquid Chromatography). Different HuIFN-α samples (natural or recombinant, approximately one million antiviral units/ ml) of 20 to 40 μl were applied to the Phenomenex, Aeris peptide column 3.6 μm XB-C18, 250 × 4.6 mm and eluted with the linear gradient of solvent A=water+0.1% TFA and solvent C=acetonitrile+0.1% TFA for 20 minutes with a flow rate of 0.8 ml/min. and pressure of 139-140 bar. The course of RP-HPLC chromatography of different IFN samples is shown in Table 1. The temperature of the column was 40°C. The absorbance was monitored at 214 and 280 nm. HuIFN-α interferon species in different IFN compositions were separated according to their relative hydrophobic properties using RP-HPLC as it was described by Punainen S, et al., 1999.
| Step | Time (min) | Solvent A (%) | Solvent C (%) |
|---|---|---|---|
| 0 | 0 | 91 | 9 |
| 1 | 3 | 80 | 20 |
| 2 | 6 | 50 | 50 |
| 3 | 12 | 50 | 50 |
| 4 | 15 | 91 | 9 |
| 5 | 20 | 91 | 9 |
Table 1: The course of Reversed-Phase High-Performance Liquid (RP- HPLC) chromatography of different Interferons (IFN) samples
HuIFN- γ mini ELISA development kit (peprotech)
The measurement of the amount of HuIFN- γ (pg/ml) in the cell (TLT with PBMC) supernatant was performed according to the manufacturer instructions. The capture antibodies (1.0 μg/ml) were bound to the Nunc Maxisorp plates, and 300.0 μl of the blocking buffer was added.
Standard/sample: Standard (HuIFN- γ) was diluted from 300.0 pg/ml to zero in diluents. Immediately, 100.0 μl of standard or sample was added to each well in triplicate and the plates were incubated for six hours at 37°C.
Detection: After the plates were washed, the detection antibodies were diluted in diluent to a concentration of 1.0 μg/ml and, 100.0 μl/well was added and incubated at room temperature for two hours.
Avidin-Horseradish Peroxidase (HRP) conjugate: After washing the plates, 5.5μl of avidin-Horseradish Peroxidase (HRP) Conjugate 1:2000 was diluted in diluent for total volume of 11.0 ml, 100 μl/well was added. The plates were incubated for one hour at room temperature.
ABTS liquid substrate: The plates were washed and aspirated two times. 100.0 μl of substrate solution was added to each well on an empty plate, and colour development was monitored. Afterwards, 10.0 μl of 1.0% Sodium Dodecyl Sulfate (SDS) was added to each well and the plate was measured using the ELISA reader at 405 nm with the wavelength correction at 650 nm. The reliable standard curves are obtained when either OD readings does not exceed 0.2 units for the zero standard concentration, or 1.2 units for the highest standard.
GM-CSF mini ELISA development kit (peprotech)
The measurement of the amount of GM-CSF (pg/ml) in the cell (TLT with PBMC) supernatant was performed in the same way as HuIFN- γ, using the GM-CSF specific capture and detection antibodies, as well as specific avidin-HRP (Horse-radish Peroxidase) conjugate.
TNF-α mini ELISA development kit (peprotech)
The measurement of the amount of TNF-α (pg/ml) in the cell (TLT with PBMC) supernatant was performed in the same way as HuIFN- γ, using the TNF-α specific capture and detection antibodies, as well as specific avidin-HRP (Horse-radish Peroxidase) conjugate.
IL-1α mini ELISA development kit (peprotech)
The measurement of the amount of IL-1α (pg/ml) in the cell (TLT with PBMC) supernatant was performed in the same way as HuIFN- γ, using the IL-1α specific capture and detection antibodies, as well as specific avidin-HRP (Horse-radish Peroxidase) conjugate.
IL-2 mini ELISA development kit (peprotech)
The measurement of the amount of IL-2 (pg/ml) in the cell (TLT with PBMC) supernatant was performed in the same way as HuIFN- γ, using the IL-2 specific capture and detection antibodies, as well as specific avidin-HRP (Horse-radish Peroxidase) conjugate.
IL-4 mini ELISA development kit (peprotech)
The measurement of the amount of IL-4 (pg/ml) in the cell (TLT with PBMC) supernatant was performed in the same way as HuIFN- γ, using the IL-4 specific capture and detection antibodies, as well as specific avidin-HRP (Horse-radish Peroxidase) conjugate.
Antibacterial activity
The antibacterial (gram-positive and gram-negative bacteria) screening was carried out by agar diffusion method described by Lino A and Deogracious O, 2006. According to their protocols, the suspensions of different bacteria in saline with McFarland 0, 5 were swabbed over the surface of the MH (Mueller Hinton) agar plate, using a sterile cotton swab. Wells of 6.0 mm diameter were bored in the medium with the help of a sterile cork-borer with a 6.0 mm diameter. 70 μl of different samples were added to the wells using a micropipette. On a separate plate 70 μl of the control antibiotic in a 10% concentration was added. The plates were left sitting with the lid closed until the extracts diffused into the medium, and incubated at 37°C for 72 hours. The zone of the inhibition was measured in millimetres using a scale.
Statistics
All the treatments were performed in triplicate and repeated three to four times. The average values ± SD (Standard Deviation) were recorded. The obtained data were analysed by the Two-tailed unpaired Student’s t test for the groups comparing NDV1999HDS to La Sota group. The Stat graphics stratus online statistics software (www.statgraphicsstratus.com) from Stat point Technologies Inc., USA was used. The differences with the p values *=< 0.05and **=<0.01 were statistically different.
Results
Determining NO (Nitric oxide), H2O2 (Hydrogen peroxide) and lysozyme
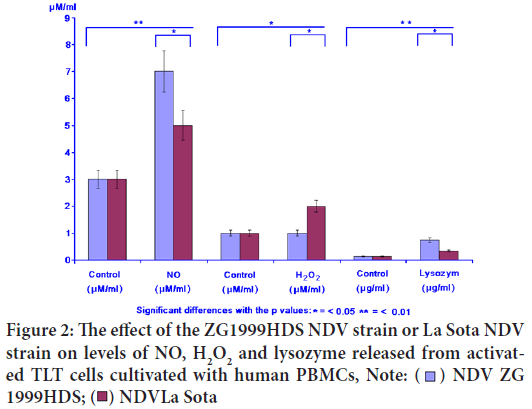
In the case of NO, the releasing enhancement was found (7.12 μM/ml) when NDV ZG1999HDS was used. The levels of H2O2 and lysozyme were increased when the same virus was used (1.82 μM and 0.748 μg). The lysozyme is the indicator of the antimicrobial activity induction. The results are presented in Figure 2 and Table 2.
| Virus | NO (μM/ml) | H2O2 (μM/ml) | Lysozyme (μM/ml) | |||
|---|---|---|---|---|---|---|
| Control | NO | Control | H2O2 | Control | Lysozyme | |
| NDV ZG1999HDS | 3 ± 0.66 | 7 ± 1,12 | 1 ± 0.17 | 1 ± 0.082 | 0.14 ± 0.05 | 0.74 ± 0.08 |
| NDV La Sota | 3 ± 0.66 | 5 ± 0.86 | 1 ± 0.17 | 2 ± 0.065 | 0.14 ± 0.05 | 0.34 ± 0.05 |
Table 2: The effect of ZG1999HDS NDV (Newcastle Disease Virus) strain or La Sota NDV strain on the NO, H2O2 and lysozyme levels
Figure 2: The effect of the ZG1999HDS NDV strain or La Sota NDV
strain on levels of NO, H2O2 and lysozyme released from activated TLT cells cultivated with human PBMCs, Note: 
Activity of GM-CSF and TNF-α
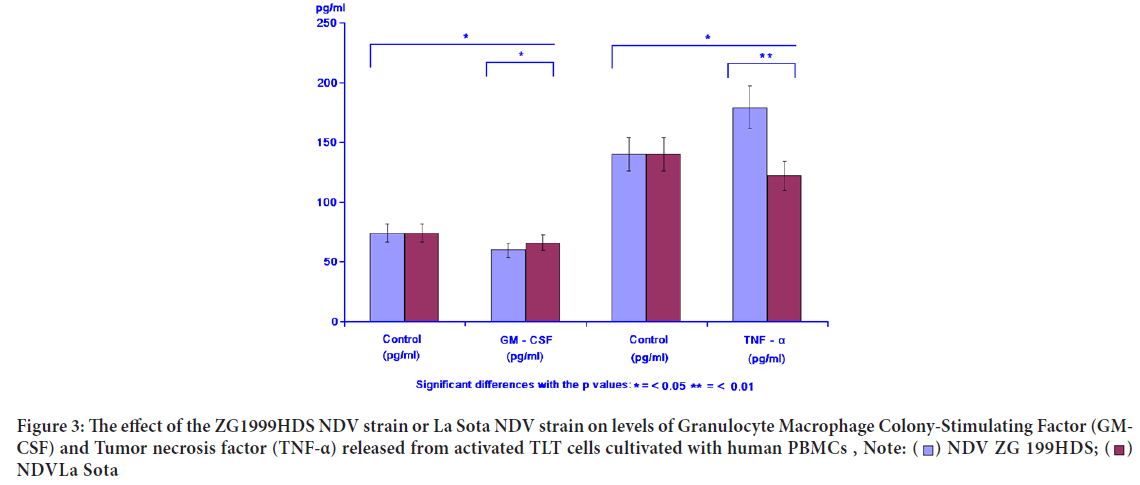
GM-CSF as proliferators of different tumour cells in vitro and in vivo was inhibited by either NDV ZG1999HDS or NDV La Sota. The opponent TNF-α was increased when the NDV ZG1999HDS was used. In case of NDV La Sota, the level of TNF-α was decreased. Table 3 and Figure 3 show the data on GM-CSF and TNF-α analysis.
| Virus | GM-CSF (pg/ml) | TNF-α (pg/ml) | ||
|---|---|---|---|---|
| Control | GM-CSF | Control | TNF-α | |
| NDV ZG1999HDS | 74 ± 4.21 | 63 ± 4.10 | 140 ± 22 | 179 ± 34 |
| NDV La Sota | 74 ± 4.21 | 66 ± 4.25 | 140 ± 22 | 122 ± 18 |
Table 3: The effect of ZG1999HDS NDV strain or La Sota NDV strain on levels of Granulocyte Macrophage Colony-Stimulating Factor (GM-CSF) and Tumor necrosis factor (TNF-α)
Figure 3: The effect of the ZG1999HDS NDV strain or La Sota NDV strain on levels of Granulocyte Macrophage Colony-Stimulating Factor (GM-
CSF) and Tumor necrosis factor (TNF-α) released from activated TLT cells cultivated with human PBMCs , 
Activity of IFN- γ and IFN-α
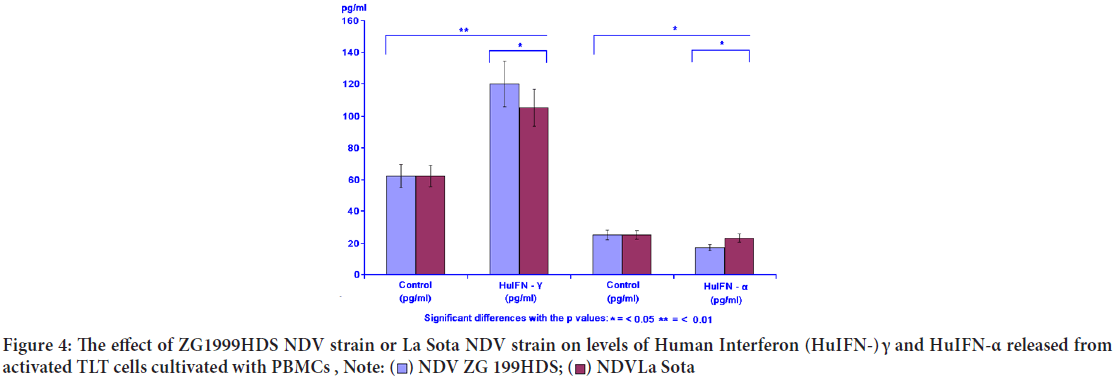
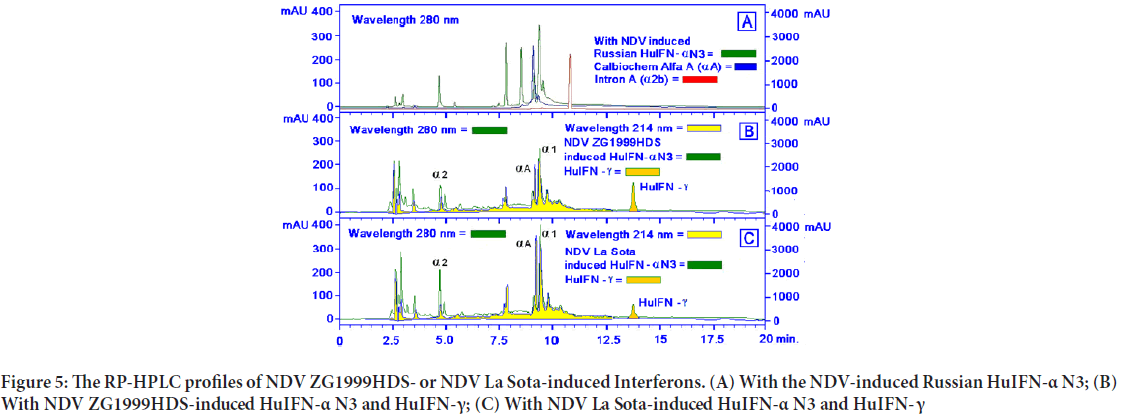
When the levels of two different IFNs were studied, the following data were obtained: Increase of IFN- γ and decrease of pro-inflammatory IFN-α. NDV ZG1999HDS caused a higher increase of HuIFN- γ and a higher decrease of HuIFN-α. Data on levels of IFN-α and IFN- γ are shown in Table 4 and Figure 4. The RP-HPLC profile of NDV ZG1999HDS-induced interferons show a higher decrease of α 2, α A and α 1 and total absence of α 2b along with an increase in HuIFN- γ compared to the NDV La Sota-in duced interferons (Figure 5).
| Virus | HuIFN- γ (pg/ml) | HuIFN- α (pg/ml) | ||
|---|---|---|---|---|
| Control | HuIFN- γ | Control | HuIFN- α | |
| NDV ZG1999HDS | 62 ± 3.10 | 120 ± 9.88 | 25 ± 1.39 | 19 ± 1.22 |
| NDV La Sota | 62 ± 3.10 | 105 ± 7.35 | 25 ± 1.39 | 23 ± 1.98 |
Table 4: The effect of ZG1999HDS NDV strain or La Sota NDV strain on the level of Human Interferon (HuIFN-) γ and HuIFN-α
Figure 4: The effect of ZG1999HDS NDV strain or La Sota NDV strain on levels of Human Interferon (HuIFN-) γ and HuIFN-α released from activated TLT cells cultivated with PBMCs ,
Figure 5: The RP-HPLC profiles of NDV ZG1999HDS- or NDV La Sota-induced Interferons. (A) With the NDV-induced Russian HuIFN-α N3; (B) With NDV ZG1999HDS-induced HuIFN-α N3 and HuIFN-γ; (C) With NDV La Sota-induced HuIFN-α N3 and HuIFN- γ
Activity of IL-1α, IL-2 and IL-4
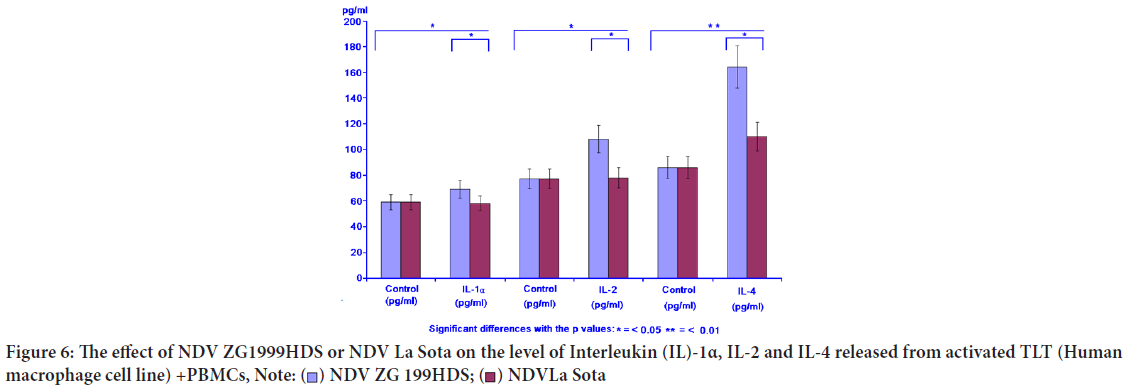
The level of IL-1α, the cytokine inducing the "Programmed cell death" (apoptosis) in various tumour cells was increased, similar to IL-2 and IL-4, when NDV (ZG1999HDS strain) was used (Table 5, Figure 6).
| Virus | IL-1 α (pg/ml) | IL-2 (pg/ml) | IL-4 (pg/ml) | |||
|---|---|---|---|---|---|---|
| Control | IL-1α | Control | IL-2 | Control | IL-4 | |
| NDV ZG1999HDS | 59 ± 6.33 | 68 ± 2.11 | 77 ± 4.17 | 108 ± 8.42 | 86 ± 9.13 | 164 ± 6.11 |
| NDV La Sota | 59 ± 6.33 | 62 ± 1.86 | 77 ± 4.17 | 79 ± 6.15 | 86 ± 9.13 | 110 ± 5.14 |
Table 5: The effect of ZG1999HDS NDV strain or La Sota NDV strain on the level of Interleukin (IL-)1α, IL-2 and IL-4
Figure 6: The effect of NDV ZG1999HDS or NDV La Sota on the level of Interleukin (IL)-1α, IL-2 and IL-4 released from activated TLT (Human
macrophage cell line) +PBMCs, 
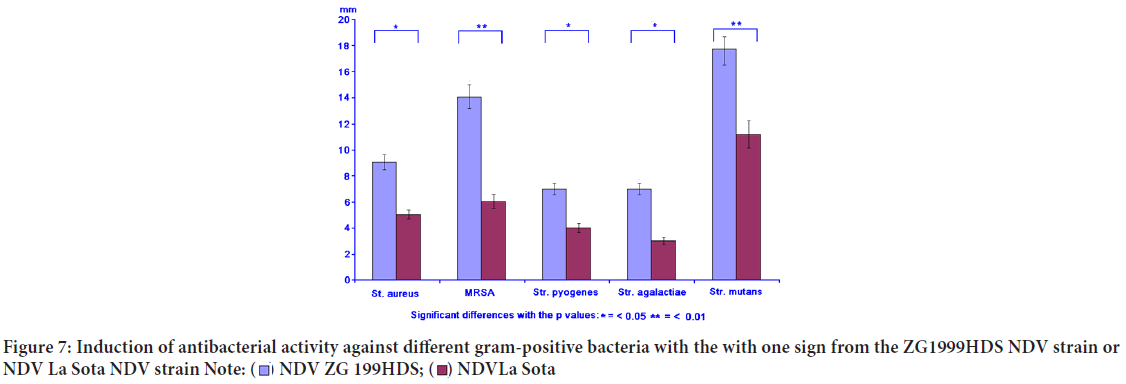
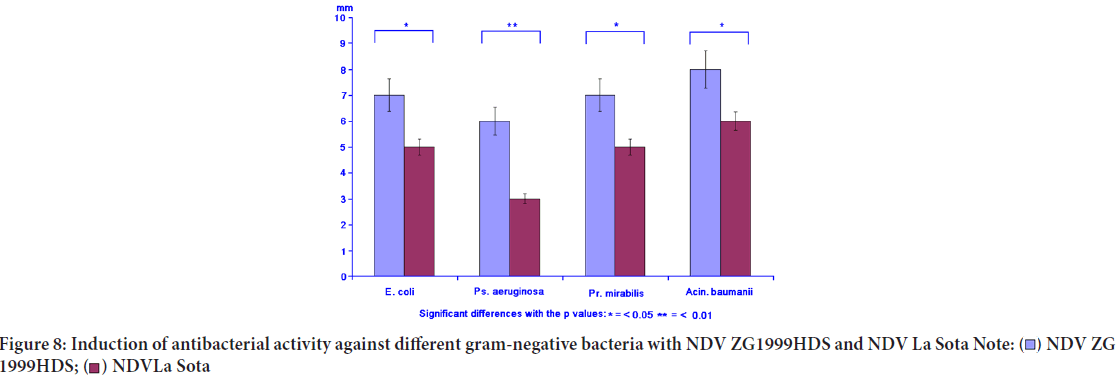
Antibacterial activity
Concomitantly with the immunomodulation, the induced antibacterial activity against different gram-positive and gram-negative bacteria was studied. The induced antibacterial activity was compared to the NDV La Sota. In general, it can be concluded that NDV ZG1999HDS induced higher antibacterial activity against gram-positive bacteria, than against gram-negative ones. It seems that such activity could be connected to the lysozyme content (Table 6, Figures 7 and 8).
| Gram-positive bacteria | NDV ZG1999HDS (mm) | NDV La Sota (mm) | Penicillin (mm) | Streptomycin (mm) | Gentamycin (mm) |
|---|---|---|---|---|---|
| Staphylococcus aureus | 9.0 ± 1.6 | 4.0 ± 0.5 | 8.0 ± 1.3 | 6.0 ± 0.3 | 8.0 ± 1.3 |
| MRSA | 14.0 ± 2.5 | 6.0 ± 0.3 | 4.0 ± 0.5 | 3.0 ± 0.3 | 13.0 ± 2.5 |
| Streptococcus pyogenes | 7.0 ± 1.4 | 4.0 ± 0.5 | 12.0 ± 2.5 | 14.0 ± 2.5 | 18.0 ± 3.2 |
| Streptococcus agalactiae | 7.0 ± 1.4 | 3.0 ± 0.3 | 8.0 ± 1.3 | 16.0 ± 2.5 | 14.0 ± 2.5 |
| Streptococcus mutants | 19.0 ± 2.5 | 13.0 ± 2.5 | 6.0 ± 0.3 | 12.0 ± 2.5 | 14.0 ± 2.5 |
| Gram-negative bacteria | |||||
| Escherichia coli | 7.0 ± 1.3 | 5.0 ± 0.5 | 8.0 ± 1.3 | 14.0 ± 2.5 | 18.0 ± 2.5 |
| Pseudomonas aeruginosa | 6.0 ± 0.5 | 3.0 ± 0.3 | 4.0 ± 0.3 | 7.0 ± 1.3 | 8.0 ± 1.3 |
| Proteus mirabilis | 7.0 ± 0.5 | 6.0 ± 0.5 | 5.0 ± 0.5 | 9.0 ± 1.3 | 8.0 ± 1.3 |
| Acinetobacter baumanii | 8.0 ± 0.7 | 6.0 ± 0.5 | 3.0 ± 0.3 | 18.0 ± 2.5 | 16.0 ± 2.5 |
Note: MRSA=Metycillin Resistant Staphylococcus aureus
Table 6: The induced antibacterial activity against various gram-positive and gram-negative bacteria by ZG1999HDS NDV strain or La Sota NDV strain
Figure 7: Induction of antibacterial activity against different gram-positive bacteria with the with one sign from the ZG1999HDS NDV strain or
NDV La Sota NDV strain 
Figure 8: Induction of antibacterial activity against different gram-negative bacteria with NDV ZG1999HDS and NDV La Sota 

Discussion
The results of the experiments comparing the immunomodulatory activity of low amount (10 ha/ml) of the ZG1999HDS or La Sota NDV strains in general show the priority of the ZG1999HDS NDV strain. At first, after the TLT cells activation the NO "burst", H2O2 and lysozyme level increased. After the binding of both NDV viruses separately to the TLT cells and their interaction with the PBMCs, the decrease of GM-CSF and increase of TNF-α and IFN-γ was observed. Concomitantly, a decrease in pro-inflammatory cytokines (IFN-α) and a differential increase in IL-1 α, IL-2 and IL-4 were observed. During the induction of the antibacterial response, it was 1/3 higher when it was induced by NDV ZG1999HDS against gram-positive bacteria (Staphylococcus aureus, MRSA,Streptococcus pyogenes, Streptococcus agalactiae and Streptococcus mutants). This effect was not so clear against gram-negative bacteria (Escherichia coli, Pseudomonas aeruginosa, Proteus mirabilis and Acinetobacter baumanii) (Figures 7 and 8). A possible cause could be the level of lysozyme induced by the ZG1999HDS NDV strain compared to La Sota. Although NDV causes direct oncolytic effects on tumour cells, it also has the ability to modulate the human immune system. Wolska K, et al., 2019, showed that cellular cytotoxicity of PBMC was enhanced significantly after co-incubation of NDV with the effectors cells. Throughout the study, NK cells were found to be the predominant mediator of lysis. Indeed, NDV was found tostimulate the host immune system to produce NO and cytokines, such as IFN-α, IFN-γ, TNF-α, and IL-1, which in turn leads to the activation of NK cells, macrophages, and sensitized T cells as it was shown by Avaki S, et al., 2004. Therefore, the activated NK cells are important contributors to innate defence against viral infections and by stimulating the secretion of cytokines, such as IL-2, IFN-γ, and TNF-α, further influence and activate the function of other immune cells related to cytolysis, thereby affecting the tumour cells.
Conclusions
The immunotherapy has raised the attention of scientists because it holds promise to be an attractive therapeutic strategy for treating different medical disorders. In this study, the immunomodulatory effects of low titres (10 ha/ml) of the ZG1999HDS NDV strain compared to La Sota on mixture of TLT cells with PBMC were analysed. The TLT activation by the NO "burst", H2O2 and lysozyme levels increased. After the binding to the TLT cells and their interaction with the PBMC, a decrease in GM-CSF and an increase in TNF-α and IFN-γ was observed. Concomitantly, the decrease in pro-inflammatory cytokines (IFN-α) and a selective increase in IL-1 α, IL-2 and IL-4 were observed. The antibacterial response was 1/3 higher when it was induced by the ZG1999HDS NDV strain against gram-positive bacteria (Staphylococcus aureus, MRSA,Streptococcus pyogenes, Streptococcus agalactiae and Streptococcus mutants). This effect was not so apparent against gram-negative bacteria (Escherichia coli, Pseudomonas aeruginosa, Proteus mirabilis and Acinetobacter baumanii). In general, the immunomodulatory effect of the ZG1999HDS NDV strain was stronger than that of the La Sota in vitro.
Declarations
Authors’ contributions
F.B., L.G. and H.M. prepared and performed the experiments and wrote the article text. A.P. performed the RP-HPLC analyses of different samples.
Funding
The research was performed in the frame of CIETO (Croatian Institute for Experimental and Translational Oncology, Koledinečka 03, 10040 Zagreb, Croatia). It was included into the Project: “Oncolytic Newcastle Disease Virus in the Veterinary Medicine.” The research was partially supported by Ivan Čermak and by Crodux, Kaptol 19, 10000 Zagreb, Croatia.
Acknowledgments
Authors are indebted to Slaven Kalebić for English suggestions.
References
- Ortuño-Sahagún D, Zänker K, Rawat AK, Kaveri SV, Hegde P. Natural immunomodulators.Immunol Res. 2017; 7529408.
[CrossRef] [Google Scholar] [Pubmed]
- Stanilova SA, Dobreva ZG, Slavov ES, Miteva LD. C3 binding glycoprotein from Cuscuta europea induced different cytokine profiles from human Peripheral Blood Mononuclear Cells (PBMC) compared to other plant and bacterial immunomodulators. Intern Immunopharm. 2005; 5(4): 723-734.
[CrossRef] [Google Scholar] [Pubmed]
- Utoh-Nedosa AU, Akah PA, Okoye TC, Okoli CO. Evaluation of the toxic effects of dihydroartemisinin on the vital organs of Wistar albino rats. Am J Pharmacol Toxicol. 2009; 4(4): 169-173.
- Tzianabos AO. Polysaccharide immunomodulators as therapeutic agents: Structural aspects and biologic function. Clin Microbiol Rev. 2000; 13(4): 523-533.
[CrossRef] [Google Scholar] [Pubmed]
- Ooi VE, Liu F. Immunomodulation and anti-cancer activity of polysaccharide-protein complexes. Curr Med Chem. 2000; 7(7): 715.
[CrossRef] [Google Scholar] [Pubmed]
- Kobayashi S, Sato R, Aoki T, Omoe K, Inanami O, Hankanga C, et al. Effect of bovine lactoferrin on functions of activated feline Peripheral Blood Mononuclear Cells (PBMC) during chronic feline immunodeficiency virus infection. J Vet Med Sci. 2008; 70(5): 429-435.
[CrossRef] [Google Scholar] [Pubmed]
- Schirrmacher V, Ahlert T, Pröbstle T, Steiner HH, Herold-Mende C, Gerhards R, et al. Immunization with virus-modified tumor cells. Sem Oncol. 1998; 25(6): 677-696.
[Google Scholar] [Pubmed]
- Schirrmacher V, Haas C, Bonifer R, Ahlert T, Gerhards R, Ertel C. Human tumor cell modification by virus infection: An efficient and safe way to produce cancer vaccine with pleiotropic immune stimulatory properties when using Newcastle Disease Virus. Gene Ther. 1999; 6(1): 63-73.
[CrossRef] [Google Scholar] [Pubmed]
- Schirrmacher V, Bai L, Umansky V, Yu LI, Xing YO, Qian ZH. Newcastle Disease Virus activates macrophages for anti-tumor activity. Int J Oncology. 2000; 16(2): 363-436.
[CrossRef] [Google Scholar] [Pubmed]
- Zorn U, Dallmann I, Große J, Kirchner H, Poliwoda H, Atzpodien J. Induction of cytokines and cytotoxicity against tumor cells by Newcastle Disease Virus. Cancer Biother Radiopharm. 1994; 9(3): 225-235.
[CrossRef] [Google Scholar] [Pubmed]
- Burke S, Shergold A, Elder MJ, Whitworth J, Cheng X, Jin H, et al. Oncolytic Newcastle Disease Virus activation of the innate immune response and priming of antitumor adaptive responses in vitro. Cancer Immunol Immunother. 2020; 69(6): 1015.
[CrossRef] [Google Scholar] [Pubmed]
- Mazija H, Gottstein Ž, Ivanković S, Čović D. Lentogenic cytolitic strain of the Newcastle Disease Virus isolated in Croatia. IX Poultry days, Proceedings, Poultry Centre Zagreb. 2011; 48-58.
- Mazija H. Pneumotropic lenthogenic strain of Newcastle Disease Virus ZG1999HDS isolated in Croatia. State intellectual property office. 2011.
- Nedeljković G. Genomic characterization and phylogenetic analysis of Newcastle Disease Virus isolate ZG1999HDS from the outbreak in 1999 in Croatia. 2011.
- Collection National de Cultures de Microorganismes (CNCM). Virus NDV (strain ZG1999HDS). CNCM. 2013.
- Biđin M, Mazija H. Immunogenicity of field strain of Newcastle Disease Virus ND Zg-2000 applied to SPF (Specific Pathogen Free) chickens. VIII Symposium of Poultry Days. 2009; 241-245.
- Ginting TE, Christian S, Larasati YO, Suryatenggara J, Suriapranata IM, Mathew G. Antiviral Interferons induced by Newcastle Disease Virus (NDV) drive a tumor-selective apoptosis. Sci Rep. 2019; 9(1): 1-10.
[CrossRef] [Google Scholar] [Pubmed]
- Antonelli G. Biological basis for a proper clinical application of alpha Interferons. New Microbiol. 2008; 31(3): 305.
[Google Scholar] [Pubmed]
- Orsi RO, Funari SR, Soares AM, Calvi SA, Oliveira SL, Sforcin JM, et al. Immunomodulatory action of propolis on macrophage activation. J Venom Anim Toxins. 2000; 6: 205-219.
- Nash JA, Ballard TN, Weaver TE, Akinbi HT. The peptidoglycan-degrading property of lysozyme is not required for bactericidal activity in vivo. J Immunol. 2006; 177(1): 519-526.
[CrossRef] [Google Scholar] [Pubmed]
- More P, Pai K. Immunomodulatory effects of Tinospora cordifolia (Guduchi) on macrophage activation. Biol Med. 2011; 3(2): 134-140.
- Punainen S, Veripavel UR, Tőlő HJ, Parkkinen J. Alpha Interferon manufacturing process using immunoadsorbtion and virus removal filtration. 1999.
- Lino A, Deogracious O. The in-vitro antibacterial activity of Annona senegalensis, Securidacca longipendiculata and Steganotaenia araliacea-Ugandan medicinal plants. Afr Health Sci. 2006; 6(1): 31-35.
[CrossRef] [Google Scholar] [Pubmed]
- Wolska K, Gorska A, Antosik K, Lugowska K. Immunomodulatory effects of propolis and its components on basic immune cell functions. Indian J Pharm Sci. 2019; 81(4): 575-588.
- Avki S, Turutoglu H, Simsek A, Unsal A. Clinical and immunological effects of Newcastle Disease Virus vaccine on bovine papillomatosis. Vet Immunol Immunopathol. 2004; 98(1-2): 9-16. [CrossRef]
[Google Scholar] [Pubmed]
Author Info
Bratko Filipič1*, Lidija Gradišnik2, Adriana Pereyra3 and Hrvoje Mazija12Department of Biomedical Sciences, Institute of Biomedical Sciences, University of Maribor, Maribor, Slovenia
3Department of Biomedical Sciences, MEDEX D.o.o. Company, Ljubljana, Slovenia
Citation: Filipi? B: The Immunomodulatory Activity In-vitro of NDV ZG1999HDS in Comparison to NDV La Sota
Received: 07-Feb-2022 Accepted: 21-Feb-2022 Published: 27-Feb-2023, DOI: 10.31858/0975-8453.13.2.108-115
Copyright: This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
ARTICLE TOOLS
- Dental Development between Assisted Reproductive Therapy (Art) and Natural Conceived Children: A Comparative Pilot Study Norzaiti Mohd Kenali, Naimah Hasanah Mohd Fathil, Norbasyirah Bohari, Ahmad Faisal Ismail, Roszaman Ramli SRP. 2020; 11(1): 01-06 » doi: 10.5530/srp.2020.1.01
- Psychometric properties of the World Health Organization Quality of life instrument, short form: Validity in the Vietnamese healthcare context Trung Quang Vo*, Bao Tran Thuy Tran, Ngan Thuy Nguyen, Tram ThiHuyen Nguyen, Thuy Phan Chung Tran SRP. 2020; 11(1): 14-22 » doi: 10.5530/srp.2019.1.3
- A Review of Pharmacoeconomics: the key to “Healthcare for All” Hasamnis AA, Patil SS, Shaik Imam, Narendiran K SRP. 2019; 10(1): s40-s42 » doi: 10.5530/srp.2019.1s.21
- Deuterium Depleted Water as an Adjuvant in Treatment of Cancer Anton Syroeshkin, Olga Levitskaya, Elena Uspenskaya, Tatiana Pleteneva, Daria Romaykina, Daria Ermakova SRP. 2019; 10(1): 112-117 » doi: 10.5530/srp.2019.1.19
- Dental Development between Assisted Reproductive Therapy (Art) and Natural Conceived Children: A Comparative Pilot Study Norzaiti Mohd Kenali, Naimah Hasanah Mohd Fathil, Norbasyirah Bohari, Ahmad Faisal Ismail, Roszaman Ramli SRP. 2020; 11(1): 01-06 » doi: 10.5530/srp.2020.1.01
- Manilkara zapota (L.) Royen Fruit Peel: A Phytochemical and Pharmacological Review Karle Pravin P, Dhawale Shashikant C SRP. 2019; 10(1): 11-14 » doi: 0.5530/srp.2019.1.2
- Pharmacognostic and Phytopharmacological Overview on Bombax ceiba Pankaj Haribhau Chaudhary, Mukund Ganeshrao Tawar SRP. 2019; 10(1): 20-25 » doi: 10.5530/srp.2019.1.4
- A Review of Pharmacoeconomics: the key to “Healthcare for All” Hasamnis AA, Patil SS, Shaik Imam, Narendiran K SRP. 2019; 10(1): s40-s42 » doi: 10.5530/srp.2019.1s.21
- A Prospective Review on Phyto-Pharmacological Aspects of Andrographis paniculata Govindraj Akilandeswari, Arumugam Vijaya Anand, Palanisamy Sampathkumar, Puthamohan Vinayaga Moorthi, Basavaraju Preethi SRP. 2019; 10(1): 15-19 » doi: 10.5530/srp.2019.1.3