Review Article - (2023) Volume 14, Issue 7
Abstract
The description of a scarce brain disease, including its clinical and pathological features, was first made by Alois Alzheimer over 100 years ago. Earlier it was the concept of dementia only before the discovery of the first case of Alzheimer’s disease. Senile plaque formation was recognized as important in the early 1900s, and further investigation was conducted in the 1990s after the identification of beta-amyloid protein. Studies of the brains of both non-demented and demented individuals using neuropathological techniques demonstrated the progression from normal aging to Alzheimer-type dementia. To understand disease progression diagnostic methods developed over 100 years till now. Numerous obstacles still need to be overcome to provide a definite diagnosis of dementia. These include the inconsistency of initial symptoms and their similarity to other disorders, as well as the potential occurrence of mixed etiologies in certain cases. This review aims to summarize all the development of diagnostic methods over the years along with their use.
Keywords
Alzheimer’s disease, Diagnostic methods, Dementia, Disease progression
Introduction
Diagnostic method development to detect early and accurately has evolved over the years for diagnosis of Alzheimer’s Disease (AD) and it has a major impact on research of disease progression as well as different treatment modalities (Khachaturian ZS, 1985).
Impairments in cognition, memory which are age-related, and changes in behaviors have been known and clinical pathological features of the disease, termed “Alzheimer’s Disease” (AD) (Sisodia SS, 1999). Alzheimer’s disease is a slowly progressive and irreversible neurological condition that gradually impairs memory, cognitive abilities, and basic task performance (Calabrò M, et al., 2021). It is the leading cause of dementia among the elderly population. In the early phase of disease evolution symptomatically it is difficult to extricate normal versus diseased elderly persons (Beck JC, et al., 1982). Due to its progressive nature AD development has divided into different phases:
Phase 1
This can be termed the pre-clinical or the asymptomatic phase, it will last for many years with or without diagnosis for the start of dementia. At this phase, it is observed mild memory loss and initial pathological changes in the cortex and hippocampus if identified.
Phase 2
The defining stage was a mild or initial phase of AD. Under this phase, observant symptoms start to be seen in patients like loss of memory, disorientation of things and situations, mood swings, and somewhat depression-like situation.
Phase 3
This is called as moderate AD phase. In this phase changes in the brain occur rapidly which leads to the memory-deficient condition, aggressive behavior, difficulty in speaking, writing, etc.
Phase 4
This phase is the prominent phase of the AD identifying stage which is called as severe AD. Changes in the brain become prominent like accumulation of plaques, neurofibrillary tangles, etc. in the cortex area. Patients become cognitively impaired and difficult or seem impossible to perform routine activities (Anwal L, et al., 2021).Literature Review
Background check on AD identification
To learn about Alzheimer’s disease it is required to understand dementia. Over 100 years evolution of diagnostic techniques has been done from dementia to AD. A collaborative study on neurosyphilis, a recognized contributor to dementia, was conducted by Otto Ludwig Binswanger (1852-1929), a Swiss researcher, along with Alois Alzheimer (1864-1915). He reported many forms of dementia in academia in 1894. The term, ‘presenile dementia’, was first identified in this report. A doctor from Germany named Emil Kraepelin explained dementia into senile and presenile in 1910. Later on, he named the disease ‘Alzheimer’s disease’ after Alois Alzheimer who identified a pathological condition in presenile dementia (Yang HD, et al., 2016).
Pathophysiology
Pathology of Alzheimer’s Disease (AD) on a macro level is the progressive damage of brain tissue. Over the year of disease progression, neurons perish in a form that supports the decline of memory and cognition slowly to a major extent (Morrison AS and Lyketsos C, 2005). Areas of the brain involved in the memory process are cortex and hippocampus. In the early phase of AD, neuronal loss has been observed and later on, cerebrospinal fluid takes up the space of the brain which was covered by the brain tissue (Fox NC, et al., 2001; Kaye JA, et al., 1997).
Mainly three neuropathologic hallmarks are involved in AD categorization which includes plaques of β-amyloid protein, neurofibrillary tangles, and neuronal deterioration (Meyer MA, 2004) (Figure 1).
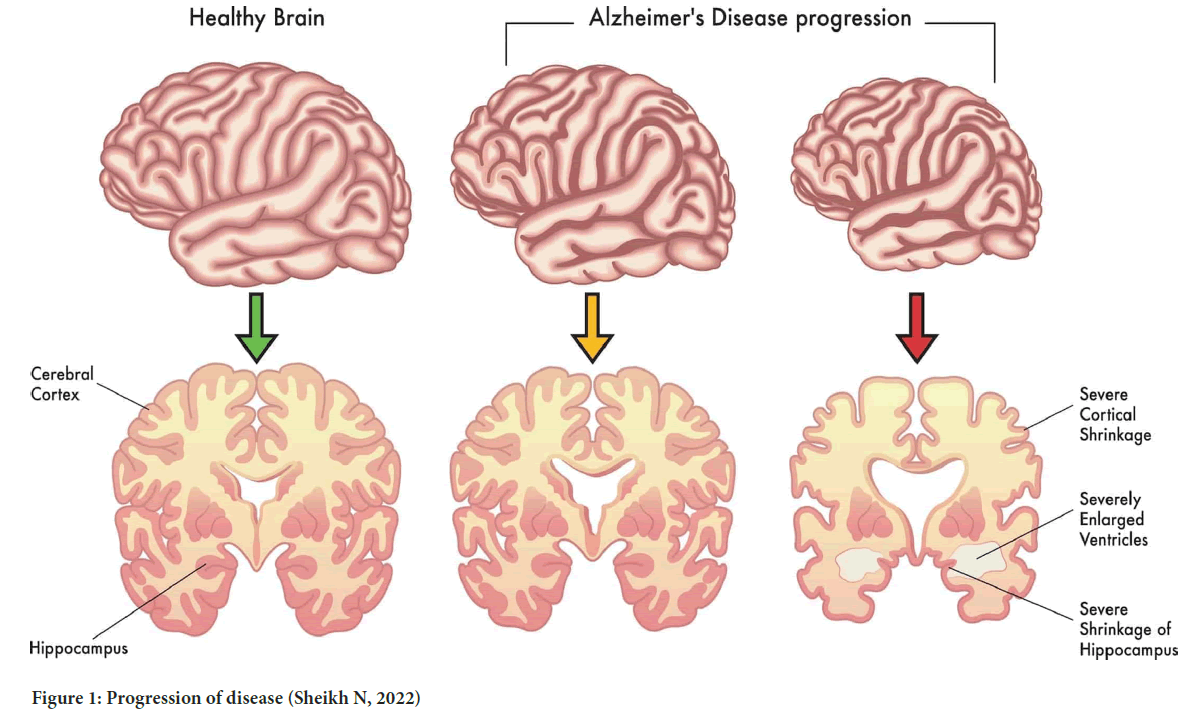
Figure 1: Progression of disease (Sheikh N, 2022)
Pathology of AD is intricate, there is the involvement of neurotransmitters and different pathologic pathways/hypothesis which helps in the identification of therapeutic targets (Morrison AS and Lyketsos C, 2005). To reach the target there should be an understanding of the diagnostic process.
AD pathology is divided into the different hypothesis that evaluates different aspects of disease progression, loss of neurons, imbalance of neurotransmitters, and genetic factors.
β-amyloid hypothesis (β-amyloid protein plaques formation): Amyloid plaque formation occurs when protein beta-amyloid accumulates. Aggregation of beta-Amyloid (Aβ) protein arises when Amyloid Precursor Protein (APP) is fragmented. Aβ is broken by APP by two enzymes which are β- and γ-secretase and upon excising Aβ it is removed from the cell. How- ever, in abnormal conditions degradation of Aβ declines which leads to the accumulation of Aβ peptides. Accumulation of Aβ develops neurotoxicity, neurodegeneration, and neuronal cell death (Kametani F and Hasegawa M, 2018) (Figure 2).
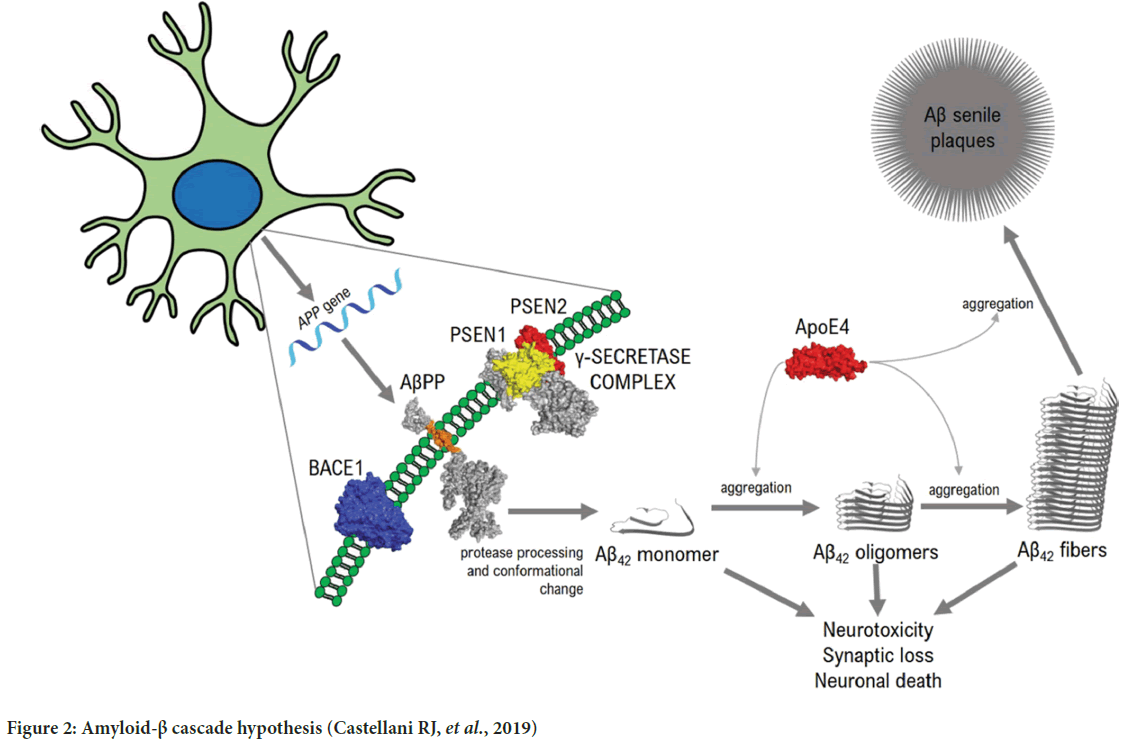
Figure 2: Amyloid-β cascade hypothesis (Castellani RJ, et al., 2019)
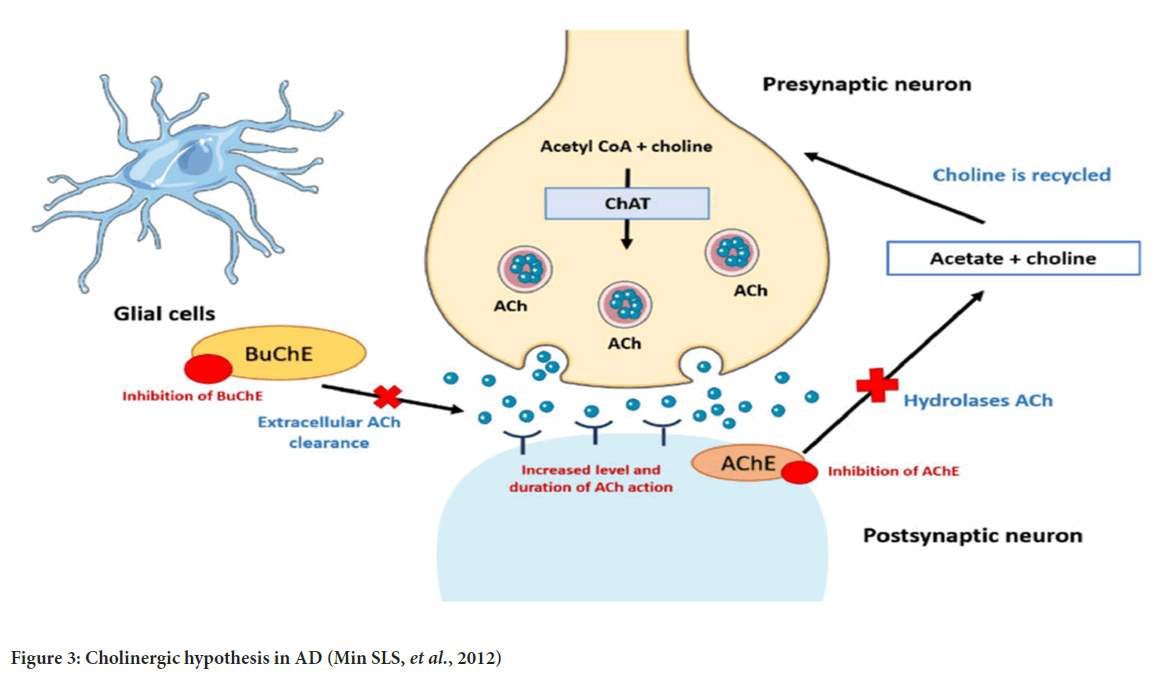
Cholinergic hypothesis: The degeneration of the cholinergic connection from the nucleus of Meynert to the cortex and hippocampus is often associated with Alzheimer’s disease (Contestabile A, 2011). In the brains of individuals with Alzheimer’s disease, there is often a decrease in the activity of choline Acetyltransferase (ChAT), which is responsible for synthesizing acetylcholine. This reduction is observed selectively in the hippocampus, a region that plays a key role in memory function, as well as in the cortex and amygdala. Observations of reduced activity in the Acetylcholinesterase (AChE) enzyme led to the hypothesis that dysfunction in the cholinergic system may be a contributing factor to the development of Alzheimer’s disease (Chen XQ and Mobley WC, 2019). Two types of postsynaptic receptors, muscarinic and nicotinic, are bound by acetylcholine. The influence of presynaptic nicotinic receptors on the release of neurotransmitters, including acetylcholine, glutamate, serotonin, and norepinephrine, is important for memory and mood, and these neurotransmitters have all been implicated in the pathology of Alzheimer’s disease (Morrison AS and Lyketsos C, 2005) (Figure 3).
Figure 3: Cholinergic hypothesis in AD (Min SLS, et al., 2012)
Oxidative stress theory: Age-related neurodegenerative conditions, such as Alzheimer’s Disease (AD), are characterized by an imbalance between oxidative radicals and antioxidant defenses. This imbalance can lead to cellular and molecular damage in various forms (Min SLS, et al., 2022; Zhu X, et al., 2005). Free radical damage can pose a particular threat to the Central Nervous System (CNS) due to the high oxygen consumption rate of the brain, its high lipid content, and the relatively low abundance of antioxidant enzymes compared to other tissues. Reactive Oxygen Species (ROS) include several types of molecules, such as oxygen free radicals (OH), hydrogen peroxide (H2O2), singlet oxygen (1O2), and hypochlorous acid (HOCl) (Markesbery WR, 1997). Increased production of reactive oxygen species, which is often observed in cases of dementia, can lead to excessive consumption of antioxidants. As a result, the capacity of the antioxidant system to protect the body against oxidative damage may be reduced. An excess of free radicals can lead to pathological changes associated with neurodegeneration (Padurariu M, et al., 2013).
Tau hypothesis: Tau, the microtubule‐associated protein, the protein tau has a normal role in regulating microtubules and is primarily concentrated in axons, but it is also present in dendrites (Arnsten AF, et al., 2021). In healthy brains, only 2-3 residues on the protein tau are phosphorylated. However, in conditions like Alzheimer’s disease and other tauopathies, the level of tau phosphorylation is significantly higher, with up to nine phosphates per molecule. This hyperphosphorylation can occur at various putative serine, threonine, and tyrosine residues, resulting from an imbalance in the activity of tau kinases and tau phosphatases. As a consequence, tau’s affinity for microtubules is reduced, leading to its eventual fibrillation and aggregation into neurofibrillary tangles (Medeiros R, et al., 2011) (Figure 4).
Figure 4: Tau phosphorylation (Gorantla NV and Chinnathambi S, 2018)
The clinical need for AD
Studies conducted in Europe indicate that the prevalence of dementia and AD among people aged 65+ years is 6.4% and 4.4%, respectively. In the United States, a national representation study found that the prevalence of AD is 9.7% among individuals aged >70 years. The overall prevalence of dementia in individuals aged 60+ years is 3.9%, with varying regional prevalence rates, such as 1.6% in Africa, 4.0% in China and the Western Pacific regions, 4.6% in Latin America, 5.4% in Western Europe, and 6.4% in North America. Approximately 25 million people worldwide currently suffer from dementia, predominantly AD, and this number is projected to double in the next 20 years (Qiu C, et al., 2022).
According to the Dementia India Report 2010 by the Alzheimer’s and Related Disorders Society of India (ARDSI), about 3.7 million Indians had dementia in 2010, and this number is expected to rise to 7.6 million by 2030 (Brahma D, 2019).
History of Alzheimer’s diagnosis over the years
Dr. Alois Alzheimer discovered the disease that now bears his name. In 1906, while studying the brain tissue of a deceased woman who exhibited symptoms of memory loss, language problems, and unpredictable behavior, he observed significant changes in her brain tissue. The term ‘Alzheimer’s disease’ was first introduced by Dr. Kraepelin in his eighth edition of Handbook of Psychiatry in 1910 as a distinct disease entity with specific clinical and pathological features (Ryan NS, et al., 2015).
Around 1900s: During the 1900s, the diagnosis of AD was only possible based on symptomatic observations and post-mortem brain autopsies of patients.
Alzheimer’s detailed the pathology of senile plaques and neurofibrillary tangles in 1911. He used the Bielshowsky silver method to illustrate the process of neurofibrillary tangle formation in the AD brain, which included extracellular tangles infiltrated by glial processes (Yamaguchi H, 2007).
Bielshowsky silver stain: To stain neurofibrils, researchers use the Bielschowsky method which involves silver nitrate sensitization and secondary impregnation with an ammoniacal silver solution. The silver deposited on Neurofibrillary Tangles (NFT) and axonal or dendritic processes of senile plaques can be reduced to visible metallic silver by formalin. This results in the appearance of black neurofibrils, NFT, and neuronal processes (LabCE, 2022).
Different researchers in 1910, 1912, 1915, etc. examined the cases of AD and demonstrated senile dementia with an abundance of senile plaques and tangles formation. Illustration of this formation in brain tissue was determined using Bielshowsky silver.
During the 1960s, researchers used electron microscopy to conduct ultra-structural studies. Through this method, they were able to identify the precise structure of plaques, which consisted of amyloid fibrils (measuring 8-10 nm in diameter), swollen neuritis, and glial proliferation. Additionally, neurofibrillary tangles were found to be composed of paired helical filaments or straight tubules (measuring 10-20 nm) (Terry RD, et al. 1964).
Contextual on microscopy: The Berlin Technische Hochschule scientists Max Knoll and Ernst Ruska developed the electron microscope in 1931, which ultimately overcame the restrictions of visible light and allowed for higher-resolution imaging. During the period spanning from the 1960s to the 1990s, numerous advancements in technology and methodologies emerged. One such development was the availability of the first Scanning Electron Microscope (SEMs) for commercial use, which was used to examine brain tissues to understand AD pathology (Palucka T, 2002).
In the 1980’s neuropsychological examinations were developed to identify AD: Alzheimer’s disease is known to cause significant deficits in the cognitive processes that are essential for everyday functioning and to identify those deficits psychological evaluations evolved. The assessment of an individual’s orientation to time and place is commonly conducted using a standardized test, such as the Mini-Mental State Examination, which can help determine the extent of cognitive impairment (McKhann G, et al., 1984).
Mini-Mental State Examination (MMSE): Despite the passage of time, the Mini-Mental State Examination (MMSE) continues to be a widely-used and efficient tool for evaluating mental status, thanks to its ease of administration and scoring, with a typical duration of around 10 minutes. Additionally, the availability of authorized translated versions of the MMSE in multiple languages makes it a versatile tool for global use. With a maximum score of 30, a result of 23 or less on the assessment typically indicates impairment in cognitive functioning. The Mini-Mental State Examination (MMSE) evaluates various cognitive domains, such as orientation (with a total of 10 points), the ability to register three words (3 points), attention (5 points), recall of the registered words (3 points), language (8 points), and visuoconstruction (1 point). A decline of approximately 3 points per year on the Mini-Mental State Examination (MMSE) is typically observed in individuals with Alzheimer’s disease. However, as low MMSE scores can also result from non-neurological factors such as limited education, language difficulties, and sensory impairments, the test is not used as the sole criterion for dementia diagnosis. Instead, the MMSE score is considered along with the patient’s clinical history, neurological examination, and other neuropsychological tests to arrive at an accurate diagnosis (Bernard BA and Goldman JG, 2010).
To assess neuropsychologically different criteria are set having compatibility with the current Diagnostic and Statistical Manual of Mental Disorders (DSM V). It is important to recognize that the criteria used in the diagnosis of Alzheimer’s disease are not absolute and may evolve. To validate the accuracy of these criteria, further long-term studies that are corroborated by autopsy findings are required (Palucka T, 2002).
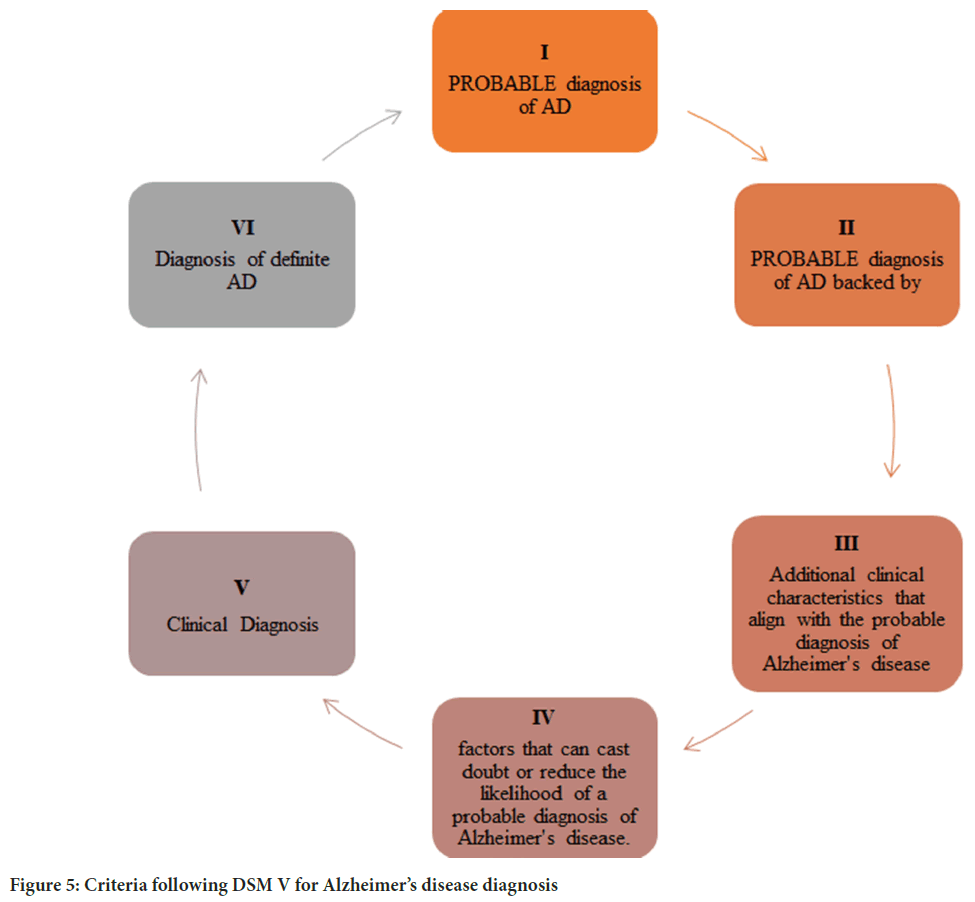
Criteria following Diagnostic and statistical manual of mental disorders (DSM-V) for Alzheimer’s disease diagnosis: In that era, Electrophysiologic methods were introduced to analyze brain activity (Figure 5). Electroencephalography (EEG) is done to measure the electrical activity of the brain. The presence of heightened slow-wave activity has been observed in individuals with Alzheimer’s disease, and this phenomenon may intensify as the condition advances. There is a significant relationship between the degree of EEG abnormalities and cognitive impairment, as evidenced by research findings. According to the prevailing view, the initial alterations in EEG activity associated with Alzheimer’s disease involve heightened theta activity and reduced beta activity. Subsequently, a decline in alpha activity follows, and later in the disease progression, there is an increase in delta activity. Individuals with advanced dementia typically display decreased alpha activity and increased delta activity (Jeong J, 2004).
Figure 5: Criteria following DSM V for Alzheimer’s disease diagnosis
The evolution of ultrastructural methods supports to provide brain imaging and the identification of variable brain injuries or damage.
Computerized Tomography (CT): The use of this technique allows for the exclusion of other conditions such as subdural hematoma, brain tumor, hydrocephalus, and vascular dementia. Furthermore, it enables the visualization and measurement of brain structures such as gyri, sulci, tissue densities, ventricular size, and CSF volume. In Alzheimer’s disease, there is often an enlargement of the ventricular system and the third ventricle, along with a narrowing of gyri and widening of sulci. However, these broad patterns may not be sufficient as standalone diagnostic criteria for individual cases.
Positron Emission Tomography (PET): Positron Emission Tomography (PET) is a research tool that permits the quantitative evaluation of glucose utilization, oxygen consumption, and regional Cerebral Blood Flow (rCBF). Brain glucose metabolism can be monitored using the glucose analog 2-[18F]-fluoro-2-deoxy-D-glucose (18F-FDG). Studies have indicated that a gradual decline in glucose metabolism, as detected by 18F-FDG PET, can occur several years before the onset of clinical symptoms in individuals with confirmed Alzheimer’s disease pathology (Nordberg A, et al., 2010).
Magnetic Resonance Imaging (MRI): MRI is capable of measuring brain morphometry, allowing for the detection of gray matter atrophy associated with neuronal loss, synapse depletion, and dendritic de-arborization that occur on a microscopic scale in Alzheimer’s disease. It can also detect white matter atrophy linked to the deterioration of the structural integrity of white matter tracts, which is likely due to demyelination and dying back of axonal processes, as well as the ex-vacuo expansion of Cerebrospinal Fluid (CSF) spaces.
1990s phase: In this phase of a diagnostic evaluation of AD took a turn for improvement and assessment of regional cerebral blood flow, body fluids testing/non-neural tissues testing started (McKhann G, et al., 1984).
Genetic exploration: A specific genetic mutation in the Amyloid Precursor Protein (APP) leads to large amounts of Aβ42 protein deposition in the brain. This mutation involves the substitution of glutamic acid with glutamine at position 693 of the APP protein sequence, concerning the longest form of APP, which is known as APP-770. This alteration affects how APP is processed by enzymes called secretases, ultimately leading to the accumulation of Aβ42 protein and the development of amyloid plaques in the brain (Hardy JA and Higgins GA, 1992). The occurrence of hereditary, early-onset Alzheimer’s disease is linked to mutations in the COOH-terminal portion of the Amyloid Precursor Protein (APP). These mutations affect the production of the Aβ42 protein, which can build up in the brain and lead to the formation of amyloid plaques. The inheritance of these mutations follows an autosomal dominant pattern, which means that only one copy of the mutated gene from one parent is enough to cause the disease. While these mutations are rare, they are known to be a significant cause of early-onset Alzheimer’s disease, which typically affects individuals under the age of 65 (Toney MF, et al., 1994).
Analysis of the APP gene was done with the help of Polymerase Chain Reaction (PCR) direct sequencing with intronic primers. PCR technique started in the use of identifying the altered gene or the genetic changes causing AD.
Polymerase Chain Reaction (PCR): It is a laboratory technique used to produce specific DNA sequences in vitro. This method employs two short, synthetic DNA primers that bind to complementary strands and bracket the region of interest within the target DNA molecule. By using PCR, researchers can amplify DNA sequences from a tiny amount of starting material, generating enough copies for downstream analysis and manipulation (Erlich HA, 1989). Back then the process was not that simplified and specificity (amplification of target) was less compared to today’s evolution.
Cerebrospinal Fluid (CSF) testing: In definite AD patients a relation of tau concentration in brain CSF. CSF sample was taken from the lumbar region and analyzed by the Enzyme-Linked Immunosorbent Assay (ELISA). Tau levels in the CSF are higher in the case of definite AD patients (Tapiola T, et al., 1997).
Since the initial identification of Alzheimer’s disease, various diagnostic methods have been developed over time. These methods have become more accurate as technology has improved. From the early 1900s through the 1990s, many diagnostic methods were developed, but conclusive results for Alzheimer’s disease diagnosis were not definitive. However, since the 2000s, more advanced diagnostic methods have emerged, which have helped to identify and target specific areas for Alzheimer’s disease treatment.
After 2000: The identification of Alzheimer’s disease requires the use of several biomarkers, including structural Magnetic Resonance Imaging (MRI), genetic markers, and analysis of clinical and biological samples. By combining these different approaches, researchers and clinicians can more accurately detect and diagnose Alzheimer’s disease. Apart from modification of the above-mentioned techniques and methods, there are some newly identified methods for the diagnosis.
Modification of bielschowsky silver stain: To reduce the high background staining commonly observed in silver nitrate staining, it is recommended to pretreat the sample with oxidizing agents before the initial incubation. This pretreatment step effectively eliminates artifacts that may arise during the staining procedure, resulting in a clearer and more accurate visualization of the target molecules (Intorcia AJ, et al., 2019).
Fluid Attenuated Inversion Recovery (FLAIR): Flare images, also known as Fluid-Attenuated Inversion Recovery (FLAIR) images, are a type of diagnostic tool used to detect Alzheimer’s disease. This technique can reveal the presence of abnormal brain lesions or lesions that interfere with the normal role of cerebrospinal fluid in the brain, providing valuable information for Alzheimer’s disease detection and diagnosis.
Diffusion Tensor Imaging (DTI): Diffusion Tensor Imaging (DTI) is a technique that employs isotropic diffusion to analyze the structure of the brain’s white matter. By measuring the diffusivity of water molecules in the tissue, DTI can help identify the fiber bundles affected in the brain regions of Alzheimer’s disease patients. This technique has proven a useful tool for investigating the changes in white matter microstructure associated with Alzheimer’s disease (Shukla A, et al., 2023).
Blood-based biomarkers: While only small amounts of brain proteins or substances can cross the blood-brain barrier and enter the bloodstream, it is still possible to detect specific biomarkers in the blood that originate exclusively from the brain or systemic pathologies. This phenomenon can provide valuable insights into the pathophysiology of neurological diseases and may facilitate earlier detection and treatment. Several studies have explored blood-based levels of Tau, a biomarker for Alzheimer’s disease, and have observed elevated levels in the plasma of AD patients. This finding suggests that blood-based Tau levels may serve as a useful diagnostic tool for detecting Alzheimer’s disease (Ausó E, et al., 2020).
Many practical approaches have evolved for the diagnosis of symptomatic AD features. To initiate the diagnostic process, gather all pertinent medical and clinical information systematically. This step involves a comprehensive evaluation of the patient’s medical history, along with a thorough physical examination and assessment of any relevant clinical data.
DemTect: The DemTect assessment tool features a ten-word list for delayed recall, which is longer than the list used in the MMSE, and avoids direct questions related to time and place. This moderately time-consuming test can be completed within 8 to 12 minutes and does not include a drawing task. Its sensitivity is noteworthy, with a validation study demonstrating an 85% sensitivity (with a cutoff of 13 points) for mild cognitive impairment and an 83% sensitivity (with a cutoff of 11 points) for Alzheimer’s dementia (Eschweiler GW, et al., 2010; Turner RS, et al., 2020).
Discussion and Conclusion
The clinical diagnosis of AD relies on a comprehensive evaluation of clinical signs and symptoms, including a detailed medical history, neurological and psychiatric assessments, and laboratory tests that analyze blood and other biological fluids. Additional diagnostic procedures may include neuropsychological testing, brain neuroimaging, and genetic testing. Brain function and physiology can be assessed through various techniques, such as Positron Emission Tomography (PET), single photon emission computed tomography, Magnetic Resonance Imaging (MRI), and blood biomarkers.
The onset of dementia in Alzheimer’s disease occurs several years after the initial presentation of the disease in the individual. Over 100 years of Alzheimer’s disease, the diagnostic approach has been changed, evolved, and modified. The diagnostic path requires modification to establish early detection of AD. Recent developments in early detection techniques have enabled the identification of Alzheimer’s disease in certain individuals with Mild Cognitive Impairment (MCI) before the onset of dementia. Innovative biomarkers for diagnosis, especially those that are affordable and non-intrusive, have the potential to significantly enhance the current practice in terms of screening, prognosis, accurate provisional diagnosis, and assessing the effectiveness of novel treatments being developed for dementia, including Alzheimer’s disease.
References
- Khachaturian ZS. Diagnosis of Alzheimer's disease. Arch Neurol. 1985; 42(11): 1097-1105.
[Crossref] [Google Scholar] [Pubmed]
- Sisodia SS. Series introduction: Alzheimer’s disease: Perspectives for the new millennium. J Clin Invest. 1999; 104(9): 1169-1170.
[Crossref] [Google Scholar] [Pubmed]
- Calabrò M, Rinaldi C, Santoro G, Crisafulli C. The biological pathways of Alzheimer disease: A review. AIMS Neurosci. 2021; 8(1): 86-132.
[Crossref] [Google Scholar] [Pubmed]
- Beck JC, Benson DF, Scheibel AB, Spar JE, Rubenstein LZ. Dementia in the elderly: The silent epidemic. Ann Intern Med. 1982; 97(2): 231-241.
[Crossref] [Google Scholar] [Pubmed]
- Anwal L, Chandakavate S, Lalitha S, Thilagasundari MK. A comprehensive review on alzheimer’s disease. World J Pharm Pharm Sci. 2021; 10(7): 1170.
- Yang HD, Lee SB, Young LD. History of Alzheimer's disease. Dement Neurocogn Disord. 2016; 15(4): 115-121.
[Crossref] [Google Scholar] [Pubmed]
- Morrison AS, Lyketsos C. The pathophysiology of Alzheimer’s disease and directions in treatment. Adv Stud Nurs. 2005; 3(8): 256-270.
- Fox NC, Crum WR, Scahill RI, Stevens JM, Janssen JC, Rossor MN. Imaging of onset and progression of Alzheimer's disease with voxel-compression mapping of serial magnetic resonance images. Lancet. 2001; 358(9277): 201-205.
[Crossref] [Google Scholar] [Pubmed]
- Kaye JA, Swihart T, Howieson D, Dame A, Moore MM, Karnos T, et al. Volume loss of the hippocampus and temporal lobe in healthy elderly persons destined to develop dementia. Neurology. 1997; 48(5): 1297-1304.
[Crossref] [Google Scholar] [Pubmed]
- Meyer MA. Drug therapy in Alzheimer's disease. N Engl J Med. 2004; 351(18): 1911-1913.
[Crossref] [Google Scholar] [Pubmed]
- Sheikh N. Advanced stages of Alzheimer's: Understanding the stages. Altoida. 2022.
- Kametani F, Hasegawa M. Reconsideration of amyloid hypothesis and tau hypothesis in Alzheimer's disease. Front Neurosci. 2018; 12: 25.
[Crossref] [Google Scholar] [Pubmed]
- Castellani RJ, Plascencia-Villa G, Perry G. The amyloid cascade and Alzheimer's disease therapeutics: Theory versus observation. Lab Invest. 2019; 99(7): 958-970.
[Crossref] [Google Scholar] [Pubmed]
- Contestabile A. The history of the cholinergic hypothesis. Behav Brain Res. 2011; 221(2): 334-340.
[Crossref] [Google Scholar] [Pubmed]
- Chen XQ, Mobley WC. Exploring the pathogenesis of Alzheimer disease in basal forebrain cholinergic neurons: Converging insights from alternative hypotheses. Front Neurosci. 2019; 13: 446.
[Crossref] [Google Scholar] [Pubmed]
- Min SLS, Liew SY, Chear NJ, Goh BH, Tan WN, Khaw KY. Plant terpenoids as the promising source of cholinesterase inhibitors for anti-AD therapy. Biology. 2022; 11(2): 307.
[Crossref] [Google Scholar] [Pubmed]
- Zhu X, Lee HG, Casadesus G, Avila J, Drew K, Perry G, Smith MA. Oxidative imbalance in Alzheimer’s disease. Mol Neurobiol. 2005; 31: 205-217.
[Crossref] [Google Scholar] [Pubmed]
- Markesbery WR. Oxidative stress hypothesis in Alzheimer's disease. Free Radic Biol Med. 1997; 23(1): 134-147.
[Crossref] [Google Scholar] [Pubmed]
- Padurariu M, Ciobica A, Lefter R, Serban IL, Stefanescu C, Chirita R. The oxidative stress hypothesis in Alzheimer’s disease. Psychiatr Danub. 2013; 25(4): 401-409.
[Google Scholar] [Pubmed]
- Arnsten AF, Datta D, Tredici KD, Braak H. Hypothesis: Tau pathology is an initiating factor in sporadic Alzheimer's disease. Alzheimers Dement. 2021; 17(1): 115-124.
[Crossref] [Google Scholar] [Pubmed]
- Medeiros R, Baglietto‐Vargas D, LaFerla FM. The role of tau in Alzheimer's disease and related disorders. CNS Neurosci Ther. 2011; 17(5): 514-524.
[Crossref] [Google Scholar] [Pubmed]
- Gorantla NV, Chinnathambi S. Tau protein squired by molecular chaperones during Alzheimer’s disease. J Mol Neurosci. 2018; 66(3): 356-368.
[Crossref] [Google Scholar] [Pubmed]
- Qiu C, Kivipelto M, von Strauss E. Epidemiology of Alzheimer's disease: Occurrence, determinants, and strategies toward intervention. Dialogues Clin Neurosci. 2022; 11(2): 111-128.
[Crossref] [Google Scholar] [Pubmed]
- Brahma D. The persistence of memory: The burden of Alzheimer’s disease in India. Brookings. 2019.
- Ryan NS, Rossor MN, Fox NC. Alzheimer’s disease in the 100 years since Alzheimer’s death. Brain. 2015; 138(12): 3816-3821.
[Crossref] [Google Scholar] [Pubmed]
- Yamaguchi H. Alzheimer pathology during the past 100 years. Psychogeriatrics. 2007; 7(3): 109-113.
- LabCE. Bielschowsky silver staining-Chemistry. LabCE. 2022.
- Terry RD, Gonatas NK, Weiss M. Ultrastructural studies in Alzheimer's presenile dementia. Am J Pathol. 1964; 44(2): 269-297.
[Google Scholar] [Pubmed]
- Palucka T. Overview of electron microscopy. History of Science and Technology. 2002.
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: Report of the NINCDS‐ADRDA Work Group* under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984; 34(7): 939-944.
[Crossref] [Google Scholar] [Pubmed]
- Bernard BA, Goldman JG. MMSE-Mini-Mental State Examination. Encyclopedia of movement disorders. 2010: 187-189.
- Jeong J. EEG dynamics in patients with Alzheimer's disease. Clin Neurophysiol. 2004; 115(7): 1490-1505.
[Crossref] [Google Scholar] [Pubmed]
- Nordberg A, Rinne JO, Kadir A, Långström B. The use of PET in Alzheimer disease. Nat Rev Neurol. 2010; 6(2): 78-87.
[Crossref] [Google Scholar] [Pubmed]
- Hardy JA, Higgins GA. Alzheimer's disease: The amyloid cascade hypothesis. Science. 1992; 256(5054): 184-185.
[Crossref] [Google Scholar] [Pubmed]
- Toney MF, Howard JN, Richer J, Borges GL, Gordon JG, Melroy OR, et al. Voltage-dependent ordering of water molecules at an electrode-electrolyte interface. Nature. 1994; 368(6470): 444-446.
- Erlich HA. Polymerase chain reaction. J Clin Immunol. 1989; 9(6): 437-447.
[Crossref] [Google Scholar] [Pubmed]
- Tapiola T, Overmyer M, Lehtovirta M, Helisalmi S, Ramberg J, Alafuzoff I, et al. The level of cerebrospinal fluid tau correlates with neurofibrillary tangles in Alzheimer's disease. Neuroreport. 1997; 8(18): 3961-3963.
[Crossref] [Google Scholar] [Pubmed]
- Intorcia AJ, Filon JR, Hoffman B, Serrano GE, Sue LI, Beach TG. A modification of the Bielschowsky silver stain for Alzheimer neuritic plaques: Suppression of artifactual staining by pretreatment with oxidizing agents. BioRxiv. 2019: 570093.
- Shukla A, Tiwari R, Tiwari S. Review on alzheimer disease detection methods: Automatic pipelines and machine learning techniques. Sci. 2023; 5(1): 13.
- Ausó E, Gómez-Vicente V, Esquiva G. Biomarkers for Alzheimer’s disease early diagnosis. J Pers Med. 2020; 10(3): 114.
[Crossref] [Google Scholar] [Pubmed]
- Eschweiler GW, Leyhe T, Klöppel S, Hüll M. New developments in the diagnosis of dementia. Dtsch Arztebl Int. 2010; 107(39): 677-683.
[Crossref] [Google Scholar] [Pubmed]
- Turner RS, Stubbs T, Davies DA, Albensi BC. Potential new approaches for diagnosis of Alzheimer's disease and related dementias. Front Neurol. 2020; 11: 496.
[Crossref] [Google Scholar] [Pubmed]
Author Info
Ashima Sharma*Citation: Sharma A: Understanding the Diagnostic Evolution of Alzheimer’s Disease Over the Phase of 100 Years
Received: 26-Jun-2023 Accepted: 21-Jul-2023 Published: 28-Jul-2023, DOI: 10.31858/0975-8453.14.7.457-464
Copyright: This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
ARTICLE TOOLS
- Dental Development between Assisted Reproductive Therapy (Art) and Natural Conceived Children: A Comparative Pilot Study Norzaiti Mohd Kenali, Naimah Hasanah Mohd Fathil, Norbasyirah Bohari, Ahmad Faisal Ismail, Roszaman Ramli SRP. 2020; 11(1): 01-06 » doi: 10.5530/srp.2020.1.01
- Psychometric properties of the World Health Organization Quality of life instrument, short form: Validity in the Vietnamese healthcare context Trung Quang Vo*, Bao Tran Thuy Tran, Ngan Thuy Nguyen, Tram ThiHuyen Nguyen, Thuy Phan Chung Tran SRP. 2020; 11(1): 14-22 » doi: 10.5530/srp.2019.1.3
- A Review of Pharmacoeconomics: the key to “Healthcare for All” Hasamnis AA, Patil SS, Shaik Imam, Narendiran K SRP. 2019; 10(1): s40-s42 » doi: 10.5530/srp.2019.1s.21
- Deuterium Depleted Water as an Adjuvant in Treatment of Cancer Anton Syroeshkin, Olga Levitskaya, Elena Uspenskaya, Tatiana Pleteneva, Daria Romaykina, Daria Ermakova SRP. 2019; 10(1): 112-117 » doi: 10.5530/srp.2019.1.19
- Dental Development between Assisted Reproductive Therapy (Art) and Natural Conceived Children: A Comparative Pilot Study Norzaiti Mohd Kenali, Naimah Hasanah Mohd Fathil, Norbasyirah Bohari, Ahmad Faisal Ismail, Roszaman Ramli SRP. 2020; 11(1): 01-06 » doi: 10.5530/srp.2020.1.01
- Manilkara zapota (L.) Royen Fruit Peel: A Phytochemical and Pharmacological Review Karle Pravin P, Dhawale Shashikant C SRP. 2019; 10(1): 11-14 » doi: 0.5530/srp.2019.1.2
- Pharmacognostic and Phytopharmacological Overview on Bombax ceiba Pankaj Haribhau Chaudhary, Mukund Ganeshrao Tawar SRP. 2019; 10(1): 20-25 » doi: 10.5530/srp.2019.1.4
- A Review of Pharmacoeconomics: the key to “Healthcare for All” Hasamnis AA, Patil SS, Shaik Imam, Narendiran K SRP. 2019; 10(1): s40-s42 » doi: 10.5530/srp.2019.1s.21
- A Prospective Review on Phyto-Pharmacological Aspects of Andrographis paniculata Govindraj Akilandeswari, Arumugam Vijaya Anand, Palanisamy Sampathkumar, Puthamohan Vinayaga Moorthi, Basavaraju Preethi SRP. 2019; 10(1): 15-19 » doi: 10.5530/srp.2019.1.3